Commercial Production of Highly Rehydrated Soy Protein Powder by the Treatment of Soy Lecithin Modification Combined with Alcalase Hydrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparations
2.2.1. Preparation of SPI
2.2.2. Preparation of SPI–SL
2.2.3. Preparation of SPH–SL
2.3. Determination of the Degree of Hydrolysis (DH)
2.4. Average Particle Size and Zeta Potential
2.5. Turbidity
2.6. Scanning Electron Microscopy
2.7. FTIR Analysis
2.8. Surface Hydrophobicity
2.9. Bulk Density
2.10. Rehydration Properties
2.10.1. Wettability
2.10.2. Dispersibility
2.10.3. Solubility
2.11. Viscosity
2.12. Emulsify Property
2.13. Statistical Analysis
3. Results
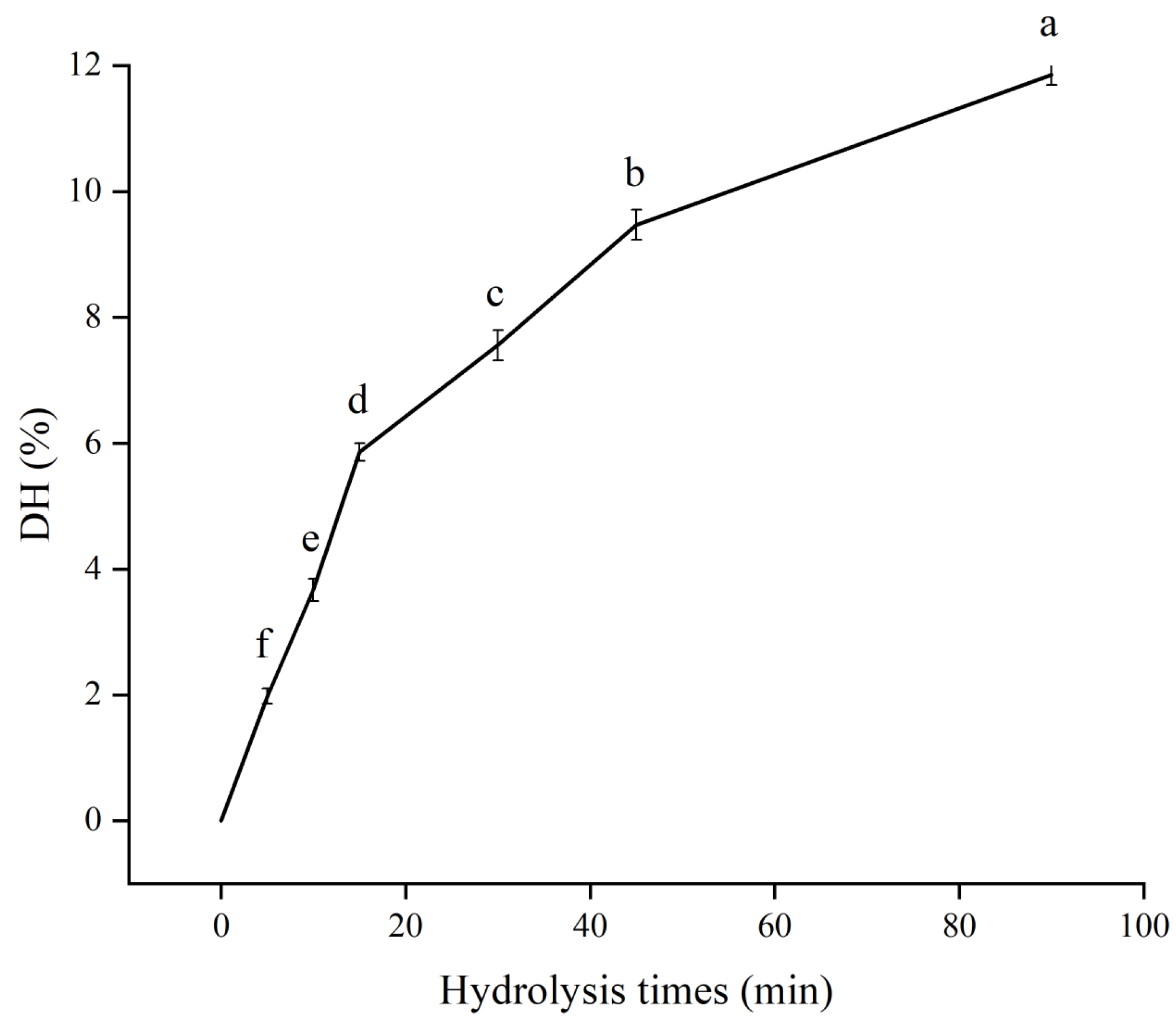
3.1. The DH of SPH−SL at Different Hydrolysis Times
3.2. The Particle Size of SPH−SL at Different Hydrolysis Times
3.3. The Turbidity of SPH−SL at Different Hydrolysis Times
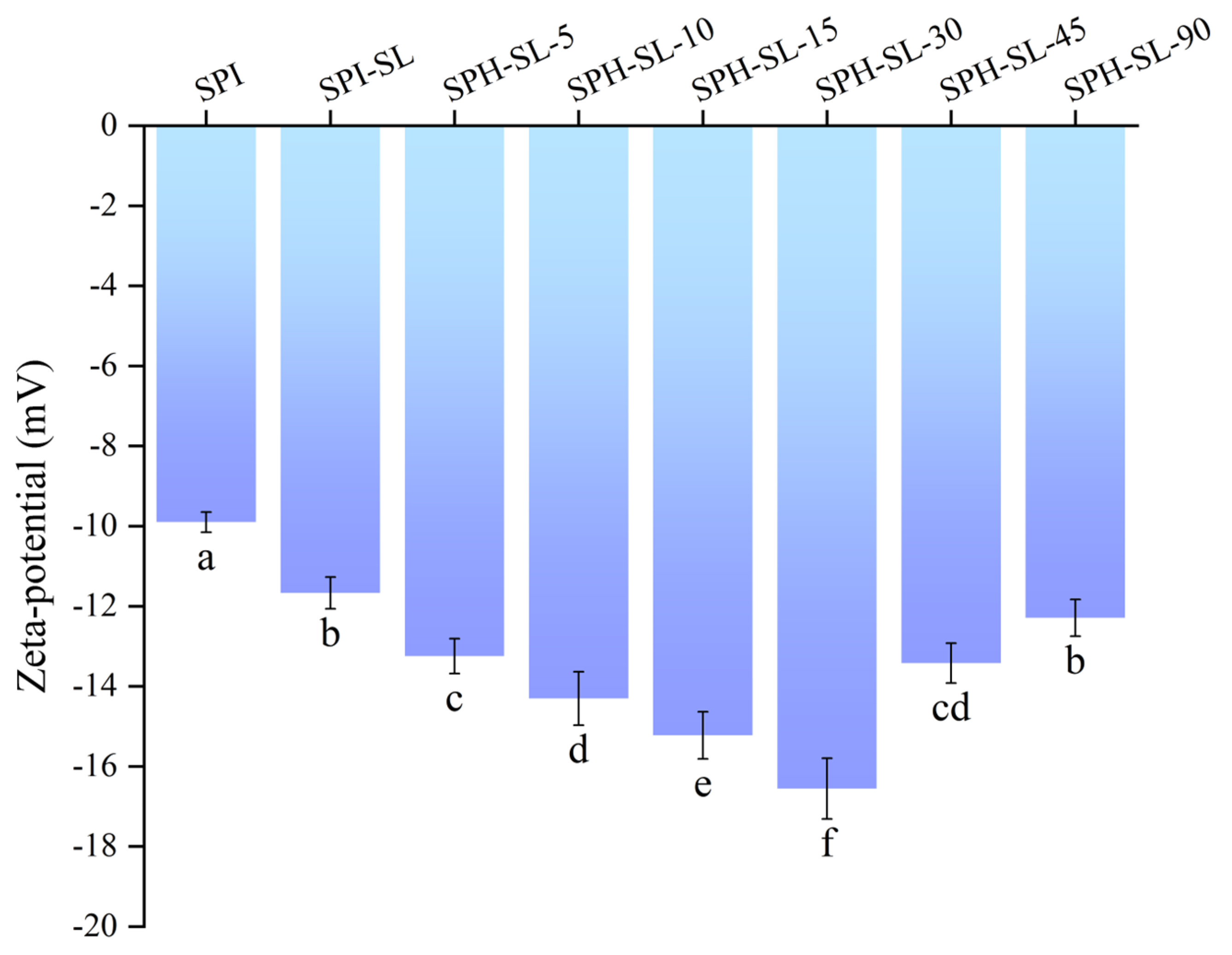
3.4. The Zeta of SPH−SL at Different Hydrolysis Times
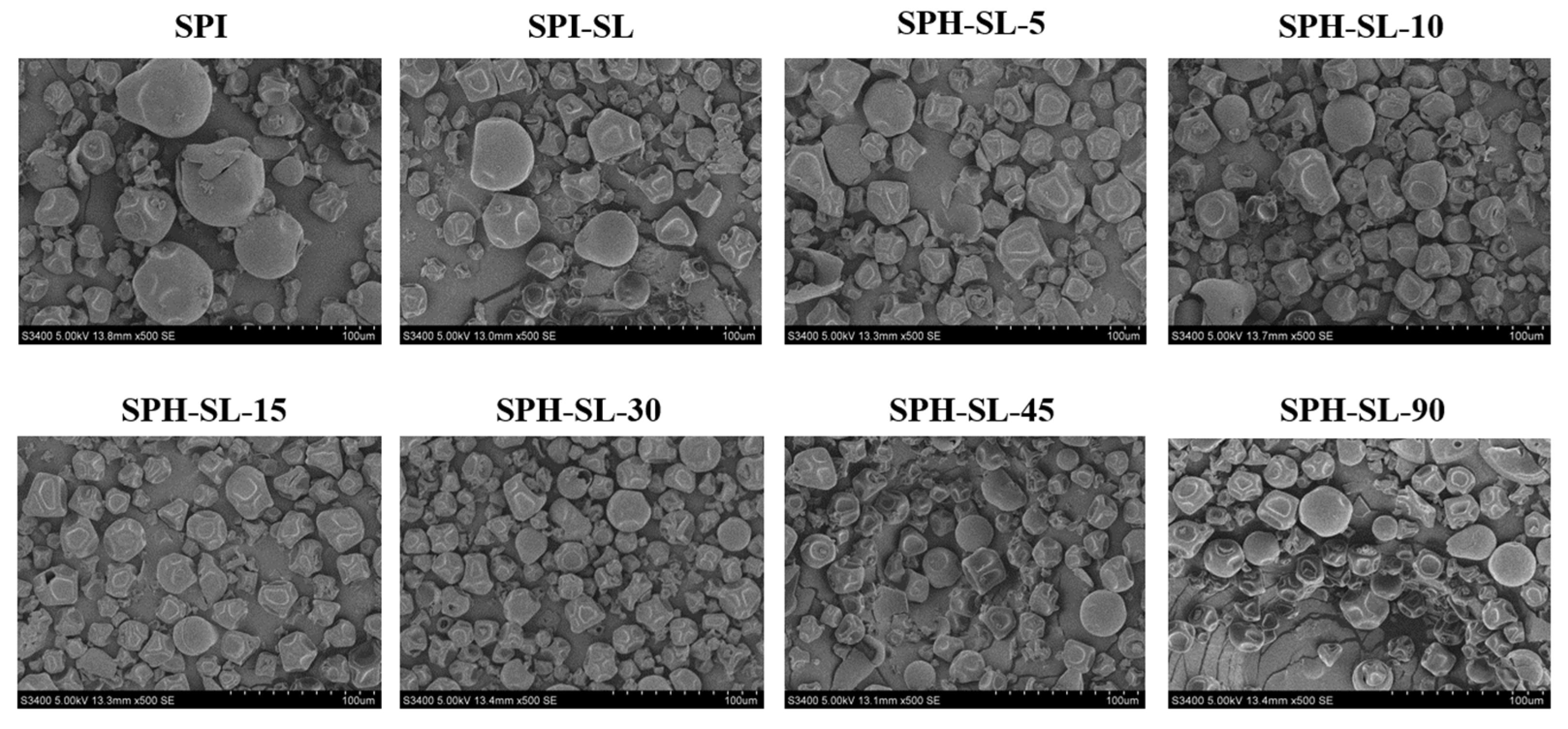
3.5. The Morphology of SPH−SL at Different Hydrolysis Times
3.6. The FTIR Spectra of SPH−SL at Different Hydrolysis Times
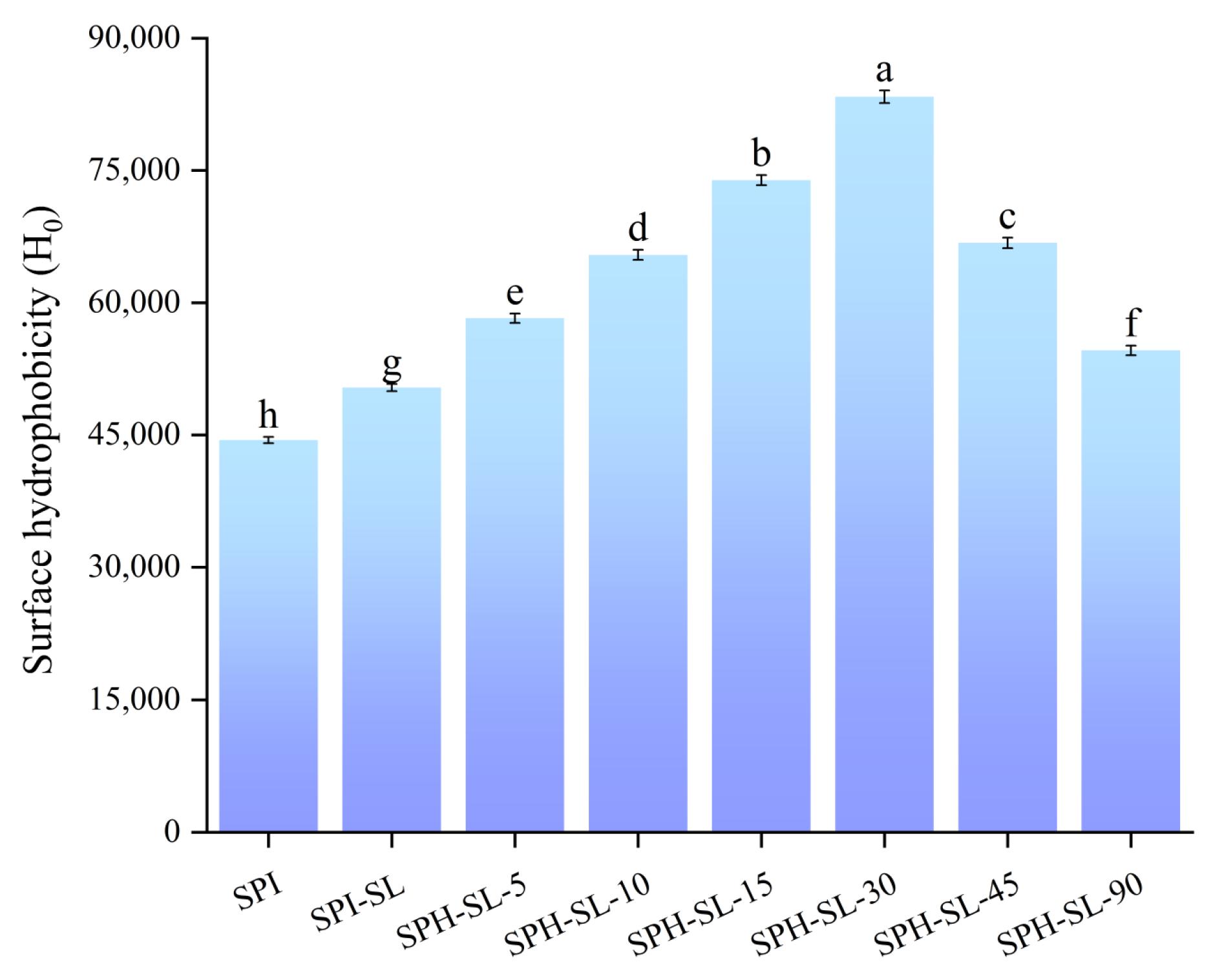
3.7. The Surface Hydrophobicity of SPH−SL at Different Hydrolysis Times
3.8. The Bulk Density of SPH−SL at Different Hydrolysis Times
3.9. The Wettability of SPH−SL at Different Hydrolysis Times
3.10. The Dispersibility of SPH−SL at Different Hydrolysis Times
3.11. The Solubility of SPH−SL at Different Hydrolysis Times
3.12. The Viscosity of SPH−SL at Different Hydrolysis Times
3.13. The EAI and ESI of SPH−SL at Different Hydrolysis Times
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aydemir, L.Y.; Yemenicioğlu, A. Potential of Turkish Kabuli type chickpea and green and red lentil cultivars as source of soy and animal origin functional protein alternatives. LWT 2013, 50, 686–694. [Google Scholar] [CrossRef]
- de Paiva Gouvêa, L.; Caldeira, R.; de Lima Azevedo, T.; Galdeano, M.C.; Felberg, I.; Lima, J.R.; Grassi Mellinger, C. Physical and technofunctional properties of a common bean protein concentrate compared to commercial legume ingredients for the plantbased market. Food Hydrocoll. 2023, 137, 108351. [Google Scholar] [CrossRef]
- Watanabe, K.; Igarashi, M.; Li, X.; Nakatani, A.; Miyamoto, J.; Inaba, Y.; Sutou, A.; Saito, T.; Sato, T.; Tachibana, N.; et al. Dietary soybean protein ameliorates highfat dietinduced obesity by modifying the gut microbiotadependent biotransformation of bile acids. PLoS ONE 2018, 13, e0202083. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Ma, T.; Zhao, J. Physicochemical and functional properties of γaminobutyric acidtreated soy proteins. Food Chem. 2019, 295, 267–273. [Google Scholar] [CrossRef]
- Syll, O.; Khalloufi, S.; Méjean, S.; Schuck, P. The effects of total protein/total solid ratio and pH on the spray drying process and rehydration properties of soy powder. Powder Technol. 2016, 289, 60–64. [Google Scholar] [CrossRef]
- Yang, Q.; Venema, P.; van der Linden, E.; de Vries, R. Soluble protein particles produced directly from mung bean flour by simple coacervation. Food Hydrocoll. 2023, 139, 108541. [Google Scholar] [CrossRef]
- Hellborg, D.; Bergenståhl, B.; Trägårdh, C. A method for measuring the imbibation rate of powder in a liquid. Chem. Eng. Process. 2010, 49, 599–604. [Google Scholar] [CrossRef]
- Bernardo, C.O.; Ascheri, J.L.R.; Carvalho, C.W.P.; Chávez, D.W.H.; Martins, I.B.A.; Deliza, R.; Freitas, D.d.G.C.; Queiroz, V.A.V. Impact of extruded sorghum genotypes on the rehydration and sensory properties of soluble beverages and the Brazilian consumers’ perception of sorghum and cereal beverage using word association. J. Cereal Sci. 2019, 89, 102793. [Google Scholar] [CrossRef]
- Wu, S.; Fitzpatrick, J.; Cronin, K.; Maidannyk, V.; Miao, S. Effects of spraying surfactants in a fluidised bed on the rehydration behaviour of milk protein isolate powder. J. Food Eng. 2020, 266, 109694. [Google Scholar] [CrossRef]
- Miyasaki, E.K.; Luccas, V.; Kieckbusch, T.G. Modified soybean lecithins as inducers of the acceleration of cocoa butter crystallization. Eur. J. Lipid Sci. Technol. 2016, 118, 1539–1549. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Y.; Zhang, M.; Jiang, H.; Yan, Z.; Liu, J.; Liu, X. Effect of soy lecithin concentration on physiochemical properties and rehydration behavior of egg white protein powder: Role of dry and wet mixing. J. Food Eng. 2022, 328, 111062. [Google Scholar] [CrossRef]
- Lallbeeharry, P.; Tian, Y.; Fu, N.; Wu, W.D.; Woo, M.W.; Selomulya, C.; Chen, X.D. Effects of ionic and nonionic surfactants on milk shell wettability during co−spray−drying of whole milk particles. J. Dairy Sci. 2014, 97, 5303–5314. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, J.; Li, J.; Sun, H.; Liu, Y. Physicochemical and antioxidative characteristics of black bean protein hydrolysates obtained from different enzymes. Food Hydrocoll. 2019, 97, 105222. [Google Scholar] [CrossRef]
- Fu, X.; Huang, X.; Jin, Y.; Zhang, S.; Ma, M. Characterization of enzymatically modified liquid egg yolk: Structural, interfacial and emulsifying properties. Food Hydrocoll. 2020, 105, 105763. [Google Scholar] [CrossRef]
- Cornelly, V.D.V.; Gruppen, H.; De Bont, D.B.A.; Voragen, A.G.J. Emulsion properties of casein and whey protein hydrolysates and the relation with other hydrolysate characteristics. J. Agric. Food. Chem. 2001, 49, 5005–5012. [Google Scholar]
- Lian, Z.; Yang, S.; Cheng, L.; Liao, P.; Dai, S.; Tong, X.; Tian, T.; Wang, H.; Jiang, L. Emulsifying properties and oil–water interface properties of succinylated soy protein isolate: Affected by conformational flexibility of the interfacial protein. Food Hydrocoll. 2023, 136, 108224. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, Z.; Yang, M.; Zhang, Y.; Wu, T.; Liu, R.; Sui, W.; Zhang, M. Micronization using combined alkaline protease hydrolysis and highspeed shearing homogenization for improving the functional properties of soy protein isolates. Bioresour. Bioprocess. 2022, 9, 77. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, T.; Liu, C.; Guo, F.; Zhao, J. lHistidine improves solubility and emulsifying properties of soy proteins under various ionic strengths. LWT 2021, 152, 112382. [Google Scholar] [CrossRef]
- Lee, J.D.; Shannon, J.G.; Choung, M.G. Selection for protein content in soybean from single F2 seed by near infrared reflectance spectroscopy. Euphytica 2010, 172, 117–123. [Google Scholar] [CrossRef]
- Beck, S.M.; Knoerzer, K.; Arcot, J. Effect of low moisture extrusion on a pea protein isolate’s expansion, solubility, molecular weight distribution and secondary structure as determined by Fourier Transform Infrared Spectroscopy (FTIR). J. Food Eng. 2017, 214, 166–174. [Google Scholar] [CrossRef]
- Vidović, S.S.; Vladić, J.Z.; Vaštag, Ž.G.; Zeković, Z.P.; Popović, L.M. Maltodextrin as a carrier of health benefit compounds in Satureja montana dry powder extract obtained by spray drying technique. Powder Technol. 2014, 258, 209–215. [Google Scholar] [CrossRef]
- Gong, Q.; Liu, C.; Tian, Y.; Zheng, Y.; Wei, L.; Cheng, T.; Wang, Z.; Guo, Z.; Zhou, L. Effect of cavitation jet technology on instant solubility characteristics of soymilk flour: Based on the change of protein conformation in soymilk. Ultrason. Sonochem. 2023, 96, 106421. [Google Scholar] [CrossRef]
- Felix da Silva, D.; Ahrné, L.; Larsen, F.H.; Hougaard, A.B.; Ipsen, R. Physical and functional properties of cheese powders affected by sweet whey powder addition before or after spray drying. Powder Technol. 2018, 323, 139–148. [Google Scholar] [CrossRef]
- Tan, L.; Hong, P.; Yang, P.; Zhou, C.; Xiao, D.; Zhong, T. Correlation Between the Water Solubility and Secondary Structure of TilapiaSoybean Protein CoPrecipitates. Molecules 2019, 24, 4337. [Google Scholar] [CrossRef]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Wang, B.; Meng, T.; Ma, H.; Zhang, Y.; Li, Y.; Jin, J.; Ye, X. Mechanism study of dualfrequency ultrasound assisted enzymolysis on rapeseed protein by immobilized Alcalase. Ultrason. Sonochem. 2016, 32, 307–313. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, L.; Shi, Y.; Akhtar, M.; Chen, J.; Ettelaie, R. Emulsifying and emulsion stabilizing properties of soy protein hydrolysates, covalently bonded to polysaccharides: The impact of enzyme choice and the degree of hydrolysis. Food Hydrocoll. 2021, 113, 106519. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Qu, W.; Ren, X.; Lu, F.; Tian, W.; Quaisie, J.; Azam, S.M.R.; Ma, H. Effect of innovative ultrasonic frequency excitation modes on rice protein: Enzymolysis and structure. LWT 2022, 153, 112435. [Google Scholar] [CrossRef]
- Surówka, K.; Żmudziński, D.; Surówka, J. Enzymic modification of extruded soy protein concentrates as a method of obtaining new functional food components. Trends Food Sci. Technol. 2004, 15, 153–160. [Google Scholar] [CrossRef]
- Xie, Y.; Min, S.; Harte, N.P.; Kirk, H.; O’Brien, J.E.; Voorheis, H.P.; Svanborg, C.; Hun Mok, K. Electrostatic interactions play an essential role in the binding of oleic acid with αlactalbumin in the HAMLETlike complex: A study using chargespecific chemical modifications. Proteins 2013, 81, 1–17. [Google Scholar] [CrossRef]
- Schmid, E.M.; Farahnaky, A.; Adhikari, B.; Savadkoohi, S.; Torley, P.J. Investigation into the physiochemical properties of soy protein isolate and concentrate powders from different manufacturers. Int. J. Food Sci. Technol. 2024, 59, 1679–1693. [Google Scholar] [CrossRef]
- He, X.; Chen, J.; He, X.; Feng, Z.; Li, C.; Liu, W.; Dai, T.; Liu, C. Industryscale microfluidization as a potential technique to improve solubility and modify structure of pea protein. Innov. Food Sci. Emerg. Technol. 2021, 67, 102582. [Google Scholar] [CrossRef]
- Lawler, D. Encyclopedia of Analytical Science, 3rd ed.; Academic Press: New York, NY, USA, 2016; pp. 152–163. [Google Scholar]
- Hall, D.; Zhao, R.; Dehlsen, I.; Bloomfield, N.; Williams, S.R.; Arisaka, F.; Goto, Y.; Carver, J.A. Protein aggregate turbidity: Simulation of turbidity profiles for mixed aggregation reactions. Anal. Biochem. 2016, 498, 78–94. [Google Scholar] [CrossRef]
- Dabbour, M.I.; Xiang, J.; Mintah, B.; He, R.; Ma, H. Localized enzymolysis and sonochemically modified sunflower protein: Physical, functional and structure attributes. Ultrason. Sonochem. 2020, 63, 104957. [Google Scholar] [CrossRef]
- Shen, Y.; Chang, C.; Shi, M.; Su, Y.; Gu, L.; Li, J.; Yang, Y. Interactions between lecithin and yolk granule and their influence on the emulsifying properties. Food Hydrocoll. 2020, 101, 105510. [Google Scholar] [CrossRef]
- Guo, Z.; Huang, Z.; Guo, Y.; Li, B.; Yu, W.; Zhou, L.; Jiang, L.; Teng, F.; Wang, Z. Effects of highpressure homogenization on structural and emulsifying properties of thermally soluble aggregated kidney bean (Phaseolus vulgaris L.) proteins. Food Hydrocoll. 2021, 119, 106835. [Google Scholar] [CrossRef]
- Xue, J.; Zhong, Q. Blending Lecithin and Gelatin Improves the Formation of Thymol Nanodispersions. J. Agric. Food Chem. 2014, 62, 2956–2962. [Google Scholar] [CrossRef]
- Pinto, I.; Buss, A. ζ Potential as a Measure of Asphalt Emulsion Stability. Energy Fuels 2020, 34, 2143–2151. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, J.; Yu, X.; Yagoub, E.G.A.; Zhang, Y.; Ma, H.; Gao, X.; Otu, P.N.Y. Heat and/or ultrasound pretreatments motivated enzymolysis of corn gluten meal: Hydrolysis kinetics and protein structure. LWT 2016, 77, 488–496. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, L.; Zhang, X.; Liu, H.; Zhu, Y.J.C.; Biointerfaces, S.B. Effects of ultrafine grinding time on the functional and flavor properties of soybean protein isolate. Colloids Surf. B 2020, 196, 111345. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, X.Y.; Zhong, N.J.; Xu, J.R. Study on the Conformation of Soybean Selenoprotein Solution with Spectroscopy Methods. Spectrosc. Spect. Anal. 2016, 36, 2874–2878. [Google Scholar]
- Zhang, Q.; Li, L.; Chen, L.; Liu, S.; Cui, Q.; Qin, W. Effects of Sequential Enzymolysis and Glycosylation on the Structural Properties and Antioxidant Activity of Soybean Protein Isolate. Antioxidants 2023, 12, 430. [Google Scholar] [CrossRef]
- Chen, X.; Ru, Y.; Chen, F.; Wang, X.; Zhao, X.; Ao, Q. FTIR spectroscopic characterization of soy proteins obtained through AOT reverse micelles. Food Hydrocoll. 2013, 31, 435–437. [Google Scholar] [CrossRef]
- Fan, M.; Hu, T.; Zhao, S.; Xiong, S.; Xie, J.; Huang, Q. Gel characteristics and microstructure of fish myofibrillar protein/cassava starch composites. Food Chem. 2017, 218, 221–230. [Google Scholar] [CrossRef]
- Xie, H.; Liu, C.; Gao, J.; Shi, J.; Ni, F.; Luo, X.; He, Y.; Ren, G.; Luo, Z. Fabrication of ZeinLecithinEGCG complex nanoparticles: Characterization, controlled release in simulated gastrointestinal digestion. Food Chem. 2021, 365, 130542. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Deng, X.; Li, Z.; Lian, W.; Zhang, G.; Zhu, Y.; Zhu, X. Effect of enzymatic hydrolysis followed after extrusion pretreatment on the structure and emulsibility of soybean protein. Process Biochem. 2022, 116, 173–184. [Google Scholar]
- Pan, K.M.; Baldwin, M.; Nguyen, J.; Gasset, M.; Serban, A.N.A.; Groth, D.; Mehlhorn, I.; Huang, Z.; Fletterick, R.J.; Cohen, F.E. Conversion of alphahelices into betasheets features in the formation of the scrapie prion proteins. Proc. Natl. Acad. Sci. USA 1993, 90, 10962–10966. [Google Scholar] [CrossRef]
- Zhu, Y.; Fu, S.; Wu, C.; Qi, B.; Teng, F.; Wang, Z.; Li, Y.; Jiang, L. The investigation of protein flexibility of various soybean cultivars in relation to physicochemical and conformational properties. Food Hydrocoll. 2020, 103, 105709. [Google Scholar] [CrossRef]
- Yang, H.; Gao, Y.; Sun, S.; Qu, Y.; Ji, S.; Wu, R.; Wu, J. Formation, characterization, and antigenicity of lecithinβconglycinin complexes. Food Chem. 2022, 407, 135178. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, X.; Tang, S.; Mi, S.; Lu, L.; Zeng, Q.; Xia, M.; Cai, Z. Improved effect of ultrasoundassisted enzymolysis on egg yolk powder: Structural properties, hydration properties and stability characteristics. Food Chem. 2022, 382, 132549. [Google Scholar] [CrossRef]
- Zou, H.; Zhao, N.; Li, S.; Sun, S.; Yu, C. Physicochemical and emulsifying properties of mussel watersoluble proteins as affected by lecithin concentration. Int. J. Biol. Macromol. 2020, 163, 180–189. [Google Scholar] [CrossRef]
- Shuai, X.; Gao, L.; Geng, Q.; Li, T.; He, X.; Chen, J.; Liu, C.; Dai, T. Effects of Moderate Enzymatic Hydrolysis on Structure and Functional Properties of Pea Protein. Foods 2022, 11, 2368. [Google Scholar] [CrossRef]
- Amiri, A.; Sharifian, P.; Morakabati, N.; MousakhaniGanjeh, A.; Mirtaheri, M.; Nilghaz, A.; Guo, Y.G.; PratapSingh, A. Modification of functional, rheological and structural characteristics of myofibrillar proteins by highintensity ultrasonic and papain treatment. Innov. Food Sci. Emerg. Technol. 2021, 72, 102748. [Google Scholar] [CrossRef]
- Schulze, D.; Schwedes, J.; Carson, J.W. Powders and Bulk Solids: Behavior, Characterization, Storage and Flow; Springer: Cham, Germany, 2008; pp. 1–511. [Google Scholar]
- Hazlett, R.; Schmidmeier, C.; O’Mahony, J.A. Influence of mechanical integrity during pneumatic conveying on the bulk handling and rehydration properties of agglomerated dairy powders. J. Food Eng. 2021, 288, 110103. [Google Scholar] [CrossRef]
- Silva, J.V.; O’Mahony, J.A. Flowability and wetting behaviour of milk protein ingredients as influenced by powder composition, particle size and microstructure. Int. J. Dairy Technol. 2017, 70, 277–286. [Google Scholar] [CrossRef]
- Hailu, Y.; Maidannyk, V.A.; Murphy, E.G.; Mccarthy, N.A. Improving the physical and wettability properties of skim milk powders through agglomeration and lecithination. J. Food Eng. 2023, 357, 111597. [Google Scholar] [CrossRef]
- Wilde, P.; Mackie, A.; Husband, F.; Gunning, P.; Morris, V. Proteins and emulsifiers at liquid interfaces. Adv. Colloid Interface Sci. 2004, 108–109, 63–71. [Google Scholar] [CrossRef]
- Fitzpatrick, J.J.; van Lauwe, A.; Coursol, M.; O’Brien, A.; Fitzpatrick, K.L.; Ji, J.; Miao, S. Investigation of the rehydration behaviour of food powders by comparing the behaviour of twelve powders with different properties. Powder Technol. 2016, 297, 340–348. [Google Scholar] [CrossRef]
- Freudig, B.; Hogekamp, S.; Schubert, H. Dispersion of powders in liquids in a stirred vessel. Chem. Eng. Process. 1999, 38, 525–532. [Google Scholar] [CrossRef]
- Ji, J.; Fitzpatrick, J.; Cronin, K.; Maguire, P.; Zhang, H.; Miao, S. Rehydration behaviours of high protein dairy powders: The influence of agglomeration on wettability, dispersibility and solubility. Food Hydrocoll. 2016, 58, 194–203. [Google Scholar] [CrossRef]
- Hu, Z.; Qiu, L.; Sun, Y.; Xiong, H.; Ogra, Y. Improvement of the solubility and emulsifying properties of rice bran protein by phosphorylation with sodium trimetaphosphate. Food Hydrocoll. 2019, 96, 288–299. [Google Scholar] [CrossRef]
- Pelegrine, D.H.G.; Gasparetto, C.A. Whey proteins solubility as function of temperature and pH. LWT 2005, 38, 77–80. [Google Scholar] [CrossRef]
- Bista, A.; Mccarthy, N.; O’Donnell, C.P.; O’Shea, N. Key parameters and strategies to control milk concentrate viscosity in milk powder manufacture. Int. Dairy J. 2021, 121, 105120. [Google Scholar] [CrossRef]
- Song, X.; Zhou, C.; Fu, F.; Chen, Z.; Wu, Q. Effect of highpressure homogenization on particle size and film properties of soy protein isolate. Ind. Crops Prod. 2013, 43, 538–544. [Google Scholar] [CrossRef]
- Chang, C.; Tu, S.; Ghosh, S.; Nickerson, M.T. Effect of pH on the interrelationships between the physicochemical, interfacial and emulsifying properties for pea, soy, lentil and canola protein isolates. Food Res. Int. 2015, 77, 360–367. [Google Scholar] [CrossRef]
- O’Flynn, T.D.; Hogan, S.A.; Daly, D.F.; O’Mahony, J.A.; McCarthy, N.A. Rheological and Solubility Properties of Soy Protein Isolate. Molecules 2021, 26, 3015. [Google Scholar] [CrossRef]
- Wang, S.; Shi, Y.; Tu, Z.; Zhang, L.; Wang, H.; Tian, M.; Zhang, N. Influence of soy lecithin concentration on the physical properties of whey protein isolate−stabilized emulsion and microcapsule formation. J. Food Eng. 2017, 207, 73–80. [Google Scholar] [CrossRef]





| Sample | α−Helix (%) | β−Sheet (%) | β−Turn (%) | Random Coil (%) | α−Helix/β−Sheet (%) |
|---|---|---|---|---|---|
| SPI | 21.18 ± 0.23 bc | 38.89 ± 0.22 f | 23.65 ± 0.12 b | 17.28 ± 0.54 cd | 54.46 ± 0.28 b |
| SPI−SL | 20.85 ± 0.22 c | 39.67 ± 0.25 e | 19.86 ± 0.20 e | 19.59 ± 0.29 a | 52.56 ± 0.21 c |
| SPH−SL−5 | 20.29 ± 0.23 d | 40.88 ± 0.18 d | 20.95 ± 0.21 d | 17.88 ± 0.38 b | 49.64 ± 0.70 d |
| SPH−SL−10 | 19.94 ± 0.20 d | 41.28 ± 0.14 c | 21.22 ± 0.21 cd | 17.57 ± 0.13 bc | 48.30 ± 0.33 e |
| SPH−SL−15 | 19.32 ± 0.20 e | 42.44 ± 0.24 b | 21.44 ± 0.22 c | 16.80 ± 0.19 d | 45.54 ± 0.72 f |
| SPH−SL−30 | 18.52 ± 0.24 f | 43.40 ± 0.23 a | 22.44 ± 0.28 b | 15.65 ± 0.19 e | 42.67 ± 0.35 g |
| SPH−SL−45 | 21.44 ± 0.20 b | 39.45 ± 0.22 e | 23.82 ± 0.15 a | 15.29 ± 0.36 e | 54.35 ± 0.27 b |
| SPH−SL−90 | 22.83 ± 0.30 a | 37.95 ± 0.20 g | 23.99 ± 0.22 a | 15.23 ± 0.28 e | 60.16 ± 0.45 a |
| Sample | Bulk Density (Kg/m3) | Wettability (min) | Dispersibility (s) | Solubility (%) |
|---|---|---|---|---|
| SPI | 0.2046 ± 0.0007 g | 7.06 ± 0.10 a | 31.28 ± 2.03 a | 54.15 ± 0.60 f |
| SPI−SL | 0.2095 ± 0.0013 f | 6.22 ± 0.06 b | 26.12 ± 1.92 b | 57.94 ± 0.23 e |
| SPH−SL−5 | 0.2137 ± 0.0015 e | 4.04 ± 0.05 d | 23.69 ± 1.98 b | 63.19 ± 0.57 d |
| SPH−SL−10 | 0.2285 ± 0.0020 c | 3.29 ± 0.04 e | 19.29 ± 1.52 c | 71.70 ± 1.36 c |
| SPH−SL−15 | 0.2318 ± 0.0028 bc | 3.24 ± 0.03 e | 13.51 ± 1.35 d | 85.30 ± 0.65 b |
| SPH−SL−30 | 0.2629 ± 0.0040 a | 3.04 ± 0.02 f | 12.29 ± 1.58 d | 93.17 ± 0.74 a |
| SPH−SL−45 | 0.2331 ± 0.0029 b | 3.26 ± 0.05 e | 18.17 ± 1.57 c | 86.22 ± 0.27 b |
| SPH−SL−90 | 0.2194 ± 0.0015 d | 5.19 ± 0.07 c | 20.25 ± 1.75 c | 71.08 ± 1.00 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, S.; Du, Y.; Zhang, J.; Zhao, K.; Guo, Z.; Wang, Z. Commercial Production of Highly Rehydrated Soy Protein Powder by the Treatment of Soy Lecithin Modification Combined with Alcalase Hydrolysis. Foods 2024, 13, 1800. https://doi.org/10.3390/foods13121800
Ren S, Du Y, Zhang J, Zhao K, Guo Z, Wang Z. Commercial Production of Highly Rehydrated Soy Protein Powder by the Treatment of Soy Lecithin Modification Combined with Alcalase Hydrolysis. Foods. 2024; 13(12):1800. https://doi.org/10.3390/foods13121800
Chicago/Turabian StyleRen, Shuanghe, Yahui Du, Jiayu Zhang, Kuangyu Zhao, Zengwang Guo, and Zhongjiang Wang. 2024. "Commercial Production of Highly Rehydrated Soy Protein Powder by the Treatment of Soy Lecithin Modification Combined with Alcalase Hydrolysis" Foods 13, no. 12: 1800. https://doi.org/10.3390/foods13121800
APA StyleRen, S., Du, Y., Zhang, J., Zhao, K., Guo, Z., & Wang, Z. (2024). Commercial Production of Highly Rehydrated Soy Protein Powder by the Treatment of Soy Lecithin Modification Combined with Alcalase Hydrolysis. Foods, 13(12), 1800. https://doi.org/10.3390/foods13121800






