Application of PLA-Based Films to Preserve Strawberries’ Bioactive Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Experimental Design
2.3. Weight Loss
2.4. pH, Total Titratable Acidity, and Total Soluble Solids
2.5. Determination of Vitamin C Content
2.6. Extraction and Determination of Total Phenol Content
2.7. Total Anthocyanin Content
2.8. Determination of DPPH Radical Scavenging Capacity
2.9. Howard Mold Count
2.10. Statistical Analysis
3. Results and Discussion
3.1. Weight Loss
3.2. pH, Total Titratable Acidity, and Total Soluble Solids
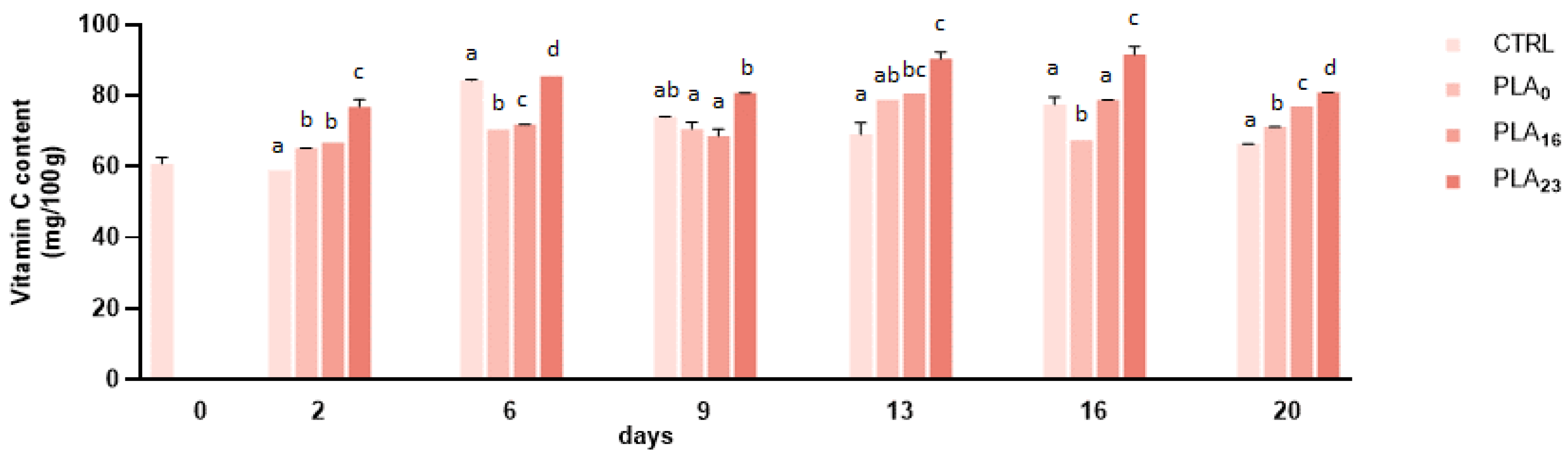
3.3. Vitamin C Content
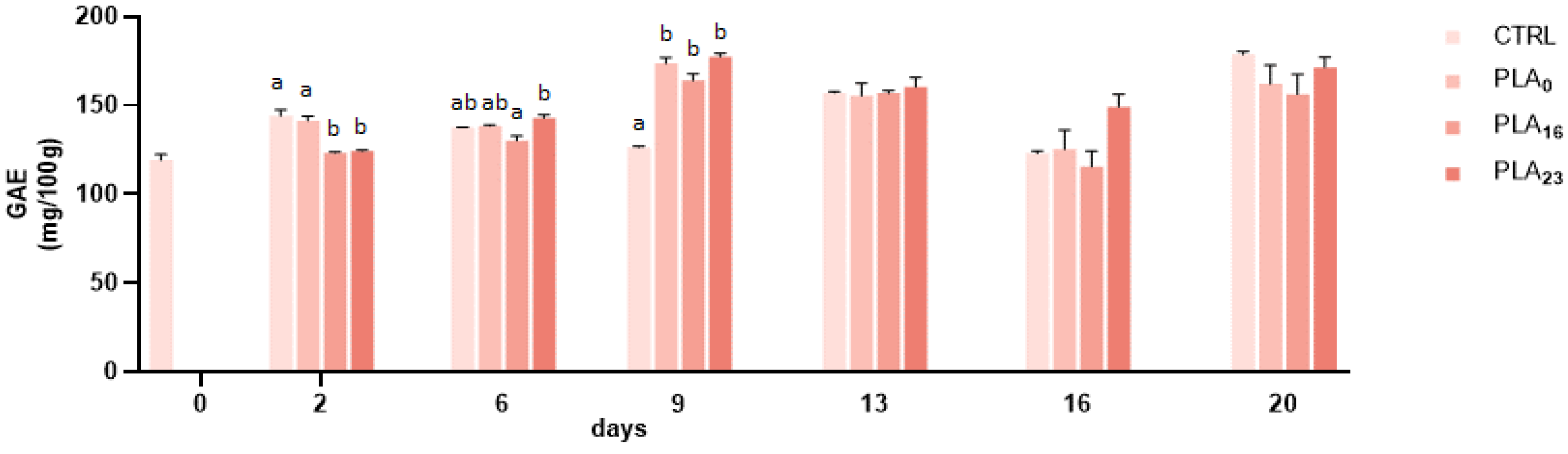
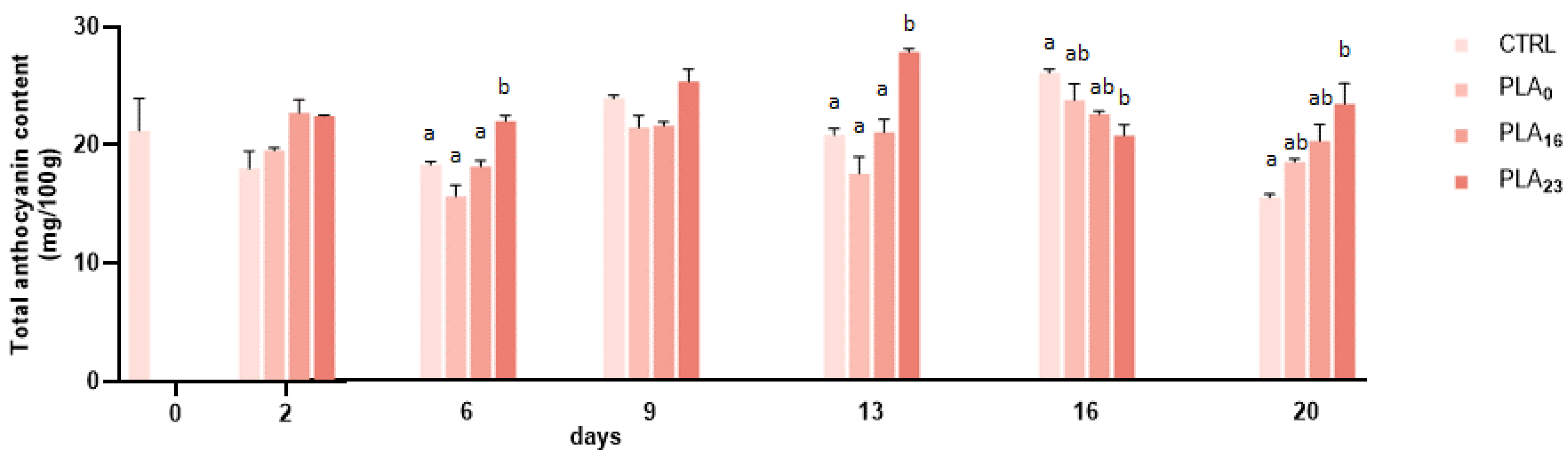
3.4. Effect of Different Packaging on TPC and TAC
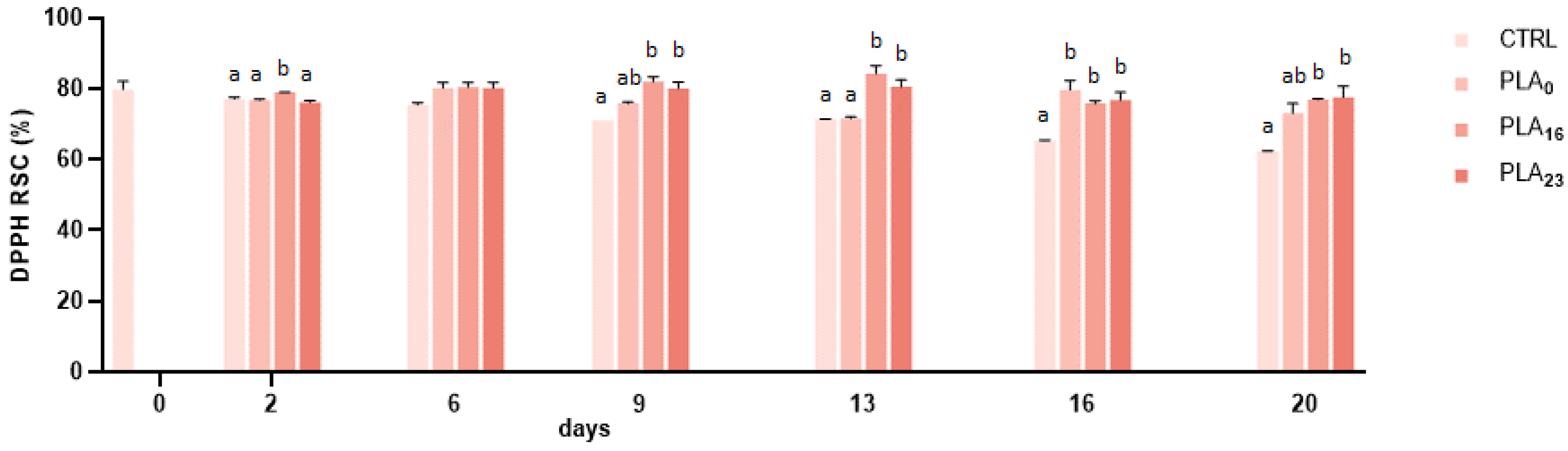
3.5. Antioxidant Activity
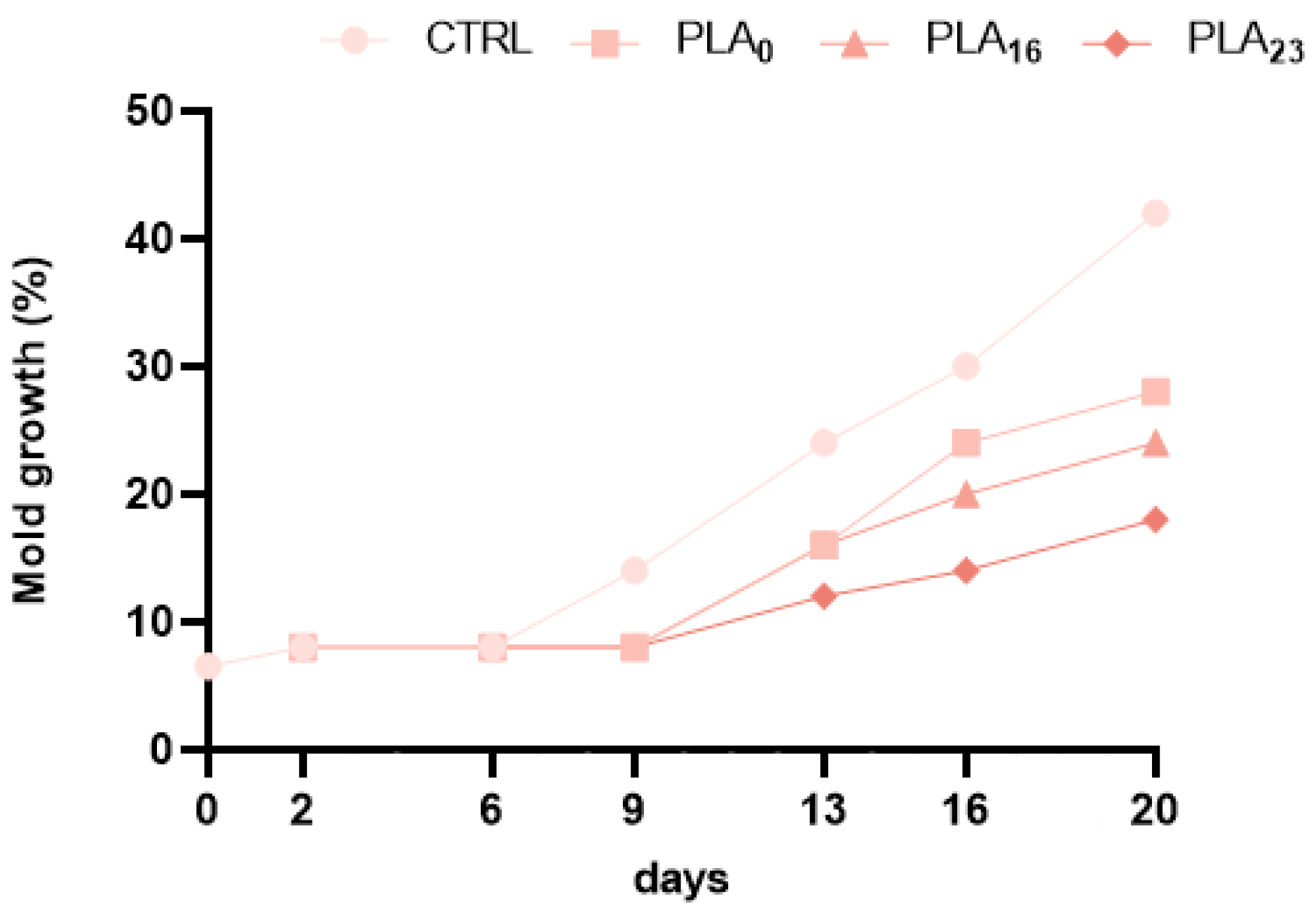
3.6. Effect of Different Packaging on Molds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albuquerque, T.G.; Silva, M.A.; Oliveira, M.B.P.; Costa, H.S. Analysis, identification, and quantification of anthocyanins in fruit juices. In Fruit Juices; Rajauria, G., Tiwari, B.K., Eds.; Academic Press: San Diego, CA, USA, 2018; pp. 693–737. [Google Scholar]
- Mir, H.; Patel, V.B. Genetic engineering of temperate fruit crops. In Genetic Engineering of Horticultural Crops; Rout, G.R., Peter, K.V., Eds.; Academic Press: San Diego, CA, USA, 2018; pp. 89–119. [Google Scholar]
- Giampieri, F.; Alvarez-Suarez, J.M.; Mazzoni, L.; Romandini, S.; Bompadre, S.; Diamanti, J.; Capocasa, F.; Mezzetti, B.; Quiles, J.L.; Ferreiro, M.S.; et al. The potential impact of strawberry on human health. Nat. Prod. Res. 2013, 27, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Ladika, G.; Strati, I.F.; Tsiaka, T.; Cavouras, D.; Sinanoglou, V.J. On the Assessment of Strawberries’ Shelf-Life and Quality, Based on Image Analysis, Physicochemical Methods, and Chemometrics. Foods 2024, 13, 234. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Stamminger, R. Preserving Strawberry Quality by Employing Novel Food Preservation and Processing Techniques—Recent Updates and Future Scope—An Overview: Preservation and Processing Techniques for Strawberries. J. Food Process Eng. 2015, 38, 536–554. [Google Scholar] [CrossRef]
- Afrin, S.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Reboredo-Rodriguez, P.; Mezzetti, B.; Varela-Lόpez, A.; Giampieri, F.; Battino, M. Promising health benefits of the strawberry: A focus on clinical studies. J. Agric. Food Chem. 2016, 64, 4435–4449. [Google Scholar] [CrossRef]
- Tulipani, S.; Mezzetti, B.; Battino, M. Impact of strawberries on human health: Insight into marginally discussed bioactive compounds for the Mediterranean diet. Public Health Nutr. 2009, 12, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Wani, I.A. Postharvest biology and technology of strawberry. In Postharvest Biology and Technology of Temperate Fruits; Mir, S., Shah, M., Mir, M., Eds.; Springer: Cham, Switzerland, 2018; pp. 331–348. [Google Scholar]
- Keshri, N.; Weltzien, C.; Mahajan, P.V. Sensors for measurement of respiratory gases in fresh produce packaging and storage. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–5. [Google Scholar]
- Iversen, L.J.L.; Rovina, K.; Vonnie, J.M.; Matanjun, P.; Erna, K.H.; ‘Aqilah, N.M.N.; Felicia, W.X.L.; Funk, A.A. The Emergence of Edible and Food-Application Coatings for Food Packaging: A Review. Molecules 2022, 27, 5604. [Google Scholar] [CrossRef] [PubMed]
- Perera, K.Y.; Jaiswal, A.K.; Jaiswal, S. Biopolymer-Based Sustainable Food Packaging Materials: Challenges, Solutions, and Applications. Foods 2023, 12, 2422. [Google Scholar] [CrossRef] [PubMed]
- Abang, S.; Wong, F.; Sarbatly, R.; Sariau, J.; Baini, R.; Besar, N. Bioplastic classifications and innovations in antibacterial, antifungal, and antioxidant applications. J. Bioresour. Bioprod. 2023, 8, 361–387. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, X.; Wu, J.; Zhou, T.; Nguyen, T.T.; Wang, Y. Biodegradable Polylactic Acid and Its Composites: Characteristics, Processing, and Sustainable Applications in Sports. Polymers 2023, 15, 3096. [Google Scholar] [CrossRef]
- Mulla, M.Z.; Rahman, M.R.T.; Marcos, B.; Tiwari, B.; Pathania, S. Poly Lactic Acid (PLA) Nanocomposites: Effect of Inorganic Nanoparticles Reinforcement on Its Performance and Food Packaging Applications. Molecules 2021, 26, 1967. [Google Scholar] [CrossRef] [PubMed]
- Yuvaraj, D.; Iyyappan, J.; Gnanasekaran, R.; Ishwarya, G.; Harshini, R.P.; Dhithya, V.; Chandran, M.; Kanishka, V.; Gomathi, K. Advances in bio food packaging—An overview. Heliyon 2021, 7, e07998. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, G.; Arvindh Seshadri, S.; Devnani, G.L.; Sanjay, M.R.; Siengchin, S.; Prakash Maran, J.; Al-Dhabi, N.A.; Karuppiah, P.; Mariadhas, V.A.; Sivarajasekar, N.; et al. Environment friendly, renewable and sustainable poly lactic acid (PLA) based natural fiber reinforced composites—A comprehensive review. J. Clean. Prod. 2021, 310, 127483. [Google Scholar] [CrossRef]
- Almenar, E.; Samsudin, H.; Auras, R.; Harte, B.; Rubino, M. Postharvest Shelf Life Extension of Blueberries Using a Biodegradable Package. Food Chem. 2008, 110, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Kawamura, S.; Koseki, S.; Kimura, T. Comparative Quality Changes of Fresh-Cut Melon in Bio-Based and Petroleum-Based Plastic Containers during Storage. Environ. Control Biol. 2016, 54, 93–99. [Google Scholar] [CrossRef]
- Botondi, R.; Bartoloni, S.; Baccelloni, S.; Mencarelli, F. Biodegradable PLA (Polylactic Acid) Hinged Trays Keep Quality of Fresh-Cut and Cooked Spinach. J. Food Sci. Technol. 2015, 52, 5938–5945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, W.; Zhu, B.; Chen, H.; Chi, H.; Li, L.; Qin, Y.; Xue, J. The Quality Evaluation of Postharvest Strawberries Stored in Nano-Ag Packages at Refrigeration Temperature. Polymers 2018, 10, 894. [Google Scholar] [CrossRef] [PubMed]
- Subbuvel, M.; Kavan, P. Preparation and characterization of polylactic acid/fenugreek essential oil/curcumin composite films for food packaging applications. Int. J. Biol. Macromol. 2022, 194, 470–483. [Google Scholar] [CrossRef]
- Mistriotis, A.; Giannoulis, A.; Giannopoulos, D.; Briassoulis, D. Analysis of the effect of perforation on the permeability of biodegradable non-barrier films. Procedia Food Sci. 2011, 1, 32–38. [Google Scholar] [CrossRef]
- Qu, P.; Zhang, M.; Fan, K.; Guo, Z. Microporous modified atmosphere packaging to extend shelf life of fresh foods: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 51–65. [Google Scholar] [CrossRef]
- Win, N.N.C.; Soe, T.T.; Kar, A.; Soe, Y.Y.; Lin, M. Effects of Syrup Solution with Different Concentrations of Citric Acid on Quality and Storage Life of Canned Litchi. Open Access Libr. J. 2021, 8, 1–16. [Google Scholar] [CrossRef]
- Pagliarulo, C.; Sansone, F.; Moccia, S.; Russo, G.L.; Aquino, R.P.; Salvatore, P.; Di Stasio, M.; Volpe, M.G. Preservation of strawberries with an antifungal edible coating using peony extracts in chitosan. Food Bioprocess Technol. 2016, 9, 1951–1960. [Google Scholar] [CrossRef]
- Lee, S.G.; Vance, T.M.; Nam, T.G.; Kim, D.O.; Koo, S.I.; Chun, O.K. Evaluation of pH differential and HPLC methods expressed as cyanidin-3-glucoside equivalent for measuring the total anthocyanin contents of berries. Food Meas. 2016, 10, 562–568. [Google Scholar] [CrossRef]
- Cascone, G.; Crescente, G.; Sorrentino, A.; Volpe, M.G.; Moccia, S. Physicochemical characterization of a functional chestnut sweet cream enriched with carotenoids and fiber. LWT 2023, 177, 114583. [Google Scholar] [CrossRef]
- Gourama, H.; Bullerman, L.B. Detection of molds in foods and feeds: Potential rapid and selective methods. J. Food Prot. 1995, 58, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Baylis, C.L. Food Spoilage Microorganisms; Blackburn, C.W., Ed.; CRC Press LLC: Cambridge, UK, 2006; Part 5; p. 635. [Google Scholar]
- Sukhavattanakul, P.; Thanyacharoen, T.; Chuysinuan, P.; Techasakul, S.; Ummartyotin, S. Influence of a Transparent and Edible Coating of Encapsulated Cannabidiol Nanoparticles on the Quality and Shelf Life of Strawberries. ACS Appl. Mater. Interfaces 2023, 15, 23834–23843. [Google Scholar] [CrossRef] [PubMed]
- Muncan, J.; Tsenkova, R. Aquaphotomics-From Innovative Knowledge to Integrative Platform in Science and Technology. Molecules 2019, 24, 2742. [Google Scholar] [CrossRef] [PubMed]
- Castelló, M.L.; Fito, P.J.; Chiralt, A. Changes in respiration rate and physical properties of strawberries due to osmotic dehydration and storage. J. Food Eng. 2010, 97, 64–71. [Google Scholar] [CrossRef]
- Nunes, M.C.N.; Brecht, J.K.; Morais, A.M.M.B.; Sargent, S.A. Physicochemical changes during strawberry development in the field compared with those that occur in harvested fruit during storage. J. Sci. Food Agric. 2006, 86, 180–190. [Google Scholar] [CrossRef]
- Musto, M.; Satriano, M.L. Fruit responses to postharvest heat treatment time: Characterisation of heat-treated strawberry (Fragaria × ananassa) cv. ‘Candonga’ fruits. Agron. Res. 2010, 8, 815–826. [Google Scholar]
- Koyuncu, M.A.; Dilmacunal, T. Determination of Vitamin C and Organic Acid Changes in Strawberry by HPLC during Cold Storage. Not. Bot. Horti Agrobot. Cluj 2010, 38, 95–98. [Google Scholar]
- Green, A. Soft fruits. In The Biochemistry of Fruits and Their Products; Hulme, A.C., Ed.; Academic Press: London, UK; New York, NY, USA, 1971; Volume 2, pp. 375–410. [Google Scholar]
- Cordenunsi, B.R.; Nascimento, J.R.O.; Lajolo, F.M. Physicochemical changes related to quality of five strawberry fruit cultivars during cool-storage. Food Chem. 2003, 83, 167–173. [Google Scholar] [CrossRef]
- Mirahmadi, F.; Hanafi, Q.M.; Alizadeh, M.; Mohamadi, H.; Sarsaifee, M. Effect of low temperature on physico-chemical properties of different strawberry cultivars. Afr. J. Food Sci. Technol. 2011, 2, 109–115. [Google Scholar]
- Pineli, L.D.L.D.O.; Moretti, C.L.; dos Santos, M.S.; Campos, A.B.; Brasileiro, A.V.; Córdova, A.C.; Chiarello, M.D. Antioxidants and other chemical and physical characteristics of two strawberry cultivars at different ripeness stages. J. Food Compos. Anal. 2011, 24, 11–16. [Google Scholar] [CrossRef]
- Simkova, K.; Veberic, R.; Hudina, M.; Grohar, M.C.; Pelacci, M.; Smrke, T.; Ivancic, T.; Cvelbar Weber, N.; Jakopic, J. Non-destructive and destructive physical measurements as indicators of sugar and organic acid contents in strawberry fruit during ripening. Sci. Hortic. 2024, 327, 112843. [Google Scholar] [CrossRef]
- Sapei, L.; Hwa, L. Study on the Kinetics of Vitamin C Degradation in Fresh Strawberry Juices. Procedia Chem. 2014, 9, 62–68. [Google Scholar] [CrossRef]
- Feszterová, M.; Kowalska, M.; Mišiaková, M. Stability of Vitamin C Content in Plant and Vegetable Juices under Different Storing Conditions. Appl. Sci. 2023, 13, 10640. [Google Scholar] [CrossRef]
- Cervantes, L.; Ariza, M.T.; Miranda, L.; Lozano, D.; Medina, J.J.; Soria, C.; Martínez-Ferri, E. Stability of Fruit Quality Traits of Different Strawberry Varieties under Variable Environmental Conditions. Agronomy 2020, 10, 1242. [Google Scholar] [CrossRef]
- Lee, S.K.; Kader, A.A. Preharvest and Postharvest Factors Influencing Vitamin C Content of Horticultural Crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef]
- Shin, Y.; Liu, R.H.; Nock, J.F.; Holliday, D.; Watkins, C.B. Temperature and relative humidity effects on quality, total ascorbic acid, phenolics and flavonoid concentrations, and antioxidant activity of strawberry. Postharvest Biol. Technol. 2007, 45, 349–357. [Google Scholar] [CrossRef]
- Koyama, R.; Ishibashi, M.; Fukuda, I.; Okino, A.; Osawa, R.; Uno, Y. Pre- and Post-Harvest Conditions Affect Polyphenol Content in Strawberry (Fragaria × ananassa). Plants 2022, 11, 2220. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Zavala, J.F.; Wang, S.Y.; Wang, C.Y.; González-Aguilar, G.A. Effect of storage temperatures on antioxidant capacity and aroma compounds in strawberry fruit. LWT 2004, 37, 687–695. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, S.Y.; Wang, C.Y.; Zheng, W. Changes in strawberry phenolics, anthocyanins, and antioxidant capacity in response to high oxygen treatments. LWT 2007, 40, 49–57. [Google Scholar] [CrossRef]
- Clifford, M.N. Anthocyanins—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1063–1072. [Google Scholar] [CrossRef]
- Bal, E.; Bahtiyar, A.Ü. Effects of chitosan coating with putrescine on bioactive compounds and quality of strawberry cv. san andreas during cold storage. Erwerbs-Obstbau 2021, 63, 1–8. [Google Scholar] [CrossRef]
- Aaby, K.; Skrede, G.; Wrolstad, R.E. Phenolic composition and antioxidant activities in flesh and achenes of strawberries (Fragaria ananassa). J. Agric. Food Chem. 2005, 53, 4032–4040. [Google Scholar] [CrossRef]
- Tulipani, S.; Mezzetti, B.; Capocasa, F.; Bompadre, S.; Beekwilder, J.; de Vos, C.H.; Capanoglu, E.; Bovy, A.; Battino, M. Antioxidants, phenolic compounds, and nutritional quality of different strawberry genotypes. J. Agric. Food Chem. 2008, 56, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Vischetti, C.; Feliziani, E.; Landi, L.; De Bernardi, A.; Marini, E.; Romanazzi, G. Effectiveness of Four Synthetic Fungicides in the Control of Post-Harvest Gray Mold of Strawberry and Analyses of Residues on Fruit. Agronomy 2024, 14, 65. [Google Scholar] [CrossRef]
- Bhujel, A.; Khan, F.; Basak, J.K.; Jaihuni, M.; Sihalath, T.; Moon, B.E.; Park, J.; Kim, H.T. Detection of gray mold disease and its severity on strawberry using deep learning networks. J. Plant Dis. Prot. 2022, 129, 579–592. [Google Scholar] [CrossRef]
- Guan, Y.; Ji, Y.; Yang, X.; Pang, L.; Cheng, J.; Lu, X.; Zheng, J.; Yin, L.; Hu, W. Antioxidant activity and microbial safety of fresh-cut red cabbage stored in different packaging films. LWT 2023, 175, 114478. [Google Scholar] [CrossRef]
| Weight Loss (%) | ||||
|---|---|---|---|---|
| Days | CTRL | PLA0 | PLA16 | PLA23 |
| 2 | 2.57 ± 0.25 a | 0.63 ± 0.03 b | 0.61 ± 0.01 b | 0.60 ± 0.04 b |
| 6 | 8.54 ± 0.03 a | 3.73 ± 0.11 b | 2.70 ± 0.05 c | 2.69 ± 0.18 c |
| 9 | 13.43 ± 0.04 a | 6.07 ± 0.21 b | 4.31 ± 0.07 c | 4.18 ± 0.29 c |
| 13 | 17.98 ± 0.05 a | 8.39 ± 0.32 | 5.39 ± 0.19 c | 5.49 ± 0.38 c |
| 16 | 20.42 ± 0.03 a | 9.46 ± 0.36 b | 6.23 ± 0.19 c | 6.17 ± 0.42 c |
| 20 | 25.81 ± 0.16 a | 11.36 ± 0.45 b | 8.03 ± 0.18 c | 7.65 ± 0.52 c |
| (a) | ||||
| pH | ||||
| Days | CTRL | PLA0 | PLA16 | PLA23 |
| 0 | 3.50 ± 0.01 | |||
| 2 | 3.38 ± 0.01 a | 3.49 ± 0.01 b | 3.63 ± 0.01 c | 3.61 ± 0.01 c |
| 6 | 3.52 ± 0.01 a | 3.62 ± 0.01 b | 3.42 ± 0.01 c | 3.49 ± 0.01 ac |
| 9 | 3.47 ± 0.00 ab | 3.44 ± 0.01 a | 3.51 ± 0.01 bc | 3.57 ± 0.01 c |
| 13 | 3.32 ± 0.01 a | 3.52 ± 0.00 b | 3.73 ± 0.01 c | 3.54 ± 0.00 b |
| 16 | 3.60 ± 0.01 a | 3.53 ± 0.01 b | 3.50 ± 0.01 b | 3.62 ± 0.01 a |
| 20 | 3.46 ± 0.01 a | 3.77 ± 0.00 b | 3.51 ± 0.00 a | 3.57 ± 0.01 c |
| (b) | ||||
| TTA | ||||
| Days | CTRL | PLA0 | PLA16 | PLA23 |
| 0 | 0.35 ± 0.00 | |||
| 2 | 0.81 ± 0.00 a | 0.67 ± 0.00 b | 0.43 ± 0.02 c | 0.56 ± 0.01 b |
| 6 | 0.81 ± 0.00 a | 0.63 ± 0.01 b | 0.47 ± 0.00 c | 0.84 ± 0.02 a |
| 9 | 0.63 ± 0.02 | 0.78 ± 0.00 | 0.65 ± 0.00 | 0.68 ± 0.04 |
| 13 | 0.95 ± 0.00 a | 0.69 ± 0.01 b | 0.47 ± 0.00 c | 0.42 ± 0.00 d |
| 16 | 0.79 ± 0.02 a | 0.61 ± 0.01 b | 0.76 ± 0.01 a | 0.59 ± 0.00 b |
| 20 | 0.88 ± 0.02 a | 0.62 ± 0.01 b | 0.70 ± 0.01 b | 0.69 ± 0.00 b |
| (c) | ||||
| TSS | ||||
| Days | CTRL | PLA0 | PLA16 | PLA23 |
| 0 | 8.63 ± 0.09 | |||
| 2 | 10.50 ± 0.00 a | 9.38 ± 0.09 b | 9.38 ± 0.09 b | 8.38 ± 0.09 c |
| 6 | 10.25 ± 0.25 a | 8.25 ± 0.25 b | 10.75 ± 0.18 a | 10.60 ± 0.07 a |
| 9 | 11.63 ± 0.09 a | 8.50 ± 0.00 b | 10.13 ± 0.37 ab | 9.62 ± 0.13 b |
| 13 | 8.75 ± 0.00 a | 9.13 ± 0.09 a | 10.13 ± 0.09 b | 10.61 ± 0.08 b |
| 16 | 8.50 ± 0.00 a | 9.25 ± 0.00 b | 8.63 ± 0.09 a | 8.59 ± 0.06 a |
| 20 | 8.38 ± 0.09 a | 7.13 ± 0.09 bc | 7.25 ± 0.00 b | 6.60 ± 0.07 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crescente, G.; Cascone, G.; Volpe, M.G.; Moccia, S. Application of PLA-Based Films to Preserve Strawberries’ Bioactive Compounds. Foods 2024, 13, 1844. https://doi.org/10.3390/foods13121844
Crescente G, Cascone G, Volpe MG, Moccia S. Application of PLA-Based Films to Preserve Strawberries’ Bioactive Compounds. Foods. 2024; 13(12):1844. https://doi.org/10.3390/foods13121844
Chicago/Turabian StyleCrescente, Giuseppina, Giovanni Cascone, Maria Grazia Volpe, and Stefania Moccia. 2024. "Application of PLA-Based Films to Preserve Strawberries’ Bioactive Compounds" Foods 13, no. 12: 1844. https://doi.org/10.3390/foods13121844







