Recent Trends in the Pre-Drying, Drying, and Post-Drying Processes for Cassava Tuber: A Review
Abstract
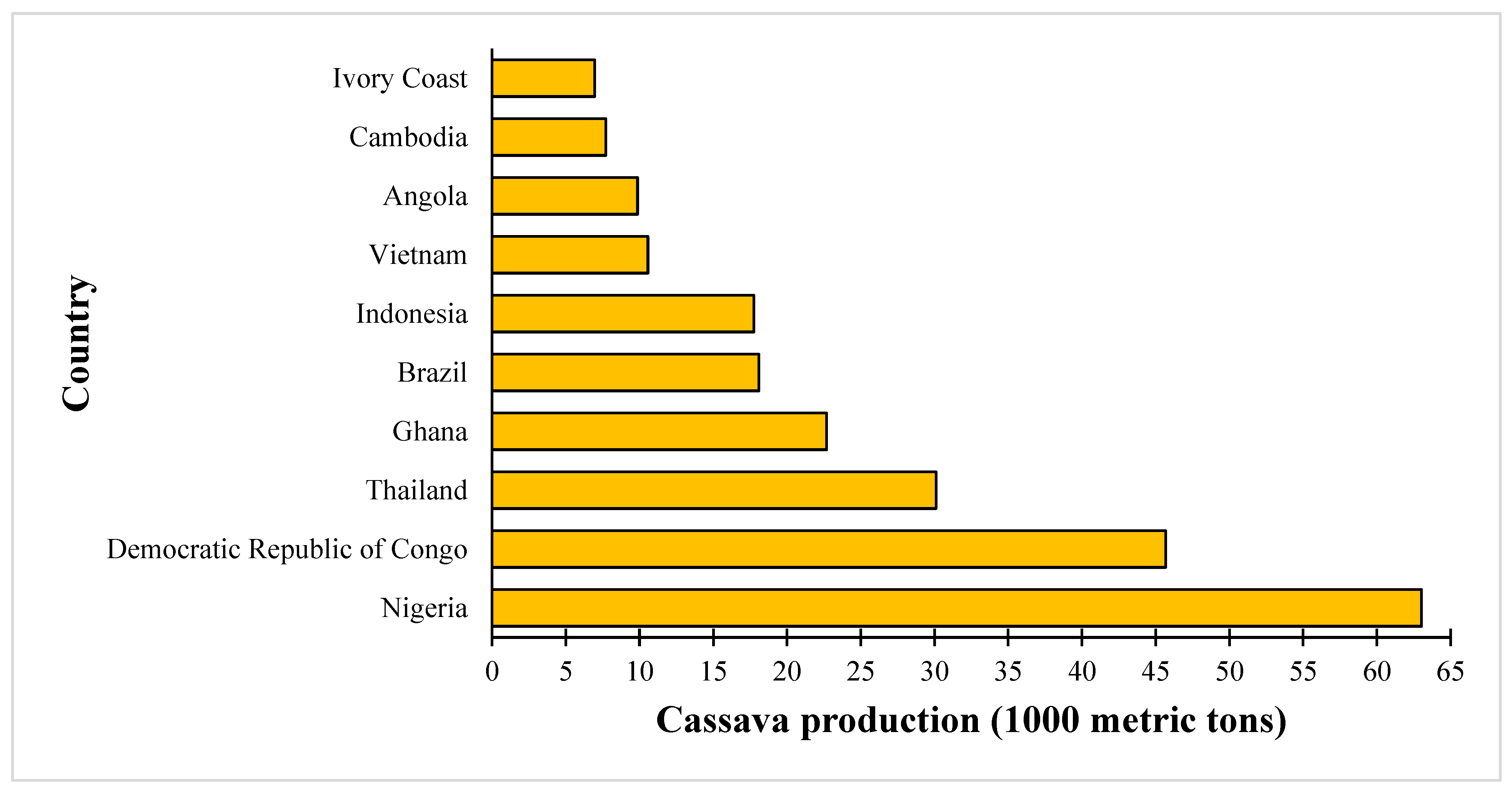
1. Introduction
2. Developments in Cassava Tuber Pre-Drying and Drying Research
3. Advancements in the Research of Pre-Drying Techniques for Cassava Tubers
3.1. Hot Water Blanching
3.2. Steam Blanching
3.3. Sulfite Solution
3.4. Acid Solution
3.5. Ultrasonic Field
3.6. Alternative Prospective Pre-Drying Techniques for Cassava Tubers
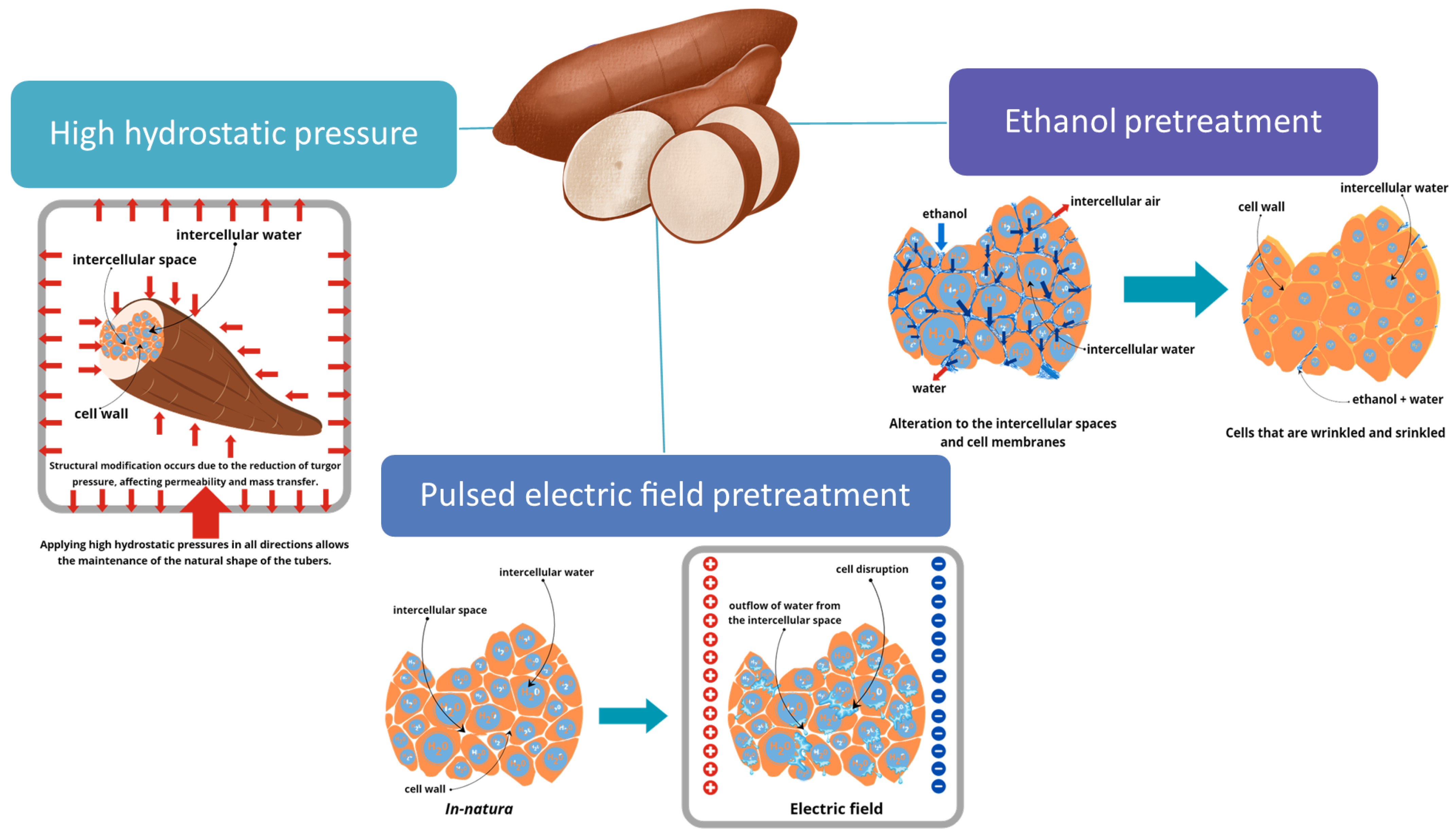
3.6.1. Ethanol Solution
3.6.2. Pulsed Electric Field
3.6.3. High Hydrostatic Pressure
4. Advancements in the Research of Drying Methods for Cassava Tubers
4.1. Sun Drying
4.2. Solar Drying
4.3. Hot Air Oven Drying
4.4. Vacuum Drying
4.5. Freeze Drying
4.6. Fluidized Bed Drying
4.7. Solar-Assisted Drying
4.8. Microwave-Assisted Drying
4.9. Alternative Prospective Drying Methods for Cassava Tubers
4.9.1. Infrared-Assisted Drying
4.9.2. Ultrasound-Assisted Drying
4.9.3. Refractance Window Drying
5. Post-Drying Operations for Dried Cassava Tubers
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Crops and Livestock Products. In Statistics Division Food and Agriculture Organization of the United Nations; FAOSTAT: Rome, Italy, 2021; Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 10 October 2023).
- Parmar, A.; Sturm, B.; Hensel, O. Crops That Feed the World: Production and Improvement of Cassava for Food, Feed, and Industrial Uses. Food Secur. 2017, 9, 907–927. [Google Scholar] [CrossRef]
- Market Data Forecast. Cassava Starch Market Size, Share, Growth 2023–2028. Available online: https://www.marketdataforecast.com/market-reports/cassava-flour-market (accessed on 27 September 2023).
- Iyer, S.; Mattinson, D.S.; Fellman, J.K. Study of the Early Events Leading to Cassava Root Postharvest Deterioration. Trop. Plant Biol. 2010, 3, 151–165. [Google Scholar] [CrossRef]
- Li, S.; Cui, Y.; Zhou, Y.; Luo, Z.; Liu, J.; Zhao, M. The Industrial Applications of Cassava: Current Status, Opportunities and Prospects. J. Sci. Food Agric. 2017, 97, 2282–2290. [Google Scholar] [CrossRef]
- Oliveira, L.A.; Motta, J.S.; Jesus, J.L.; Sasaki, F.F.C.; Viana, E.S. Processing of Sweet and Bitter Cassava; Embrapa: Brasília, Brazil, 2020. [Google Scholar]
- Omolara, G.M.; Adunni, A.A.; Omotayo, A.O. Cost and return analysis of cassava flour (Lafun) production among the women of Osun state, Nigeria. Sci. Res. 2017, 5, 72. [Google Scholar] [CrossRef]
- Morante, N.; Sánchez, T.; Ceballos, H.; Calle, F.; Pérez, J.C.; Egesi, C.; Cuambe, C.E.; Escobar, A.F.; Ortiz, D.; Chávez, A.L.; et al. Tolerance to Postharvest Physiological Deterioration in Cassava Roots. Crop Sci. 2010, 50, 1333–1338. [Google Scholar] [CrossRef]
- Wang, S. Starch Structure, Functionality and Application in Foods; Springer Nature: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Falade, K.O.; Akingbala, J.O. Utilization of Cassava for Food. Food Rev. Int. 2010, 27, 51–83. [Google Scholar] [CrossRef]
- Szadzińska, J.; Mierzwa, D.; Pawłowski, A.; Musielak, G.; Pashminehazar, R.; Kharaghani, A. Ultrasound- and Microwave-Assisted Intermittent Drying of Red Beetroot. Dry. Technol. 2019, 38, 93–107. [Google Scholar] [CrossRef]
- Buzera, A.; Gikundi, E.; Orina, I.; Sila, D. Effect of Pretreatments and Drying Methods on Physical and Microstructural Properties of Potato Flour. Foods 2022, 11, 507. [Google Scholar] [CrossRef] [PubMed]
- Utomo, J.S.; Che Man, Y.B.; Rahman, R.A.; Said Saad, M. The Effect of Shape, Blanching Methods and Flour on Characteristics of Restructured Sweetpotato Stick. Int. J. Food Sci. Technol. 2008, 43, 1896–1900. [Google Scholar] [CrossRef]
- Malakar, S.; Arora, V.K.; Munshi, M.; Yadav, D.K.; Pou, K.R.J.; Deb, S.; Chandra, R. Application of novel pretreatment technologies for intensification of drying performance and quality attributes of food commodities: A review. Food Sci. Biotechnol. 2023, 32, 1303–1335. [Google Scholar] [CrossRef] [PubMed]
- Melese, A.D.; Keyata, E.O. Impacts of Pretreatment Techniques on the Quality of Tuber Flours. Sci. World J. 2022, 2022, 9323694. [Google Scholar] [CrossRef]
- Vera, F.H.C.-D.; Soriano, A.N.; Dugos, N.P.; Rubi, R.V.C. A Comprehensive Review on the Drying Kinetics of Common Tubers. Appl. Sci. Eng. Prog. 2021, 14, 146–155. [Google Scholar] [CrossRef]
- Carvalho, G.R.; Santos, K.C.; Guedes, J.S.; Bitencourt, B.S.; Rojas, M.L.; Augusto, P.E.D. Chapter 17—Drying of roots and tubers. In Drying Technology in Food Processing; Jafari, S.M., Malekjani, N., Eds.; Woodhead Publishing: Witney, Oxford, UK, 2023; pp. 587–628. [Google Scholar]
- Mousakhani-Ganjeh, A.; Amiri, A.; Nasrollahzadeh, F.; Wiktor, A.; Nilghaz, A.; Pratap-Singh, A.; Mousavi Khaneghah, A. Electro-Based Technologies in Food Drying—A Comprehensive Review. LWT 2021, 145, 111315. [Google Scholar] [CrossRef]
- Acurio, L.; Baquerizo, A.; Borja, A.; Vayas, M.; García-Segovia, P.; Martínez-Monzó, J.; Igual, M. Water-Sorption Isotherms and Air-Drying-Kinetics Modelling of Andean Tubers and Tuberous Roots. Biol. Life Sci. Forum 2023, 26, 71. [Google Scholar] [CrossRef]
- Guiné, R.P.F. The Drying of Foods and Its Effect on the Physical-Chemical, Sensorial and Nutritional Properties. ETP Int. J. Food Eng. 2018, 4, 93–100. [Google Scholar] [CrossRef]
- Moses, J.A.; Norton, T.; Alagusundaram, K.; Tiwari, B.K. Novel Drying Techniques for the Food Industry. Food Eng. Rev. 2014, 6, 43–55. [Google Scholar] [CrossRef]
- Mukhtar, A.; Latif, S.; Barati, Z.; Müller, J. Valorization of Cassava By-Products: Cyanide Content and Quality Characteristics of Leaves and Peel. Appl. Sci. 2023, 13, 6340. [Google Scholar] [CrossRef]
- Quinn, A.A.; Myrans, H.; Gleadow, R.M. Cyanide Content of Cassava Food Products Available in Australia. Foods 2022, 11, 1384. [Google Scholar] [CrossRef]
- Abass, A.B.; Awoyale, W.; Sulyok, M.; Alamu, E.O. Occurrence of Regulated Mycotoxins and Other Microbial Metabolites in Dried Cassava Products from Nigeria. Toxins 2017, 9, 207. [Google Scholar] [CrossRef]
- Precoppe, M.; Komlaga, G.A.; Chapuis, A.; Müller, J. Comparative Study between Current Practices on Cassava Drying by Small-Size Enterprises in Africa. Appl. Sci. 2020, 10, 7863. [Google Scholar] [CrossRef]
- Gonçalves, L.T.; Pereira, N.R.; Almeida, S.B.; Freitas, S.d.J.; Waldman, W.R. Microwave–Hot Air Drying Applied to Selected Cassava Cultivars: Drying Kinetics and Sensory Acceptance. Int. J. Food Sci. Technol. 2016, 52, 389–397. [Google Scholar] [CrossRef]
- Akonor, P.T.; Tutu, C.O.; Affrifah, N.S.; Budu, A.S.; Saalia, F.K. Effect of Different Drying Techniques on the Functionality and Digestibility of Yellow-Fleshed Cassava Flour and Its Performance in Food Application. J. Food Process. Preserv. 2023, 2023, 1775604. [Google Scholar] [CrossRef]
- Ekeledo, E.; Latif, S.; Abass, A.; Müller, J. Amylose, Rheological and Functional Properties of Yellow Cassava Flour as Affected by Pretreatment and Drying Methods. Food Humanit. 2023, 1, 57–63. [Google Scholar] [CrossRef]
- Nainggolan, E.A.; Banout, J.; Urbanova, K. Chemical and Thermal Treatment for Drying Cassava Tubers: Optimization, Microstructure, and Dehydration Kinetics. Life 2023, 13, 2355. [Google Scholar] [CrossRef] [PubMed]
- Oladejo, A.O.; Ekpene, M.M.; Onwude, D.I.; Assian, U.E.; Nkem, O.M. Effects of Ultrasound Pretreatments on the Drying Kinetics of Yellow Cassava during Convective Hot Air Drying. J. Food Process. Preserv. 2021, 45, e15251. [Google Scholar] [CrossRef]
- Wahab, B.A.; Adebowale, A.A.; Sanni, S.A.; Sobukola, O.P.; Obadina, A.O.; Kajihausa, O.E.; Adegunwa, M.O.; Sanni, L.O.; Tomlins, K. Effect of species, pretreatments, and drying methods on the functional and pasting properties of high-quality yam flour. Food Sci. Nutr. 2015, 4, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Bikila, A.M.; Tola, Y.B.; Esho, T.B.; Forsido, S.F.; Mijena, D.F. Starch composition and functional properties of raw and pretreated anchote (Coccinia abyssinica (Lam.) Cogn.) tuber flours dried at different temperatures. Food Sci. Nutr. 2021, 10, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.K. Food Processing Technologies; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Filho, L.M.; Frascareli, E.C.; Mauro, M.A. Effect of an Edible Pectin Coating and Blanching Pretreatments on the Air-Drying Kinetics of Pumpkin (Cucurbita moschata). Food Bioprocess Technol. 2016, 9, 859–871. [Google Scholar] [CrossRef]
- Ando, Y.; Maeda, Y.; Mizutani, K.; Wakatsuki, N.; Hagiwara, S.; Nabetani, H. Impact of Blanching and Freeze-Thaw Pretreatment on Drying Rate of Carrot Roots in Relation to Changes in Cell Membrane Function and Cell Wall Structure. LWT—Food Sci. Technol. 2016, 71, 40–46. [Google Scholar] [CrossRef]
- Nainggolan, E.A.; Banout, J.; Urbanova, K. Application of Central Composite Design and Superimposition Approach for Optimization of Drying Parameters of Pretreated Cassava Flour. Foods 2023, 12, 2101. [Google Scholar] [CrossRef]
- Garba, U.; Kaur, S.; Gurumayum, S.; Rasane, P. Effect of Hot Water Blanching Time and Drying Temperature on The Thin Layer Drying Kinetics and Anthocyanin Degradation of Black Carrot (Daucus carota L.) Shreds. Food Technol. Biotechnol. 2015, 53, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Badwaik, L.S.; Gautam, G.; Deka, S.C. Influence of Blanching on Antioxidant, Nutritional and Physical Properties of Bamboo Shoot. J. Agric. Sci.—Sri Lanka 2015, 10, 140–150. [Google Scholar] [CrossRef]
- Lin, S.; Brewer, M.S. Effects of Blanching Method on the Quality Characteristics of Frozen Peas. J. Food Qual. 2005, 28, 350–360. [Google Scholar] [CrossRef]
- Marzuki, S.U.; Fardenan, D.; Nguyen, L.T. Drying Characteristic of Blanched Purple-Fleshed Sweet Potato Under Microwave Vacuum Drying. IOP Conf. Ser. Earth Environ. Sci. 2019, 309, 012059. [Google Scholar] [CrossRef]
- Miranda, G.; Berna, À.; Salazar, D.; Mulet, A. Sulphur Dioxide Evolution during Dried Apricot Storage. LWT—Food Sci. Technol. 2009, 42, 531–533. [Google Scholar] [CrossRef]
- van Hal, M. Quality of Sweetpotato Flour During Processing and Storage. Food Rev. Int. 2000, 16, 1–37. [Google Scholar] [CrossRef]
- García-Martínez, E.; Igual, M.; Martín-Esparza, M.E.; Martínez-Navarrete, N. Assessment of the Bioactive Compounds, Color, and Mechanical Properties of Apricots as Affected by Drying Treatment. Food Bioprocess Technol. 2012, 6, 3247–3255. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Toydemir, G.; Boyacioglu, D.; Beekwilder, J.; Hall, R.D.; Capanoglu, E. A Review on the Effect of Drying on Antioxidant Potential of Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2015, 56 (Suppl. S1), S110–S129. [Google Scholar] [CrossRef]
- Hiranvarachat, B.; Devahastin, S.; Chiewchan, N. Effects of Acid Pretreatments on Some Physicochemical Properties of Carrot Undergoing Hot Air Drying. Food Bioprod. Process. 2011, 89, 116–127. [Google Scholar] [CrossRef]
- Wang, L.; Xu, B.; Wei, B.; Zeng, R. Low Frequency Ultrasound Pretreatment of Carrot Slices: Effect on the Moisture Migration and Quality Attributes by Intermediate-Wave Infrared Radiation Drying. Ultrason. Sonochem. 2018, 40, 619–628. [Google Scholar] [CrossRef]
- Miano, A.C.; Ibarz, A.; Augusto, P.E.D. Mechanisms for Improving Mass Transfer in Food with Ultrasound Technology: Describing the Phenomena in Two Model Cases. Ultrason. Sonochem. 2016, 29, 413–419. [Google Scholar] [CrossRef]
- Tao, Y.; Sun, D.-W. Enhancement of Food Processes by Ultrasound: A Review. Crit. Rev. Food Sci. Nutr. 2014, 55, 570–594. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of Ultrasound in Food Technology: Processing, Preservation and Extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Kehinde, B.A.; Sharma, P.; Kaur, S. Recent Nano-, Micro- and Macrotechnological Applications of Ultrasonication in Food-Based Systems. Crit. Rev. Food Sci. Nutr. 2020, 61, 599–621. [Google Scholar] [CrossRef] [PubMed]
- Amaral, R.D.A.; Benedetti, B.C.; Pujolà, M.; Achaerandio, I.; Bachelli, M.L.B. A First Approach of Using Ultrasound as an Alternative for Blanching in Vacuum-Packaged Potato Strips. Food Bioprocess Technol. 2016, 9, 1794–1801. [Google Scholar] [CrossRef]
- Peshkovsky, A.S.; Peshkovsky, S.L.; Bystryak, S. Scalable High-Power Ultrasonic Technology for the Production of Translucent Nanoemulsions. Chem. Eng. Process. Process Intensif. 2013, 69, 77–82. [Google Scholar] [CrossRef]
- Álvarez-Arenas, T.E.G. Simultaneous Determination of the Ultrasound Velocity and the Thickness of Solid Plates from the Analysis of Thickness Resonances Using Air-Coupled Ultrasound. Ultrasonics 2010, 50, 104–109. [Google Scholar] [CrossRef]
- Carvalho, G.R.; Massarioli, A.P.; Alvim, I.D.; Augusto, P.E.D. Iron-Fortified Pineapple Chips Produced Using Microencapsulation, Ethanol, Ultrasound and Convective Drying. Food Eng. Rev. 2020, 13, 726–739. [Google Scholar] [CrossRef]
- Rojas, M.L.; Augusto, P.E.D.; Cárcel, J.A. Ethanol Pre-Treatment to Ultrasound-Assisted Convective Drying of Apple. Innov. Food Sci. Emerg. Technol. 2020, 61, 102328. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Zhou, C.; Sun, Y.; Wu, B.; Yagoub, A.E.A.; Aboagarib, E.A.A. Effect of Vacuum and Ethanol Pretreatment on Infrared-Hot Air Drying of Scallion (Allium fistulosum). Food Chem. 2019, 295, 432–440. [Google Scholar] [CrossRef]
- Guedes, J.S.; Santos, K.C.; Castanha, N.; Rojas, M.L.; Matta Junior, M.D.; Lima, D.C.; Augusto, P.E.D. Structural Modification on Potato Tissue and Starch Using Ethanol Pre-Treatment and Drying Process. Food Struct. 2021, 29, 100202. [Google Scholar] [CrossRef]
- Santos, K.C.; Guedes, J.S.; Rojas, M.L.; Carvalho, G.R.; Augusto, P.E.D. Enhancing Carrot Convective Drying by Combining Ethanol and Ultrasound as Pre-Treatments: Effect on Product Structure, Quality, Energy Consumption, Drying and Rehydration Kinetics. Ultrason. Sonochem. 2021, 70, 105304. [Google Scholar] [CrossRef]
- Rojas, M.L.; Augusto, P.E.D. Ethanol Pre-Treatment Improves Vegetable Drying and Rehydration: Kinetics, Mechanisms and Impact on Viscoelastic Properties. J. Food Eng. 2018, 233, 17–27. [Google Scholar] [CrossRef]
- Arshad, R.N.; Abdul-Malek, Z.; Munir, A.; Buntat, Z.; Ahmad, M.H.; Jusoh, Y.M.M.; Bekhit, A.E.-D.; Roobab, U.; Manzoor, M.F.; Aadil, R.M. Electrical Systems for Pulsed Electric Field Applications in the Food Industry: An Engineering Perspective. Trends Food Sci. Technol. 2020, 104, 1–13. [Google Scholar] [CrossRef]
- Ade-Omowaye, B.I.O.; Angersbach, A.; Taiwo, K.A.; Knorr, D. Use of Pulsed Electric Field Pre-Treatment to Improve Dehydration Characteristics of Plant Based Foods. Trends Food Sci. Technol. 2001, 12, 285–295. [Google Scholar] [CrossRef]
- Deng, L.-Z.; Mujumdar, A.S.; Zhang, Q.; Yang, X.-H.; Wang, J.; Zheng, Z.-A.; Gao, Z.-J.; Xiao, H.-W. Chemical and Physical Pretreatments of Fruits and Vegetables: Effects on Drying Characteristics and Quality Attributes—A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2017, 59, 1408–1432. [Google Scholar] [CrossRef]
- Wiktor, A.; Śledź, M.; Nowacka, M.; Chudoba, T.; Witrowa-Rajchert, D. Pulsed Electric Field Pretreatment for Osmotic Dehydration of Apple Tissue: Experimental and Mathematical Modeling Studies. Dry. Technol. 2014, 32, 408–417. [Google Scholar] [CrossRef]
- Liu, C.; Pirozzi, A.; Ferrari, G.; Vorobiev, E.; Grimi, N. Impact of Pulsed Electric Fields on Vacuum Drying Kinetics and Physicochemical Properties of Carrot. Food Res. Int. 2020, 137, 109658. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, K.; Subramanian, V.; Shanmugam, N. Effect of Thermal and Nonthermal Processing on Textural Quality of Plant Tissues. Crit. Rev. Food Sci. Nutr. 2015, 56, 2665–2694. [Google Scholar] [CrossRef]
- Llavata, B.; García-Pérez, J.V.; Simal, S.; Cárcel, J.A. Innovative Pre-Treatments to Enhance Food Drying: A Current Review. Curr. Opin. Food Sci. 2020, 35, 20–26. [Google Scholar] [CrossRef]
- Swami Hulle, N.R.; Rao, P.S. Effect of High-Pressure Pretreatments on Structural and Dehydration Characteristics of Aloe Vera (Aloe barbadensis Miller) Cubes. Dry. Technol. 2015, 34, 105–118. [Google Scholar] [CrossRef]
- de Oliveira, M.M.; Tribst, A.A.L.; Júnior, B.R.D.C.L.; de Oliveira, R.A.; Cristianini, M. Effects of High-Pressure Processing on Cocoyam, Peruvian Carrot, and Sweet Potato: Changes in Microstructure, Physical Characteristics, Starch, and Drying Rate. Innov. Food Sci. Emerg. Technol. 2015, 31, 45–53. [Google Scholar] [CrossRef]
- Li, X.; Farid, M. A Review on Recent Development in Non-Conventional Food Sterilization Technologies. J. Food Eng. 2016, 182, 33–45. [Google Scholar] [CrossRef]
- Jermann, C.; Koutchma, T.; Margas, E.; Leadley, C.; Ros-Polski, V. Mapping Trends in Novel and Emerging Food Processing Technologies around the World. Innov. Food Sci. Emerg. Technol. 2015, 31, 14–27. [Google Scholar] [CrossRef]
- Alamu, E.O.; Manda, N.; Ntawuruhunga, P.; Abass, A.; Maziya-Dixon, B. Elite Cassava Clones (Manihot esculenta) Grown in Zambia: Effects of Drying Techniques on Their Chemical, Functional, and Pasting Properties. Front. Sustain. Food Syst. 2023, 7, 1129779. [Google Scholar] [CrossRef]
- Okonkwo, C.E.; Olaniran, A.F.; Adeyi, O.; Adeyi, A.J.; Ojediran, J.O.; Adewumi, A.D.; Iranloye, Y.M.; Erinle, O.C. Drying Characteristics of Fermented-cooked Cassava Chips Used in the Production of Complementary Food: Mathematical and Gaussian Process Regression Modeling Approaches. J. Food Process Eng. 2021, 44, e13715. [Google Scholar] [CrossRef]
- Handojo, L.A.; Zefanya, S.; Christanto, Y. Drying Performance of Fermented Cassava (Fercaf) Using a Convective Multiple Flash Dryer. AIP Conf. Proc. 2017, 1840, 060002. [Google Scholar] [CrossRef]
- Suherman; Trisnaningtyas, R. Thin Layer Drying of Cassava Starch Using Continuous Vibrated Fluidized Bed Dryer. AIP Conf. Proc. 2015, 1699, 060021. [Google Scholar] [CrossRef]
- Elisabeth, D.A.A.; Utomo, J.S.; Byju, G.; Ginting, E. Cassava Flour Production by Small Scale Processors, Its Quality and Economic Feasibility. Food Sci. Technol. 2022, 42, e41522. [Google Scholar] [CrossRef]
- Yahya, M.; Fudholi, A.; Hafizh, H.; Sopian, K. Comparison of Solar Dryer and Solar-Assisted Heat Pump Dryer for Cassava. Sol. Energy 2016, 136, 606–613. [Google Scholar] [CrossRef]
- Suherman, S.; Susanto, E.E.; Zardani, A.W.; Dewi, N.H.R. Performance Study of Hybrid Solar Dryer for Cassava Starch. In Proceedings of the 2nd International Conference on Chemical Process and Product Engineering (ICCPPE), Semarang, Indonesia, 25–26 September 2019. [Google Scholar] [CrossRef]
- Dahal, P.; Tamang, M.K. Effects of different processing methods on anti-nutritional factors of cassava (Manihot esculenta crantz). J. Food Nutr. Disord. 2021, 10, 5. [Google Scholar]
- Nebiyu, A.; Getachew, E. Soaking and drying of cassava roots reduced cyanogenic potential of three cassava varieties at Jimma, Southwest Ethiopia. Afr. J. Biotechnol. 2011, 10, 13465–13469. [Google Scholar] [CrossRef]
- Montagnac, J.A.; Davis, C.R.; Tanumihardjo, S.A. Processing Techniques to Reduce Toxicity and Antinutrients of Cassava for Use as a Staple Food. Compr. Rev. Food Sci. Food Saf. 2008, 8, 17–27. [Google Scholar] [CrossRef]
- Brimer, L. Chapter 10— Cassava Production and Processing and Impact on Biological Compounds. In Processing and Impact on Active Components in Food; Preedy, V., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 81–87. [Google Scholar]
- Perera, C.O. Removal of Cyanogenic Glycoside from Cassava during Controlled Drying. Dry. Technol. 2009, 28, 68–72. [Google Scholar] [CrossRef]
- Kehinde, A.T.; Udoro, E.O. Studies on the Physicochemical, Functional and Sensory Properties of Gari Processed from Dried Cassava Chips. J. Food Process. Technol. 2013, 5, 1000293. [Google Scholar] [CrossRef]
- Silayo, V.C.K.; Lazaro, E.L.; Yustas, Y.; Laswai, H.S. Cassava Sun Drying Performance on Various Surfaces and Drying Bed Depths. Tanzan. J. Agric. Sci. 2013, 1, 31–36. [Google Scholar]
- Vijaya Venkata Raman, S.; Iniyan, S.; Goic, R. A Review of Solar Drying Technologies. Renew. Sustain. Energy Rev. 2012, 16, 2652–2670. [Google Scholar] [CrossRef]
- Suherman, S.; Susanto, E.E.; Busairi, A. Applications of solar dryer for seaweed and cassava starch. J. Phys. Conf. Ser. 2019, 1295, 012001. [Google Scholar] [CrossRef]
- El-Beltagy, A.; Gamea, G.R.; Essa, A.H.A. Solar Drying Characteristics of Strawberry. J. Food Eng. 2007, 78, 456–464. [Google Scholar] [CrossRef]
- Akonor, P.T.; Tortoe, C.; Buckman, E.S.; Hagan, L. Proximate Composition and Sensory Evaluation of Root and Tuber Composite Flour Noodles. Cogent Food Agric. 2017, 3, 1292586. [Google Scholar] [CrossRef]
- Famurewa, J.; Oluwamukomi, M.; Alaba, J. Effect of Different Drying Methods on the Physicochemical Characteristics of Cassava Flour (“Pupuru”). Int. J. Biol. Chem. Sci. 2013, 7, 832–839. [Google Scholar] [CrossRef]
- Nwafor, J. Effect of drying methods on the nutritional composition of D. alata and D. rotundata yam varieties. J. Food Sci. Nutr. 2022, 5, 102. [Google Scholar]
- Balzarini, M.F.; Reinheimer, M.A.; Ciappini, M.C.; Scenna, N.J. Comparative study of hot air and vacuum drying on the drying kinetics and physicochemical properties of chicory roots. J. Food Sci. Technol. 2018, 55, 4067–4078. [Google Scholar] [CrossRef] [PubMed]
- Van ’t Land, C.M. Drying in the Process Industry; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Rashid, M.T.; Liu, K.; Jatoi, M.A.; Safdar, B.; Lv, D.; Li, Q. Energy Efficient Drying Technologies for Sweet Potatoes: Operating and Drying Mechanism, Quality-Related Attributes. Front. Nutr. 2022, 9, 1040314. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wu, G.; Li, P.; Qi, X.; Zhang, H.; Wang, X.; Jin, Q. Effect of microwave heating and vacuum oven drying of potato strips on oil uptake during deep-fat frying. Food Res. Int. 2020, 137, 109338. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.; Lu, C.; Xu, S.; Fu, Z.; Lin, D.; Zheng, Y. Appearance, Microstructure, and Bioactive Components of Bletilla striata Tuber as Affected by Different Drying Methods. Food Bioprocess Technol. 2024. [Google Scholar] [CrossRef]
- Fatimah, S.; Hafied, M.A.; Indiasih, P.A.Y.; Airlangga, B.; Rahmawati, Y.; Roesyadi, A.; Sumarno, S. Amylose Isolation of Cassava Starch with the Combination of High Shear Mixer and Centrifugation Treatment to Improve the Quality of Resistant Starch Type 3 (RS-3) Products. Adv. Sci. Technol. 2024, 138, 13–19. [Google Scholar] [CrossRef]
- Sivakumar, R.; Saravanan, R.; Elaya Perumal, A.; Iniyan, S. Fluidized Bed Drying of Some Agro Products—A Review. Renew. Sustain. Energy Rev. 2016, 61, 280–301. [Google Scholar] [CrossRef]
- Famurewa, J.A.V.; Emuekele, P.O. Cyanide reduction pattern of cassava (mannihot Esculenta) as affected by variety and air velocity using fluidized bed dryer. Afr. J. Food Sci. Technol. 2014, 5, 75–80. [Google Scholar] [CrossRef]
- Bakal, S.B.; Sharma, G.P.; Sonawane, S.P.; Verma, R.C. Kinetics of Potato Drying Using Fluidized Bed Dryer. J. Food Sci. Technol. 2011, 49, 608–613. [Google Scholar] [CrossRef]
- Lozano-Acevedo, A.; Jimenez-Fernández, M.; Ragazzo-Sánchez, A.; Urrea-Garcia, G.R.; Luna-Solano, G. Fluidized Bed Drying Process of Thinly Sliced Potato (Solanum tuberosum). Am. J. Potato Res. 2011, 88, 360–366. [Google Scholar] [CrossRef]
- Okoronkwo, C.A.; Nwufo, O.C.; Nwaigwe, K.N.; Ogueke, N.V.; Anyanwu, E.E. Experimental evaluation of a fluidized bed dryer performance. Int. J. Eng. Sci. 2013, 2, 45–53. [Google Scholar]
- Şevik, S. Experimental Investigation of a New Design Solar-Heat Pump Dryer under the Different Climatic Conditions and Drying Behavior of Selected Products. Sol. Energy 2014, 105, 190–205. [Google Scholar] [CrossRef]
- Prasanna, N.S.; Manjula, B. Review on drying of agricultural produce using solar assisted heat pump drying. Int. J. Agric. Eng. 2018, 11, 409–420. [Google Scholar] [CrossRef]
- Hasibuan, R.; Yahya, M.; Fahmi, H.; Edison, E. Comparative performance of a solar assisted heat pump dryer with a heat pump dryer for Curcuma. Int. J. Power Electron. Drive Syst. 2020, 11, 1617. [Google Scholar] [CrossRef]
- Loemba, A.B.T.; Kichonge, B.; Kivevele, T. Comprehensive Assessment of Heat Pump Dryers for Drying Agricultural Products. Energy Sci. Eng. 2022, 11, 2985–3014. [Google Scholar] [CrossRef]
- Monteiro, R.L.; De Moraes, J.O.; Domingos, J.D.; Carciofi, B.A.M.; Laurindo, J.B. Evolution of the physicochemical properties of oil-free sweet potato chips during microwave vacuum drying. Innov. Food Sci. Emerg. Technol. 2020, 63, 102317. [Google Scholar] [CrossRef]
- Regier, M.; Mayer-Miebach, E.; Behsnilian, D.; Neff, E.; Schuchmann, H.P. Influences of Drying and Storage of Lycopene-Rich Carrots on the Carotenoid Content. Dry. Technol. 2005, 23, 989–998. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.; Wang, W. A Novel Low-Frequency Microwave Assisted Pulse-Spouted Bed Freeze-Drying of Chinese Yam. Food Bioprod. Process. 2019, 118, 217–226. [Google Scholar] [CrossRef]
- Song, X.; Zhang, M.; Mujumdar, A.S.; Fan, L. Drying Characteristics and Kinetics of Vacuum Microwave–Dried Potato Slices. Dry. Technol. 2009, 27, 969–974. [Google Scholar] [CrossRef]
- Lech, K.; Figiel, A.; Wojdyło, A.; Korzeniowska, M.; Serowik, M.; Szarycz, M. Drying Kinetics and Bioactivity of Beetroot Slices Pretreated in Concentrated Chokeberry Juice and Dried with Vacuum Microwaves. Dry. Technol. 2015, 33, 1644–1653. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, M.; Huang, L.; Tang, J.; Mujumdar, A.S.; Sun, J. Studies on Different Combined Microwave Drying of Carrot Pieces. Int. J. Food Sci. Technol. 2010, 45, 2141–2148. [Google Scholar] [CrossRef]
- Pawar, S.B.; Pratape, V.M. Fundamentals of Infrared Heating and Its Application in Drying of Food Materials: A Review. J. Food Process Eng. 2015, 40, e12308. [Google Scholar] [CrossRef]
- Doymaz, İ. Infrared Drying Kinetics and Quality Characteristics of Carrot Slices. J. Food Process. Preserv. 2015, 39, 2738–2745. [Google Scholar] [CrossRef]
- Doymaz, İ. Infrared Drying of Sweet Potato (Ipomoea batatas L.) Slices. J. Food Sci. Technol. 2011, 49, 760–766. [Google Scholar] [CrossRef]
- Onwude, D.I.; Hashim, N.; Abdan, K.; Janius, R.; Chen, G. Investigating the Influence of Novel Drying Methods on Sweet Potato (Ipomoea batatas L.): Kinetics, Energy Consumption, Color, and Microstructure. J. Food Process Eng. 2018, 41, e12686. [Google Scholar] [CrossRef]
- Guo, J.; Huang, K.; Wang, J. Bactericidal Effect of Various Non-Thermal Plasma Agents and the Influence of Experimental Conditions in Microbial Inactivation: A Review. Food Control 2015, 50, 482–490. [Google Scholar] [CrossRef]
- Onwude, D.I.; Hashim, N.; Abdan, K.; Janius, R.; Chen, G. The Effectiveness of Combined Infrared and Hot-Air Drying Strategies for Sweet Potato. J. Food Eng. 2019, 241, 75–87. [Google Scholar] [CrossRef]
- Lee, S.-H.; Ko, S.-C.; Kang, S.-M.; Cha, S.H.; Ahn, G.-N.; Um, B.-H.; Jeon, Y.-J. Antioxidative Effect of Ecklonia Cava Dried by Far Infrared Radiation Drying. Food Sci. Biotechnol. 2010, 19, 129–135. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, M.; Ye, Y.; Yu, D. Influence of ultrasonic pretreatments on drying kinetics and quality attributes of sweet potato slices in infrared freeze drying (IRFD). LWT 2020, 131, 109801. [Google Scholar] [CrossRef]
- Nowacka, M.; Wedzik, M. Effect of Ultrasound Treatment on Microstructure, Colour and Carotenoid Content in Fresh and Dried Carrot Tissue. Appl. Acoust. 2016, 103, 163–171. [Google Scholar] [CrossRef]
- Mulet, A.; Cárcel, J.A.; Sanjuán, N.; Bon, J. New Food Drying Technologies—Use of Ultrasound. Food Sci. Technol. Int. 2003, 9, 215–221. [Google Scholar] [CrossRef]
- Kroehnke, J.; Szadzińska, J.; Stasiak, M.; Radziejewska-Kubzdela, E.; Biegańska-Marecik, R.; Musielak, G. Ultrasound- and Microwave-Assisted Convective Drying of Carrots—Process Kinetics and Product’s Quality Analysis. Ultrason. Sonochem. 2018, 48, 249–258. [Google Scholar] [CrossRef]
- Cárcel, J.A.; Garcia-Perez, J.V.; Riera, E.; Mulet, A. Improvement of Convective Drying of Carrot by Applying Power Ultrasound—Influence of Mass Load Density. Dry. Technol. 2011, 29, 174–182. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Yu, H.; Yin, Y.; Li, X.; Duan, X. Hot Air Drying of Purple-Fleshed Sweet Potato with Contact Ultrasound Assistance. Dry. Technol. 2016, 35, 564–576. [Google Scholar] [CrossRef]
- Ortiz-Jerez, M.J.; Gulati, T.; Datta, A.K.; Ochoa-Martínez, C.I. Quantitative Understanding of Refractance WindowTM Drying. Food Bioprod. Process. 2015, 95, 237–253. [Google Scholar] [CrossRef]
- Bernaert, N.; Van Droogenbroeck, B.; Van Pamel, E.; De Ruyck, H. Innovative Refractance Window Drying Technology to Keep Nutrient Value during Processing. Trends Food Sci. Technol. 2019, 84, 22–24. [Google Scholar] [CrossRef]
- Raghavi, L.M.; Moses, J.A.; Anandharamakrishnan, C. Refractance Window Drying of Foods: A Review. J. Food Eng. 2018, 222, 267–275. [Google Scholar] [CrossRef]
- Nayak, B.; Berrios, J.D.J.; Powers, J.R.; Tang, J.; Ji, Y. Colored potatoes (Solanum tuberosum L.) Dried for Antioxidant-rich Value-added Foods. J. Food Process. Preserv. 2011, 35, 571–580. [Google Scholar] [CrossRef]
- Duarte-Correa, Y.; Vargas-Carmona, M.I.; Vásquez-Restrepo, A.; Ruiz Rosas, I.D.; Pérez Martínez, N. Native Potato (Solanum phureja) Powder by Refractance Window Drying: A Promising Way for Potato Processing. J. Food Process Eng. 2021, 44, e13819. [Google Scholar] [CrossRef]
- Ueda, J.M.; Morales, P.; Fernández-Ruiz, V.; Ferreira, A.; Barros, L.; Carocho, M.; Heleno, S.A. Powdered Foods: Structure, Processing, and Challenges: A Review. Appl. Sci. 2023, 13, 12496. [Google Scholar] [CrossRef]
- Amelework, A.B.; Bairu, M.W. Advances in Genetic Analysis and Breeding of Cassava (Manihot esculenta Crantz): A Review. Plants 2022, 11, 1617. [Google Scholar] [CrossRef]
- Maulida, Y.F.; Subejo; Hardyastuti, S. The Urgency of Institutional Development of Cassava Industry in Daerah Istimewa Yogyakarta and Jawa Tengah. Sodality J. Sosiol. Pedesaan 2021, 9, e33369. [Google Scholar] [CrossRef]
- Shittu, T.A.; Alimi, B.A.; Wahab, B.; Sanni, L.O.; Abass, A.B. Cassava flour and starch: Processing technology and utilization. In Tropical Roots and Tubers; Sharma, H.K., Njintang, N.Y., Singhal, R.S., Kaushal, P., Eds.; John Wiley & Sons: Chichester, UK, 2016; pp. 415–450. [Google Scholar]
- Breuninger, W.F.; Piyachomkwan, K.; Sriroth, K. Tapioca/cassava starch: Production and use. In Starch: Chemistry and Technology, 3rd ed.; BeMiller, J., Whistler, R., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 541–568. [Google Scholar]
- Neves, E.C.A.; Neves, D.A.; Lobato, K.B.d.S.; Nascimento, G.C.d.; Clerici, M.T.P.S. Technological aspects of processing of cassava derivatives. In Handbook on Cassava: Production, Potential Uses and Recent Advances; Klein, C., Ed.; Nova Science Publishers: New York, NY, USA, 2017; pp. 105–127. [Google Scholar]
- Pornpraipech, P.; Khusakul, M.; Singklin, R.; Sarabhorn, P.; Areeprasert, C. Effect of Temperature and Shape on Drying Performance of Cassava Chips. Agric. Nat. Resour. 2017, 51, 402–409. [Google Scholar] [CrossRef]
- Veeramanipriya, E.; Umayal Sundari, A.R. Performance Evaluation of Hybrid Photovoltaic Thermal (PVT) Solar Dryer for Drying of Cassava. Sol. Energy 2021, 215, 240–251. [Google Scholar] [CrossRef]
- Akinwande, B.A.; Ade-Omowaye, B.I.O.; Olaniyan, S.A.; Akintaro, O.O. Quality Evaluation of Ginger-flavoured Soy-cassava Biscuit. Nutr. Food Sci. 2008, 38, 473–481. [Google Scholar] [CrossRef]
- Ammar, A.; Abd El-Razik, M. Quality Characteristics of Gluten Free Cake Produced from Cassava, Pumpkin and Potato Flours. J. Food Dairy Sci. 2013, 4, 401–412. [Google Scholar] [CrossRef]
- Olapade, A.A.; Adeyemo, M.A. Evaluation of Cookies Produced from Blends of Wheat, Cassava and Cowpea Flours. Int. J. Food Stud. 2014, 3, 175–185. [Google Scholar] [CrossRef]
- Aly, M.M.A.; Seleem, H.A. Gluten-Free Flat Bread and Biscuits Production by Cassava, Extruded Soy Protein and Pumpkin Powder. Food Nutr. Sci. 2015, 6, 660–674. [Google Scholar] [CrossRef]
- Jensen, S.; Skibsted, L.H.; Kidmose, U.; Thybo, A.K. Addition of Cassava Flours in Bread-Making: Sensory and Textural Evaluation. LWT—Food Sci. Technol. 2015, 60, 292–299. [Google Scholar] [CrossRef]
- Adeboye, A.S.; Babajide, J.M.; Shittu, T.A.; Omemu, A.M.; Oluwatola, O.J. Effect of Honey as Partial Sugar Substitute on Pasting Properties, Consumer Preference and Shelf Stability of Cassava-Wheat Composite Bread. Niger. Food J. 2013, 31, 13–22. [Google Scholar] [CrossRef]
- Nwabueze, T.U.; Anoruoh, G.A. Evaluation of Flour and Extruded Noodles from Eight Cassava Mosaic Disease (CMD)-Resistant Varieties. Food Bioprocess Technol. 2009, 4, 80–91. [Google Scholar] [CrossRef]
- Ogugbue, C.J.; Gloria, O. Bioburden of garri stored in different packaging materials undertropical market conditions. Middle-East J. Sci. Res. 2011, 7, 741–745. [Google Scholar]
- Ogiehor, I.; Ikenebomeh, M. The effects of different packaging materials on the shelf stability of garri. Afr. J. Biotechnol. 2006, 23, 2412–2416. [Google Scholar]
- Opara, U.L.; Caleb, O.J.; Uchechukwu-Agua, A.D. Evaluating the Impacts of Selected Packaging Materials on the Quality Attributes of Cassava Flour (Cvs. TME 419 and UMUCASS 36). J. Food Sci. 2016, 81, C324–C331. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, H.M.; Kowalczewski, P.Ł. Low-Field NMR Analyses of Gels and Starch-Stabilized Emulsions with Modified Potato Starches. Processes 2022, 10, 2109. [Google Scholar] [CrossRef]
- Walkowiak, K.; Przybył, K.; Baranowska, H.M.; Koszela, K.; Masewicz, Ł.; Piątek, M. The Process of Pasting and Gelling Modified Potato Starch with LF-NMR. Polymers 2022, 14, 184. [Google Scholar] [CrossRef] [PubMed]

| Pre-Drying Technique | Pre-Drying Condition | Drying Method | Major Observations | References |
|---|---|---|---|---|
| Hot Water Blanching | 70 °C for 6 min Sample-to-water ratio 1:5 (w:w) | Microwave–hot air drying | Pre-drying and drying process had uniform effects on cassava regardless of cultivar variation. Starch changes during cooking did not affect drying kinetics, resulting in consistent texture and sensory scores. | [26] |
| Steam Blanching | 110 °C for 5 min | Solar drying Hot air oven drying Drum drying | Pre-drying with steam blanching followed by drum drying yields higher digestibility and preferable sensory attributes, while hot-air oven drying enhances β-carotene concentration. Cultivar and drying method influence flour characteristics, highlighting the potential of drum drying for optimal product quality. | [27] |
| Sulfite Solution | 0.3% sodium metabisulfite solution for 7 min | Flash drying | Pre-drying with sodium metabisulfite followed by flash-drying enhances rheological properties and water absorption capacity of cassava flour, making it suitable for formulations requiring good pasting quality and moderately high gel strength. | [28] |
| Acid Solution | 0.3% citric acid solution for 7 min | Cabinet drying | Citric acid-treated flour exhibits inferior rheological traits and water absorption capacity compared to sodium metabisulfite-treated counterparts. | [28] |
| Combined | 1.31% citric acid and 1.03% sodium metabisulfite for 20 min Steam blanching at 80 °C for 1.01 min. | Hot-air oven drying | Combined pre-drying conditions enhance cassava drying efficiency, yielding moisture content of 6.19% and whiteness index of 92.00. The logarithmic model best describes dehydration kinetics, crucial for tuber processing improvements. | [29] |
| Ultrasonic Field | Distilled water with ultrasound (DWU), osmotic dehydration with ultrasound (ODU) Frequency of 20 kHz at ultrasound power Pretreatment time of 600 W and 10 min | Hot-air oven drying | Ultrasound pretreatments, especially ODU, significantly reduce drying time of yellow cassava, enhancing effective moisture diffusivity and reducing energy costs. Cavitation effects create microscopic pathways, influencing both internal and external resistances. Parabolic model fits best for DWU, while Page model suits ODU and untreated samples. Thus, ultrasound pretreatment proves beneficial for hot air drying of yellow cassava. | [30] |
| Drying Method | Drying Condition | Drying Characteristics and Quality | References |
|---|---|---|---|
| Sun Drying | Temperatures: 18–25 °C; Humidity: 60–75%. | Sun drying significantly influenced the color, proximate, functional, and pasting properties of cassava flour, impacting its quality and industrial applicability. Despite its simplicity and cost-effectiveness, sun drying demonstrates varying effects on different properties compared to other drying methods, emphasizing its importance in cassava processing. | [71] |
| Solar Drying | Drying time: 40 h; Average drying temperature: 52 °C. | Solar drying of cassava leads to the formation of coarser particles as a consequence of prolonged drying periods and exposure to light, consequently resulting in a reduced β-carotene content. Additionally, this drying method yields fufu with distinctive texture attributes, thereby affecting its stickiness and softness. | [27] |
| Hot Air Oven Drying | Load: max 120 kg; Drying time: 660 min; Drying temperature: 50, 60, 70 °C; Fan speed: 0.5, 0.9, and 1.3 m/s. | Hot air oven drying effects on fermented-cooked cassava chips revealed that increasing temperature and fan speed reduced drying time. Effective diffusivity rose with temperature and fan speed, affecting proximate compositions and functional characteristics. Gaussian process regression (GPR)-based modeling proved superior for optimization and control monitoring, vital for product standardization. | [72] |
| Vacuum Drying | Drying time: 10 h; Drying air temperature 50, 60, and 70 °C; Drying pressure: −60 cmHg. | Vacuum drying significantly influences dried cassava quality. Convective multiple flash drying (CMFD), a novel drying method, achieves desired moisture content in 5–6 h, yielding harder chips compared to convective and vacuum methods. Operating conditions affect moisture content. | [73] |
| Freeze Drying | Vacuum: Hi MBars pressure, wait for the collector at 22 °C; Run collector: −41 °C for 48 cumulative hours. | Freeze drying significantly preserved color and quality of cassava flour compared to other methods. It yields superior moisture, ash, fat, and protein content, ensuring high-quality flour. Functional properties like swelling power and water absorption are favorable. Pasting properties also indicate its suitability for various food products. | [71] |
| Fluidized Bed Drying | Drying air temperature: 50, 55, 60, 65, and 70 °C; Solid feed flow rate: 10 g/min and 30 g/min; Airflow rate: 0.012 m3/min. | In the continuous vibrated fluidized bed drying of cassava starch, air temperature significantly influences drying, while weir height and solid feed rate have minimal impact. The Page model proves most accurate in describing the drying kinetics. | [74] |
| Sun Drying and Oven Drying | Sun drying for 4–6 h, followed by oven drying at 55 °C for 24 h. | Drying cassava chips followed by milling into flour reduces hydrogen cyanide (HCN) content by up to 81%, enhancing safety. However, slow drying during rainy seasons can lead to higher acidity, compromising taste and favoring spoilage. Whiteness may be affected by ash content and water quality, influencing product quality. | [75] |
| Solar-Assisted Heat Pump Drying | Load: 30.8 kg; Drying time: 9 h; Drying temperature: 45 °C; Specific moisture extraction ratio (SMER): 0.38 kg/kWh; Coefficient of performance (COP): 3.38; Thermal efficiency (η): 30.9. | Both solar drying (SD) and solar-assisted heat pump drying (SAHPD) significantly reduced cassava mass and moisture content. SAHPD demonstrated higher drying rate, specific moisture extraction rate, thermal efficiency, and pick-up efficiency compared to SD, indicating its superior performance in drying cassava. | [76] |
| Hybrid Solar Drying | Load: 300 g Drying time: 3 h Drying temperature: 40, 50, 60 °C | Higher drying temperatures lead to faster and more effective drying of cassava starch. The fastest moisture reduction occurs initially, with the drying process mainly happening during the falling rate period. Hybrid solar dryers, especially when combined with liquefied petroleum gas (LPG), are more effective compared to open sun drying, with an effectivity factor reaching up to 6.4. | [77] |
| Microwave–Hot Air Drying (MHAD) | Drying temperature: 70 °C; Airflow rate: 1.9 m3 min−1; Microwave power: 95 W. | MHAD affected the physicochemical properties, drying kinetics, and sensory acceptance of dried cassava. MHAD resulted in similar drying kinetics across cultivars, with no significant differences in moisture removal rates. | [26] |
| Products | Compositions | Key Highlights | References |
|---|---|---|---|
| Biscuits | Cassava flour, full-fat soy-flour, wheat flour, sugar, margarine, egg, baking powder, ginger flour, sugar, margarine and egg | The protein enrichment of cassava flour increased the nutrient content of the biscuits, potentially meeting the nutrient requirements of school children. | [138] |
| Gluten-free cup cakes | Cassava flour, shortening, egg, milk powder, baking powder and vanilla | The use of cassava flour, along with pumpkin and potato flours, or their mixture, tailored for celiac patients. | [139] |
| Cookies | Cassava flour, cowpea flour, salt, sugar, skimmed milk, baking fat, and water | A decrease in protein content occurred with the increase in cassava flour substitution, but the addition of cowpea flour led to an overall elevation in protein content. | [140] |
| Gluten-free flat bread and biscuits | Flat bread: Cassava flour, rice flour, extruded soy protein, xanthan, salt, butter, and water. Biscuits: Cassava flour, rice flour, extruded soy protein, xanthan, sugar, egg, baking powder and butter. | Incorporating cassava flour and extruded soy protein led to an improvement in the nutritional content of both flat bread and biscuits. | [141] |
| Bread | Cassava flour, wheat flour, fiber, yeast, water, salt and sucrose. | Cassava flour presents a promising solution for replacing up to 30% of wheat flour without significant differences in the final product. | [142] |
| Bread | Cassava flour, wheat flour, honey, baking fat, yeast, bread improver, water, and salt. | The substitution of sugar with liquid honey in cassava-wheat composite flour formulations led to notable effects on both the pasting properties of cooked dough and the characteristics of the resulting bread. | [143] |
| Noodles | Cassava flour, water, and alum. | Cassava flour presents a promising solution for replacing wheat flour in noodle manufacturing. | [144] |
| Noodles | Cassava flour, wheat flour, salt and water. | Noodles made from a composite flour of cassava and wheat showed promising results in terms of acceptability. | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nainggolan, E.A.; Banout, J.; Urbanova, K. Recent Trends in the Pre-Drying, Drying, and Post-Drying Processes for Cassava Tuber: A Review. Foods 2024, 13, 1778. https://doi.org/10.3390/foods13111778
Nainggolan EA, Banout J, Urbanova K. Recent Trends in the Pre-Drying, Drying, and Post-Drying Processes for Cassava Tuber: A Review. Foods. 2024; 13(11):1778. https://doi.org/10.3390/foods13111778
Chicago/Turabian StyleNainggolan, Ellyas Alga, Jan Banout, and Klara Urbanova. 2024. "Recent Trends in the Pre-Drying, Drying, and Post-Drying Processes for Cassava Tuber: A Review" Foods 13, no. 11: 1778. https://doi.org/10.3390/foods13111778
APA StyleNainggolan, E. A., Banout, J., & Urbanova, K. (2024). Recent Trends in the Pre-Drying, Drying, and Post-Drying Processes for Cassava Tuber: A Review. Foods, 13(11), 1778. https://doi.org/10.3390/foods13111778






