Selenium-Chelating Peptide Derived from Wheat Gluten: In Vitro Functional Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of WPH-Se
2.3. Analysis of Free Amino Acids after In Vitro Digestion of WPH-Se
2.4. Effect of Temperature and pH on In Vitro Antioxidant Activity
2.4.1. The DPPH Radical Scavenging Activity
2.4.2. The OH Radical Scavenging Activity
2.4.3. The ABTS Radical Scavenging Activity
2.4.4. Reducing Capacity
2.5. Determination of Antioxidant Activity of WPH-Se In Vitro Digestion
2.6. Statistical Analysis
3. Results and discussion
3.1. Fabrication of WPH-SE
3.1.1. Changes in Free Amino Acids of WPH-Se In Vitro Digestion
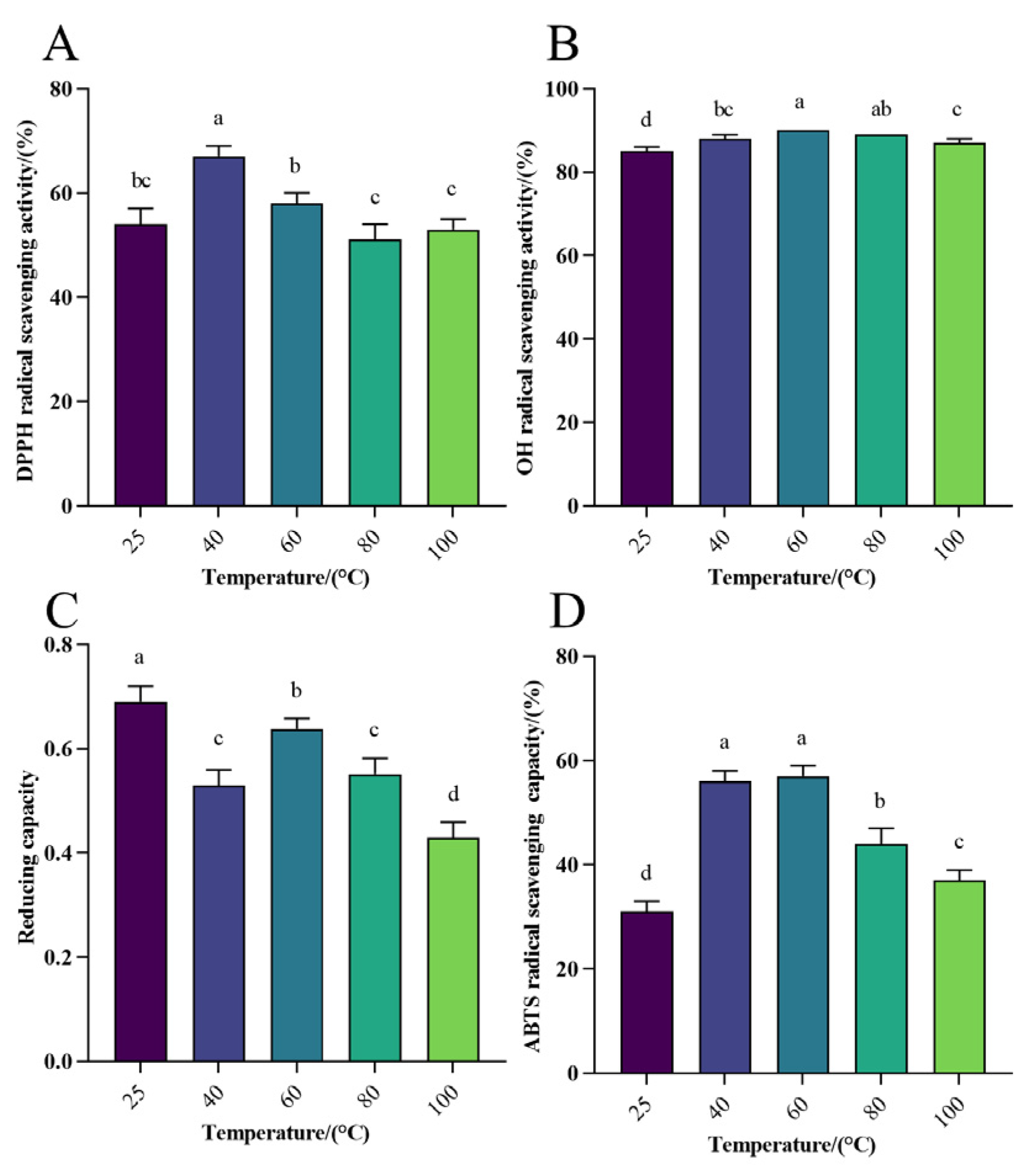
3.1.2. Antioxidant Activity Analysis of WHP-SE at Different Temperatures
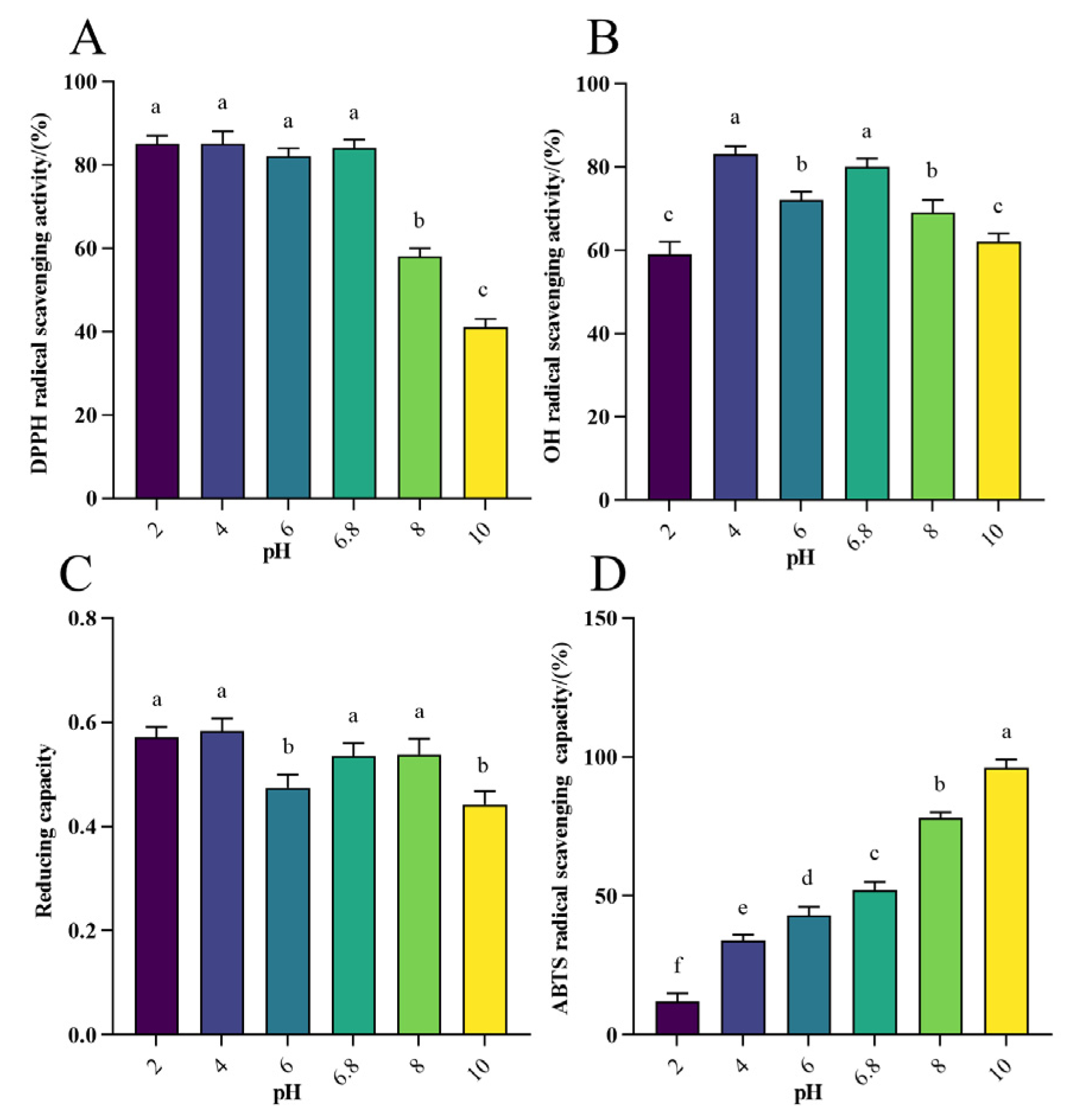
3.1.3. Antioxidant Activity Analysis of WHP-Se at Different pH
3.2. Physicochemical Property Analysis of WPH-Se
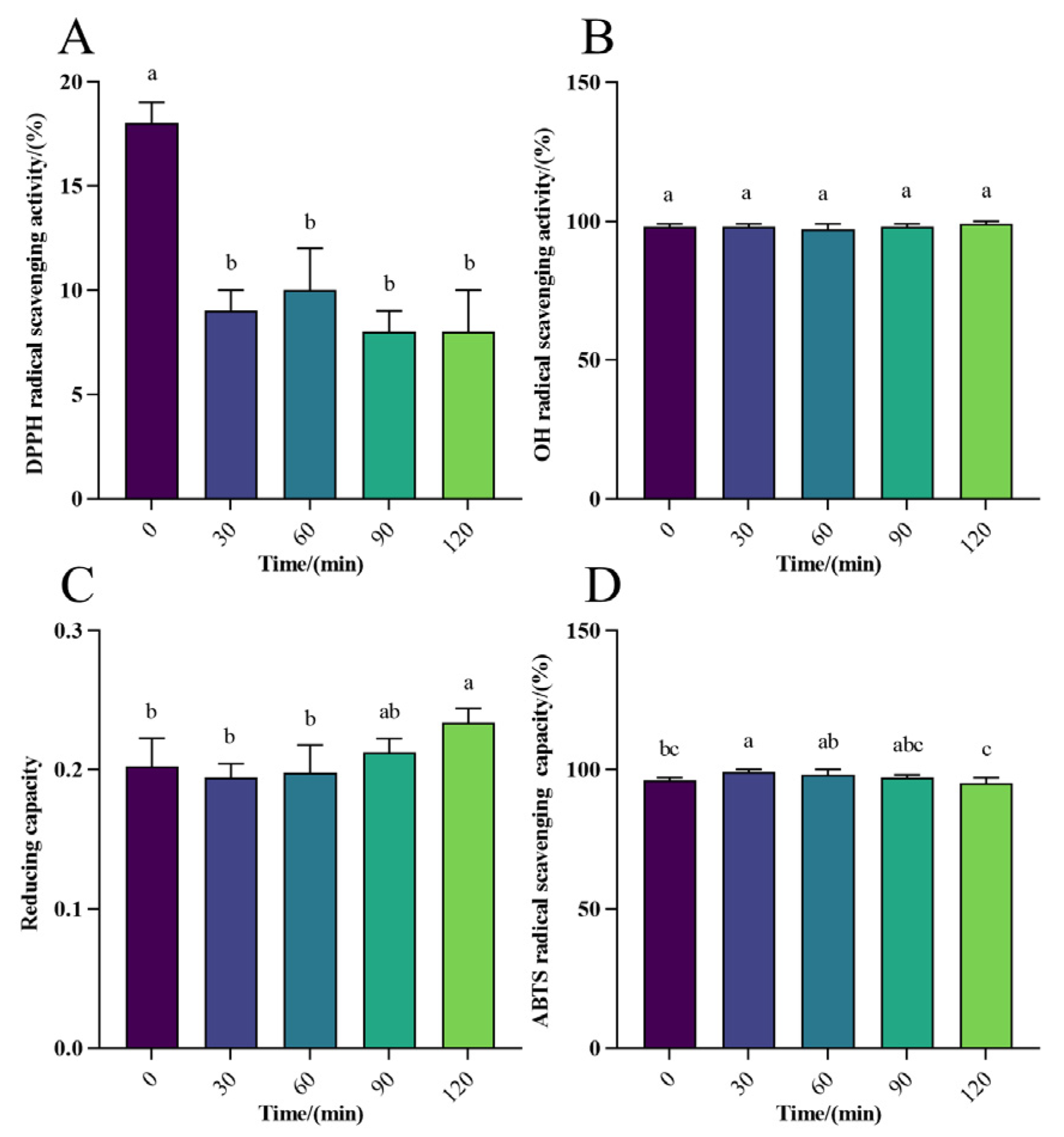
3.2.1. Antioxidant Activity Analysis of WPH-Se In Vitro Digestion
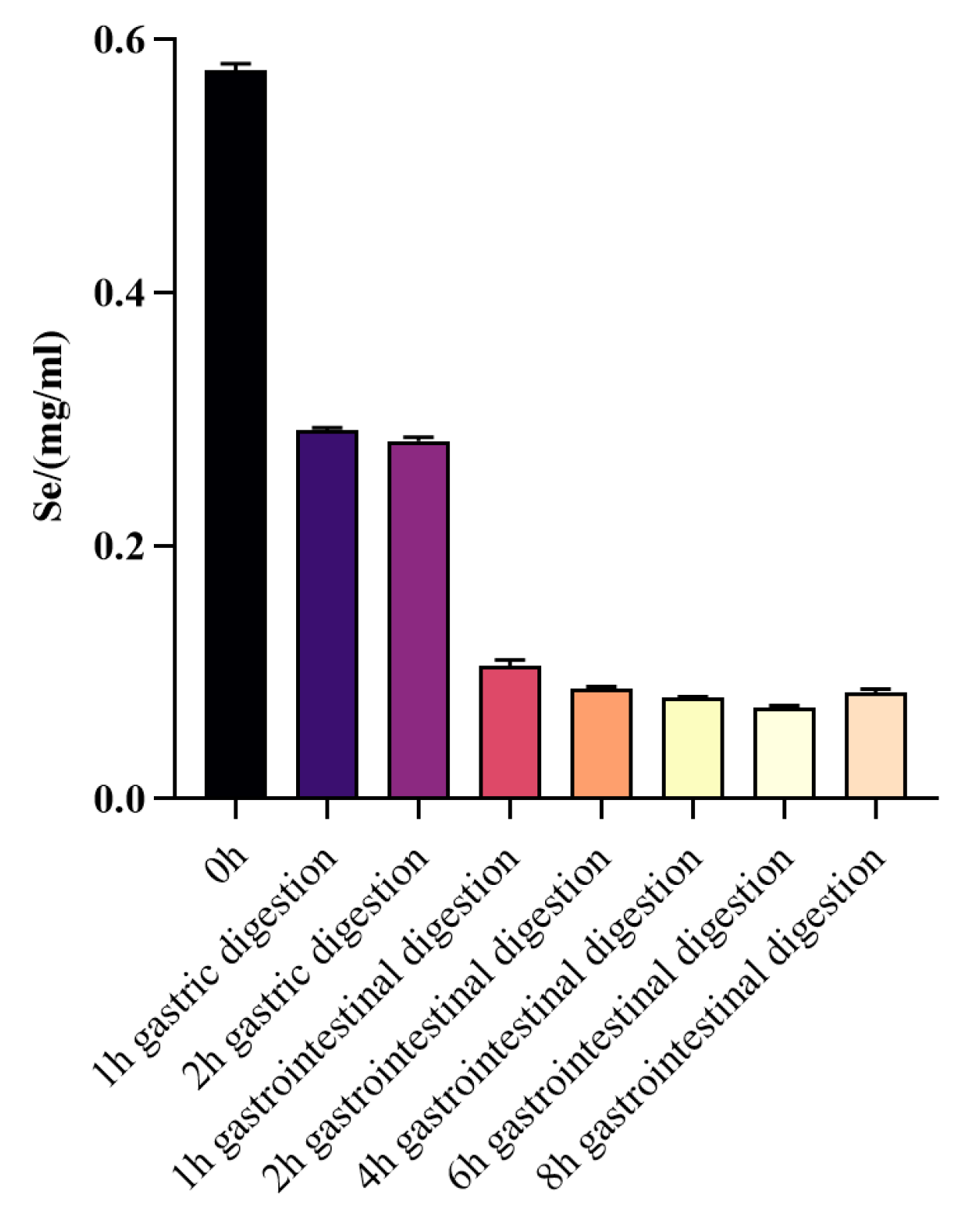
3.2.2. Changes in Selenium Content before and after WPH-Se In Vitro Digestion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hong, H.; Fan, H.; Chalamaiah, M.; Wu, J. Preparation of low-molecular-weight, collagen hydrolysates (peptides): Current progress, challenges, and future perspectives. Food Chem. 2019, 301, 125222. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Pan, X.; Zhao, E.; Shi, Y.; Shen, X.; Wu, J.; Pei, F.; Hu, Q.; Qiu, W. Isolation and identification of immunomodulatory selenium-containing peptides from selenium-enriched rice protein hydrolysates. Food Chem. 2019, 275, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, C.; Tamura, S.; Tohse, R.; Fujita, S.; Kikuchi, M.; Asada, C.; Nakamura, Y. Isolation and identification of an angiotensin I-converting enzyme inhibitory peptide from pearl oyster (Pinctada fucata) shell protein hydrolysate. Process Biochem. 2019, 77, 137–142. [Google Scholar] [CrossRef]
- Stührwohldt, N.; Schaller, A. Regulation of plant peptide hormones and growth factors by post-translational modification. Plant Biol. 2019, 21 (Suppl. S1), 49–63. [Google Scholar] [CrossRef] [PubMed]
- de Veer, S.J.; Kan, M.W.; Craik, D.J. Cyclotides: From Structure to Function. Chem. Rev. 2019, 119, 12375–12421. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Wei, H.; Yu, C.Y. Peptide-functionalized delivery vehicles for enhanced cancer therapy. Int. J. Pharm. 2021, 593, 120141. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, J.; Chen, L.; Zhou, Y.; Wu, J. Preparation, isolation and hypothermia protection activity of antifreeze peptides from shark skin collagen. LWT 2014, 55, 210–217. [Google Scholar] [CrossRef]
- Patil, S.P.; Goswami, A.; Kalia, K.; Kate, A.S. Plant-Derived Bioactive Peptides: A Treatment to Cure Diabetes. Int. J. Pept. Res. Ther. 2020, 26, 955–968. [Google Scholar] [CrossRef]
- Escudero, E.; Mora, L.; Toldra, F. Stability of ACE inhibitory ham peptides against heat treatment and in vitro digestion. Food Chem. 2014, 161, 305–311. [Google Scholar] [CrossRef]
- Reich, H.J.; Hondal, R.J. Why Nature Chose Selenium. ACS Chem. Biol. 2016, 11, 821–841. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, H.; Li, H.; Ying, Z.; Liu, X. Research progress on separation of selenoproteins/Se-enriched peptides and their physiological activities. Food Funct. 2021, 12, 1390–1401. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Qi, Z.; Li, M.; Ahammed, G.J.; Chu, X.; Zhou, J. Selenium forms and methods of application differentially modulate plant growth, photosynthesis, stress tolerance, selenium content and speciation in Oryza sativa L. Ecotoxicol. Environ. Saf. 2019, 169, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, T.; Li, Q.; Li, D. Prevention of Keshan Disease by Selenium Supplementation: A Systematic Review and Meta-analysis. Biol. Trace Elem. Res. 2018, 186, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Khanam, A.; Platel, K. Bioaccessibility of selenium, selenomethionine and selenocysteine from foods and influence of heat processing on the same. Food Chem. 2016, 194, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.J.; Reul, B.; Ozcelikay, A.T.; Buchet, J.P.; Henquin, J.C.; Brichard, S.M. Oral selenate improves glucose homeostasis and partly reverses abnormal expression of liver glycolytic and gluconeogenic enzymes in diabetic rats. Diabetologia 1996, 39, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.Q.; Huang, K.X.; Xu, H.B. New experimental observation on the relationship of selenium and diabetes mellitus. Biol. Trace Elem. Res. 2004, 99, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Fang, Y.; Chen, Y.; Yang, W.; Ma, N.; Pei, F.; Kimatu, B.M.; Hu, Q.; Qiu, W. Protective effects of Se-containing protein hydrolysates from Se-enriched rice against Pb2+-induced cytotoxicity in PC12 and RAW264.7 cells. Food Chem. 2016, 202, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Meizoso, I.; Jaime, L.; Santoyo, S.; Cifuentes, A.; García-Blairsy Reina, G.; Señoráns, F.J.; Ibáñez, E. Pressurized fluid extraction of bioactive compounds from Phormidium species. J. Agric. Food Chem. 2008, 28, 3517–3523. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, Y.; Matsuhashi, K.; Tanaka, Y.-K.; Suzuki, N.; Ogra, Y. Band 3/anion exchanger 1/solute carrier family 4 member 1 expression as determinant of cellular sensitivity to selenite exposure. Biochem. Biophys. Rep. 2022, 29, 101223. [Google Scholar] [CrossRef]
- Spallholz, J.E.; Hoffman, D.J. Selenium toxicity: Cause and effects in aquatic birds. Aquat. Toxicol. 2002, 57, 27–37. [Google Scholar] [CrossRef]
- Chen, N.; Yao, P.; Zhang, W.; Zhang, Y.T.; Xin, N.C.; Wei, H.D.; Zhang, T.H.; Zhao, C.H. Selenium nanoparticles: Enhanced nutrition and beyond. Crit. Rev. Food Sci. Nutr. 2023, 63, 12360–12371. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Chen, Z.H.; Zhang, F.L.; Yu, Z.Y.; Liu, B.; Zhang, C.; Zhao, L.N. A specific selenium-chelating peptide isolated from the protein hydrolysate of Grifola frondosa. RSC Adv. 2021, 11, 10272–10284. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Liu, G.; Xu, Y.; Wu, X.; Yi, R.; Jin, F.; Sa, C.; Su, X. Antioxidant stress and anticancer activity of peptide-chelated selenium in vitro. Int. J. Mol. Med. 2021, 48, 153. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zheng, Y.; He, S.; Su, D.; Nag, A.; Zeng, Q.; Yuan, Y. Novel Zn-Binding Peptide Isolated from Soy Protein Hydrolysates: Purification, Structure, and Digestion. J. Agric. Food Chem. 2021, 69, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Dong, W.; Zhang, Z.; Xu, J.; Li, H.; Zhang, J.; Dai, L.; Wang, S. A new iron supplement: The chelate of pig skin collagen peptide and Fe2+ can treat iron-deficiency anemia by modulating intestinal flora. Front. Nutr. 2022, 9, 1055725. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.J.; Janisse, S.E.; Heffern, M.C. Plant-derived chelators and ionophores as potential therapeutics for metabolic diseases. Chem. Soc. Rev. 2023, 52, 3927–3945. [Google Scholar] [CrossRef] [PubMed]
- Udechukwu, M.C.; Collins, S.A.; Udenigwe, C.C. Prospects of enhancing dietary zinc bioavailability with food-derived zinc-chelating peptides. Food Funct. 2016, 7, 4137–4144. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.R.; Hou, Y.C.; Huang, J.H.; Zhang, Q.S.; Liao, A.M.; Shen, X.L.; Yuan, X.Q.; Lu, M.X. Preparation, structural characterization and functional properties of a novel selenium chelating peptide derived from the hydrolyzate of wheat protein. Int. J. Food Prop. 2023, 26, 3241–3260. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Reig, M.; Toldrá, F. Stability of the potent antioxidant peptide SNAAC identified from Spanish dry-cured ham. Food Res. Int. 2018, 105, 873–879. [Google Scholar] [CrossRef]
- Wong, F.C.; Xiao, J.B.; Ong, M.G.L.; Pang, M.J.; Wong, S.J.; Teh, L.K.; Chai, T.T. Identification and characterization of antioxidant peptides from hydrolysate of blue-spotted stingray and their stability against thermal, pH and simulated gastrointestinal digestion treatments. Food Chem. 2019, 271, 614–622. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Fogliano, V. Food matrix interaction and bioavailability of bioactive peptides: Two faces of the same coin? J. Funct. Foods 2017, 35, 9–12. [Google Scholar] [CrossRef]
- Liu, K.; Du, R.; Chen, F. Stability of the antioxidant peptide SeMet-Pro-Ser identified from selenized brown rice protein hydrolysates. Food Chem. 2020, 319, 126540. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium biochemistry and its role for human health. Metallomics 2014, 6, 25–54. [Google Scholar] [CrossRef]
- Saidi, S.; Deratani, A.; Belleville, M.P.; Amar, R.B. Production and fractionation of tuna by-product protein hydrolysate by ultrafiltration and nanofiltration: Impact on interesting peptides fractions and nutritional properties. Food Res. Int. 2014, 65, 453–461. [Google Scholar] [CrossRef]
- Feng, Y.Z.; Wu, W.Y.; Chen, T.; Huang, M.T.; Zhao, M.M. Exploring the core functional microbiota related with flavor compounds in fermented soy sauce from different sources. Food Res. Int. 2023, 173, 113456. [Google Scholar] [CrossRef]
- Ren, Y.; Wu, H.; Li, X.; Lai, F.; Zhao, G.; Xiao, X. A Two-Step, One-Pot Enzymatic Method for Preparation of Duck Egg White Protein Hydrolysates with High Antioxidant Activity. Appl. Biochem. Biotechnol. 2014, 172, 1227–1240. [Google Scholar] [CrossRef] [PubMed]
- Homayouni-Tabrizi, M.; Asoodeh, A.; Soltani, M. Cytotoxic and antioxidant capacity of camel milk peptides: Effects of isolated peptide on superoxide dismutase and catalase gene expression. J. Food Drug Anal. 2017, 25, 567. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Yang, X.Q.; Kou, M.; Lu, C.Y.; Wang, Y.Y.; Peng, J.; Chen, P.; Jiang, J.H. Selenylation of Polysaccharide from the Sweet Potato and Evaluation of Antioxidant, Antitumor, and Antidiabetic Activities. J. Agric. Food Chem. 2017, 65, 605. [Google Scholar] [CrossRef] [PubMed]
- Siddhuraju, P.; Mohan, P.S.; Becker, K. Studies on the antioxidant activity of Indian Laburnum (Cassia fistula L.): A preliminary assessment of crude extracts from stem bark, leaves, flowers and fruit pulp. Food Chem. 2002, 79, 61–67. [Google Scholar] [CrossRef]
- Minekus, M.; Smeets-Peeters, M.; Bernalier, A.; Marol-Bonnin, S.; Havenaar, R.; Marteau, P.; Alric, M.; Fonty, G.; Huis in’t Veld, J.H. A computer-controlled system to simulate conditions of the large intestine with peristaltic mixing, water absorption and absorption of fermentation products. Appl. Biochem. Biotechnol. 1999, 53, 108–114. [Google Scholar] [CrossRef]
- Minekus, M.; Marteau, P.; Havenaar, R.; Huis in ‘t Veld, J.H.H. multicompartmental dynamic computer-controlled model simulating the stomach and small intestine. Altern. Lab. Anim. 1995, 23, 197–209. [Google Scholar] [CrossRef]
- Oded, S.; Amit, Y. Collagen Structures and Method of Fabricating the Same. EP2640877A2, 25 September 2013. [Google Scholar]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Brodkorb, A. A standardised static in-vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Gao, J.; Xue, Z.; Zhang, Z.; Wang, H.; Wang, X. Purification and identification of antioxidant peptides from chickpea (Cicer arietinum L.) albumin hydrolysates. LWT Food Sci. Technol. 2013, 50, 591–598. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Bioactive properties of milk proteins in humans: A review. Peptides 2015, 73, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Mao, X.; Wu, Q. Purification, Identification and Molecular Docking of Novel Antioxidant Peptides from Walnut (Juglans regia L.) Protein Hydrolysates. Molecules 2022, 27, 8423. [Google Scholar] [CrossRef]
- Li, H.; Aluko, R.E. Identification and inhibitory properties of multifunctional peptides from pea protein hydrolysate. J. Agric. Food Chem. 2010, 58, 11471–11476. [Google Scholar] [CrossRef] [PubMed]
- Ng, Z.X.; Kuppusamy, U.R. Effects of different heat treatments on the antioxidant activity and ascorbic acid content of bitter melon, Momordica charantia. Braz. J. Food Technol. 2019, 22, e2018283. [Google Scholar] [CrossRef]
- Arnal, M.; Gallego, M.; Talens, P.; Mora, L. Impact of thermal treatments and simulated gastrointestinal digestion on the α-amylase inhibitory activity of different legumes. Food Chem. 2023, 418, 135884. [Google Scholar] [CrossRef]
- Sun, X.M.; Wang, L.; Wang, S.; Tan, Y.X.; Zhu, Y.J.; Wang, Q.Y.; Tao, W.B.; Qin, L.X. Effects of thermal treatments on physicochemical properties and peptide profiles of infant formula protein model based on peptidomics. Int. J. Dairy Technol. 2024; Epub ahead of printing. [Google Scholar] [CrossRef]
- Singh, B.P.; Vij, S. In vitro stability of bioactive peptides derived from fermented soy milk against heat treatment, pH and gastrointestinal enzymes. LWT Food Sci. Technol. 2018, 91, 303–307. [Google Scholar] [CrossRef]
- Asaduzzaman, A.K.M.; Chun, B.S. Recovery of functional materials with thermally stable antioxidative properties in squid muscle hydrolyzates by subcritical water. J. Food Sci. Technol. 2015, 52, 793–802. [Google Scholar] [CrossRef]
- Jang, H.L.; Liceaga, A.M.; Yoon, K.Y. Purification, characterisation and stability of an antioxidant peptide derived from sandfish (Arctoscopus japonicus) protein hydrolysates. J. Funct. Foods 2016, 20, 433–442. [Google Scholar] [CrossRef]
- Lin, S.Y.; Wang, C.C.; Lu, Y.L.; Wu, W.C.; Hou, W.C. Antioxidant, anti-semicarbazide-sensitive amine oxidase, and anti-hypertensive activities of geraniin isolated from Phyllanthus urinaria. Food Chem. Toxicol. 2008, 46, 2485–2492. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Wan, S.Y.; Liu, J.; Zou, Y.X.; Liao, S.T. Antioxidant Activity and Stability Study of Peptides from Enzymatically Hydrolyzed Male Silkmoth. J. Food Process. Preserv. 2017, 41, e13081. [Google Scholar] [CrossRef]
- Zheng, L.; Lin, L.Z.; Su, G.W.; Zhao, Q.Z.; Zhao, M.M. Pitfalls of using 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay to assess the radical scavenging activity of peptides: Its susceptibility to interference and low reactivity towards peptides. Food Res. Int. 2015, 76, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-L.; Ye, J.; Yu, H.; Su, L.-J. Optimization of the Enzymatic Hydrolysis Process and Analysis of the Antioxidant Activity of Polypeptide from Apostichopus japonicus Gonads. Sci. Technol. Food Ind. 2018, 39, 181–187. [Google Scholar]
- Zheng, L.; Zhao, M.M.; Xiao, C.Q.; Zhao, Q.Z.; Su, G.W. Practical problems when using ABTS assay to assess the radical-scavenging activity of peptides: Importance of controlling reaction pH and time. Food Chem. 2016, 192, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [CrossRef]
- Ma, J.; Zeng, X.; Zhou, M.; Cheng, L.; Ren, D. Inhibitory effect of low-molecular-weight peptides (0–3 kDa) from Spirulina platensis on H2O2-induced oxidative damage in L02 human liver cells. Bioresour. Bioprocess. 2021, 8, 36. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, S.; Wang, Q.; Yuan, Q.; Li, Y.; Reboredo-Rodríguez, P.; Varela-López, A.; He, Z.; Wu, F.; Hu, H.; et al. Oxidative Stress Amelioration of Novel Peptides Extracted from Enzymatic Hydrolysates of Chinese Pecan Cake. Int. J. Mol. Sci. 2022, 23, 12086. [Google Scholar] [CrossRef]
- Moran; Edwin, T. Gastric digestion of protein through pancreozyme action optimizes intestinal forms for absorption, mucin formation and villus integrity. Anim. Feed. Sci. Technol. 2016, 221, 284–303. [Google Scholar] [CrossRef]
- Huang, Q.; Yu, H.; Ru, Q. Bioavailability and Delivery of Nutraceuticals Using Nanotechnology. J. Food Sci. 2010, 75, R50–R57. [Google Scholar] [CrossRef]
- Goodwin, D. Lipid and Carbohydrate-Based Systems to Enhance the Bioavailability and Immunogenicity of Therapeutic Peptides. Ph.D. Thesis, School of Chemistry and Molecular Biosciences, The University of Queensland, St Lucia, Australia, 2013. [Google Scholar]
- Aguilar-Toalá, J.E.; Quintanar-Guerrero, D.; Liceaga, A.M.; Zambrano-Zaragoza, M.L. Encapsulation of bioactive peptides: A strategy to improve the stability, protect the nutraceutical bioactivity and support their food applications. RSC Adv. 2022, 12, 6449–6458. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Suzuki, N.; Ogra, Y. Bioavailability Comparison of Nine Bioselenocompounds In Vitro and In Vivo. Int. J. Mol. Sci. 2017, 18, 506. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Collings, R.; Hurst, R. Selenium bioavailability: Current knowledge and future research requirements. Am. J. Clin. Nutr. 2010, 91, 1484S–1491S. [Google Scholar] [CrossRef] [PubMed]


| Amino Acid Name | WPH-Se (%) | Gastric WPH-Se (%) | Intestinal WPH-Se (%) |
|---|---|---|---|
| Aspartate | 6.3 | 4.9 | 3.4 |
| Threonine | 1.2 | 2.5 | 2.5 |
| Serine | 2.7 | 5.5 | 3.2 |
| Glutamate | 20.8 | 12.5 | 7.0 |
| Alanine | 2.4 | ||
| Glycine | 8.5 | 4.7 | 8.7 |
| Cysteine | 4.5 | 3.6 | |
| Valine | 7.6 | 5.8 | |
| Leucine | 4.9 | ||
| Phenylalanine | 5.1 | ||
| Histidine | 42.3 | 41.4 | 42.5 |
| Tryptophan | 1.2 | ||
| Lysine | 3.2 | 3.1 | 6.7 |
| Arginine | 14.9 | 5.9 | 10.4 |
| Acidic amino acids | 27.1 | 17.4 | 10.4 |
| Alkaline amino acids | 60.4 | 50.4 | 59.6 |
| Essential amino acids | 46.7 | 59.5 | 63.8 |
| Hydrophobic amino acids | 8.5 | 19.6 | 20.8 |
| Aromatic amino acids | 0 | 0 | 6.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.; Chen, X.; Zhang, M.; Yang, S.; Liao, A.; Pan, L.; Wang, Z.; Shen, X.; Yuan, X.; Huang, J. Selenium-Chelating Peptide Derived from Wheat Gluten: In Vitro Functional Properties. Foods 2024, 13, 1819. https://doi.org/10.3390/foods13121819
Hou Y, Chen X, Zhang M, Yang S, Liao A, Pan L, Wang Z, Shen X, Yuan X, Huang J. Selenium-Chelating Peptide Derived from Wheat Gluten: In Vitro Functional Properties. Foods. 2024; 13(12):1819. https://doi.org/10.3390/foods13121819
Chicago/Turabian StyleHou, Yinchen, Xinyang Chen, Mingyi Zhang, Shengru Yang, Aimei Liao, Long Pan, Zhen Wang, Xiaolin Shen, Xiaoqing Yuan, and Jihong Huang. 2024. "Selenium-Chelating Peptide Derived from Wheat Gluten: In Vitro Functional Properties" Foods 13, no. 12: 1819. https://doi.org/10.3390/foods13121819
APA StyleHou, Y., Chen, X., Zhang, M., Yang, S., Liao, A., Pan, L., Wang, Z., Shen, X., Yuan, X., & Huang, J. (2024). Selenium-Chelating Peptide Derived from Wheat Gluten: In Vitro Functional Properties. Foods, 13(12), 1819. https://doi.org/10.3390/foods13121819





