GC-MS Combined with Proteomic Analysis of Volatile Compounds and Formation Mechanisms in Green Teas with Different Aroma Types
Abstract
1. Introduction
2. Materials and Methods
2.1. Tea Samples
2.2. Sensory Evaluation
2.3. Chemicals and Standards
2.4. Chemical Composition Analyses
2.5. Determination of Volatile Compounds
2.5.1. Extraction of Aroma Compounds
2.5.2. GC-MS Analysis
2.5.3. Identification and Quantification of Aroma Compounds
2.6. Proteomic Analysis
2.6.1. Protein Extraction
2.6.2. Protein Digestion and Deionization
2.6.3. Peptide Desalination and Quantification
2.6.4. Liquid Chromatography–Mass Spectrometry Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Sensory Evaluation Results for the Baked Green Tea Samples
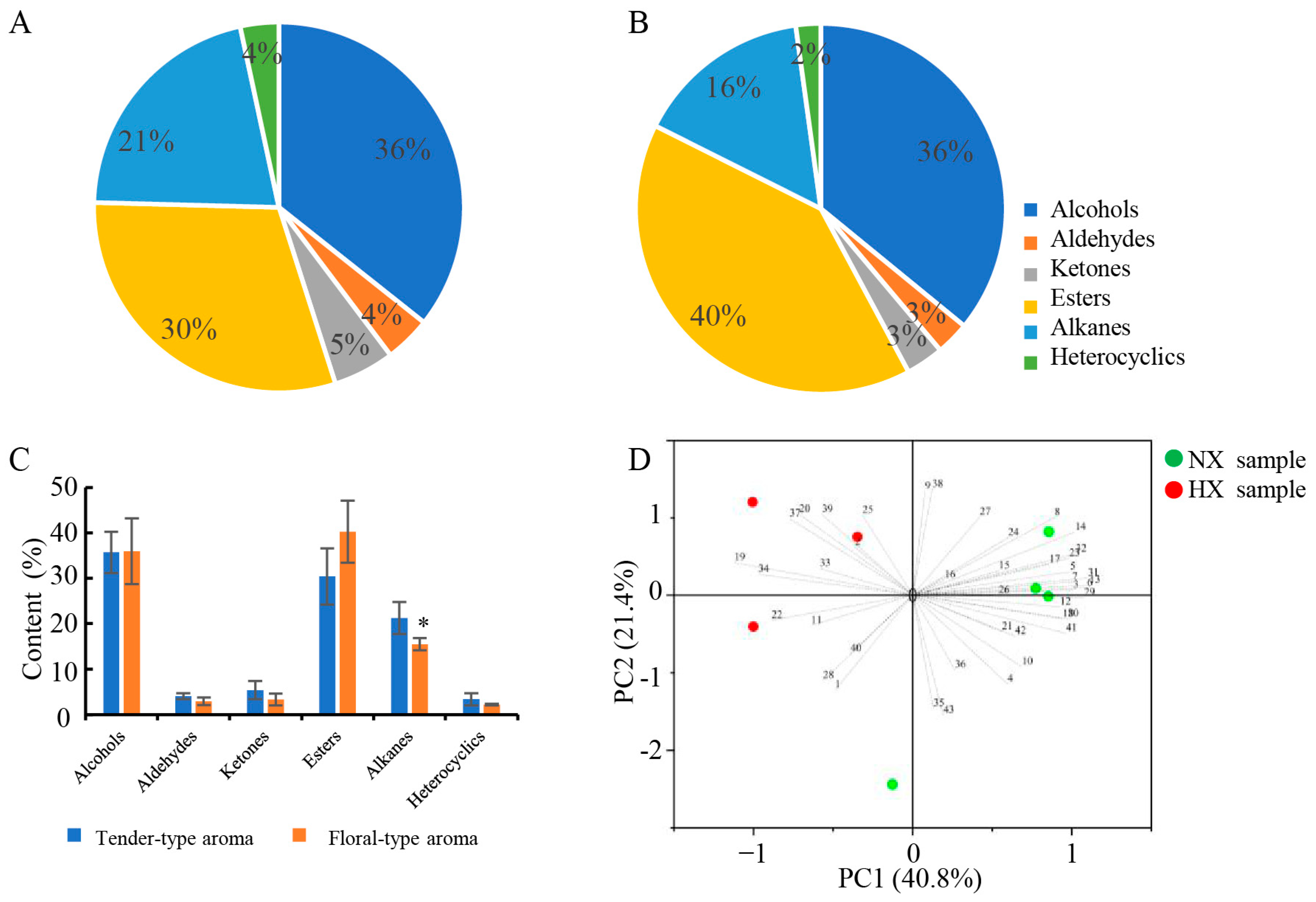
3.2. Detected Volatile Compounds in Baked Green Teas by GC-MS
3.3. PCA
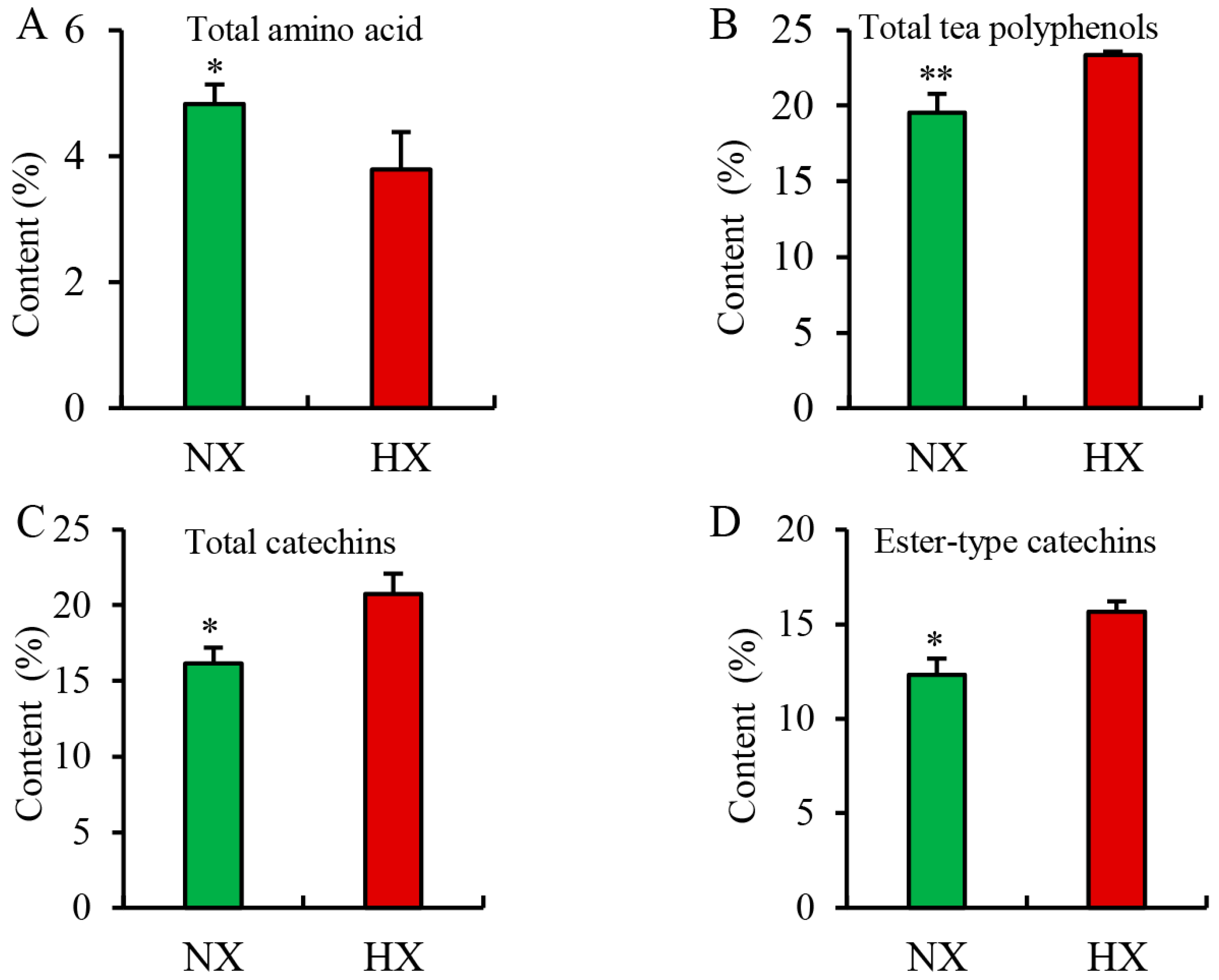
3.4. Chemical Compositions Detected in Different Flavors of Baked Green Tea
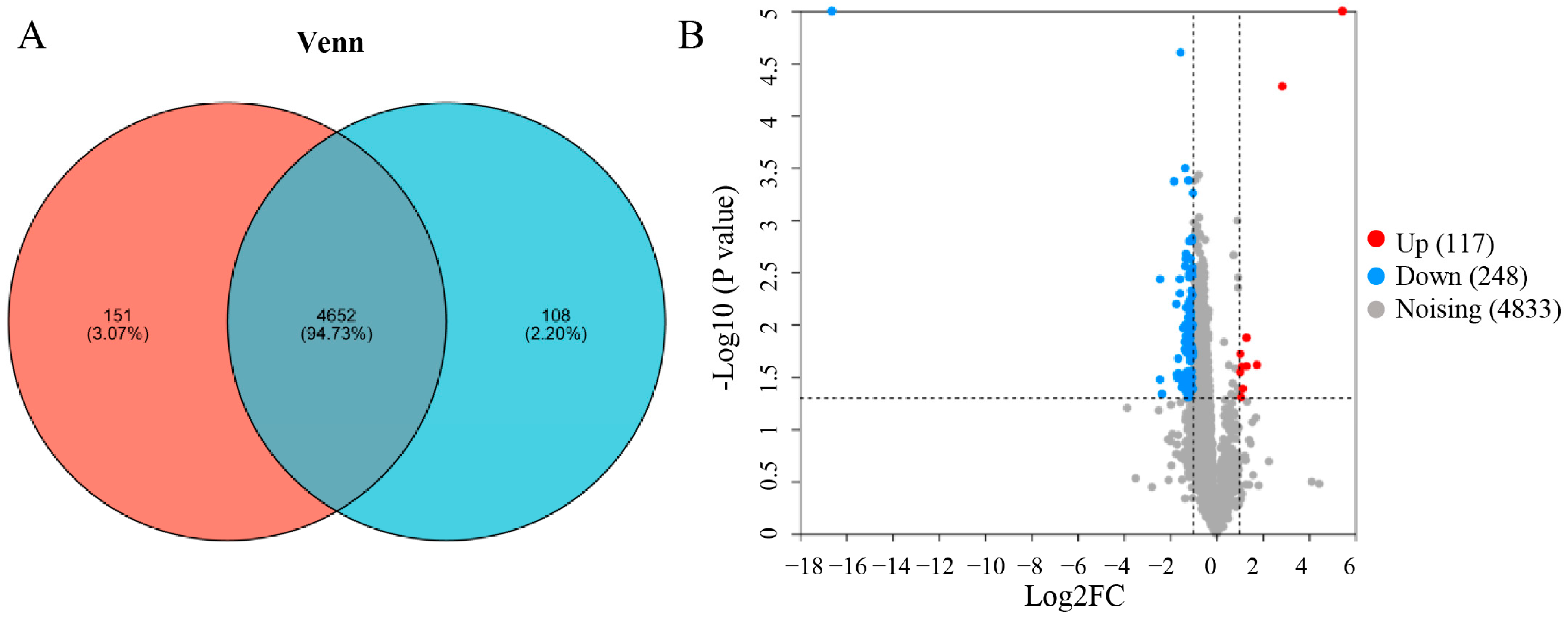
3.5. Proteomic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baba, R.; Kumazawa, K. Characterization of the potent odorants contributing to the characteristic aroma of Chinese green tea infusions by aroma extract dilution analysis. J. Agric. Food Chem. 2014, 62, 8308–8313. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Zhang, S. Analysis of China’s tea production and domestic sales in 2022. China Tea 2023, 45, 25–30. [Google Scholar]
- You, Q.; Li, Q.; Zhu, Y.; Shi, Y.; Ma, W.; Lin, Z.; Lu, H. Research progress of aroma components in green tea with different aroma types and their influencing factors. China Tea 2022, 44, 7–18. [Google Scholar]
- Ye, G.; Yuan, H.; Jiang, Y.; Yin, J.; Wang, F.; Chen, J. Application of Bayes Stepwise Discrimination Analysis on Chemical Recognition of Green Tea with Chestnut-like Aroma. J. Tea Sci. 2009, 29, 27–33. [Google Scholar]
- Yin, H.; Yang, Y.; Yao, Y.; Zhang, M.; Wang, J.; Jiang, Y.; Yuan, H. Discrimination of Different Characteristics of Chestnut-like Green Tea Based on Gas Chromatography-Mass Spectrometry and Multivariate Statistical Data Analysis. Food Sci. 2019, 40, 192–198. [Google Scholar]
- Wang, H.; Hua, J.; Jiang, Y.; Yang, Y.; Wang, J.; Yuan, H. Influence of fixation methods on the chestnut-like aroma of green tea and dynamics of key aroma substances. Food Res. Int. 2020, 136, 109479. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Watanabe, N.; Yang, Z. Understanding the biosyntheses and stress response mechanisms of aroma compounds in tea (Camellia sinensis) to safely and effectively improve tea aroma. Crit. Rev. Food Sci. Nutr. 2019, 59, 2321–2334. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ma, W.; Shi, J.; Zhu, Y.; Lin, Z.; Lv, H. Characterization of the key aroma compounds in Longjing tea using stir bar sorptive extraction (SBSE) combined with gas chromatography-mass spectrometry (GC-MS), gas chromatography-olfactometry (GC-O), odor activity value (OAV), and aroma recombination. Food Res. Int. 2020, 130, 108908. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Yu, M.; Song, H.; Xu, Y.; Lin, Y.; Granvogl, M. Characterization of key aroma-active compounds in rough and moderate fire Rougui Wuyi rock tea (Camellia sinensis) by sensory-directed flavor analysis and elucidation of the influences of roasting on aroma. J. Agric. Food Chem. 2022, 70, 267–278. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, Y.; Chen, Y.; Xiao, L.; Zhang, X.; Yang, C.; Li, Z.; Zhu, M.; Liu, Z.; Wang, Y. Discrimination and characterization of the volatile profiles of five Fu brick teas from different manufacturing regions by using HS–SPME/GC–MS and HS–GC–IMS. Curr. Res. Food Sci. 2022, 5, 1788–1807. [Google Scholar] [CrossRef]
- Yin, X.; Huang, J.A.; Huang, J.; Wu, W.; Tong, T.; Liu, S.; Zhou, L.; Liu, Z.; Zhang, S. Identification of volatile and odor-active compounds in Hunan black tea by SPME/GC-MS and multivariate analysis. LWT-Food Sci. Technol. 2022, 164, 113656. [Google Scholar] [CrossRef]
- Ma, L.; Sun, Y.; Wang, X.; Zhang, H.; Zhang, L.; Yin, Y.; Wu, Y.; Du, L.; Du, Z. The characteristic of the key aroma-active components in white tea using GC-TOF-MS and GC-olfactometry combined with sensory-directed flavor analysis. J. Sci. Food Agric. 2023, 103, 7136–7152. [Google Scholar] [CrossRef] [PubMed]
- Sílvia, P.; Marcelo, M.; Ivonne, D.; Manuel, A.; Sílvia, M. Sesquiterpenic composition of the inflorescences of Brazilian chamomile (Matricaria recutita L.): Impact of the agricultural practices. Ind. Crops Prod. 2011, 34, 1482–1490. [Google Scholar]
- Liu, N.; Shen, S.; Huang, L.; Deng, G.; Wei, Y.; Ning, J.; Wang, Y. Revelation of volatile contributions in green teas with different aroma types by GC-MS and GC-IMS. Food Res. Int. 2023, 169, 112845. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, L.; Deng, Y.; Yuan, H.; Zhu, J.; Jiang, Y.; Yang, Y. Characterization of the key odorants in floral aroma green tea based on GC-E-Nose, GC-IMS, GC-MS and aroma recombination and investigation of the dynamic changes and aroma formation during processing. Food Chem. 2023, 427, 136641. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Yu, Q.; Huang, M.; Hung, W.; Grosser, J.; Chen, S.; Wang, Y.; Gmitter, F. Proteomic and metabolomic analyses provide insight into the off-flavour of fruits from citrus trees infected with ‘Candidatus Liberibacter asiaticus’. Hortic. Res. 2019, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Sun, Z.; Gao, J.; Peng, J.; Wang, Z.; Zhao, Y.; Lin, Z.; Dai, W. Metabolomics combined with proteomics provides a novel interpretation of the compound differences among Chinese tea cultivars (Camellia sinensis var. sinensis) with different manufacturing suitabilities. Food Chem. 2022, 377, 131976. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, B.; Zheng, T.; Zhao, C.; Shen, X.; Wang, X.; Qiu, M.; Fan, J. Integrating metabolomics and proteomics technologies provides insights into the flavor precursor changes at different maturity stages of arabica coffee cherries. Foods 2023, 12, 1432. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, Y.; Liu, S.; Sun, Y.; Li, C.; Lin, J.; Wei, S. The stress-induced metabolites changes in the flavor formation of oolong tea during enzymatic-catalyzed process: A case study of Zhangping Shuixian tea. Food Chem. 2022, 391, 133192. [Google Scholar] [CrossRef]
- Zhu, Y.; Lv, H.; Shao, C.; Kang, S.; Zhang, Y.; Guo, L.; Dei, W.; Tan, J.; Peng, Q.; Lin, Z. Identification of key odorants responsible for chestnut-like aroma quality of green teas. Food Res. Int. 2018, 108, 74–82. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, X.; Mei, X.; Zhou, Y.; Cheng, S.; Zeng, L.; Dong, F.; Yang, Z. Proteolysis of chloroplast proteins is responsible for accumulation of free amino acids in dark-treated tea (Camellia sinensis) leaves. J. Proteom. 2017, 157, 10–17. [Google Scholar] [CrossRef]
- Teng, R.; Ao, C.; Huang, H.; Shi, D.; Mao, Y.; Zheng, X.; Zhao, Y. Research of Processing Technology of Longjing Tea with ‘Baiye 1’ Based on Non-Targeted Aroma Metabolomics. Foods 2024, 13, 1338. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Ou, X.; Zhang, Y.; Li, S.; Xiong, Y.; Li, Q.; Huang, J.; Liu, Z. Study on the key volatile compounds and aroma quality of jasmine tea with different scenting technology. Food Chem. 2022, 385, 132718. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shi, J.; Mu, B.; Chen, Z.; Dai, W.; Lin, Z. Metabolomics combined with proteomics provides a novel interpretation of the changes in nonvolatile compounds during white tea processing. Food Chem. 2020, 332, 127412. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Niu, Y.; Xiao, Z. Characterization of the key aroma compounds in Laoshan green teas by application of odour activity value (OAV), gas chromatography-mass spectrometry-olfactometry (GC-MS-O) and comprehensive two-dimensional gas chromatography mass spectrometry (GC×GC-qMS). Food Chem. 2021, 339, 128136. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Kong, Y.; Liu, P.; Wang, J.; Zhu, Y.; Wang, G.; Sun, M.; Chen, Y.; Guo, G.; Liu, Z. A critical review of key odorants in green tea: Identification and biochemical formation pathway. Trends Food Sci. Technol. 2022, 129, 221–232. [Google Scholar] [CrossRef]
- You, Q.; Shi, Y.; Zhu, Y.; Yang, G.; Yan, H.; Liu, Z.; Lv, H. Effect of different processing technologies on the key aroma-active compounds of green tea. Food Sci. 2023, 44, 194–200. [Google Scholar]
- Xue, J.; Liu, P.; Yin, J.; Wang, W.; Zhang, J.; Wang, W.; Le, T.; Ni, D.; Jiang, H. Dynamic Changes in Volatile Compounds of Shaken Black Tea during Its Manufacture by GC × GC-TOFMS and Multivariate Data Analysis. Foods 2022, 11, 1228. [Google Scholar] [CrossRef]
- Wang, C.; Lv, S.; Wu, Y.; Lian, M.; Gao, X.; Meng, Q. Study of aroma formation and transformation during the manufacturing process of Biluochun green tea in Yunnan Province by HS-SPME and GC-MS. J. Sci. Food Agric. 2016, 96, 4492–4498. [Google Scholar] [CrossRef]
- He, Y.; Liu, Z.; Qian, M.; Yu, X.; Xu, Y.; Chen, S. Unraveling the chemosensory characteristics of strong-aroma type Baijiu from different regions using comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry and descriptive sensory analysis. Food Chem. 2020, 331, 127335. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; Yuan, H.; Hua, J.; Deng, Y.; Jiang, Y.; Wang, J. Contribution of addition theanine/sucrose on the formation of chestnut-like aroma of green tea. LWT-Food Sci. Technol. 2020, 129, 109512. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, C.; Li, C.; Gong, S.; Guo, H.; Tong, Y.; Fan, F. Aroma compositions and comparison of their related components of Longjing tea with different aroma types. J. Zhejiang Univ. (Agric. Life Sci.) 2021, 47, 203–211. [Google Scholar]
- Zheng, L.; Liu, P.; Gong, Z.; Wang, X.; Cheng, Y.; Yang, W.; Tang, Q.; Zheng, P. Analysis on the difference of main chemical components of different aromatic green tea from Xiangyang. Sci. Technol. Food Ind. 2021, 42, 252–258. [Google Scholar]
- Nose, M.; Nakatani, Y.; Yamanishi, T. Studies on the flavor of green tea Part IX. Identification and composition of intermediate and high boiling constituents in green tea flavor. J. Agric. Chem. Soc. Jpn. 1971, 35, 261–271. [Google Scholar]
- Tan, H.; Lau, H.; Liu, S.; Tan, L.; Sakumota, S.; Lassabliere, B.; Leong, K.; Sun, J.; Yu, B. Characterisation of key odourants in Japanese green tea using gas chromatography-olfactometry and gas chromatography-mass spectrometry. LWT-Food Sci. Technol. 2019, 108, 221–232. [Google Scholar] [CrossRef]
- Hu, D.; Chen, W.; Ma, C.; Fang, Z.; Huang, H.; Miao, A. Effect of high-temperature and long-time final roasting on aroma of roasted green tea. Food Sci. 2022, 43, 255–261. [Google Scholar]
- Wang, M.; Zhu, Y.; Zhang, Y.; Shi, J.; Lin, Z.; Lv, H. Analysis of volatile composition and key aroma compounds of green teas with fresh scent flavor. Food Sci. 2019, 40, 219–228. [Google Scholar]
- Ho, C.; Zheng, X.; Li, S. Tea aroma formation. Food Sci. Hum. Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Chen, J.; Wang, F.; Du, Q.; Yin, J. Quantitative analyses of the bitterness and astringency of catechins from green tea. Food Chem. 2018, 258, 16–24. [Google Scholar] [CrossRef]
- Mei, X.; Wan, S.; Lin, C.; Zhou, C.; Hu, L.; Deng, C.; Zhang, L. Integration of metabolome and transcriptome reveals the relationship of benzenoid-phenylpropanoid pigment and aroma in Purple tea flowers. Front. Plant Sci. 2021, 12, 762330. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, X.; Liu, Y.; Chen, J.; Ma, J.; Chen, L. Identification of QTL controlling volatile terpene contents in tea plant (Camellia sinensis) using a high-aroma ‘Huangdan’ x ‘Jinxuan’ F1 population. Front. Plant Sci. 2023, 14, 1130582. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Xiao, Y.; Zhou, X.; Yu, J.; Jian, G.; Li, J.; Chen, J.; Tang, J.; Yang, Z. Uncovering reasons for differential accumulation of linalool in tea cultivars with different leaf area. Food Chem. 2021, 345, 128752. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar] [CrossRef]
- Pichersky, E.; Noel, J.; Dudareva, N. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef]
- Yang, Z.; Sakaia, M.; Sayama, H.; Shimeno, T.; Yamaguchi, K.; Watanabe, N. Elucidation of the biochemical pathway of 2-phenylethanol from shikimic acid using isolated protoplasts of rose flowers. J. Plant Physiol. 2009, 166, 887–891. [Google Scholar] [CrossRef]

| NO. | Cultival | Aroma Characteristics |
|---|---|---|
| 1 | Fuding | High brisk, with tender aroma |
| 2 | Lifeng | Clean and refreshing, tender aroma |
| 3 | 741103 | High brisk, obvious tender aroma |
| 4 | 741006 | Obvious tend aroma and tip aroma |
| 5 | J1130 | Clean and refreshing, fresh and brisk |
| 6 | 730206 | Fragrant and lasting |
| 7 | 730120 | With flowery aroma |
| 8 | 741209 | Obvious flowery aroma |
| 9 | XGL16-14 | High brisk |
| 10 | 740209 | High brisk, with tip aroma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, X.; Ao, C.; Yu, J.; Zhao, Y.; Huang, H. GC-MS Combined with Proteomic Analysis of Volatile Compounds and Formation Mechanisms in Green Teas with Different Aroma Types. Foods 2024, 13, 1848. https://doi.org/10.3390/foods13121848
Niu X, Ao C, Yu J, Zhao Y, Huang H. GC-MS Combined with Proteomic Analysis of Volatile Compounds and Formation Mechanisms in Green Teas with Different Aroma Types. Foods. 2024; 13(12):1848. https://doi.org/10.3390/foods13121848
Chicago/Turabian StyleNiu, Xiaojun, Cun Ao, Jizhong Yu, Yun Zhao, and Haitao Huang. 2024. "GC-MS Combined with Proteomic Analysis of Volatile Compounds and Formation Mechanisms in Green Teas with Different Aroma Types" Foods 13, no. 12: 1848. https://doi.org/10.3390/foods13121848
APA StyleNiu, X., Ao, C., Yu, J., Zhao, Y., & Huang, H. (2024). GC-MS Combined with Proteomic Analysis of Volatile Compounds and Formation Mechanisms in Green Teas with Different Aroma Types. Foods, 13(12), 1848. https://doi.org/10.3390/foods13121848







