Harnessing the Role of Three Lactic Acid Bacteria (LAB) Strains for Type II Sourdough Production and Influence of Sourdoughs on Bread Quality and Maillard Reaction Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. The Preparation of Sourdough with LAB Strains
2.2.2. LAB Counts, Titratable Acidity and pH of Sourdough
2.2.3. Preparation of Sourdough Breads
2.2.4. Physical Characteristics of Bread Samples and Bake Loss
2.2.5. Texture Profile of Bread Samples
2.2.6. Compositional Analysis of Bread Samples
2.2.7. Maillard Reaction Products (FAST Index)
2.2.8. Volatile Organic Compounds in Bread Samples
2.2.9. Statistical Analysis
3. Results and Discussion
3.1. LAB Count, Titratable Acidity, and pH of Sourdough and Bread Doughs
3.2. Physical Characteristics of Sourdough Breads
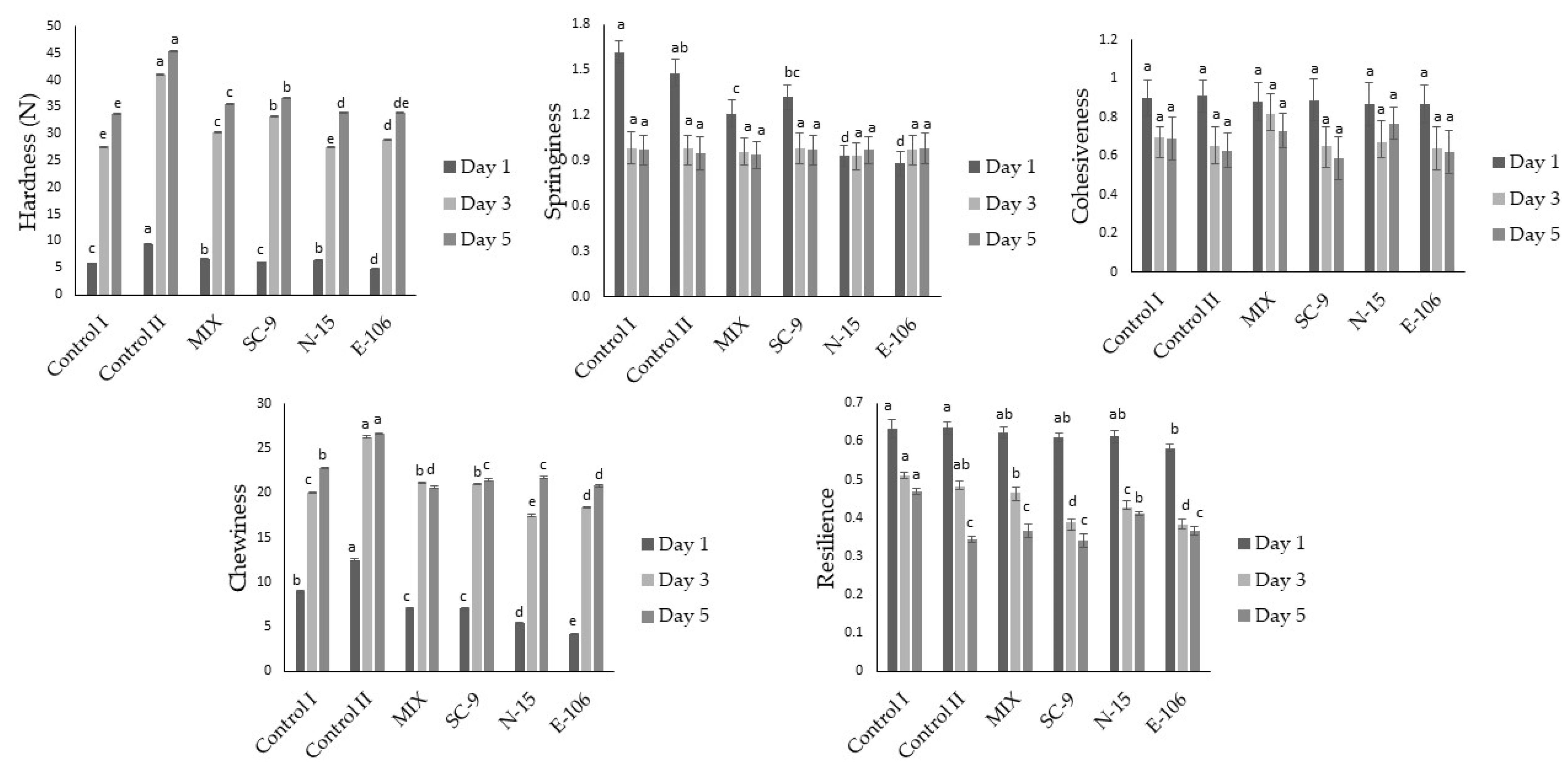
3.3. Textural Properties of Sourdough Breads
3.4. Chemical Composition of Sourdough Breads
3.5. Maillard Reaction Products (FAST Index) of Sourdough Breads
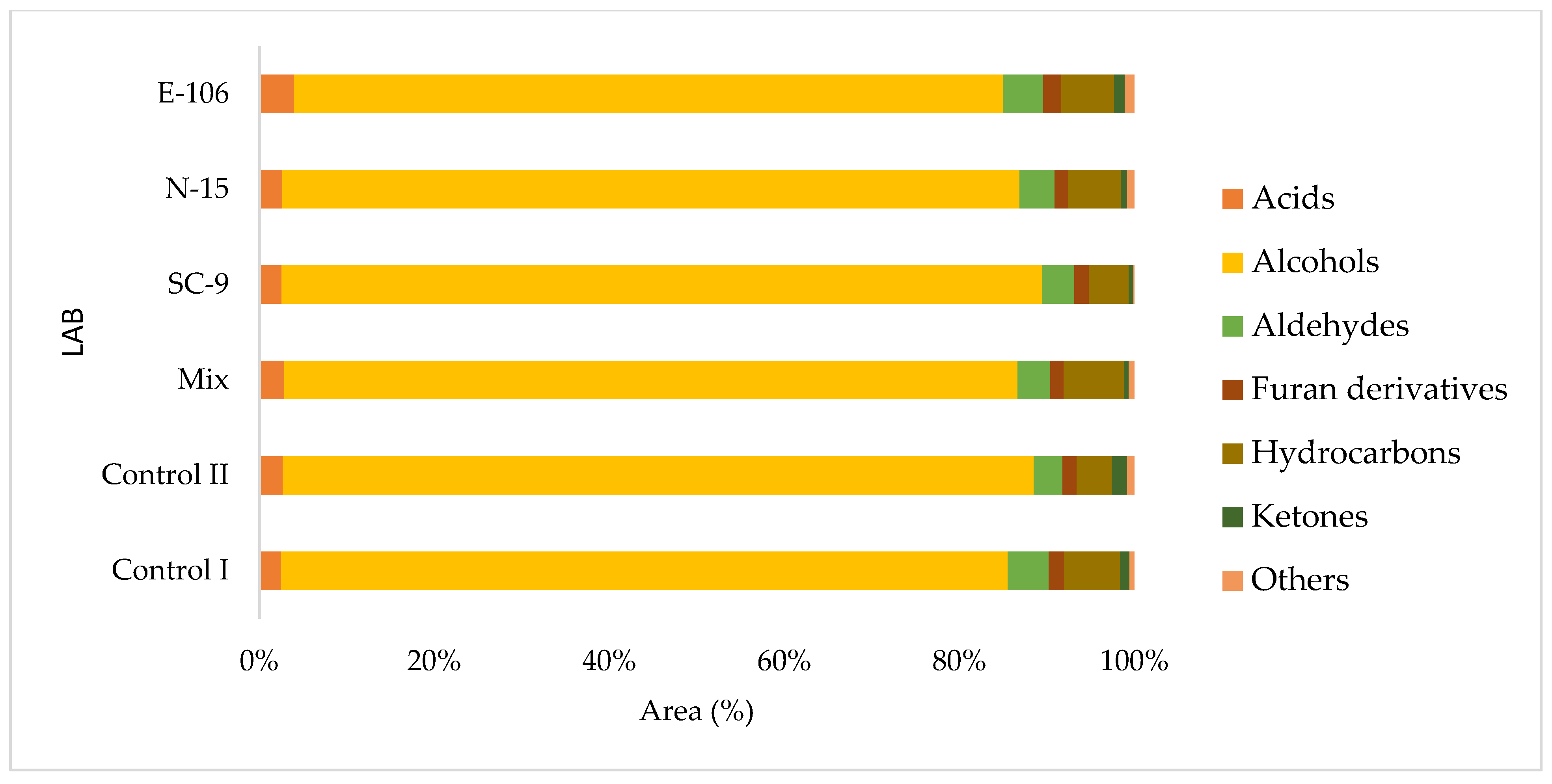
3.6. Volatile Organic Compounds of Sourdough Breads
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sakandar, H.A.; Hussain, R.; Kubow, S.; Sadiq, F.A.; Huang, W.; Imran, M. Sourdough bread: A contemporary cereal fermented product. J. Food Process. Preserv. 2019, 43, e13883. [Google Scholar] [CrossRef]
- Fernández-Peláez, J.; Paesani, C.; Gómez, M. Sourdough technology as a tool for the development of healthier grain-based products: An update. Agronomy 2020, 10, 1962. [Google Scholar] [CrossRef]
- Hayta, M.; Hendek Ertop, M. Optimisation of sourdough bread incorporation into wheat bread by response surface methodology: Bioactive and nutritional properties. Int. J. Food Sci. Technol. 2017, 52, 1828–1835. [Google Scholar] [CrossRef]
- Siepmann, F.B.; de Almeida, B.S.; Ripari, V.; da Silva, B.J.; Peralta-Zamora, P.G.; Waszczynskyj, N.; Spier, M.R. Brazilian sourdough: Microbiological, structural, and technological evolution. Eur. Food Res. Technol. 2019, 245, 1583–1594. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, J.; Manickam, S.; He, J.; Li, D.; Han, Y.; Jiang, X.; Tao, Y. Biochemical and physical investigations on detoxification of ginkgo kernel juice using probiotic fermentation with macroporous resin addition. Food Innov. Adv. 2023, 2, 324–339. [Google Scholar] [CrossRef]
- Troadec, R.; Nestora, S.; Niquet-Léridon, C.; Marier, D.; Jacolot, P.; Sarron, E.; Regnault, S.; Anton, P.M.; Jouquand, C. Effect of leavening agent on Maillard reaction and the bifidogenic effect of traditional French bread. Food Chem. 2022, 393, 133387. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Van Kerrebroeck, S.; Leroy, F. Microbial ecology and process technology of sourdough fermentation. Adv. Appl. Microbiol. 2017, 100, 49–160. [Google Scholar]
- Demirkesen-Bicak, H.; Arici, M.; Yaman, M.; Karasu, S.; Sagdic, O. Effect of different fermentation condition on estimated glycemic index, in vitro starch digestibility, and textural and sensory properties of sourdough bread. Foods 2021, 10, 514. [Google Scholar] [CrossRef] [PubMed]
- Çakır, E.; Arıcı, M.; Durak, M.Z.; Karasu, S. The molecular and technological characterization of lactic acid bacteria in einkorn sourdough: Effect on bread quality. J. Food Meas. Charact. 2020, 14, 1646–1655. [Google Scholar] [CrossRef]
- Ozgolet, M.; Yaman, M.; Durak, M.Z.; Karasu, S. The effect of five different sourdough on the formation of glyoxal and methylglyoxal in bread and influence of in vitro digestion. Food Chem. 2022, 371, 131141. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.I.; Jongen, W.M.; Van Boekel, M.A. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- Axel, C.; Brosnan, B.; Zannini, E.; Peyer, L.C.; Furey, A.; Coffey, A.; Arendt, E.K. Antifungal activities of three different Lactobacillus species and their production of antifungal carboxylic acids in wheat sourdough. Appl. Microbiol. Biotechnol. 2016, 100, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; Celano, G.; Lattanzi, A.; De Angelis, M.; Gobbetti, M. Added ingredients affect the microbiota and biochemical characteristics of durum wheat type-I sourdough. Food Microbiol. 2016, 60, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Karaman, K.; Sagdic, O.; Durak, M.Z. Use of phytase active yeasts and lactic acid bacteria isolated from sourdough in the production of whole wheat bread. LWT 2018, 91, 557–567. [Google Scholar] [CrossRef]
- Bartkiene, E.; Bartkevics, V.; Krungleviciute, V.; Pugajeva, I.; Zadeike, D.; Juodeikiene, G. Lactic acid bacteria combinations for wheat sourdough preparation and their influence on wheat bread quality and acrylamide formation. J. Food Sci. 2017, 82, 2371–2378. [Google Scholar] [CrossRef] [PubMed]
- Akcicek, A.; Özgölet, M.; Tekin-Cakmak, Z.H.; Karasu, S.; Duran, E.; Sagdic, O. Cold-Pressed Okra Seed Oil Byproduct as an Ingredient for Muffins to Decrease Glycemic Index, Maillard Reaction, and Oxidation. ACS Omega 2024, 9, 7491–7501. [Google Scholar] [CrossRef] [PubMed]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.-J. Colour measurement and analysis in fresh and processed foods: A review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Dapčević Hadnađev, T.R.; Dokić, L.P.; Hadnađev, M.S.; Pojić, M.M.; Rakita, S.M.; Torbica, A.M. Changes in quality parameters of bread supplemented with OSA starch during storage. Food Feed Res. 2013, 40, 101–108. [Google Scholar]
- Yildirim, R.M.; Arici, M. Effect of the fermentation temperature on the degradation of phytic acid in whole-wheat sourdough bread. LWT 2019, 112, 108224. [Google Scholar] [CrossRef]
- American Association of Cereal Chemists; Approved Methods Committee. Approved Methods of the American Association of Cereal Chemists; American Association of Cereal Chemists: St. Paul, MN, USA, 2000; Volume 1. [Google Scholar]
- Birlouez-Aragon, I.; Leclere, J.; Quedraogo, C.; Birlouez, E.; Grongnet, J.F. The FAST method, a rapid approach of the nutritional quality of heat-treated foods. Food/Nahrung 2001, 45, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Plessas, S.; Bekatorou, A.; Gallanagh, J.; Nigam, P.; Koutinas, A.A.; Psarianos, C. Evolution of aroma volatiles during storage of sourdough breads made by mixed cultures of Kluyveromyces marxianus and Lactobacillus delbrueckii ssp. bulgaricus or Lactobacillus helveticus. Food Chem. 2008, 107, 883–889. [Google Scholar] [CrossRef]
- Dertli, E.; Mercan, E.; Arıcı, M.; Yılmaz, M.T.; Sağdıç, O. Characterisation of lactic acid bacteria from Turkish sourdough and determination of their exopolysaccharide (EPS) production characteristics. LWT-Food Sci. Technol. 2016, 71, 116–124. [Google Scholar] [CrossRef]
- Torrieri, E.; Pepe, O.; Ventorino, V.; Masi, P.; Cavella, S. Effect of sourdough at different concentrations on quality and shelf life of bread. LWT-Food Sci. Technol. 2014, 56, 508–516. [Google Scholar] [CrossRef]
- Wolter, A.; Hager, A.-S.; Zannini, E.; Czerny, M.; Arendt, E.K. Impact of sourdough fermented with Lactobacillus plantarum FST 1.7 on baking and sensory properties of gluten-free breads. Eur. Food Res. Technol. 2014, 239, 1–12. [Google Scholar] [CrossRef]
- Mantzourani, I.; Plessas, S.; Odatzidou, M.; Alexopoulos, A.; Galanis, A.; Bezirtzoglou, E.; Bekatorou, A. Effect of a novel Lactobacillus paracasei starter on sourdough bread quality. Food Chem. 2019, 271, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Katina, K.; Sauri, M.; Alakomi, H.-L.; Mattila-Sandholm, T. Potential of lactic acid bacteria to inhibit rope spoilage in wheat sourdough bread. LWT-Food Sci. Technol. 2002, 35, 38–45. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Mohebbi, M. Evaluation of cress seed gum and xanthan gum effect on macrostructure properties of gluten-free bread by image processing. J. Food Meas. Charact. 2015, 9, 110–119. [Google Scholar] [CrossRef]
- Santos, J.G.D.; Ávila, P.M.D.; Gomes, R.A.B.; Monteiro, R.D.S.; Vieira, S.M.; Pereira, P.A.P. Use of natural yogurt, pineapple juice, and beer wort as starter cultures in sourdough combined with white and whole wheat flours to improve the technological characteristics of milk breads. Food Process. Technol. 2023, 11, 9–14. [Google Scholar] [CrossRef]
- Moroni, A.V.; Dal Bello, F.; Zannini, E.; Arendt, E.K. Impact of sourdough on buckwheat flour, batter and bread: Biochemical, rheological and textural insights. J. Cereal Sci. 2011, 54, 195–202. [Google Scholar] [CrossRef]
- Wang, H.E.; Hwang, C.F.; Tzeng, Y.M.; Hwang, W.Z.; Mau, J.L. Quality of White Bread Made from Lactic Acid Bacteria-Enriched Dough. J. Food Process. Preserv. 2012, 36, 553–559. [Google Scholar] [CrossRef]
- Yezbick, G.; Ahn-Jarvis, J.; Schwartz, S.J.; Vodovotz, Y. Physicochemical characterization and sensory analysis of yeast-leavened and sourdough soy breads. J. Food Sci. 2013, 78, C1487–C1494. [Google Scholar] [CrossRef] [PubMed]
- Caglar, N.; Ermis, E.; Durak, M.Z. Spray-dried and freeze-dried sourdough powders: Properties and evaluation of their use in breadmaking. J. Food Eng. 2021, 292, 110355. [Google Scholar] [CrossRef]
- Guiné, R.P.; Almeida, I.C.; Correia, A.C.; Gonçalves, F.J. Evaluation of the physical, chemical and sensory properties of raisins produced from grapes of the cultivar Crimson. J. Food Meas. Charact. 2015, 9, 337–346. [Google Scholar] [CrossRef]
- Jitrakbumrung, S.; Therdthai, N. Effect of addition of sourdough on physicochemical characteristics of wheat and rice flour bread. Agric. Nat. Resour. 2014, 48, 964–969. [Google Scholar]
- Katina, K.; Heiniö, R.-L.; Autio, K.; Poutanen, K. Optimization of sourdough process for improved sensory profile and texture of wheat bread. LWT-Food Sci. Technol. 2006, 39, 1189–1202. [Google Scholar] [CrossRef]
- Casado, A.; Álvarez, A.; González, L.; Fernández, D.; Marcos, J.L.; Tornadijo, M.E. Effect of fermentation on microbiological, physicochemical and physical characteristics of sourdough and impact of its use on bread quality. Czech J. Food Sci. 2017, 35, 496. [Google Scholar] [CrossRef]
- Crowley, P.; Schober, T.J.; Clarke, C.I.; Arendt, E.K. The effect of storage time on textural and crumb grain characteristics of sourdough wheat bread. Eur. Food Res. Technol. 2002, 214, 489–496. [Google Scholar] [CrossRef]
- Pourmohammadi, K.; Abedi, E. Hydrolytic enzymes and their directly and indirectly effects on gluten and dough properties: An extensive review. Food Sci. Nutr. 2021, 9, 3988–4006. [Google Scholar] [CrossRef] [PubMed]
- Paramithiotis, S.; Sofou, A.; Tsakalidou, E.; Kalantzopoulos, G. Flour carbohydrate catabolism and metabolite production by sourdough lactic acid bacteria. World J. Microbiol. Biotechnol. 2007, 23, 1417–1423. [Google Scholar] [CrossRef]
- Sahin, A.W.; Zannini, E.; Coffey, A.; Arendt, E.K. Sugar reduction in bakery products: Current strategies and sourdough technology as a potential novel approach. Food Res. Int. 2019, 126, 108583. [Google Scholar] [CrossRef] [PubMed]
- Van Boekel, M. Kinetic aspects of the Maillard reaction: A critical review. Food/Nahrung 2001, 45, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cheng, W.; Wang, X.; Wang, M.; Chen, F.; Cheng, K.-W. A novel formation pathway of Nε-(carboxyethyl) lysine from lactic acid during high temperature exposure in wheat sourdough bread and chemical model. Food Chem. 2022, 388, 132942. [Google Scholar] [CrossRef] [PubMed]
- Van Kerrebroeck, S.; Comasio, A.; Harth, H.; De Vuyst, L. Impact of starter culture, ingredients, and flour type on sourdough bread volatiles as monitored by selected ion flow tube-mass spectrometry. Food Res. Int. 2018, 106, 254–262. [Google Scholar] [CrossRef]
- Damiani, P.; Gobbetti, M.; Cossignani, L.; Corsetti, A.; Simonetti, M.S.; Rossi, J. The sourdough microflora. Characterization of hetero-and homofermentative lactic acid bacteria, yeasts and their interactions on the basis of the volatile compounds produced. LWT-Food Sci. Technol. 1996, 29, 63–70. [Google Scholar] [CrossRef]
- Xi, J.; Zhao, Q.; Xu, D.; Jin, Y.; Wu, F.; Jin, Z.; Xu, X. Volatile compounds in Chinese steamed bread influenced by fermentation time, yeast level and steaming time. LWT 2021, 141, 110861. [Google Scholar] [CrossRef]
- Pizarro, F.; Franco, F., Jr. Volatile organic compounds at early stages of sourdough preparation via static headspace and GC/MS analysis. Curr. Res. Nutr. Food Sci. J. 2017, 5, 89–99. [Google Scholar] [CrossRef]
- Mietton, L.; Samson, M.-F.; Marlin, T.; Godet, T.; Nolleau, V.; Guezenec, S.; Segond, D.; Nidelet, T.; Desclaux, D.; Sicard, D. Impact of Leavening Agent and Wheat Variety on Bread Organoleptic and Nutritional Quality. Microorganisms 2022, 10, 1416. [Google Scholar] [CrossRef] [PubMed]

| TTA | pH | |||
|---|---|---|---|---|
| LAB | 0 h | 24 h | 0 h | 24 h |
| Control I | 1.37 ± 0.08 b | 2.12 ± 0.03 d | 6.42 ± 0.02 b | 6.01 ± 0.05 b |
| Control II | 1.33 ± 0.03 b | 2.28 ± 0.03 d | 6.51 ± 0.02 a | 6.04 ± 0.05 a |
| MIX | 2.02 ± 0.03 a | 10.45 ± 0.09 b | 5.20 ± 0.02 e | 3.43 ± 0.01 e |
| SC-9 | 2.13 ± 0.06 a | 10.15 ± 0.10 c | 4.98 ± 0.04 f | 3.21 ± 0.02 f |
| N-15 | 2.05 ± 0.05 a | 10.35 ± 0.05 b | 5.49 ± 0.01 c | 3.68 ± 0.02 c |
| E-106 | 2.03 ± 0.03 a | 10.72 ± 0.10 a | 5.38 ± 0.02 d | 3.55 ± 0.03 d |
| pH | TTA | |||||
|---|---|---|---|---|---|---|
| LAB | First Dough | Last Dough | Bread Dough | First Dough | Last Dough | Bread Dough |
| Control I | 6.53 ± 0.07 a | 6.14 ± 0.04 a | 6.05 ± 0.02 a | 1.10 ± 0.05 c | 1.92 ± 0.03 d | 1.82 ± 0.06 d |
| Control II | 6.42 ± 0.03 b | 6.09 ± 0.03 b | 5.97 ± 0.02 b | 1.13 ± 0.03 c | 1.93 ± 0.03 d | 1.83 ± 0.07 d |
| MIX | 5.54 ± 0.07 c | 4.97 ± 0.08 c | 4.89 ± 0.04 c | 2.33 ± 0.03 a | 4.48 ± 0.08 a | 4.29 ± 0.07 a |
| SC-9 | 5.48 ± 0.04 e | 4.74 ± 0.06 e | 4.68 ± 0.03 e | 2.27 ± 0.03 a | 4.08 ± 0.06 b | 3.95 ± 0.03 b |
| N-15 | 5.51 ± 0.03 d | 4.68 ± 0.04 f | 4.60 ± 0.04 f | 2.13 ± 0.03 b | 4.47 ± 0.08 a | 4.27 ± 0.04 a |
| E-106 | 5.31 ± 0.01 f | 4.76 ± 0.04 d | 4.69 ± 0.03 d | 2.12 ± 0.03 b | 3.72 ± 0.03 c | 3.42 ± 0.06 c |
| LAB | Specific Volume (cm3/g) | Bake Loss (%) | Porosity (%) | Circularity | WI (Crumb) | BI (Crust) |
|---|---|---|---|---|---|---|
| Control I | 2.99 ± 0.04 cd | 13.07 ± 0.11 a | 40.69 ± 0.44 d | 0.753 ± 0.006 ab | 34.08 ± 0.10 a | 36.09 ± 0.19 a |
| Control II | 3.01 ± 0.05 bc | 12.39 ± 0.23 b | 44.74 ± 1.05 c | 0.752 ± 0.011 ab | 33.54 ± 0.60 a | 35.92 ± 0.19 a |
| MIX | 3.03 ± 0.07 ab | 12.15 ± 0.38 b | 45.58 ± 0.52 bc | 0.766 ± 0.012 a | 31.89 ± 0.08 b | 34.22 ± 0.09 c |
| SC-9 | 3.01 ± 0.06 bc | 12.64 ± 0.03 ab | 46.38 ± 0.78 abc | 0.728 ± 0.009 b | 30.20 ± 0.06 c | 32.27 ± 0.12 d |
| N-15 | 2.97 ± 0.06 d | 12.75 ± 0.09 ab | 47.42 ± 0.35 ab | 0.744 ± 0.012 ab | 32.08 ± 0.31 b | 35.12 ± 0.14 b |
| E-106 | 3.04 ± 0.04 a | 12.66 ± 0.38 ab | 47.71 ± 1.04 a | 0.754 ± 0.012 ab | 32.05 ± 0.63 b | 32.80 ± 0.48 d |
| Bread Type | Protein (%) | Fat (%) | Ash (%) | Moisture (%) | Carbohydrate (%) |
|---|---|---|---|---|---|
| Control I | 6.85 ± 0.10 a | 0.257 ± 0.003 a | 0.602 ± 0.008 a | 47.30 ± 0.10 cd | 45.01 ± 0.19 a |
| Control II | 6.83 ± 0.09 a | 0.246 ± 0.010 a | 0.606 ± 0.019 a | 47.19 ± 0.04 d | 45.15 ± 0.09 a |
| MIX | 6.85 ± 0.11 a | 0.253 ± 0.001 a | 0.605 ± 0.010 a | 47.36 ± 0.01 c | 44.95 ± 0.10 ab |
| SC-9 | 6.94 ± 0.05 a | 0.259 ± 0.010 a | 0.582 ± 0.045 a | 47.7 ± 0.01 a | 44.55 ± 0.06 c |
| N-15 | 6.81 ± 0.03 a | 0.254 ± 0.014 a | 0.608 ± 0.019 a | 47.68 ± 0.01 a | 44.66 ± 0.04 bc |
| E-106 | 6.77 ± 0.09 a | 0.260 ± 0.013 a | 0.587 ± 0.031 a | 47.56 ± 0.02 b | 44.85 ± 0.09 ab |
| LAB | TRP Fluorescence (290/340 nm) | AMP Fluorescence (340/420 nm) | FAST Index (%) |
|---|---|---|---|
| Control I | 5866.44 ± 64.59 e | 4175.27 ± 58.44 c | 71.17 ± 0.80 b |
| Control II | 5986.11 ± 52.44 e | 4862.04 ± 76.14 b | 81.22 ± 0.79 a |
| MIX | 8438.21 ± 94.53 b | 3415.15 ± 69.01 d | 40.48 ± 0.99 f |
| SC-9 | 11,201.59 ± 80.30 a | 5240.63 ± 72.46 a | 46.79 ± 0.98 e |
| N-15 | 7597.42 ± 19.50 d | 4126.39 ± 62.15 c | 54.31 ± 0.95 d |
| E-106 | 8092.97 ± 84.43 c | 4833.05 ± 95.84 b | 59.71 ± 0.61 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahin, M.; Ozgolet, M.; Cankurt, H.; Dertli, E. Harnessing the Role of Three Lactic Acid Bacteria (LAB) Strains for Type II Sourdough Production and Influence of Sourdoughs on Bread Quality and Maillard Reaction Products. Foods 2024, 13, 1801. https://doi.org/10.3390/foods13121801
Sahin M, Ozgolet M, Cankurt H, Dertli E. Harnessing the Role of Three Lactic Acid Bacteria (LAB) Strains for Type II Sourdough Production and Influence of Sourdoughs on Bread Quality and Maillard Reaction Products. Foods. 2024; 13(12):1801. https://doi.org/10.3390/foods13121801
Chicago/Turabian StyleSahin, Mustafa, Muhammed Ozgolet, Hasan Cankurt, and Enes Dertli. 2024. "Harnessing the Role of Three Lactic Acid Bacteria (LAB) Strains for Type II Sourdough Production and Influence of Sourdoughs on Bread Quality and Maillard Reaction Products" Foods 13, no. 12: 1801. https://doi.org/10.3390/foods13121801
APA StyleSahin, M., Ozgolet, M., Cankurt, H., & Dertli, E. (2024). Harnessing the Role of Three Lactic Acid Bacteria (LAB) Strains for Type II Sourdough Production and Influence of Sourdoughs on Bread Quality and Maillard Reaction Products. Foods, 13(12), 1801. https://doi.org/10.3390/foods13121801







