Procyanidin B1 and Coumaric Acid from Highland Barley Alleviated High-Fat-Diet-Induced Hyperlipidemia by Regulating PPARα-Mediated Hepatic Lipid Metabolism and Gut Microbiota in Diabetic C57BL/6J Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Raw Materials
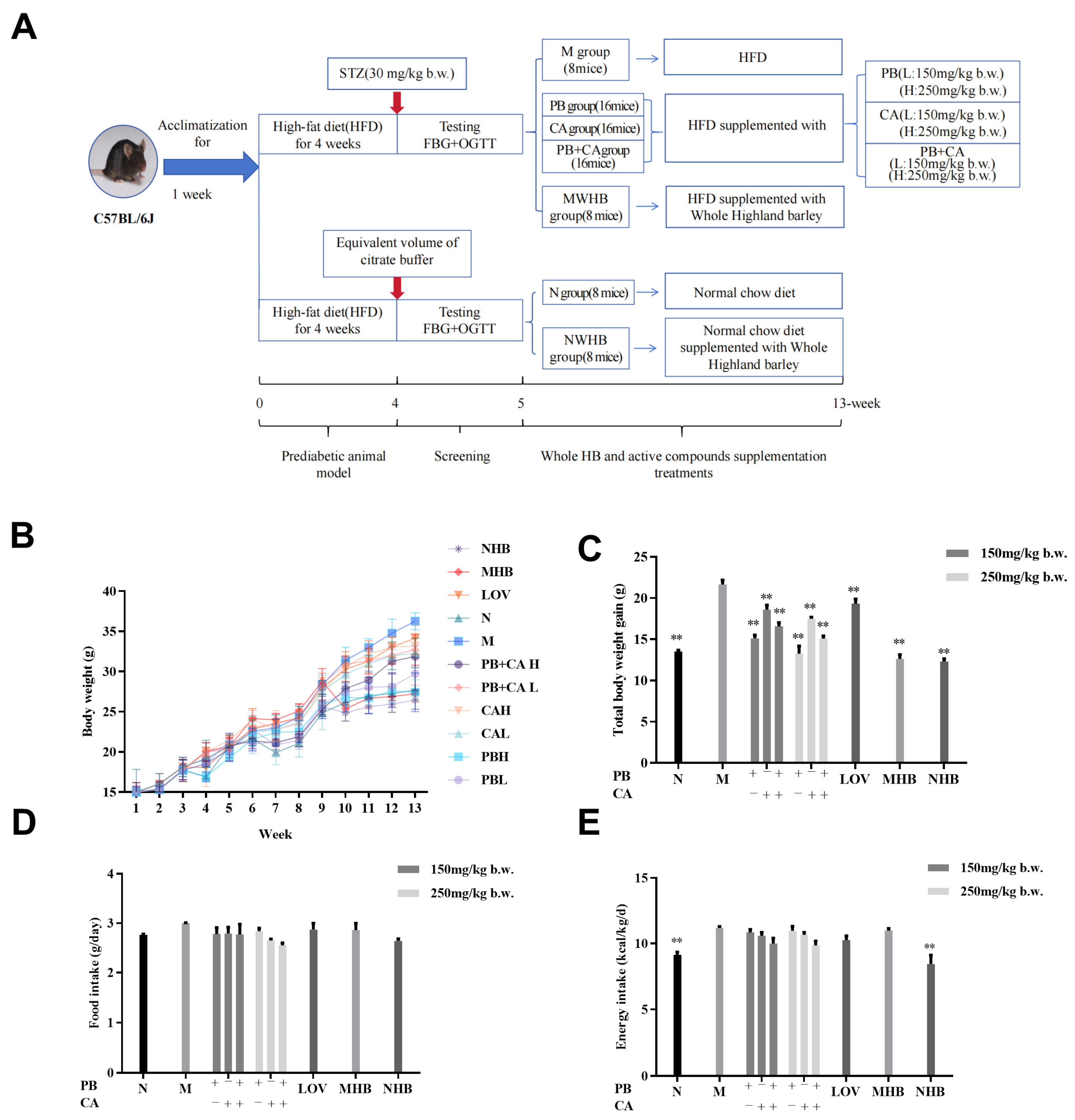
2.2. Animal Care
2.3. Dosage Information
2.4. Determination of Serum Lipids and Hepatic AST and ALT Activities
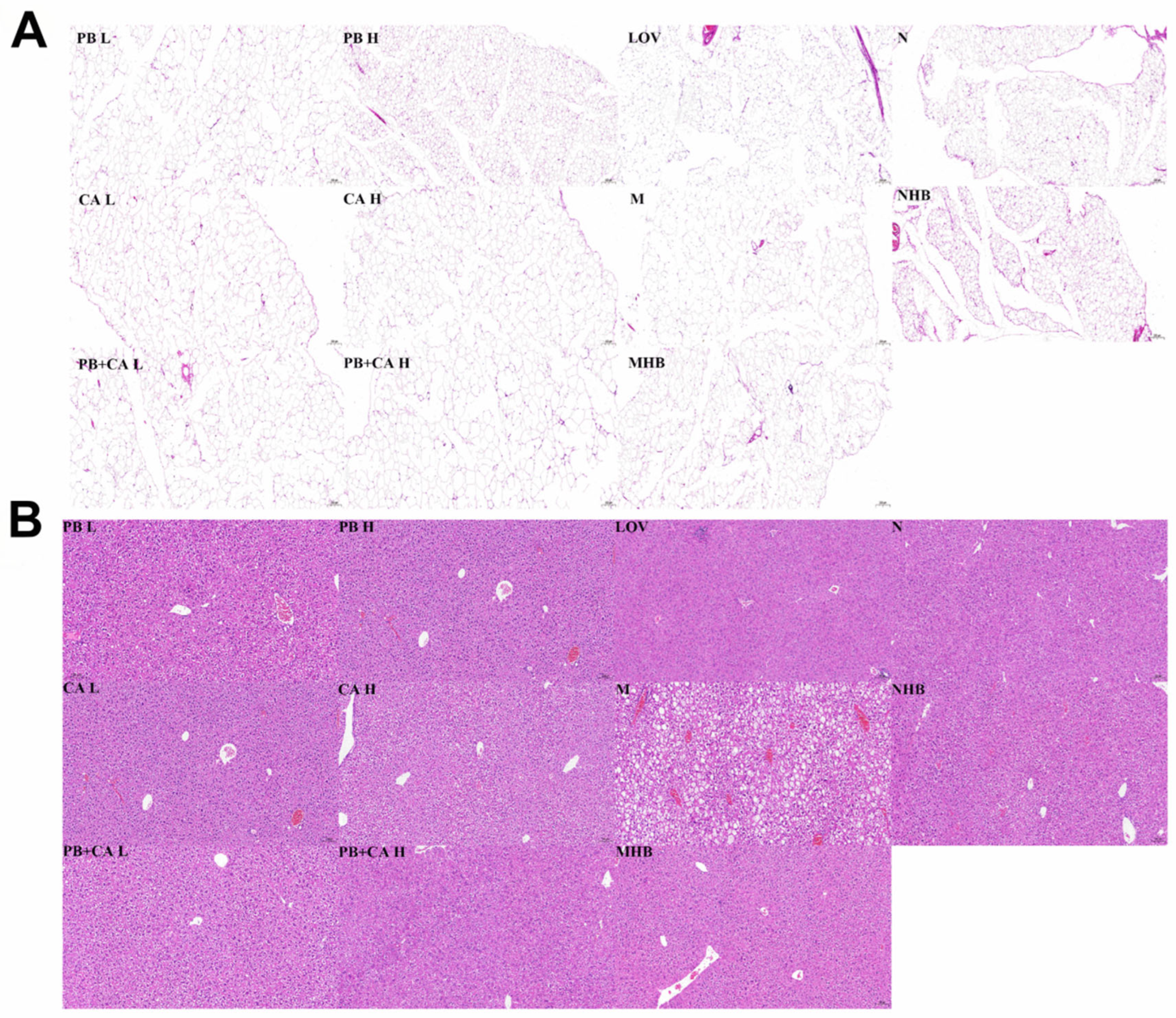
2.5. Histopathological Study
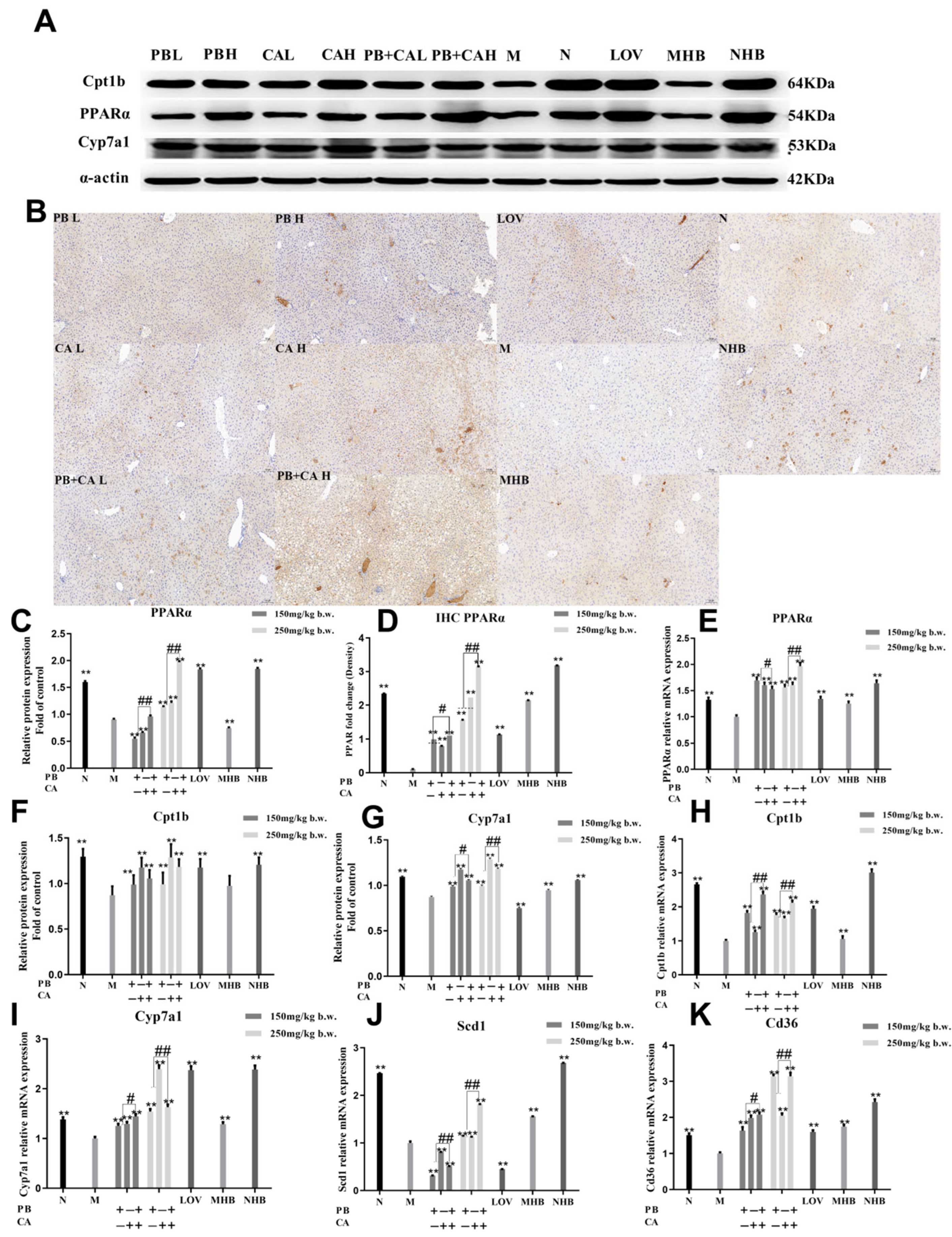
2.6. Immunohistochemistry Analysis
2.7. Western Blot Analysis
2.8. Quantitative RT-PCR Assay
2.9. Gut Microbiota Profiling
2.10. Statistical Analysis
3. Results
3.1. Procyanidin B1 and Coumaric acid Attenuated HFD-Induced Weight Gain
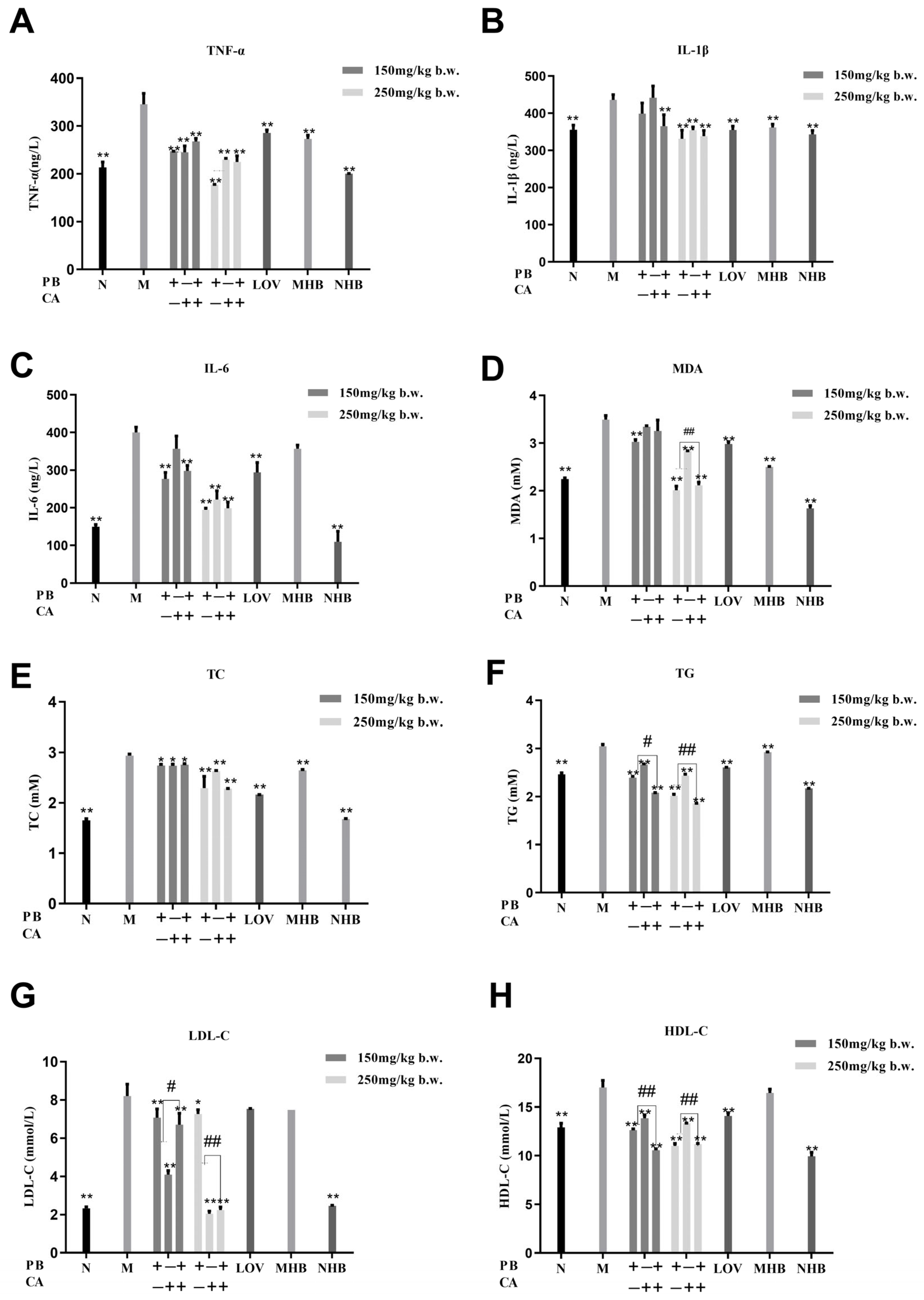
3.2. Procyanidin B1 and Coumaric Acid Ameliorated HFD-Induced Inflammation and Lipid Disorders in Serum
3.3. Procyanidin B1 and Coumaric Acid Reduced HFD-Induced Hepatic Steatosis and Adipose Tissue Hypertrophy
3.4. Procyanidin B1 and Coumaric Acid Activated PPARα Expression in Liver
3.5. Procyanidin B1 and Coumaric Acid Restored HFD-Induced Gut Microbiota Dysbiosis
3.6. Correlation among Gut Microbiota and Hyperlipidemic Parameters in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bougarne, N.; Weyers, B.; Desmet, S.J.; Deckers, J.; Ray, D.W.; Staels, B.; Bosscher, K.D. Molecular actions of PPARα in lipid metabolism and inflammation. Endocr. Rev. 2018, 39, 760–802. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Guan, X.; Huang, K.; Zhang, Y.; Li, S.; Xia, J.; Shen, M. Flavonoids from whole-grain oat alleviated high-fat diet-induced hyperlipidemia via regulating bile acid metabolism and gut microbiota in mice. J. Agric. Food Chem. 2021, 69, 7629–7640. [Google Scholar] [CrossRef] [PubMed]
- Corrales, P.; Vidal-Puig, A.; Medina-Gomez, G. PPARs and metabolic disorders associated with challenged adipose tissue plasticity. Int. J. Mol. Sci. 2018, 19, 2124. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Gao, G.; Wang, H.; Li, E.; Yuan, Y.; Xu, J.; Zhang, Z.; Wang, P.; Fu, Y.; Zeng, H.; et al. Dehydroabietic acid alleviates high fat diet-induced insulin resistance and hepatic steatosis through dual activation of PPAR-γ and PPAR-α. Biomed. Pharmacother. 2020, 127, 110155. [Google Scholar] [CrossRef] [PubMed]
- Chambers, K.F.; Day, P.E.; Aboufarrag, H.T.; Kroon, P.A. Polyphenol effects on cholesterol metabolism via bile acid biosynthesis, CYP7A1: A review. Nutrients 2019, 11, 2588. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Park, J.Y.; Freake, H.; Kim, Y. Green tea catechin enhances cholesterol 7α-hydroxylase gene expression in HepG2 cells. Br. J. Nutr. 2008, 99, 1182–1185. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Feng, S.; Liu, A.; Dai, Z.; Wang, H.; Reuhl, K.; Lu, W.; Yang, C. Green tea polyphenol EGCG alleviates metabolic abnormality and fatty liver by decreasing bile acid and lipid absorption in mice. Mol. Nutr. Food Res. 2018, 62, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Deng, Q.Y.; Zhang, Y.Q.; Wu, D.R.; Li, G.L.; Liu, J.W.; Zhang, L.Y.; Wang, H.M.D. Three novel dietary phenolic compounds from pickled Raphanus sativus L. inhibit lipid accumulation in obese mice by modulating the gut microbiota composition. Mol. Nutr. Food Res. 2021, 65, 2000780. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, S.; Holtekjolen, A. Preparation and analysis of dietary fibre constituents in whole grain from hulled and hull-less barley. Food Chem. 2007, 102, 707–715. [Google Scholar] [CrossRef]
- Liu, Z.H.; Li, B. Procyanidin B1 and p-coumaric acid from highland barley grain showed synergistic effect on modulating glucose metabolism via IRS-1/PI3K/Akt pathway. Mol. Nutr. Food Res. 2021, 65, e2100454. [Google Scholar] [CrossRef]
- Zhong, Y.; Marungruang, N.; Fak, F.; Nyman, M. Effects of two whole-grain barley varieties on caecal SCFA, gut microbiota and plasma inflammatory markers in rats consuming low- and high-fat diets. Br. J. Nutr. 2015, 113, 1558–1570. [Google Scholar] [CrossRef]
- Li, X.; Du, Y.; Zhang, C.; Tu, Z.; Wang, L. Modified highland barley regulates lipid metabolism, liver inflammation and gut microbiota in high-fat/cholesterol diet mice as revealed by LC-MS based metabonomics. Food Funct. 2022, 13, 9119–9142. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.; He, Z.; Guo, R.; Zheng, B.; Li, T.; Liu, R.H. Highland barley whole grain (Hordeum vulgare L.) ameliorates hyperlipidemia by modulating cecal microbiota, miRNAs, and AMPK pathways in leptin receptor-deficient db/db mice. J. Agric. Food Chem. 2020, 68, 11735–11746. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Li, Z.; Qin, B.; Ju, X.; Wang, L. Evaluation of the intracellular lipid-lowering effect of polyphenols extract from highland barley in HepG2 cells. Food Sci. Hum. Wellness 2024, 13, 454–461. [Google Scholar] [CrossRef]
- Gong, L.; Cao, W.; Chi, H.; Wang, J.; Zhang, H.; Liu, J.; Sun, B. Whole cereal grains and potential health effects: Involvement of the gut microbiota. Food Res. Int. 2018, 103, 84–102. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L.; Jacobs, D.; Marquart, L.; Wiemer, K. The role of whole grains in disease prevention. J. Acad. Nutr. Diet. 2001, 101, 7. [Google Scholar] [CrossRef] [PubMed]
- Belobrajdic, D.; Bird, A.R. The potential role of phytochemicals in whole grain cereals for the prevention of type-2 diabetes. Nutr. J. 2013, 12, 62. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Li, X.; Du, Y.; Tu, Z.; Zhang, C.; Wang, L. Highland barley improves lipid metabolism, liver injury, antioxidant capacities and liver functions in high-fat/cholesterol diet mice based on gut microbiota and LC-MS metabonomics. Food Biosci. 2022, 50, 102094. [Google Scholar] [CrossRef]
- Li, S.Q.; Wang, M.Q.; Li, C.; Meng, Q.J.; Meng, Y.T.; Ying, J.; Bai, S.Q.; Shen, Q.; Xue, Y. Beneficial effects of partly milled highland barley on the prevention of high-fat diet-induced glycometabolic disorder and the modulation of gut microbiota in mice. Nutrients 2022, 14, 762. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L. Highland barley attenuates high fat and cholesterol diet induced hyperlipidemia in mice revealed by 16S rRNA gene sequencing and untargeted metabolomics. Life Sci. 2023, 334, 122142. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Nie, Q.X.; Yao, H.Y.; Zhang, K.; Yin, J.Y.; Nie, S.P. Protective effects of β-glucan isolated from highland barley on ethanolinduced gastric damage in rats and its benefits to mice gut conditions. Food Res. Int. 2019, 122, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Tian, S.H.; Li, S.C.; Pang, X.Y.; Sun, J.; Zhu, X.Y.; Lv, F.X.; Lu, Z.X.; Li, X.F. β-Glucan extracted from highland barley alleviates dextran sulfate sodium-induced ulcerative colitis in C57BL/6J mice. Molecules 2021, 26, 5812. [Google Scholar] [CrossRef]
- Shen, J.; Obin, M.S.; Zhao, L. The gut microbiota, obesity and insulin resistance. Mol. Asp. Med. 2013, 34, 39–58. [Google Scholar] [CrossRef]
- Morais, C.A.; Oyama, L.M.; Conrado, R.; Rosso, V.; Nascimento, C.; Pisani, L.P. Polyphenols-rich fruit in maternal diet modulates inflammatory markers and the gut microbiota and improves colonic expression of ZO-1 in offspring. Food Res. Int. 2015, 77, 186–193. [Google Scholar] [CrossRef]
- Crakes, K.R.; Rocha, C.S.; Grishina, I.; Hirao, L.; Napoli, A.E.; Gaulke, C.A.; Dandekar, S. PPARalpha-targeted mitochondrial bioenergetics mediate repair of intestinal barriers at the host-microbe intersection during SIV infection. Proc. Natl. Acad. Sci. USA 2019, 116, 24819–24829. [Google Scholar] [CrossRef]
- Liu, Z.H.; Li, B. (-)-Epicatechin and β-glucan from highland barley grain modulated glucose metabolism and showed synergistic effect via Akt pathway. J. Funct. Food 2021, 87, 104793. [Google Scholar] [CrossRef]
- Hou, D.; Zhao, Q.; Yousaf, L.; Xue, Y.; Shen, Q. Whole mung bean (Vigna radiata L.) supplementation prevents high-fat diet-induced obesity and disorders in a lipid profile and modulates gut microbiota in mice. Eur. J. Nutr. 2020, 59, 3617–3634. [Google Scholar] [CrossRef]
- Hou, D.; Liu, F.; Ren, X.; Shen, Q.; Zhou, S.M. Protective mechanism of mung bean coat against hyperlipidemia in mice fed with a high-fat diet: Insight from hepatic transcriptome analysis. Food Funct. 2021, 12, 12434. [Google Scholar] [CrossRef]
- Zhao, G.; Teng, J.; Dong, R.; Ban, Q.; Yang, L.; Du, K.; Wang, Y.; Pu, H.; Ren, Z. Alleviating effects and mechanisms of action of large-leaf yellow tea drinking on diabetes and diabetic nephropathy in mice. Food Sci. Hum. Wellness 2023, 12, 1660–1673. [Google Scholar] [CrossRef]
- Hong, F.; Pan, S.; Guo, Y.; Xu, P.; Zhai, Y. PPARs as nuclear receptors for nutrient and energy metabolism. Molecules 2019, 24, 2545. [Google Scholar] [CrossRef]
- Preidis, G.A.; Kim, K.H.; Moore, D.D. Nutrient-sensing nuclear receptors PPARalpha and FXR control liver energy balance. J. Clin. Investig. 2017, 127, 1193–1201. [Google Scholar] [CrossRef]
- Regnier, M.; Polizzi, A.; Lippi, Y.; Fouche, E.; Michel, G.; Lukowicz, C.; Smati, S.; Marrot, A.; Lasserre, F.; Naylies, C.; et al. Insights into the role of hepatocyte PPARα activity in response to fasting. Mol. Cell. Endocrinol. 2018, 471, 75–88. [Google Scholar] [CrossRef]
- Shimada, T.; Tokuhara, D.; Tsubata, M.; Kamiya, T.; Kamiya-Sameshima, M.; Nagamine, R.; Takagaki, K.; Sai, Y.; Miyamoto, K.; Aburada, M. Flavangenol (pine bark extract) and its major component procyanidin B1 enhance fatty acid oxidation in fat-loaded models. Eur. J. Pharmacol. 2012, 677, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Dubois, V.; Eeckhoute, J.; Lefebvre, P.; Staels, B. Distinct but complementary contributions of PPAR isotypes to energy homeostasis. J. Clin. Investig. 2017, 127, 1202–1214. [Google Scholar] [CrossRef]
- Tie, F.; Wang, J.; Liang, Y.; Zhu, S.; Wang, Z.; Li, G.; Wang, H. Proanthocyanidins ameliorated deficits of lipid metabolism in type 2 diabetes mellitus via inhibiting adipogenesis and improving mitochondrial function. Int. J. Mol. Sci. 2020, 21, 2029. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Li, Y.; Hu, D.; Xie, L.; Ke, H.; Zheng, X.; Chen, W. Procyanidin B2 ameliorates free fatty acids-induced hepatic steatosis through regulating TFEB-mediated lysosomal pathway and redox state. Free Radic. Biol. Med. 2018, 126, 269–286. [Google Scholar] [CrossRef]
- Jayachandran, M.; Chung, S.S.M.; Xu, B. A critical review of the relationship between dietary components, the gut microbe Akkermansia muciniphila, and human health. Crit. Rev. Food Sci. Nutr. 2020, 60, 2265–2276. [Google Scholar] [CrossRef]
- Kameyama, K.; Itoh, K. Intestinal colonization by a Lachnospiraceae bacterium contributes to the development of diabetes in obese mice. Microbes Environ. 2014, 29, 427–430. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Huang, Q.; Fu, X. Polysaccharide from Rosa roxburghii tratt fruit attenuates hyperglycemia and hyperlipidemia and regulates colon microbiota in diabetic db/db mice. J. Agric. Food Chem. 2020, 68, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Węglarz1, L.; Parfiniewicz, B.; Mertas, A.; Kondera-Anasz, Z.; Jaworska-Kik, M.; Dzierzewicz, Z.; Swiatkowska, L. Effect of endotoxins isolated from Desulfovibrio desulfuricans soil and intestinal strain on the secretion of TNF-α by human mononuclear cells. Pol. J. Environ. Stud. 2006, 15, 615–622. [Google Scholar]
- Park, Y.H.; Kim, J.G.; Shin, Y.W.; Kim, H.S.; Kim, Y.; Chun, T.; Kim, S.H.; Whang, K.Y. Effects of Lactobacillus acidophilus 43121 and a mixture of Lactobacillus casei and Bifidobacterium longum on the serum cholesterol level and fecal sterol excretion in hypercholesterolemia-induced pigs. Biosci. Biotechnol. Biochem. 2008, 72, 595–600. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cui, H.X.; Hu, Y.N.; Li, J.W.; Yuan, K. Hypoglycemic mechanism of the berberine organic acid salt under the synergistic effect of intestinal flora and oxidative stress. Oxidative Med. Cell. Longev. 2018, 2018, 8930374. [Google Scholar] [CrossRef]
- Gomez-Gallego, C.; Pohl, S.; Salminen, S.; Vos, W.M.; Kneifel, W. Akkermansia muciniphila: A novel functional microbe with probiotic properties. Benef. Microbes 2016, 7, 571–584. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Liu, J.; Tang, R.; Zhang, Z.; Tian, S. Procyanidin B1 and Coumaric Acid from Highland Barley Alleviated High-Fat-Diet-Induced Hyperlipidemia by Regulating PPARα-Mediated Hepatic Lipid Metabolism and Gut Microbiota in Diabetic C57BL/6J Mice. Foods 2024, 13, 1843. https://doi.org/10.3390/foods13121843
Liu Z, Liu J, Tang R, Zhang Z, Tian S. Procyanidin B1 and Coumaric Acid from Highland Barley Alleviated High-Fat-Diet-Induced Hyperlipidemia by Regulating PPARα-Mediated Hepatic Lipid Metabolism and Gut Microbiota in Diabetic C57BL/6J Mice. Foods. 2024; 13(12):1843. https://doi.org/10.3390/foods13121843
Chicago/Turabian StyleLiu, Zehua, Jianshen Liu, Ruoxin Tang, Zhaowan Zhang, and Shuangqi Tian. 2024. "Procyanidin B1 and Coumaric Acid from Highland Barley Alleviated High-Fat-Diet-Induced Hyperlipidemia by Regulating PPARα-Mediated Hepatic Lipid Metabolism and Gut Microbiota in Diabetic C57BL/6J Mice" Foods 13, no. 12: 1843. https://doi.org/10.3390/foods13121843
APA StyleLiu, Z., Liu, J., Tang, R., Zhang, Z., & Tian, S. (2024). Procyanidin B1 and Coumaric Acid from Highland Barley Alleviated High-Fat-Diet-Induced Hyperlipidemia by Regulating PPARα-Mediated Hepatic Lipid Metabolism and Gut Microbiota in Diabetic C57BL/6J Mice. Foods, 13(12), 1843. https://doi.org/10.3390/foods13121843




