A Straightforward, Sensitive, and Reliable Strategy for Ethyl Carbamate Detection in By-Products from Baijiu Production by Enzyme-Linked Immunosorbent Assay
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
2.2. Collection of Samples
2.3. Preparation of Hapten
2.4. Preparation of Antigen
2.5. Sample Preparation
2.6. ELISA Testing Procedures
2.7. Optimization of Pretreatment
2.7.1. Extraction for EC
2.7.2. Reconstituted Solution
2.8. Optimization for ELISA Analysis
2.8.1. Checkboard Analysis
2.8.2. The Optical Concentration of the Secondary Antibody, the Sample, and the Primary Antibody
2.9. The Impact of Alcohol Strength
2.10. Performance Parameters for the Method
2.10.1. Sensitivity
2.10.2. Specificity
2.10.3. Accuracy and Precision
2.11. Confirmatory Analysis
3. Results and Discussion
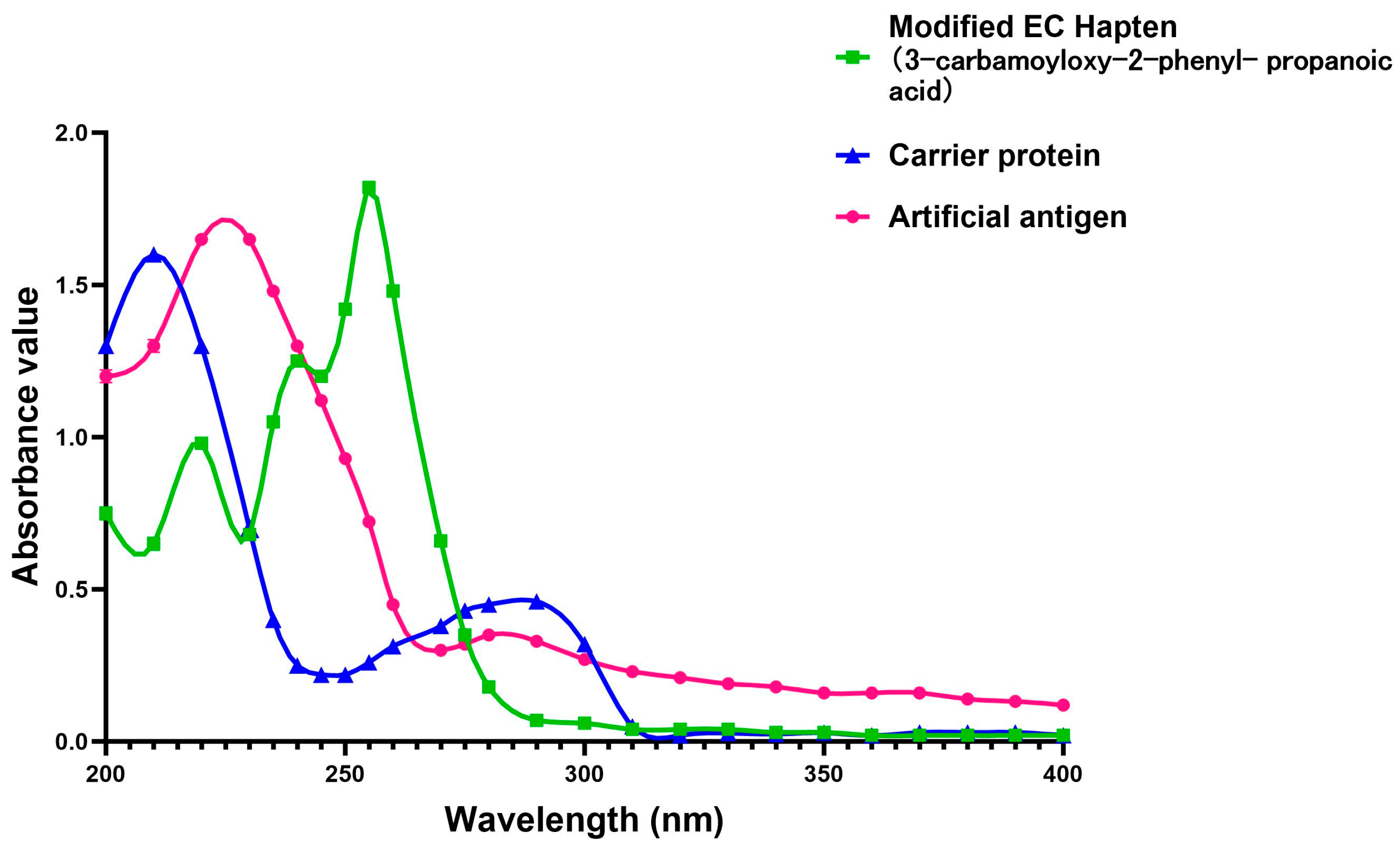
3.1. Identification of the Hapten and Antigen
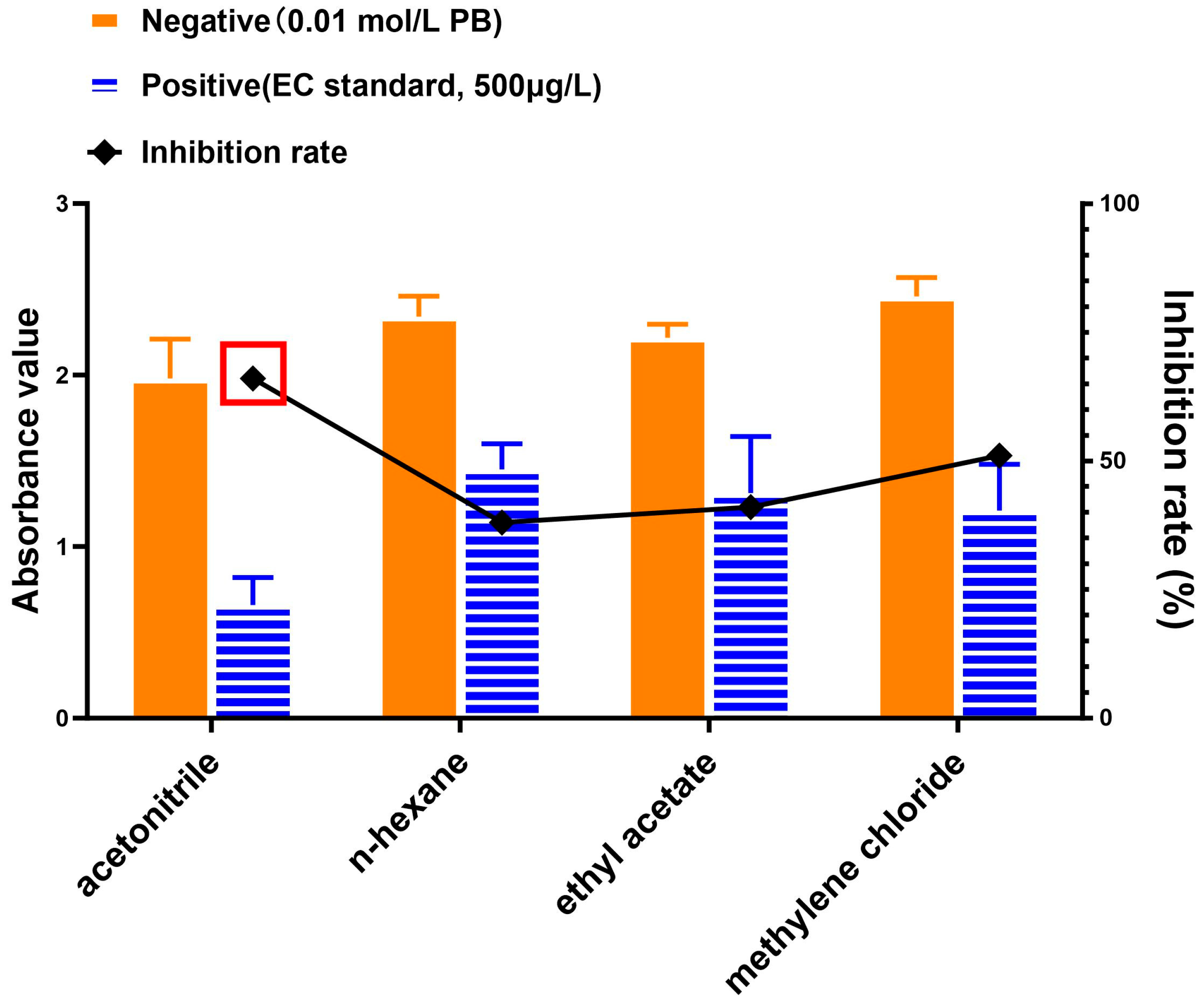
3.2. Optimization of Organic Extraction of EC from Fermented Sample
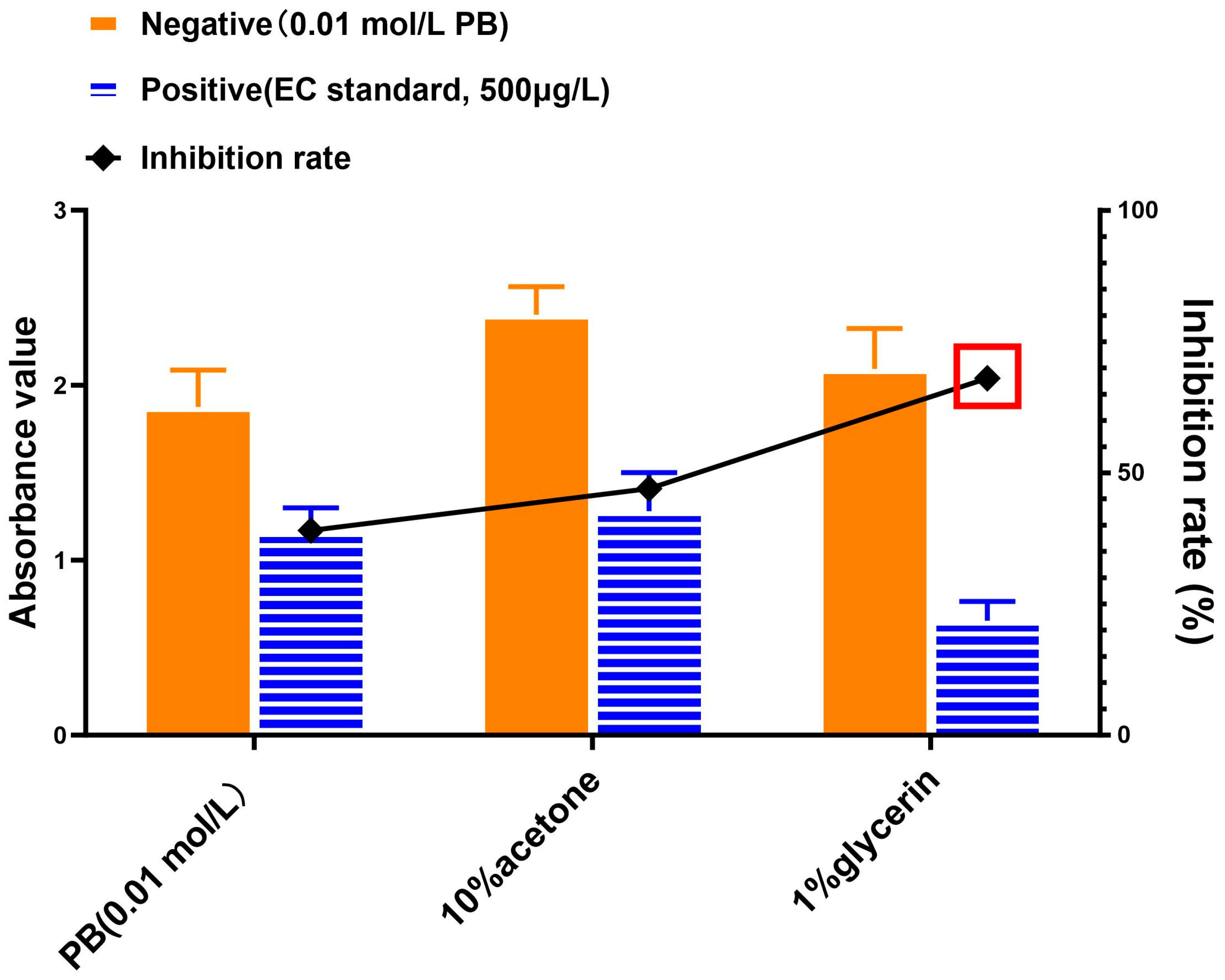
3.3. Comparison of Solutions for Reconstitution of Extracted EC
3.4. Effect of Alcohol Concentration
3.5. Optimization for ELISA Conditions
3.5.1. Checkboard Titration Test
3.5.2. Consumption of the Secondary IgG Antibody, the Sample and the Primary Antibody
3.6. Analytical Performance for ELISA
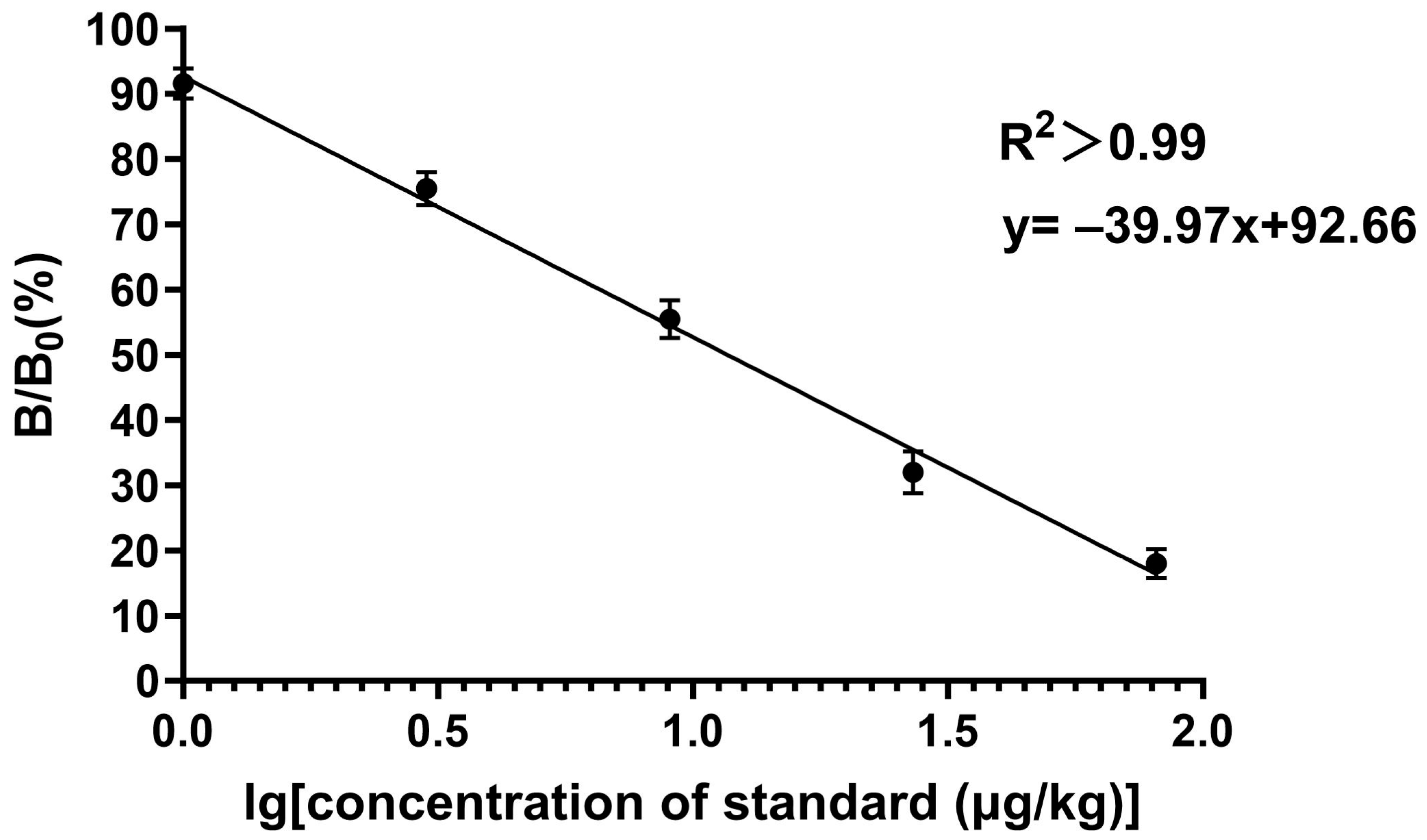
3.6.1. Sensitivity
3.6.2. Specificity
3.6.3. Accuracy and Precision
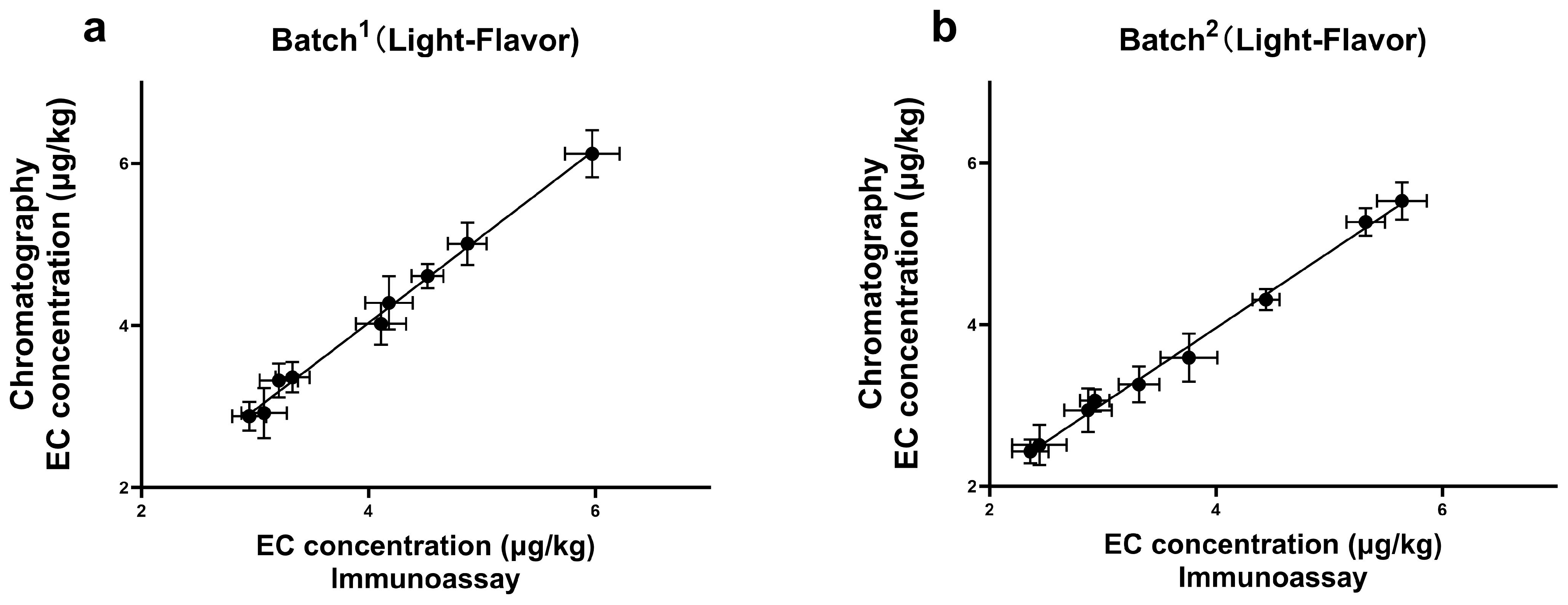
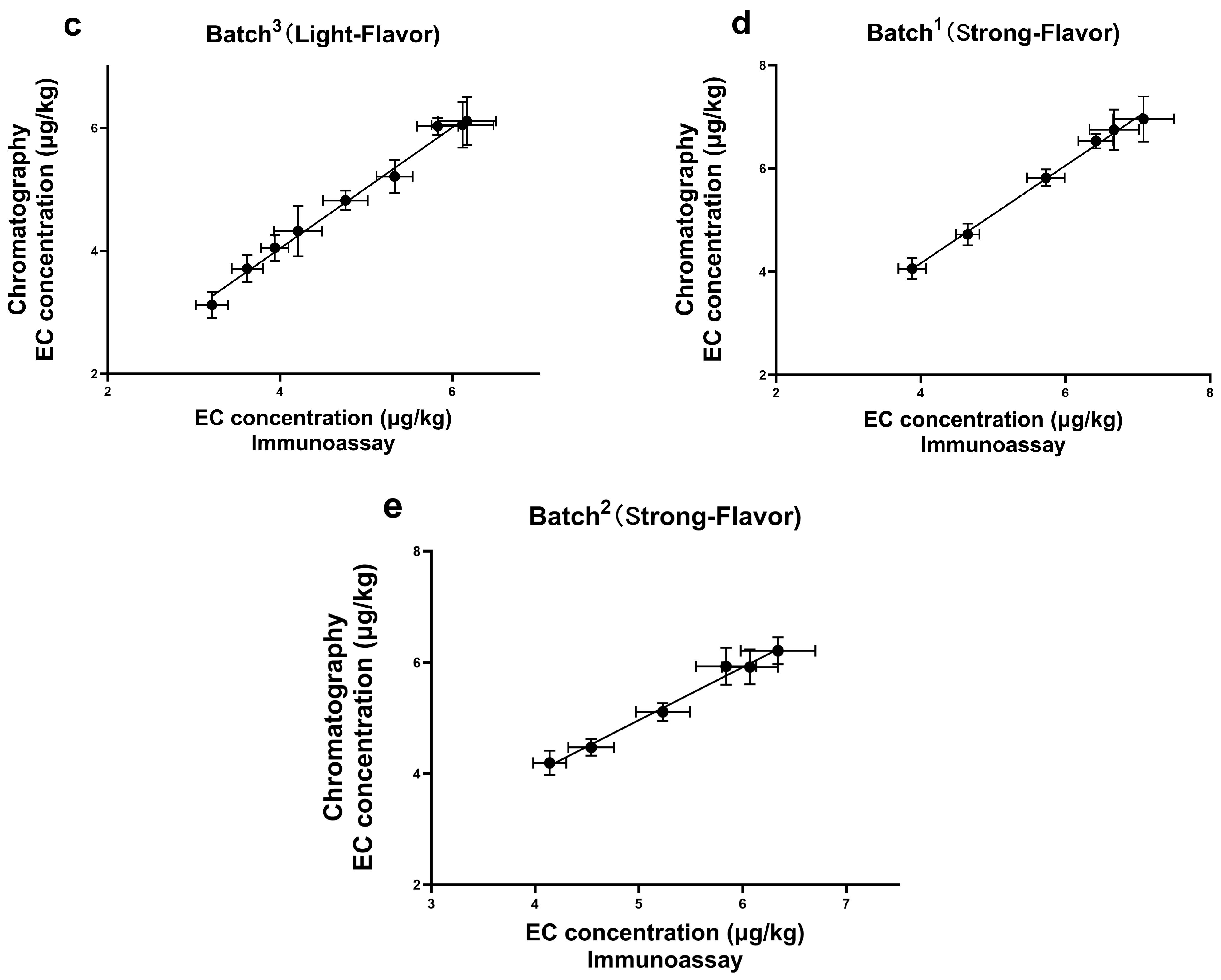
3.7. Detection of Real Samples
3.8. Comparison with Other Immunoassays
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

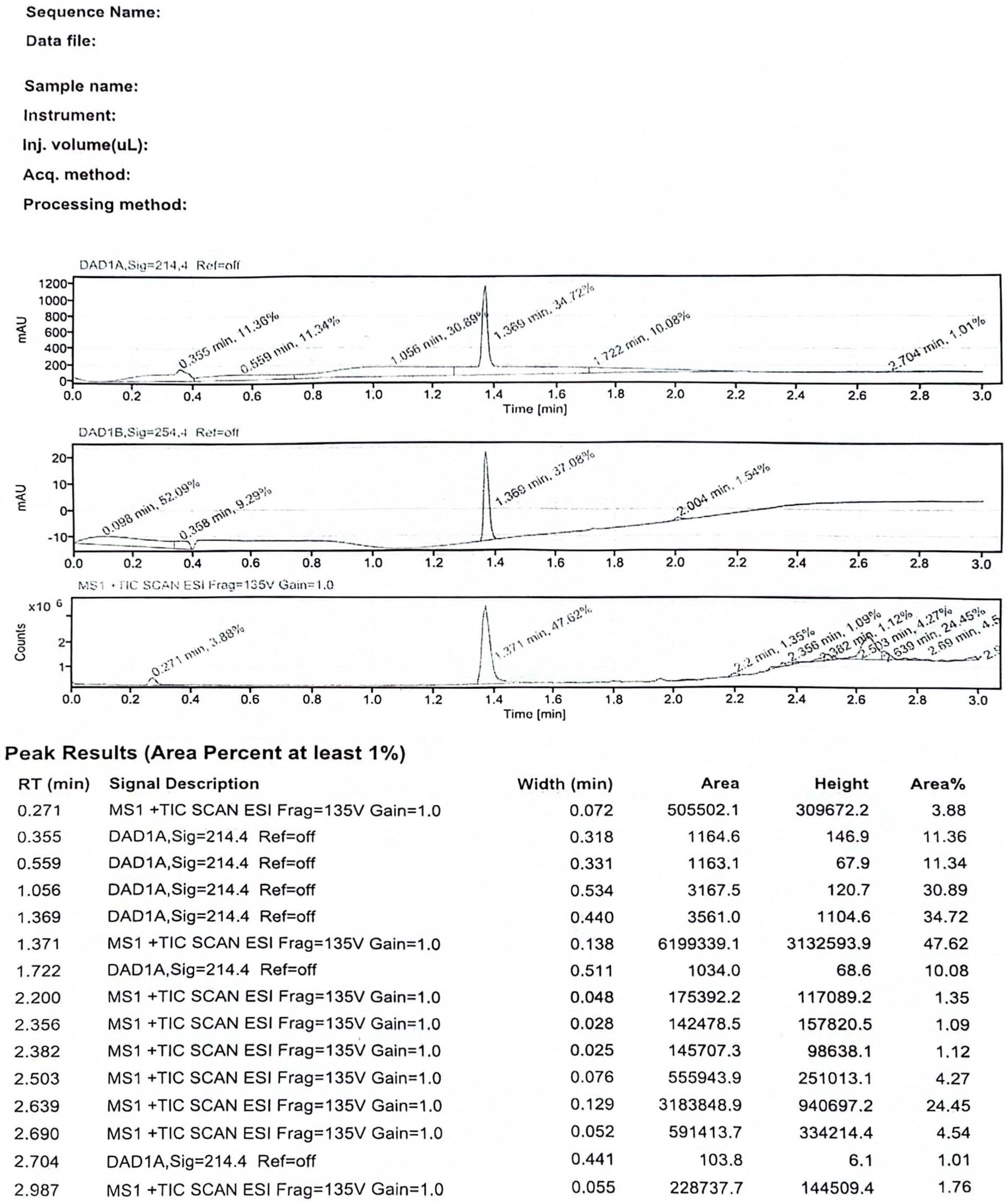
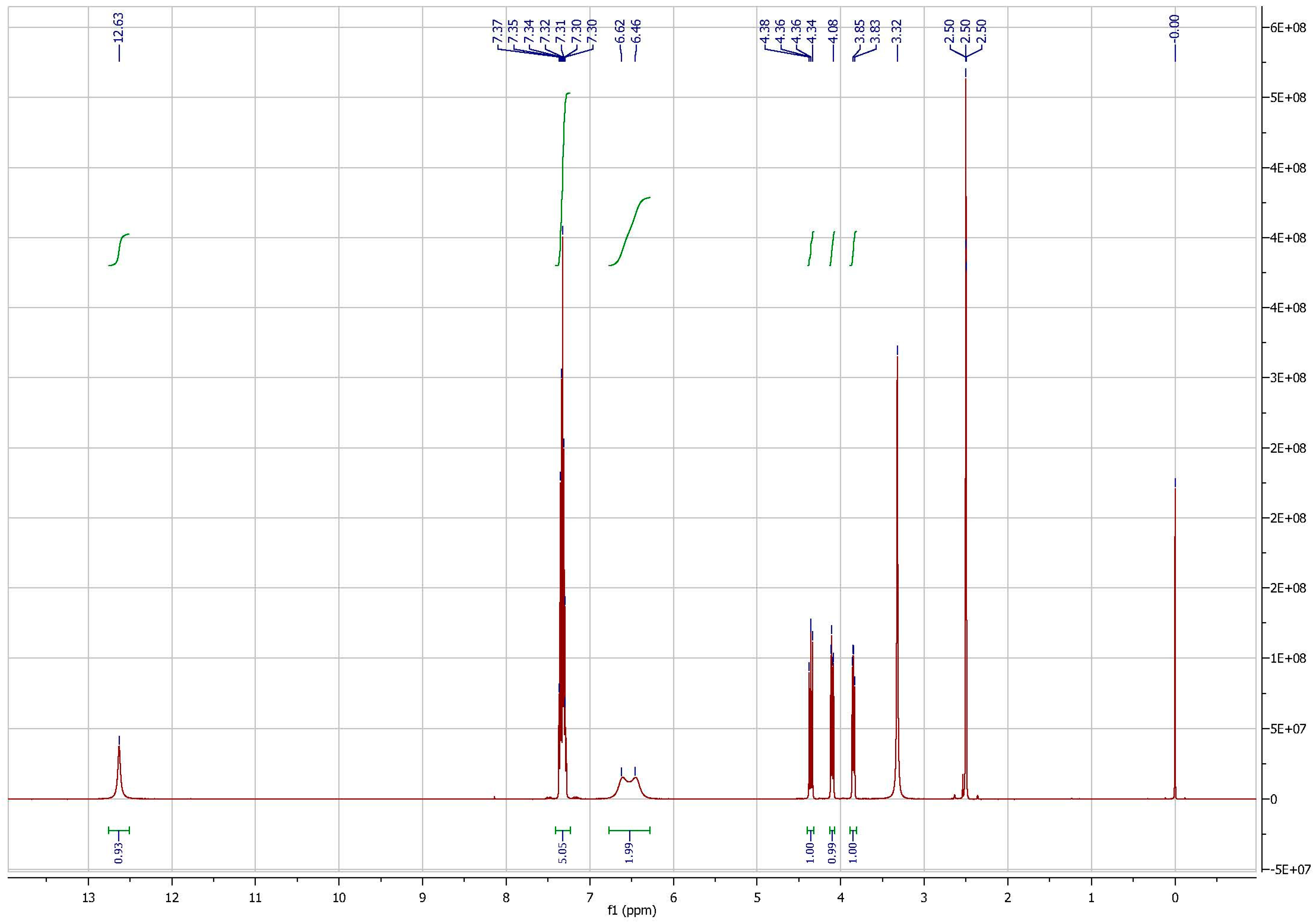
References
- Zimmerli, B.; Schlatter, J. Ethyl carbamate: Analytical methodology, occurrence, formation, biological activity and risk assessment. Mutat. Res. 1991, 259, 325–350. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Dong, Y.; Chen, Q. Ethyl Carbamate in Fermented Beverages: Presence, Analytical Chemistry, Formation Mechanism, and Mitigation Proposals. Compr. Rev. Food Sci. Food Saf. 2014, 13, 611–626. [Google Scholar] [CrossRef]
- Gowd, V.; Su, H.; Karlovsky, P.; Chen, W. Ethyl carbamate: An emerging food and environmental toxicant. Food Chem. 2018, 248, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Abt, E.; Incorvati, V.; Robin, L.P.; Redan, B.W. Occurrence of Ethyl Carbamate in Foods and Beverages: Review of the Formation Mechanisms, Advances in Analytical Methods, and Mitigation Strategies. J. Food Prot. 2021, 84, 2195–2212. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, W.; Fang, F.; Yu, S.; Zhou, J. Elimination of ethyl carbamate in fermented foods. Food Biosci. 2022, 47, 101725. [Google Scholar] [CrossRef]
- Guan, T.; Tian, X.; Wu, J.; Luo, J.; Peng, Z.; Yang, H.; Zhao, X.; Zhang, J. Investigation and risk assessment of ethyl carbamate in Chinese Baijiu. LWT 2021, 152, 112340. [Google Scholar] [CrossRef]
- Jung, S.; Kim, S.; Kim, I.; Chung, M.-S.; Moon, B.; Shin, S.; Lee, J. Risk assessment of ethyl carbamate in alcoholic beverages in Korea using the margin of exposure approach and cancer risk assessment. Food Control 2021, 124, 107867. [Google Scholar] [CrossRef]
- Leça, J.M.; Pereira, V.; Miranda, A.; Vilchez, J.L.; Marques, J.C. New insights into ethyl carbamate occurrence in fortified wines. LWT 2021, 150, 111566. [Google Scholar] [CrossRef]
- Xia, Q.; Yang, C.; Wu, C.; Zhou, R.; Li, Y. Quantitative strategies for detecting different levels of ethyl carbamate (EC) in various fermented food matrices: An overview. Food Control 2018, 84, 499–512. [Google Scholar] [CrossRef]
- Wang, Z.; Qu, P.; Zhao, Y.; Wu, Y.; Lyu, B.; Miao, H. A Nationwide Survey and Risk Assessment of Ethyl Carbamate Exposure Due to Daily Intake of Alcoholic Beverages in the Chinese General Population. Foods 2023, 12, 3129. [Google Scholar] [CrossRef]
- Jin, G.; Zhu, Y.; Xu, Y. Mystery behind Chinese liquor fermentation. Trends Food Sci. Technol. 2017, 63, 18–28. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Zhang, M.P. Ethyl carbamate in Chinese liquor (Baijiu): Presence, analysis, formation, and control. Appl. Microbiol. Biotechnol. 2021, 105, 4383–4395. [Google Scholar] [CrossRef] [PubMed]
- Di, Y.; Li, J.; Chen, J.; Zhao, X.; Du, G. Simulation and Control of the Formation of Ethyl Carbamate during the Fermentation and Distillation Processes of Chinese Baijiu. Foods 2023, 12, 821. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhong, Q.; Wang, D.; Zhang, X.; Gao, H.; Shen, S. Determination and formation of Ethyl Carbamate in Chinese spirits. Food Control 2015, 56, 169–176. [Google Scholar] [CrossRef]
- Tu, Q.; Qi, W.; Zhao, J.; Zhang, L.; Guo, Y. Quantification ethyl carbamate in wines using reaction-assisted-extraction with 9-xanthydrol and detection by heart-cutting multidimensional gas chromatography-mass spectrometry. Anal. Chim. Acta 2018, 1001, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Liu, Y.; Li, W.-Q.; Liu, G.-L.; Wei, N.; Sun, Y.-M.; Bai, W.-D.; Xu, Z.-L. An Improved HPLC-FLD for Fast and Simple Detection of Ethyl Carbamate in Soy Sauce and Prediction of Precursors. Food Anal. Methods 2017, 10, 3856–3865. [Google Scholar] [CrossRef]
- Luo, L.; Lei, H.T.; Yang, J.Y.; Liu, G.L.; Sun, Y.M.; Bai, W.D.; Wang, H.; Shen, Y.D.; Chen, S.; Xu, Z.L. Development of an indirect ELISA for the determination of ethyl carbamate in Chinese rice wine. Anal. Chim. Acta 2017, 950, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Song, Y.; Zhu, C.; Fu, S.; Shi, Q.; Sun, Y.-M.; Jia, B.; Du, D.; Xu, Z.-L.; Lin, Y. Fluorescent silicon nanoparticles-based ratiometric fluorescence immunoassay for sensitive detection of ethyl carbamate in red wine. Sens. Actuators B Chem. 2018, 255, 2742–2749. [Google Scholar] [CrossRef]
- Fu, H.J.; Chen, Z.J.; Wang, H.; Luo, L.; Wang, Y.; Huang, R.M.; Xu, Z.L.; Hammock, B. Development of a sensitive non-competitive immunoassay via immunocomplex binding peptide for the determination of ethyl carbamate in wine samples. J. Hazard. Mater. 2021, 406, 124288. [Google Scholar] [CrossRef]
- Zhang, W.; Si, G.; Ye, M.; Feng, S.; Cheng, F.; Li, J.; Mei, J.; Zong, S.; Wang, J.; Zhou, P. An Efficient Assay for Simultaneous Quantification of Ethyl Carbamate and Phthalate Esters in Chinese Liquor by Gas Chromatography-Mass Spectrometry. Food Anal. Methods 2017, 10, 3487–3495. [Google Scholar] [CrossRef]
- de Melo Abreu, S.; Alves, A.; Oliveira, B.; Herbert, P. Determination of ethyl carbamate in alcoholic beverages: An interlaboratory study to compare HPLC-FLD with GC-MS methods. Anal. Bioanal. Chem. 2005, 382, 498–503. [Google Scholar] [CrossRef]
- Xia, L.; Yang, Y.; Yang, H.; Tang, Y.; Zhou, J.; Wu, Y. Screening and identification of an aptamer as novel recognition molecule in the test strip and its application for visual detection of ethyl carbamate in liquor. Anal. Chim. Acta 2022, 1226, 340289. [Google Scholar] [CrossRef]
- Han, L.; Meng, C.; Zhang, D.; Liu, H.; Sun, B. Fabrication of a fluorescence probe via molecularly imprinted polymers on carbazole-based covalent organic frameworks for optosensing of ethyl carbamate in fermented alcoholic beverages. Anal. Chim. Acta 2022, 1192, 339381. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, Z.L.; Luo, L.; Dong, Y.Z.; Yang, J.Y.; Lei, H.T.; Wang, H.; Shen, Y.D.; Xu, Z.L. Development of Cu(II)/Cu(I)-induced quantum dot-mediated fluorescence immunoassay for the sensitive determination of ethyl carbamate. Mikrochim. Acta 2020, 187, 533. [Google Scholar] [CrossRef] [PubMed]
- ISO 6496:1999; Animal Feeding Stuffs—Determination of Moisture and Other Volatile Matter Content. International Organization for Standardization: Geneva, Switzerland, 1999.
- Xiong, Y.; Leng, Y.; Li, X.; Huang, X.; Xiong, Y. Emerging strategies to enhance the sensitivity of competitive ELISA for detection of chemical contaminants in food samples. TrAC Trends Anal. Chem. 2020, 126, 115861. [Google Scholar] [CrossRef]
- Leça, J.M.; Pereira, V.; Pereira, A.C.; Marques, J.C. A Sensitive Method for the Rapid Determination of Underivatized Ethyl Carbamate in Fortified Wine by Liquid Chromatography-Electrospray Tandem Mass Spectrometry. Food Anal. Methods 2017, 11, 327–333. [Google Scholar] [CrossRef]
- Chen, D.; Ren, Y.; Zhong, Q.; Shao, Y.; Zhao, Y.; Wu, Y. Ethyl carbamate in alcoholic beverages from China: Levels, dietary intake, and risk assessment. Food Control 2017, 72, 283–288. [Google Scholar] [CrossRef]
- Huang, Z.; Pan, X.D.; Wu, P.G.; Chen, Q.; Han, J.L.; Shen, X.H. Validation (in-house and collaboratory) of the quantification method for ethyl carbamate in alcoholic beverages and soy sauce by GC-MS. Food Chem. 2013, 141, 4161–4165. [Google Scholar] [CrossRef]
- Wu, P.; Pan, X.; Wang, L.; Shen, X.; Yang, D. A survey of ethyl carbamate in fermented foods and beverages from Zhejiang, China. Food Control 2012, 23, 286–288. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Fan, Y.; Jia, Y.; Sun, L.; Ma, S.; Ji, C.; Ma, Q.; Zhang, J. Occurrence of mycotoxins in feed ingredients and complete feeds obtained from the Beijing region of China. J. Anim. Sci. Biotechnol. 2014, 5, 37. [Google Scholar] [CrossRef]
- Zhu, Y.; Hassan, Y.I.; Watts, C.; Zhou, T. Innovative technologies for the mitigation of mycotoxins in animal feed and ingredients—A review of recent patents. Anim. Feed. Sci. Technol. 2016, 216, 19–29. [Google Scholar] [CrossRef]
- Buenavista, R.M.E.; Siliveru, K.; Zheng, Y. Utilization of Distiller’s dried grains with solubles: A review. J. Agric. Food Res. 2021, 5, 100195. [Google Scholar] [CrossRef]
- Ajtony, Z.; Szoboszlai, N.; Bencs, L.; Viszket, E.; Mihucz, V.G. Determination of ethyl carbamate in wine by high performance liquid chromatography. Food Chem. 2013, 141, 1301–1305. [Google Scholar] [CrossRef] [PubMed]
- El-Adawy, M.M.; Attia, M.M.; Elgendy, M.Y.; Abdelsalam, M.; Fadel, A. Development of silver nano-based indirect ELISA and Dot-ELISA methods for serological diagnosis of a bacterial fish pathogen Aeromonas veronii. J. Microbiol. Methods 2023, 211, 106782. [Google Scholar] [CrossRef] [PubMed]
- Poolperm, P.; Varinrak, T.; Kataoka, Y.; Tragoolpua, K.; Sawada, T.; Sthitmatee, N. Development and standardization of an in-house indirect ELISA for detection of duck antibody to fowl cholera. J. Microbiol. Methods 2017, 142, 10–14. [Google Scholar] [CrossRef]
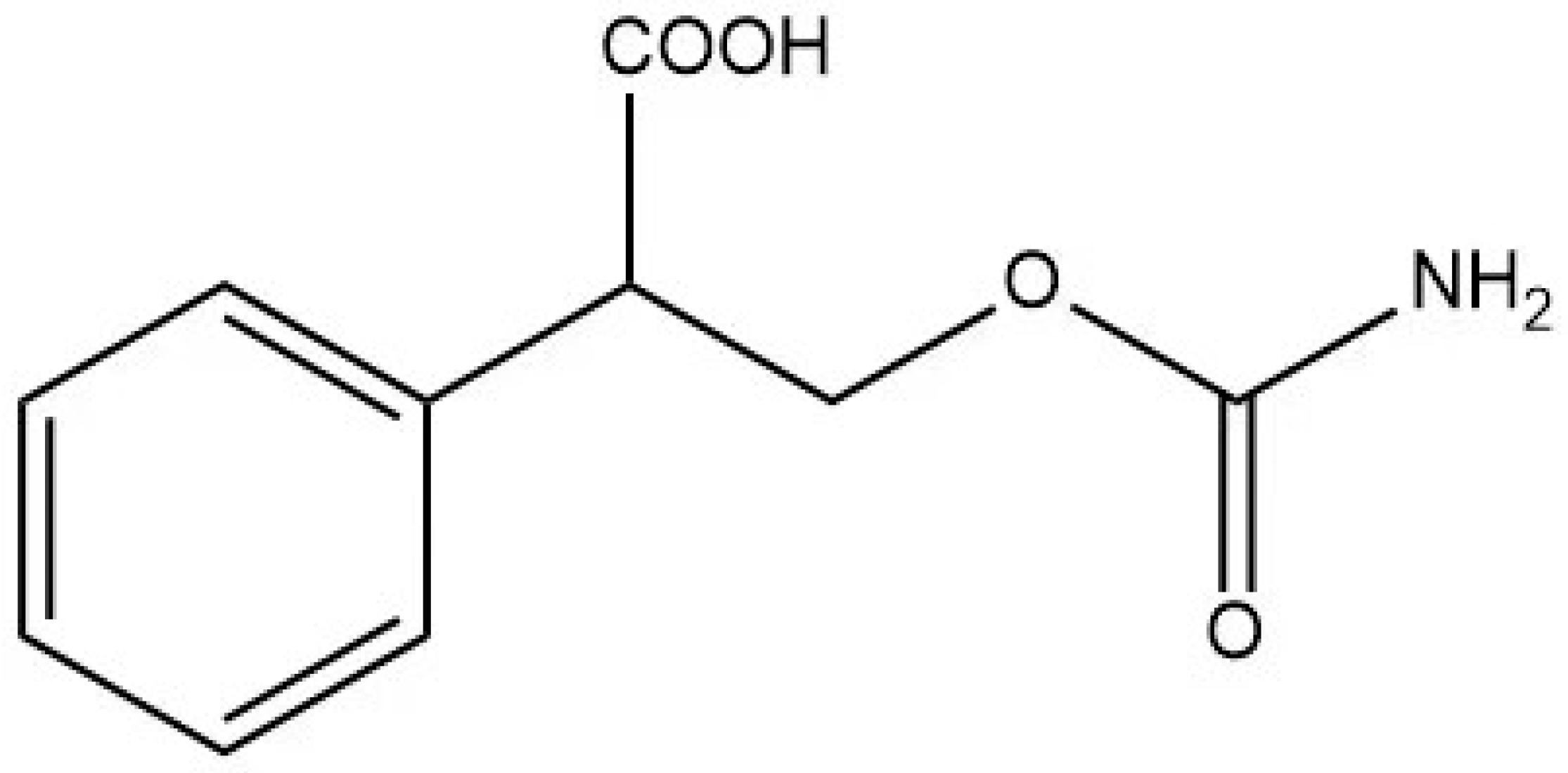
| Alcohol Concentrations (%) | Low Level | High Level | ||||
|---|---|---|---|---|---|---|
| Recoveries (%) | RSD (%) | Inhibitory Rate (%) | Recoveries (%) | RSD (%) | Inhibitory Rate (%) | |
| 2% | 94.27 | 6.44 | 30.22 | 107.72 | 3.54 | 68.25 |
| 4% | 89.25 | 6.50 | 29.65 | 92.21 | 2.28 | 67.89 |
| 20% | 92.68 | 2.24 | 35.44 | 104.21 | 4.16 | 66.68 |
| 40% | 85.90 | 3.58 | 32.11 | 90.43 | 3.60 | 67.42 |
| Dilution Rate of Primary Antibody | Dilution Rate of Coating Antigen | |||||||
|---|---|---|---|---|---|---|---|---|
| 1:5000 | 1:20,000 | 1:40,000 | 1:100,000 | |||||
| Amax | Inhibition Rate (%) | Amax | Inhibition Rate (%) | Amax | Inhibition Rate (%) | Amax | Inhibition Rate (%) | |
| 1:5000 | 2.85 | 12 | 2.26 | 10 | 2.32 | 8 | 1.56 | 28 |
| 1:10,000 | 2.33 | 18 | 1.84 | 14 | 1.72 | 54 | 1.41 | 64 |
| 1:50,000 | 2.08 | 48 | 1.63 | 58 | 1.78 | 75 | 1.23 | 77 |
| 1:100,000 | 1.56 | 57 | 1.28 | 59 | 1.15 | 78 | 0.83 | 81 |
| Analytical Performance Parameters | Dilution Rate of IgG | ||
|---|---|---|---|
| 1:1000 | 1:2000 | 1:5000 | |
| Amax | 1.98 | 2.12 | 1.35 |
| IC50 (μg/kg) | 6.21 | 11.20 | 9.12 |
| Linear (R2) | 0.9932 | 0.9711 | 0.9833 |
| Analytical Performance Parameters | Ratios | |||
|---|---|---|---|---|
| 20:80 | 40:60 | 50:50 | 70:30 | |
| Amax | 2.61 | 2.02 | 2.14 | 1.53 |
| IC50 (μg/kg) | 4.12 | 4.33 | 4.65 | 12.56 |
| Linear (R2) | 0.9812 | 0.9914 | 0.9923 | 0.9654 |
| EC and Its Analogs | Chemical Structure | Cross-Reactivity (%) |
|---|---|---|
| EC |  | 100 |
| Methyl carbamate | 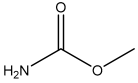 | 10.6 |
| Acrylamide | 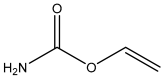 | 6.5 |
| Butyl carbamate |  | 8.8 |
| Method | Sample | Sample Pretreatment | Cut-Off Value/LOD | LOQ | Reference |
|---|---|---|---|---|---|
| Indirect ELISA | Chinese rice wine | Derivatization (ethyl carbamate with 9-xanthydrol) | 166 μg/L | / a | [17] |
| Non-competitive ELISA | Wine | Different phages that recognize XEC were constructed | 5.4 ng/mL | / | [19] |
| Dot-based fluorescence immunoassay | Chinese baijiu; Wine; Chinese rice wine; Brandy | Alkaline phosphatase-triggered Cu+ quenching of CdSe quantum dots (QDs) | 24.3 ng/mL | / | [24] |
| Silicon-based fluorescence ELISA | Red wine | A simple pre-analysis derivatization | 2.6 μg/L | [18] | |
| Indirect competitive ELISA | By-products from the Baijiu industry | IC50: 11.83 μg/kg; IC20: 2.35 μg/kg | The proposed method | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Liu, Q.; Luo, J.; Luo, P.; Wu, Y. A Straightforward, Sensitive, and Reliable Strategy for Ethyl Carbamate Detection in By-Products from Baijiu Production by Enzyme-Linked Immunosorbent Assay. Foods 2024, 13, 1835. https://doi.org/10.3390/foods13121835
Wang Z, Liu Q, Luo J, Luo P, Wu Y. A Straightforward, Sensitive, and Reliable Strategy for Ethyl Carbamate Detection in By-Products from Baijiu Production by Enzyme-Linked Immunosorbent Assay. Foods. 2024; 13(12):1835. https://doi.org/10.3390/foods13121835
Chicago/Turabian StyleWang, Zifei, Qing Liu, Jiaqi Luo, Pengjie Luo, and Yongning Wu. 2024. "A Straightforward, Sensitive, and Reliable Strategy for Ethyl Carbamate Detection in By-Products from Baijiu Production by Enzyme-Linked Immunosorbent Assay" Foods 13, no. 12: 1835. https://doi.org/10.3390/foods13121835
APA StyleWang, Z., Liu, Q., Luo, J., Luo, P., & Wu, Y. (2024). A Straightforward, Sensitive, and Reliable Strategy for Ethyl Carbamate Detection in By-Products from Baijiu Production by Enzyme-Linked Immunosorbent Assay. Foods, 13(12), 1835. https://doi.org/10.3390/foods13121835






