Chicken Egg White Gels: Fabrication, Modification, and Applications in Foods and Oral Nutraceutical Delivery
Abstract
1. Introduction
2. Gelation of EW Proteins
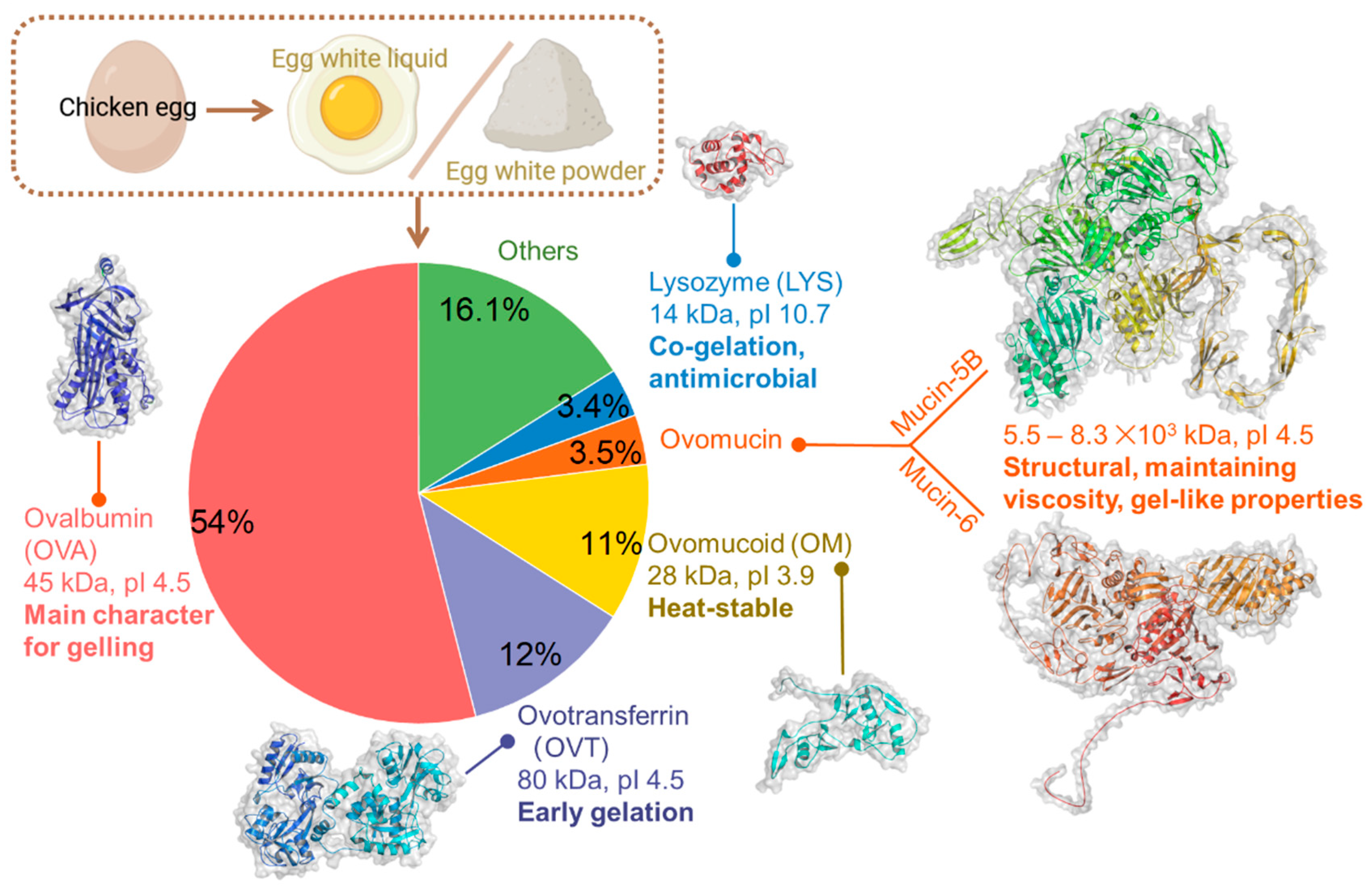
2.1. Roles of EW Proteins in Gel Formation
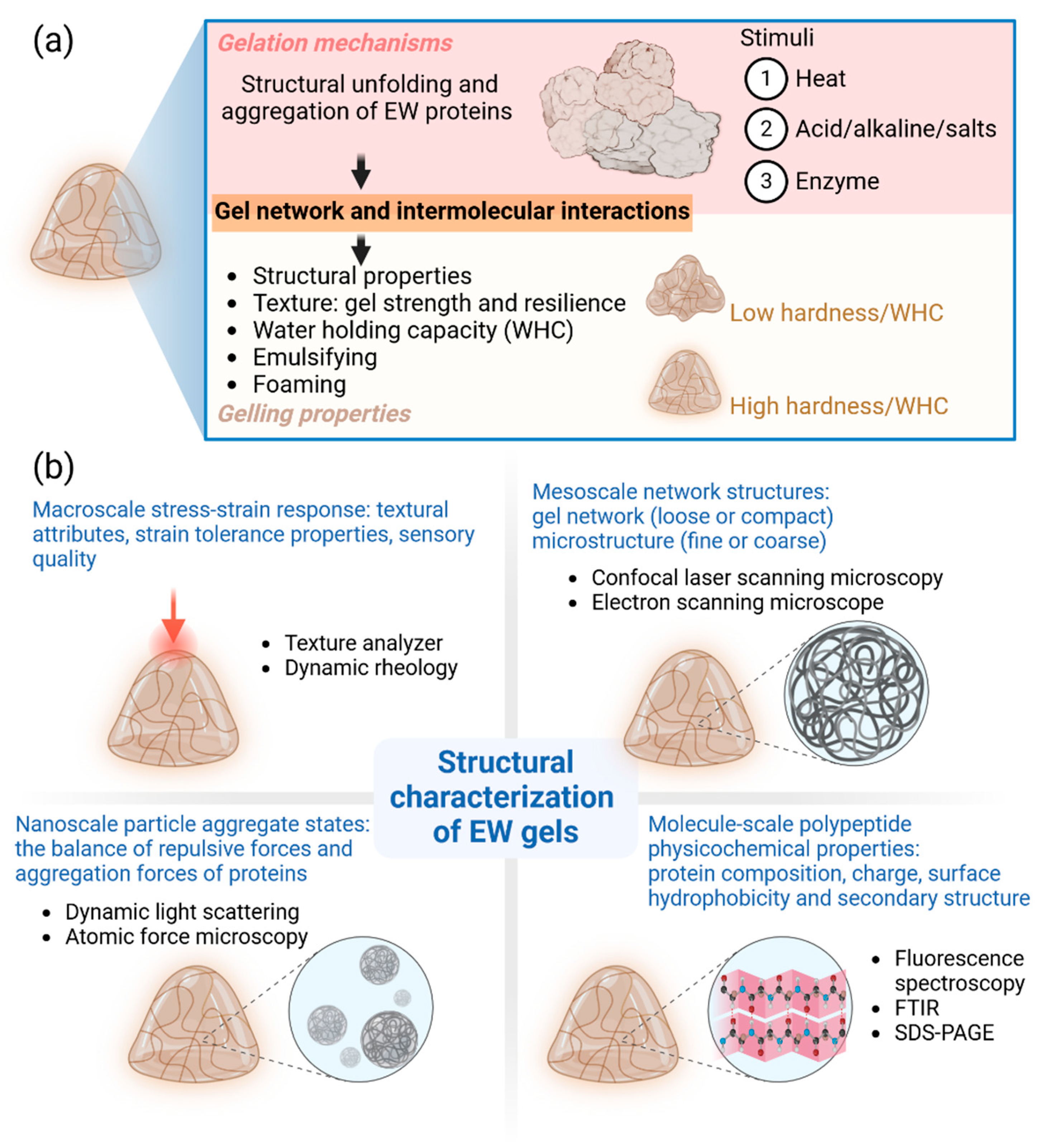
2.2. Gelation Mechanisms of EW Proteins
2.3. Properties of EW Gels
2.4. Structural Characterization of EW Gels
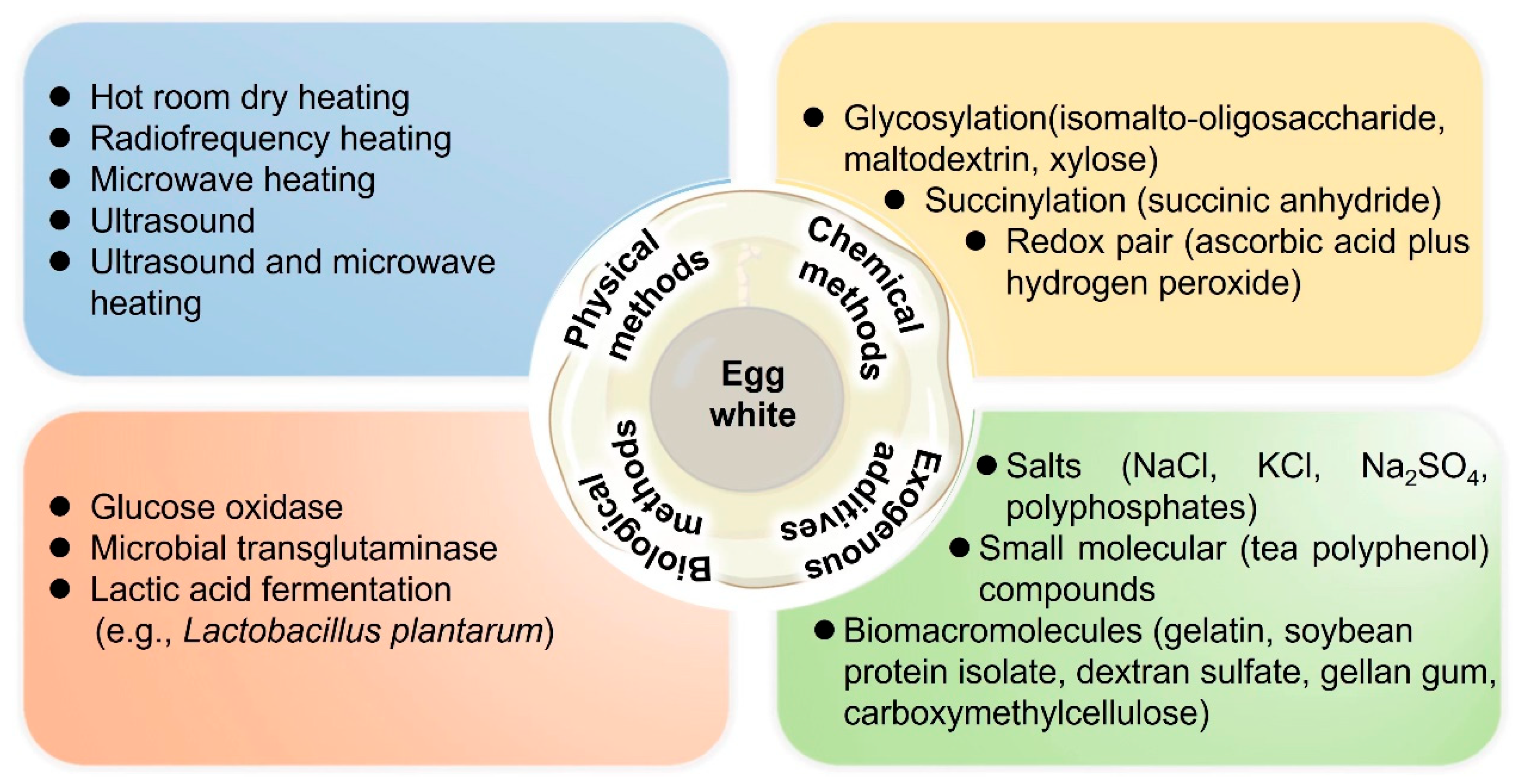
3. Strategies for Modifying the Gelling Properties of EW Proteins
3.1. Physical Methods
3.2. Chemical Methods
3.3. Biological Modification
3.4. Exogenous Additives
3.4.1. Salts
3.4.2. Small Molecular Compounds
3.4.3. Biomacromolecules
3.5. Main Differences of the Different Modification Methods
4. Application of EW Gels in the Food and Pharmaceutical Industries
4.1. Application of EW Proteins as Gelling Agents in Foods
| Application Type | Materials | Object | Key Impacts | Ref. |
|---|---|---|---|---|
| As gelling agent | Fresh EW (0–30%) | Oat noodles | Improved textural attributes and decreased cooking loss. | [83] |
| EW powder (0, 5, 10, and 15 g/100 g flour) | Banana–cassava pasta | Decreased starch digestibility, increased protein digestibility, and improved amino acid profile. | [84] | |
| EW powder | Chicken gel | Increased gel hardness, reduced cooking loss. | [85] | |
| Tea polyphenol (TP)-modified EW | Surimi gel | Significant increase in breaking force and WHC. | [87] | |
| As gel-type delivery carriers | Cold-set EW protein/dextran sulfate hydrogels | Curcumin | Showed controlled-release properties. | [88] |
| EW protein nanoparticles | Curcumin | Fibrous nanoparticles (pH 3.0) had higher curcumin loading (11.53 mg/g protein) and stability than granule nanoparticles (9.89 mg/g protein) (pH 3.8). | [89] | |
| EW protein nanoparticles | Linoleic acid (LA) | EW protein nanoparticles prepared by heating at 85 °C, 5 min of the 5% EW protein solution, pH 11.4 had average hydrodynamic diameters of 87 nm and LA loading capacities of 0.35 g LA/g nanoparticles. | [90] | |
| Acylated OVA nanogels | Curcumin | Improved encapsulation efficiency (93.63%) and sustained release of curcumin compared to non-modified OVA nanogels. | [91] | |
| OVA–pullulan (1:1) nanogels | Curcumin | Showed better encapsulation efficiency (88.38%), loading capacity (8.78%), and controlled release for curcumin than single OVA nanogel. | [92] | |
| Supercritical fluid-dried EW protein aerogel | Soybean oil | Aerogels obtained from the cold-set hydrogels had significantly higher macroporous volumes and excellent oil structuring capacities (maximal areogel-to-oil ratio of 1:15) than heat-set hydrogels (maximal areogel-to-oil ratio of 1:5). | [93] | |
| EW protein/CMC-Na conjugate aerogels | Soybean oil | Had a uniform structure, larger specific surface areas, higher mechanical strength, and oil holding capacities than those of the single EW aerogel. | [94] |
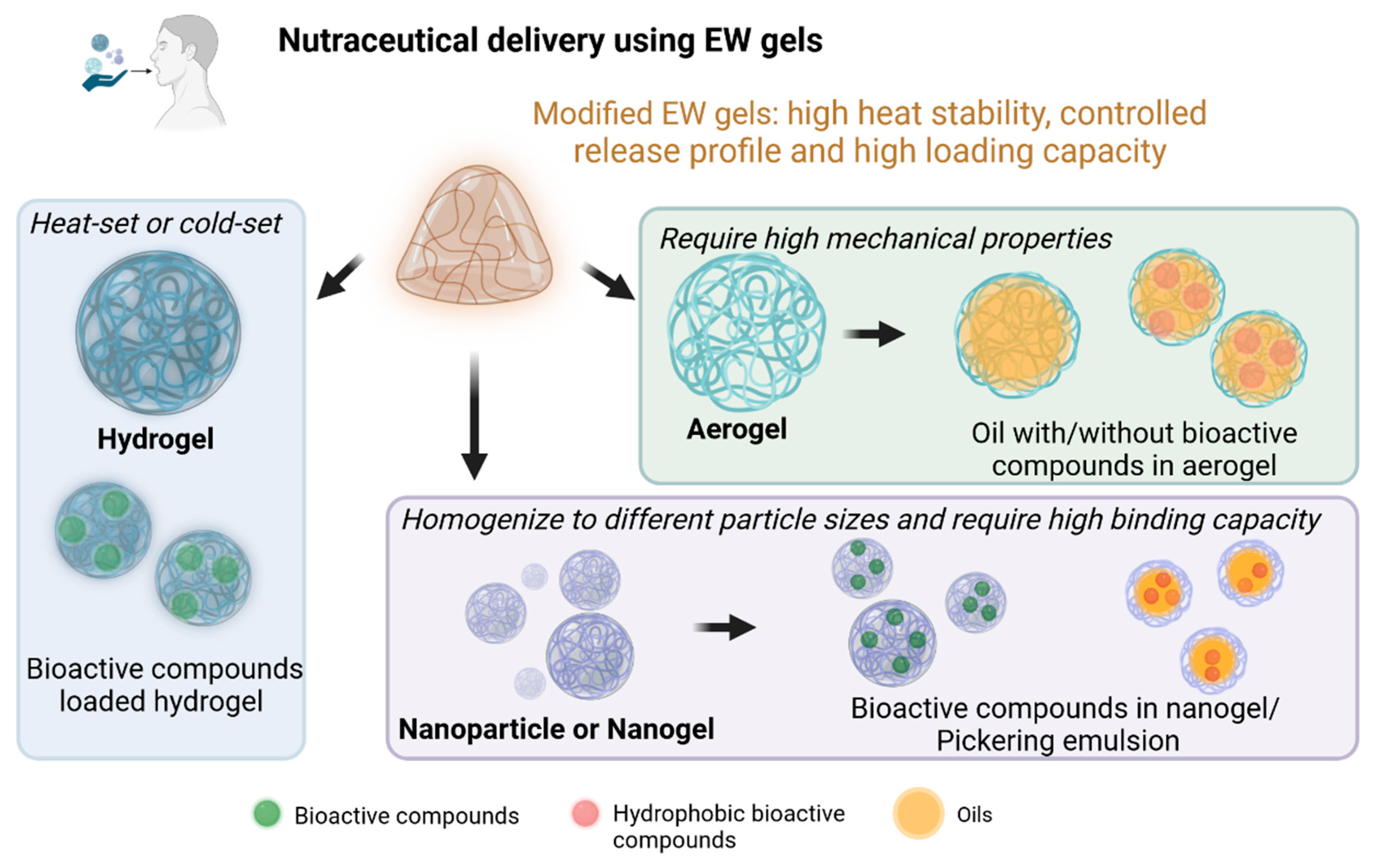
4.2. Application of EW Proteins as Gel-Type Carriers for Nutraceutical Delivery
4.3. EW Hydrogels as Delivery Carriers
4.4. EW Nanoparticles or Nanogels as Delivery Carriers
4.5. EW Aerogels as Delivery Carriers
4.6. Importance of Processing Control and Storage Conditions
5. Research Gaps between Modified EW Gels and Their Applications
6. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Razi, S.M.; Fahim, H.; Amirabadi, S.; Rashidinejad, A. An overview of the functional properties of egg white proteins and their application in the food industry. Food Hydrocoll. 2023, 135, 108183. [Google Scholar] [CrossRef]
- Zang, J.; Zhang, Y.; Pan, X.; Peng, D.; Tu, Y.; Chen, J.; Zhang, Q.; Tang, D.; Yin, Z. Advances in the formation mechanism, influencing factors and applications of egg white gels: A review. Trends Food Sci. Technol. 2023, 138, 417–432. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, Y.; Wu, N.; Chen, S.; Xu, M.; Du, H.; Yao, Y.; Tu, Y. Egg white protein-based delivery system for bioactive substances: A review. Crit. Rev. Food Sci. 2022, 64, 617–637. [Google Scholar] [CrossRef] [PubMed]
- Jalili-Firoozinezhad, S.; Filippi, M.; Mohabatpour, F.; Letourneur, D.; Scherberich, A. Chicken egg white: Hatching of a new old biomaterial. Mater. Today 2020, 40, 193–214. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, Y.Q. An insight on egg white: From most common functional food to biomaterial application. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1045–1058. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Chaudhary, V.; Chhikara, N.; Sharma, N.; Nowacka, M.; Demirkesen, I.; Rathnakumar, K.; Falsafi, S.R. Ovalbumin, an outstanding food hydrocolloid: Applications, technofunctional attributes, and nutritional facts, A systematic review. Food Hydrocoll. 2023, 139, 108514. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Li, S.; Ye, H.; Luo, W.; Huang, Q.; Geng, F. Ovomucin may be the key protein involved in the early formation of egg-white thermal gel. Food Chem. 2022, 366, 130596. [Google Scholar] [CrossRef] [PubMed]
- Yuno-Ohta, N.; Kato, T.; Ashizawa, S.; Kimura, Y.; Maruyama, N.; Nishizu, T. Role of ovomucoid in the gelation of a beta-lactoglobulin-ovomucoid mixture. Colloid. Polym. Sci. 2016, 294, 1065–1073. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, H.; Zhang, M.; Yang, M.; Zhang, T.; Du, Z.; Xu, M.; Liu, X. Relationship of co-gelation and co-aggregation on egg white ovalbumin-lysozyme heteroprotein complex: Formation and thermodynamics. Food Chem. 2022, 388, 133030. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, W.; Tang, T.; Gu, L.; Su, Y.; Yang, Y.; Chang, C.; Han, Q. Thermal gelation and digestion properties of hen egg white: Study on the effect of neutral and alkaline salts addition. Food Chem. 2023, 409, 135263. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Qing, M.; Zang, J.; Shan, A.; Zhang, H.; Chi, Y.; Chi, Y.; Gao, X. Molecular interactions in the dry heat-facilitated hydrothermal gel formation of egg white protein. Food Res. Int. 2022, 162, 112058. [Google Scholar] [CrossRef] [PubMed]
- Farjami, T.; Babaei, J.; Nau, F.; Dupont, D.; Madadlou, A. Effects of thermal, non-thermal and emulsification processes on the gastrointestinal digestibility of egg white proteins. Trends Food Sci. Technol. 2021, 107, 45–56. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Fan, Q.; Teng, C.; Xie, W.; Shi, Y.; Su, Y.; Yang, Y. Combination effects of NaOH and NaCl on the rheology and gel characteristics of hen egg white proteins. Food Chem. 2018, 250, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Famiglietti, M.; Mirpoor, S.F.; Giosafatto, C.V.L.; Mariniello, L. Enzyme assisted food processing. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Alavi, F.; Emam-Djomeh, Z.; Salami, M.; Mohammadian, M. Effect of microbial transglutaminase on the mechanical properties and microstructure of acid-induced gels and emulsion gels produced from thermal denatured egg white proteins. Int. J. Biol. Macromol. 2020, 153, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, C.; Li, X.; Su, Y.; Yang, Y.; Yu, X. Effects of pH and NaCl on the physicochemical and interfacial properties of egg white/yolk. Food Biosci. 2018, 23, 115–120. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Wang, C.; Zhang, M.; Xu, Y.; Zhou, B.; Su, Y.; Yang, Y. Characteristics of gelling and water holding properties of hen egg white/yolk gel with NaCl addition. Food Hydrocoll. 2018, 77, 887–893. [Google Scholar] [CrossRef]
- Dong, W.; Zhang, X.; Ding, L.; Liu, C.; Ai, M.; Jin, Y.; Isobe, K.; Handa, A.; Cai, Z. Enhancement of emulsification properties by modulation of egg white protein fibril structure with different heating times. Food Hydrocoll. 2023, 135, 108203. [Google Scholar] [CrossRef]
- Jin, H.; Chen, J.; Zhang, J.; Sheng, L. Impact of phosphates on heat-induced egg white gel properties: Texture, water state, micro-rheology and microstructure. Food Hydrocoll. 2021, 110, 106200. [Google Scholar] [CrossRef]
- Jiang, J.; Zang, J.; Qing, M.; Ma, Y.; Yang, X.; Chi, Y.; Chi, Y. Regulating the thermal properties of egg white by adding surfactants. J. Food Eng. 2024, 362, 111759. [Google Scholar] [CrossRef]
- Ma, Y.; Shan, A.; Chi, Y. Changes in structural, rheological, and gel properties of egg white protein induced by preheating in the dry state. Int. J. Biol. Macromol. 2023, 248, 125851. [Google Scholar] [CrossRef] [PubMed]
- Kar, A.; Guha, S.; Subbiah, J.; Majumder, K. Effect of radiofrequency processing on the structural and bio-functional properties of egg white proteins. Food Chem. 2023, 404, 134533. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, I.; Wouters, A.G.B.; Lambrecht, M.A.; Uten, L.; Van Den Bosch, W.; Vercruysse, S.A.R.; Delcour, J.A. Food protein network formation and gelation induced by conductive or microwave heating: A focus on hen egg white. Innov. Food Sci. Emerg. Technol. 2020, 66, 102484. [Google Scholar] [CrossRef]
- Jun, S.; Yaoyao, M.; Hui, J.; Obadi, M.; Zhongwei, C.; Bin, X. Effects of single- and dual-frequency ultrasound on the functionality of egg white protein. J. Food Eng. 2020, 277, 109902. [Google Scholar] [CrossRef]
- Liu, B.; Jin, F.; Li, Y.; Wang, H.; Chi, Y.; Tian, B.; Feng, Z. Pasteurization of egg white by integrating ultrasound and microwave: Effect on structure and functional properties. Innov. Food Sci. Emerg. Technol. 2022, 79, 103063. [Google Scholar] [CrossRef]
- Wei, R.; Xiao, N.; Guo, S.; Ai, M. Environmental stress-induced conformational transitions modulate foaming and gelation properties of glycosylated egg white proteins. Food Hydrocoll. 2024, 150, 109643. [Google Scholar] [CrossRef]
- Bashash, M.; Varidi, M.; Varshosaz, J. Ultrasound-triggered transglutaminase-catalyzed egg white-bovine gelatin composite hydrogel: Physicochemical and rheological studies. Innov. Food Sci. Emerg. Technol. 2022, 76, 102936. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Xu, L.; Lv, Y.; Tang, T.; Zhang, M.; Li, J.; Su, Y.; Gu, L.; Yang, Y.; et al. Study on gel properties of lysozyme-free egg white before and after Lactiplantibacillus plantarum fermentation. J. Sci. Food Agric. 2022, 102, 5618–5627. [Google Scholar] [PubMed]
- Liu, J.; Jiang, H.; Zhang, M.; Gong, P.; Yang, M.; Zhang, T.; Liu, X. Ions-regulated aggregation kinetics for egg white protein: A promising formulation with controlled gelation and rheological properties. Int. J. Biol. Macromol. 2022, 200, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Han, T.; Zhang, G.; Hu, X.; Li, R.; Liu, H.; Li, R.; Tu, Y.; Zhao, Y. Combined effects of NaOH, NaCl, and heat on the characteristics of ovalbumin gel and the exploration of the mechanism of transparent gel formation. Food Hydrocoll. 2023, 140, 108589. [Google Scholar] [CrossRef]
- Xue, H.; Zhang, G.; Han, T.; Li, R.; Liu, H.; Gao, B.; Tu, Y.; Zhao, Y. Improvement of gel properties and digestibility of the water-soluble polymer of tea polyphenol-egg white under thermal treatment. Food Chem. 2022, 372, 131319. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xiao, N.; Guo, S.; Tian, X.; Ai, M. Tea polyphenol-mediated network proteins modulate the NaOH-heat induced egg white protein gelling properties. Food Hydrocoll. 2024, 149, 109514. [Google Scholar] [CrossRef]
- Koyama, S.; Kodama, D.; Tsujii, Y.; Handa, A. Soluble-protein-aggregate-assisted improvements in heat-induced gel properties: Effect of genipin-mediated crosslinks on egg white protein. LWT 2023, 184, 115079. [Google Scholar] [CrossRef]
- Babaei, J.; Mohammadian, M.; Madadlou, A. Gelatin as texture modifier and porogen in egg white hydrogel. Food Chem. 2019, 270, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chai, J.; Yuan, Y.; Zhang, T.; Saini, R.K.; Yang, M.; Shang, X. Dextran sulfate facilitates egg white protein to form transparent hydrogel at neutral pH: Structural, functional, and degradation properties. Food Hydrocoll. 2022, 122, 107094. [Google Scholar] [CrossRef]
- Mao, Y.; Huang, M.; Bi, J.; Sun, D.; Li, H.; Yang, H. Effects of kappa-carrageenan on egg white ovalbumin for enhancing the gelation and rheological properties via electrostatic interactions. Food Hydrocoll. 2023, 134, 108031. [Google Scholar] [CrossRef]
- Yao, K.; Guo, W.; Yao, Y.; Wu, N.; Xu, M.; Zhao, Y.; Tu, Y. Properties, digestion and peptide release of heat-induced duck egg white. LWT 2022, 154, 112788. [Google Scholar] [CrossRef]
- Van der Plancken, I.; Van Loey, A.; Hendrickx, M. Effect of moisture content during dry-heating on selected physicochemical and functional properties of dried egg white. J. Agric. Food Chem. 2007, 55, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Shan, A.; Wang, R.; Zhao, Y.; Chi, Y. Characterization of egg white powder gel structure and its relationship with gel properties influenced by pretreatment with dry heat. Food Hydrocoll. 2021, 110, 106149. [Google Scholar] [CrossRef]
- Ma, Z.; Chi, Y.; Zhang, H.; Chi, Y.; Ma, Y. Inhibiting effect of dry heat on the heat-induced aggregation of egg white protein. Food Chem. 2022, 387, 132850. [Google Scholar] [CrossRef] [PubMed]
- Dag, D.; Singh, R.K.; Chen, J.; Mishra, A.; Kong, F. Radio frequency assisted thermal processing for pasteurization of packaged whole milk powder surrounded by oil. Food Control 2022, 135, 108762. [Google Scholar] [CrossRef]
- Kar, A.; Wei, X.; Majumder, K.; Eskridge, K.; Handa, A.; Subbiah, J. Effect of traditional and radiofrequency assisted thermal processing on the gel firmness of egg white powder. LWT 2020, 133, 110091. [Google Scholar] [CrossRef]
- Soni, A.; Smith, J.; Thompson, A.; Brightwell, G. Microwave-induced thermal sterilization- A review on history, technical progress, advantages and challenges as compared to the conventional methods. Trends Food Sci. Technol. 2020, 97, 433–442. [Google Scholar] [CrossRef]
- Cao, H.; Zhu, H.; Wang, Q.; Fan, D.; Huang, J.; Zhao, J.; Jiao, X.; Yan, B.; Zhou, W.; Zhang, H. Intervention on activity and structure of cathepsin L during surimi gel degradation under microwave irradiation. Food Hydrocoll. 2020, 103, 105705. [Google Scholar] [CrossRef]
- Wang, X.; Gu, L.; Su, Y.; Li, J.; Yang, Y.; Chang, C. Microwave technology as a new strategy to induce structural transition and foaming properties improvement of egg white powder. Food Hydrocoll. 2020, 101, 105530. [Google Scholar] [CrossRef]
- Jafari, Z.; Goli, M.; Toghyani, M. The effects of phosphorylation and microwave treatment on the functional characteristics of freeze-dried egg white powder. Foods 2022, 11, 2711. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, M.; Liu, X.; Hu, M. Structural characterization and functional properties of egg white protein treated by electron beam irradiation. Innov. Food Sci. Emerg. Technol. 2023, 84, 103262. [Google Scholar] [CrossRef]
- O’Sullivan, J.J.; Park, M.; Beevers, J.; Greenwood, R.W.; Norton, I.T. Applications of ultrasound for the functional modification of proteins and nanoemulsion formation: A review. Food Hydrocoll. 2017, 71, 299–310. [Google Scholar] [CrossRef]
- Xue, H.; Tu, Y.; Zhang, G.; Xin, X.; Hu, H.; Qiu, W.; Ruan, D.; Zhao, Y. Mechanism of ultrasound and tea polyphenol assisted ultrasound modification of egg white protein gel. Ultrason. Sonochem. 2021, 81, 105857. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Xu, Q.; Hu, X.; Sheng, L. Structure and properties of egg white protein films modified by high-intensity ultrasound: An effective strategy. Food Res. Int. 2022, 157, 111264. [Google Scholar] [CrossRef]
- Xue, H.; Liu, H.; Wu, N.; Zhang, G.; Tu, Y.; Zhao, Y. Improving the gel properties of duck egg white by synergetic phosphorylation/ultrasound: Gel properties, crystalline structures, and protein structure. Ultrason. Sonochem. 2022, 89, 106149. [Google Scholar] [CrossRef] [PubMed]
- Nooshkam, M.; Varidi, M.; Verma, D.K. Functional and biological properties of Maillard conjugates and their potential application in medical and food: A review. Food Res. Int. 2020, 131, 109003. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Qi, P.X.; Wickham, E.D. Interactions, induced by heating, of whey protein isolate (WPI) with sugar beet pectin (SBP) in solution: Comparisons with a dry-state Maillard reaction. Food Hydrocoll. 2018, 83, 61–71. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Li, X.; Zhang, M.; Gu, L.; Chang, C.; Su, Y.; Yang, Y. Molecular forces and gelling properties of heat-induced gel from egg white protein glycated with isomalto-oligosaccharide. Food Hydrocoll. 2020, 99, 105356. [Google Scholar] [CrossRef]
- Ma, Y.; Zang, J.; Qing, M.; Xiao, Y.; Zhang, H.; Chi, Y.; Chi, Y. Glycosylation of egg white protein with maltodextrin in the dry state: Changes in structural and gel properties. Food Chem. 2023, 401, 134113. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, H.; Xiao, L.; Chu, L.; Wang, S.; Wang, H. Comparison of ovalbumin glycation by microwave irradiation and conventional heating. LWT 2019, 116, 108560. [Google Scholar] [CrossRef]
- Chang, C.; Su, Y.; Gu, L.; Li, J.; Yang, Y. Microwave induced glycosylation of egg white protein:study on physicochemical properties and baking performance. Food Hydrocoll. 2021, 118, 106569. [Google Scholar] [CrossRef]
- Sheng, L.; Liu, Q.; Dong, W.; Cai, Z. Effect of high intensity ultrasound assisted glycosylation on the gel properties of ovalbumin: Texture, rheology, water state and microstructure. Food Chem. 2022, 372, 131215. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zheng, Z.; Zhu, G.; Luo, S.; Zhang, D.; Liu, F.; Shen, Y. Modification of the structural and functional properties of wheat gluten protein using a planetary ball mill. Food Chem. 2021, 363, 130251. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, Y.; Duan, W.; Wang, Q.; An, F.; Luo, P.; Huang, Q. Ball-milling is an effective pretreatment of glycosylation modified the foaming and gel properties of egg white protein. J. Food Eng. 2022, 319, 110908. [Google Scholar] [CrossRef]
- Basak, S.; Singhal, R.S. Succinylation of food proteins- a concise review. LWT 2022, 154, 112866. [Google Scholar] [CrossRef]
- Hu, G.; Ma, M.; Batool, Z.; Sheng, L.; Cai, Z.; Liu, Y.; Jin, Y. Gel properties of heat-induced transparent hydrogels from ovalbumin by acylation modifications. Food Chem. 2022, 369, 130912. [Google Scholar] [CrossRef] [PubMed]
- Alavi, F.; Momen, S.; Emam-Djomeh, Z.; Salami, M.; Moosavi-Movahedi, A.A. Tailoring egg white proteins by a GRAS redox pair for production of cold-set gel. LWT 2018, 98, 428–437. [Google Scholar] [CrossRef]
- Wang, T.; Chang, C.; Gu, L.; Su, Y.; Zhang, M.; Yang, Y.; Li, J. Comparison of the functionality of egg white liquid with different desugaring treatments. Int. J. Food Sci. Tech. 2022, 57, 5811–5819. [Google Scholar] [CrossRef]
- Alavi, F.; Emam-Djomeh, Z.; Chen, L. Acid-induced gelation of thermal co-aggregates from egg white and hempseed protein: Impact of microbial transglutaminase on mechanical and microstructural properties of gels. Food Hydrocoll. 2020, 107, 105960. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Dai, X.; Zhu, Y.; Hao, W.; Yang, X. Effects of synergistic modification with enzymatic hydrolysis and phosphorylation on functional and structural properties of ovalbumin. J. Food Process. Preserv. 2020, 44, e14934. [Google Scholar] [CrossRef]
- Wang, J.; Xu, L.; Lv, Y.; Su, Y.; Gu, L.; Chang, C.; Zhang, M.; Yang, Y.; Li, J. To improve the gel properties of liquid whole egg by short-term lactic acid bacteria fermentation. Innov. Food Sci. Emerg. Technol. 2022, 75, 102873. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Zhai, J.; Gu, L.; Su, Y.; Chang, C.; Yang, Y. Improving gelling properties of diluted whole hen eggs with sodium chloride and sodium tripolyphosphate: Study on intermolecular forces, water state and microstructure. Food Chem. 2021, 358, 129823. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Li, Y.; Wang, Q.; Wang, B.; Guo, L.; Sun, J.; Xiao, J.; Zhu, Y.; Zhang, X.; Huang, M.; et al. Profiles of gelling characteristics of myofibrillar proteins extracted from chicken breast: Effects of temperatures and phosphates. LWT 2020, 129, 109525. [Google Scholar] [CrossRef]
- Perez, D.; Harte, F.; Lopez-Pedemonte, T. Ionic strength and buffering capacity of emulsifying salts determine denaturation and gelation temperatures of whey proteins. J. Dairy Sci. 2022, 105, 7230–7241. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Chi, R.; Jiang, J.; Ma, J.; Zhang, Y.; Sun, W.; Zhou, Y. Insight into the mechanisms of combining direct current magnetic field with phosphate in promoting emulsifying properties of myofibrillar protein. Food Chem. 2024, 447, 138990. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Niu, F.; Li, J.; Su, Y.; Liu, Y.; Yang, Y. Interactions between tea polyphenol and two kinds of typical egg white proteins—Ovalbumin and lysozyme: Effect on the gastrointestinal digestion of both proteins in vitro. Food Res. Int. 2014, 59, 100–107. [Google Scholar] [CrossRef]
- Su, Y.; Dong, Y.; Niu, F.; Wang, C.; Liu, Y.; Yang, Y. Study on the gel properties and secondary structure of soybean protein isolate/egg white composite gels. Eur. Food Res. Technol. 2015, 240, 367–378. [Google Scholar] [CrossRef]
- Tomczyńska-Mleko, M.; Terpiłowski, K.; Pérez-Huertas, S.; Sapiga, V.; Polischuk, G.; Sołowiej, B.; Nastaj, M.; Wesołowska-Trojanowska, M.; Mleko, S. Co-gelation of pumpkin-seed protein with egg-white protein. Foods 2023, 12, 2030. [Google Scholar] [CrossRef]
- Yu, M.; Every, H.A.; Jiskoot, W.; Witkamp, G.-J.; Buijs, W. Molecular structure of dextran sulphate sodium in aqueous environment. J. Mol. Struct. 2018, 1156, 320–329. [Google Scholar] [CrossRef]
- Liu, J.; Chai, J.; Zhang, T.; Yuan, Y.; Saini, R.K.; Xu, M.; Li, S.; Shang, X. Phase behavior, thermodynamic and rheological properties of ovalbumin/dextran sulfate: Effect of biopolymer ratio and salt concentration. Food Hydrocoll. 2021, 118, 106777. [Google Scholar] [CrossRef]
- Zhang, T.; Yuan, Y.; Chai, J.; Wu, X.; Saini, R.K.; Liu, J.; Shang, X. How does dextran sulfate promote the egg white protein to form transparent hydrogel?the gelation mechanism and molecular force changes. Food Hydrocoll. 2022, 133, 107901. [Google Scholar] [CrossRef]
- Huang, M.; Mao, Y.; Li, H.; Yang, H. Kappa-carrageenan enhances the gelation and structural changes of egg yolk via electrostatic interactions with yolk protein. Food Chem. 2021, 360, 129972. [Google Scholar] [CrossRef] [PubMed]
- Vilela, J.A.P.; Perrechil, F.d.A.; Picone, C.S.F.; Sato, A.C.K.; Cunha, R.L.d. Preparation, characterization and in vitro digestibility of gellan and chitosan–gellan microgels. Carbohydr. Polym. 2015, 117, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Babaei, J.; Khodaiyan, F.; Mohammadian, M. Effects of enriching with gellan gum on the structural, functional, and degradation properties of egg white heat-induced hydrogels. Int. J. Biol. Macromol. 2019, 128, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Ren, C.; Xu, X.; Li, J.; Wang, L.; Li, B. Thermally induced gelation behavior and fractal analysis of ovalbumin-carboxymethylcellulose electrostatic complexes. Food Hydrocoll. 2019, 91, 214–223. [Google Scholar] [CrossRef]
- Xiong, W.; Li, Y.; Li, B.; Geng, F. Relationship between gel properties and water holding of ovalbumin-carboxymethylcellulose electrostatic complex hydrogels. Int. J. Biol. Macromol. 2021, 167, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-N.; Gao, F.; Zhu, K.-X. Effect of fresh egg white addition on the quality characteristics and protein aggregation of oat noodles. Food Chem. 2020, 330, 127319. [Google Scholar] [CrossRef] [PubMed]
- Rachman, A.; Brennan, M.A.; Morton, J.; Torrico, D.; Brennan, C.S. In-vitro digestibility, protein digestibility corrected amino acid, and sensory properties of banana-cassava gluten-free pasta with soy protein isolate and egg white protein addition. Food Sci. Hum. Wellness 2023, 12, 520–527. [Google Scholar] [CrossRef]
- Lv, Y.; Xu, L.; Su, Y.; Chang, C.; Gu, L.; Yang, Y.; Li, J. Effect of soybean protein isolate and egg white mixture on gelation of chicken myofibrillar proteins under salt/-free conditions. LWT 2021, 149, 111871. [Google Scholar] [CrossRef]
- Tadpitchayangkoon, P.; Park, J.W.; Yongsawatdigul, J. Gelation characteristics of tropical surimi under water bath and ohmic heating. LWT Food Sci. Technol. 2012, 46, 97–103. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, T.; Lin, H.; Chen, H.; Liu, J.; Lyu, F.; Ding, Y. Physicochemical properties and microstructure of surimi treated with egg white modified by tea polyphenols. Food Hydrocoll. 2019, 90, 82–89. [Google Scholar] [CrossRef]
- Liu, J.; Chai, J.; Yuan, Y.; Wu, X.; Gong, L.; Yu, P.; Liu, P.; Zhang, T.; Shang, X. Designation and characterization of cold-set egg white protein/dextran sulfate hydrogel for curcumin entrapment. Food Chem. 2023, 419, 136038. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Meikle, T.G.; Su, Y.; Wang, X.; Dekiwadia, C.; Drummond, C.J.; Conn, C.E.; Yang, Y. Encapsulation in egg white protein nanoparticles protects anti-oxidant activity of curcumin. Food Chem. 2019, 280, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Sponton, O.E.; Perez, A.A.; Stechina, M.F.; Santiago, L.G. Production of protein nanovehicles by heat treatment of industrial egg white in a batch reactor. J. Food Eng. 2020, 268, 109740. [Google Scholar] [CrossRef]
- Hu, G.; Batool, Z.; Cai, Z.; Liu, Y.; Ma, M.; Sheng, L.; Jin, Y. Production of self-assembling acylated ovalbumin nanogels as stable delivery vehicles for curcumin. Food Chem. 2021, 355, 129635. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Zeng, W.; Jin, Y.; Sheng, L. Construction and evaluation of ovalbumin-pullulan nanogels as a potential delivery carrier for curcumin. Food Chem. 2022, 367, 130716. [Google Scholar] [CrossRef] [PubMed]
- Alavi, F.; Ciftci, O.N. Superlight macroporous aerogels produced from cold-set egg white protein hydrogels show superior oil structuring capacity. Food Hydrocoll. 2023, 136, 108180. [Google Scholar] [CrossRef]
- Tang, S.; Jiang, Y.; Tang, T.; Du, H.; Tu, Y.; Xu, M. Effects of grafting degree on the physicochemical properties of egg white protein-sodium carboxymethylcellulose conjugates and their aerogels. Appl. Sci. 2022, 12, 2017. [Google Scholar] [CrossRef]
- Ai, M.; Zhou, Q.; Guo, S.; Ling, Z.; Zhou, L.; Fan, H.; Cao, Y.; Jiang, A. Effects of tea polyphenol and Ca(OH)2 on the intermolecular forces and mechanical, rheological, and microstructural characteristics of duck egg white gel. Food Hydrocoll. 2019, 94, 11–19. [Google Scholar] [CrossRef]
- Alavi, F.; Emam-Djomeh, Z.; Momen, S.; Hosseini, E.; Moosavi-Movahedi, A.A. Fabrication and characterization of acid-induced gels from thermally-aggregated egg white protein formed at alkaline condition. Food Hydrocoll. 2020, 99, 105337. [Google Scholar] [CrossRef]
- Chang, C.; Niu, F.; Gu, L.; Li, X.; Yang, H.; Zhou, B.; Wang, J.; Su, Y.; Yang, Y. Formation of fibrous or granular egg white protein microparticles and properties of the integrated emulsions. Food Hydrocoll. 2016, 61, 477–486. [Google Scholar] [CrossRef]
- Sponton, O.E.; Perez, A.A.; Ramel, J.V.; Santiago, L.G. Protein nanovehicles produced from egg white. Part 1: Effect of pH and heat treatment time on particle size and binding capacity. Food Hydrocoll. 2017, 73, 67–73. [Google Scholar] [CrossRef]
- Sponton, O.E.; Perez, A.A.; Ramel, J.V.; Santiago, L.G. Protein nanovehicles produced from egg white. Part 2: Effect of protein concentration and spray drying on particle size and linoleic acid binding capacity. Food Hydrocoll. 2018, 77, 863–869. [Google Scholar] [CrossRef]
| Types | Methods/Materials | Processing Conditions | Outcome | Action Mechanisms | Advantages | Drawbacks | Ref. |
|---|---|---|---|---|---|---|---|
| Physical | Hot room dry heating | 75 °C and 65% humidity for 15 d | Increased gel hardness by 2.10-fold | Appropriate heat and mechanical denaturation, changed protein structure and aggregation state | Mature and stable technology | Long-time, high-energy cost | [21] |
| Radiofrequency heating | 80 °C for 5 min | Increased gel Firmness by 48.6% | Short time | High temperature | [22] | ||
| Microwave (MW) | 100 °C for 15, 30, and 60 min | Increased gel firmness by 3–5 fold | Short time | High temperature | [23] | ||
| Ultrasound (US) | 375 W/L (dual frequency: 20/40 kHz) | Decreased the mobility of free water | Low temperature, short time | Only for liquid | [24] | ||
| US and MW heating | 57 °C for 2 min with US power of 700 W | Increased gel strength from 261 g to 338 g | Uniform, short time | Complicated instrument | [25] | ||
| Chemical | Glycosylation or succinylation | 60 °C for 3 h D-xylose: EW powder (1:2, w/w) | Increased gel hardness from 80 to 140 g | Introduce new functional groups | Increased hydrophilicity and changed structures | Additional non-egg ingredients | [26] |
| Biological | Transglutaminase | 85 °C for 30 min and then treated by MTGase at pH 7.5. | Increased hardness from 275.6 g to 419.3 g | Form additional intra- or inter-molecular bond | Suitable for preparing cold-set hydrogels | Not ideal for native EW proteins | [15,27] |
| Lactic bacteria fermentation | 37 °C for 7 h by Lactiplantibacillus plantarum. | Decreased hardness by about 1/7 | Involve acidification and hydrolysis | Improve gel appearance and decrease pH value | Partial consumption of EW proteins | [28] | |
| Exogenous additives | Salts (NaCl, KCl, Na2SO4, NaOH, and polyphophate) | e.g., sodium tripolyphosphate 0.45%, w/v | Increased hardness to 768.17 g | Change the charges and intermolecule forces of proteins | Simple operation | Changed taste, hampered by regulations | [19,29,30] |
| Small molecular compounds (Tea polyphenol and genipin) | The EW liquid containing tea polyphenol (0.01–0.05% w/w) heated at 85 °C for 20 min. | Hardness ranged from 971.43 g to 1261.63 g | Change the structure and cross-linking properties of EW proteins | Simple operation, increase the digestibility and rigidity of EW gels | Changes the sensory attributes of EW gels | [31,32,33] | |
| Biomacromolecules (Gelatin, dextran sulfate, and carrageena) | Depends on their types (e.g., dextran sulfate) | Increased hardness from about 580 g to 700 g | Form composite gel systems by non-covalent interactions | Simple operation | Additional non-egg proteins or polysaccharides | [34,35,36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, X.; Chang, C.; Gu, L.; Su, Y.; Yang, Y.; Agyei, D.; Han, Q. Chicken Egg White Gels: Fabrication, Modification, and Applications in Foods and Oral Nutraceutical Delivery. Foods 2024, 13, 1834. https://doi.org/10.3390/foods13121834
Li J, Wang X, Chang C, Gu L, Su Y, Yang Y, Agyei D, Han Q. Chicken Egg White Gels: Fabrication, Modification, and Applications in Foods and Oral Nutraceutical Delivery. Foods. 2024; 13(12):1834. https://doi.org/10.3390/foods13121834
Chicago/Turabian StyleLi, Junhua, Xuechun Wang, Cuihua Chang, Luping Gu, Yujie Su, Yanjun Yang, Dominic Agyei, and Qi Han. 2024. "Chicken Egg White Gels: Fabrication, Modification, and Applications in Foods and Oral Nutraceutical Delivery" Foods 13, no. 12: 1834. https://doi.org/10.3390/foods13121834
APA StyleLi, J., Wang, X., Chang, C., Gu, L., Su, Y., Yang, Y., Agyei, D., & Han, Q. (2024). Chicken Egg White Gels: Fabrication, Modification, and Applications in Foods and Oral Nutraceutical Delivery. Foods, 13(12), 1834. https://doi.org/10.3390/foods13121834









