Raffinose Ameliorates DSS-Induced Colitis in Mice by Modulating Gut Microbiota and Targeting the Inflammatory TLR4–MyD88–NF-κB Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
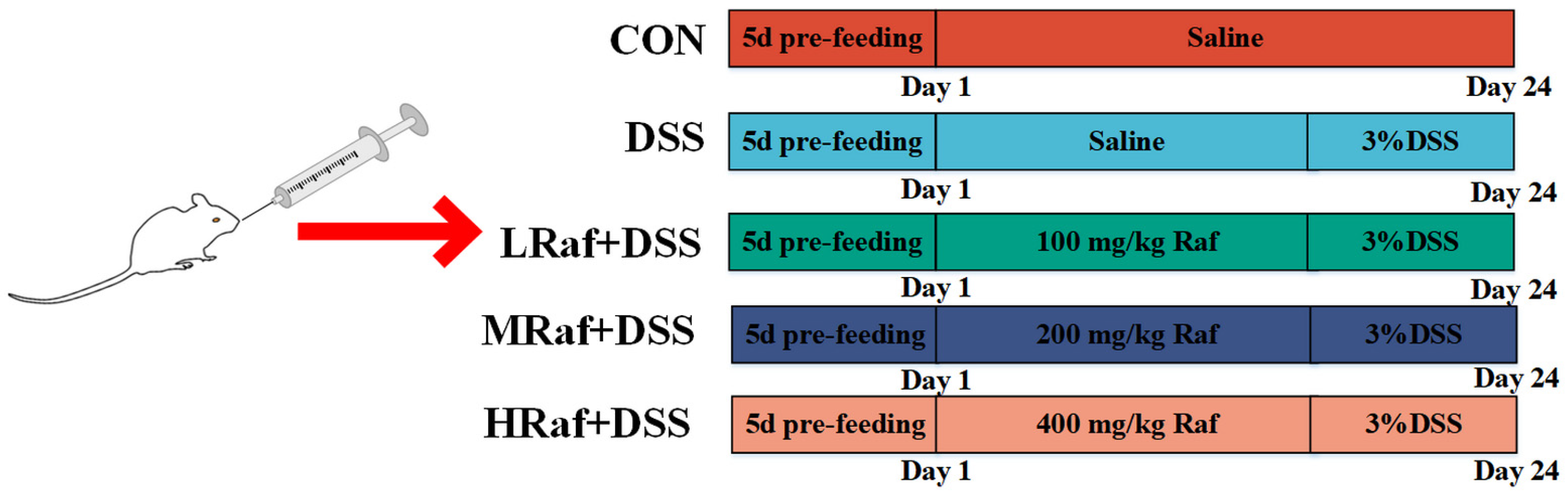
2.1. Animals and Treatments
2.2. Sample Collection
2.3. Determining Thymus and Spleen Indices
2.4. Histological Analysis
2.5. Determination of Serum Cytokines Levels
2.6. Determination of Serum Antioxidant Capacity in Serum
2.7. Determination of Immunityin Serum
2.8. qRT-PCR Analysis
2.9. Gut Microbiota Analysis
2.10. Statistical Analysis
3. Results
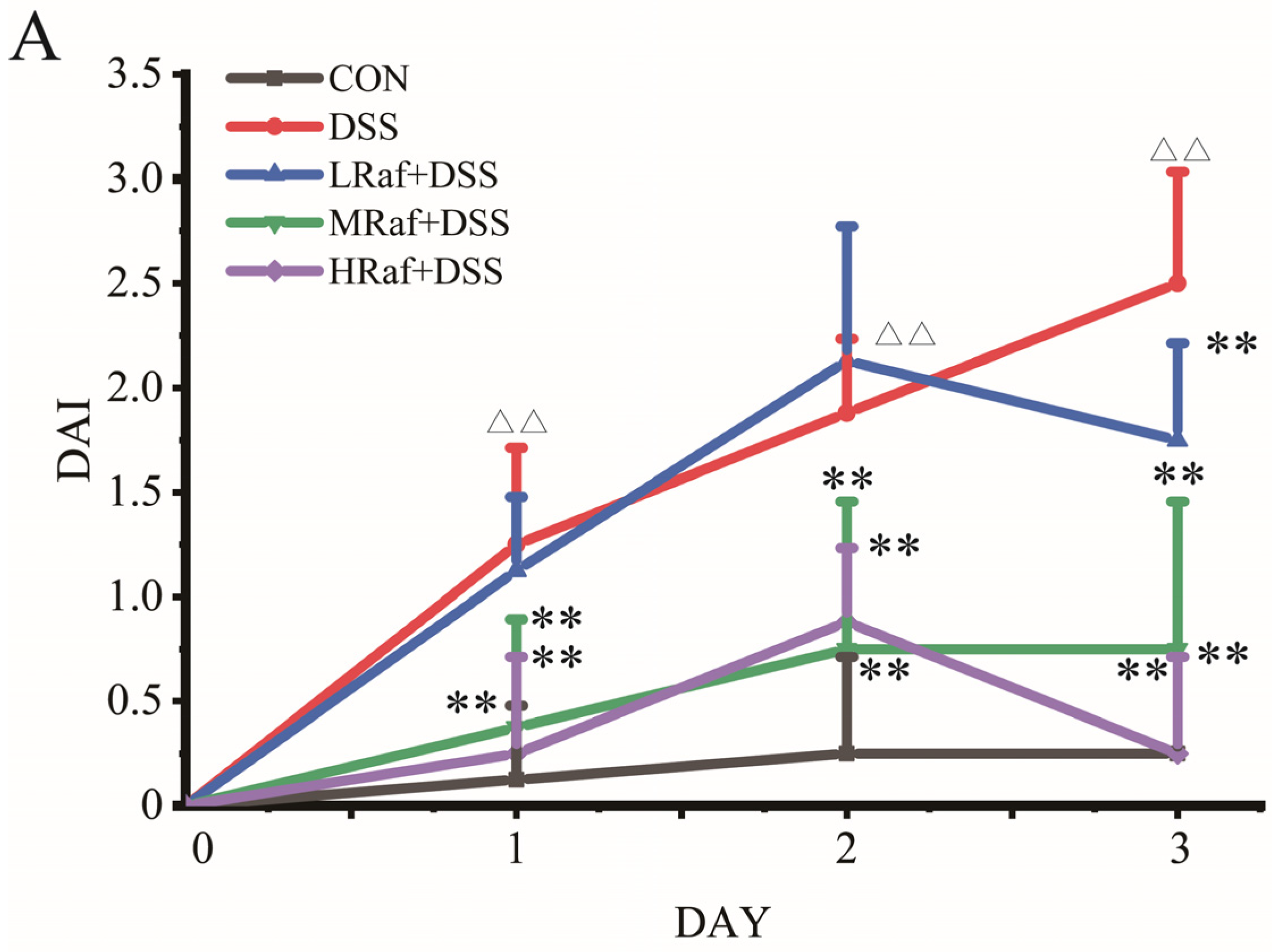
3.1. Raf Alleviated DSS-Induced Colitis Symptoms in Mice
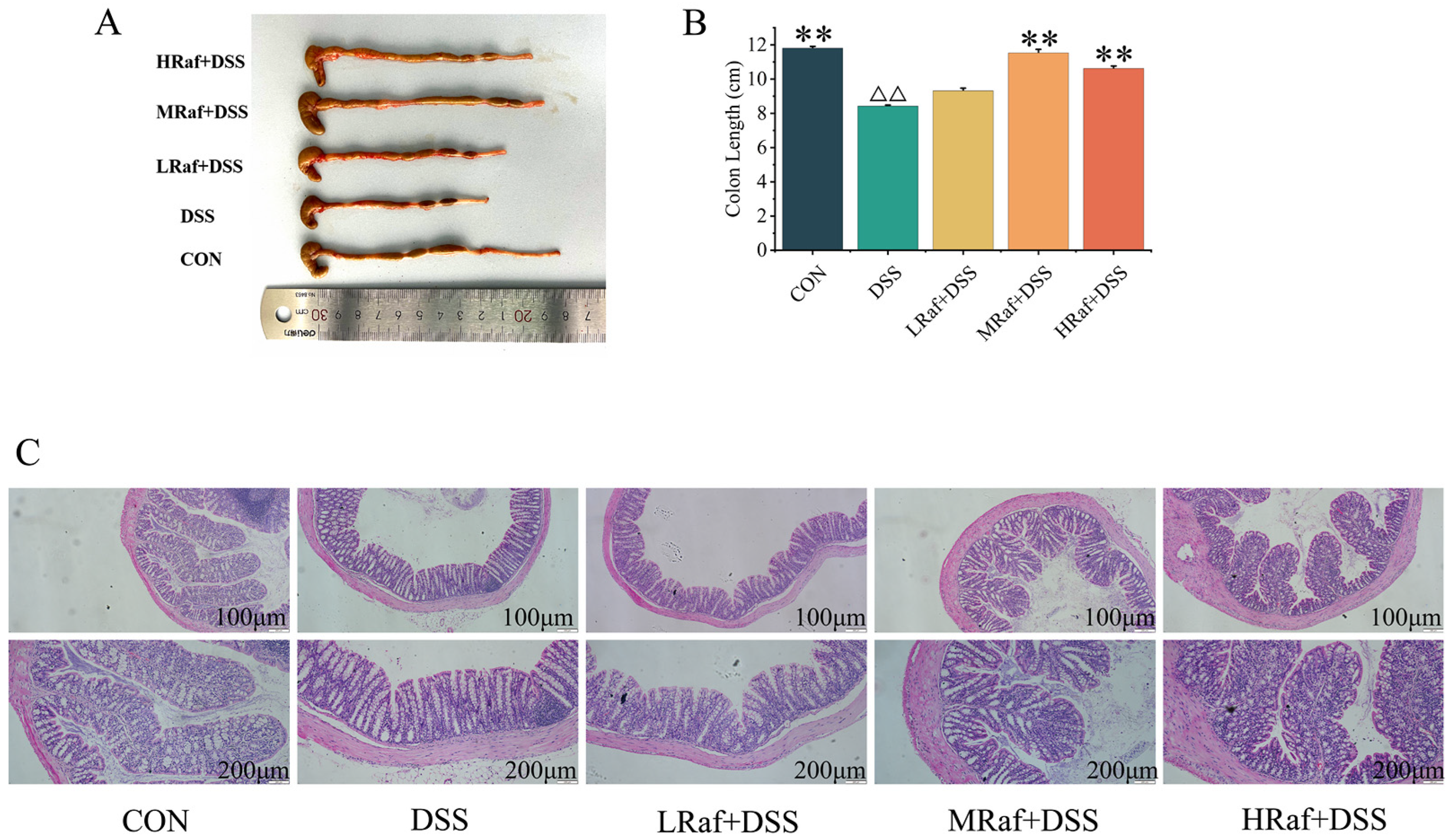
3.2. Effects of Raf on Colon Histology Examinations in ICR Mice
3.3. Effects of Raf on the Levels of Antioxidant Factors and Immunocorrelative Cytokine Levels
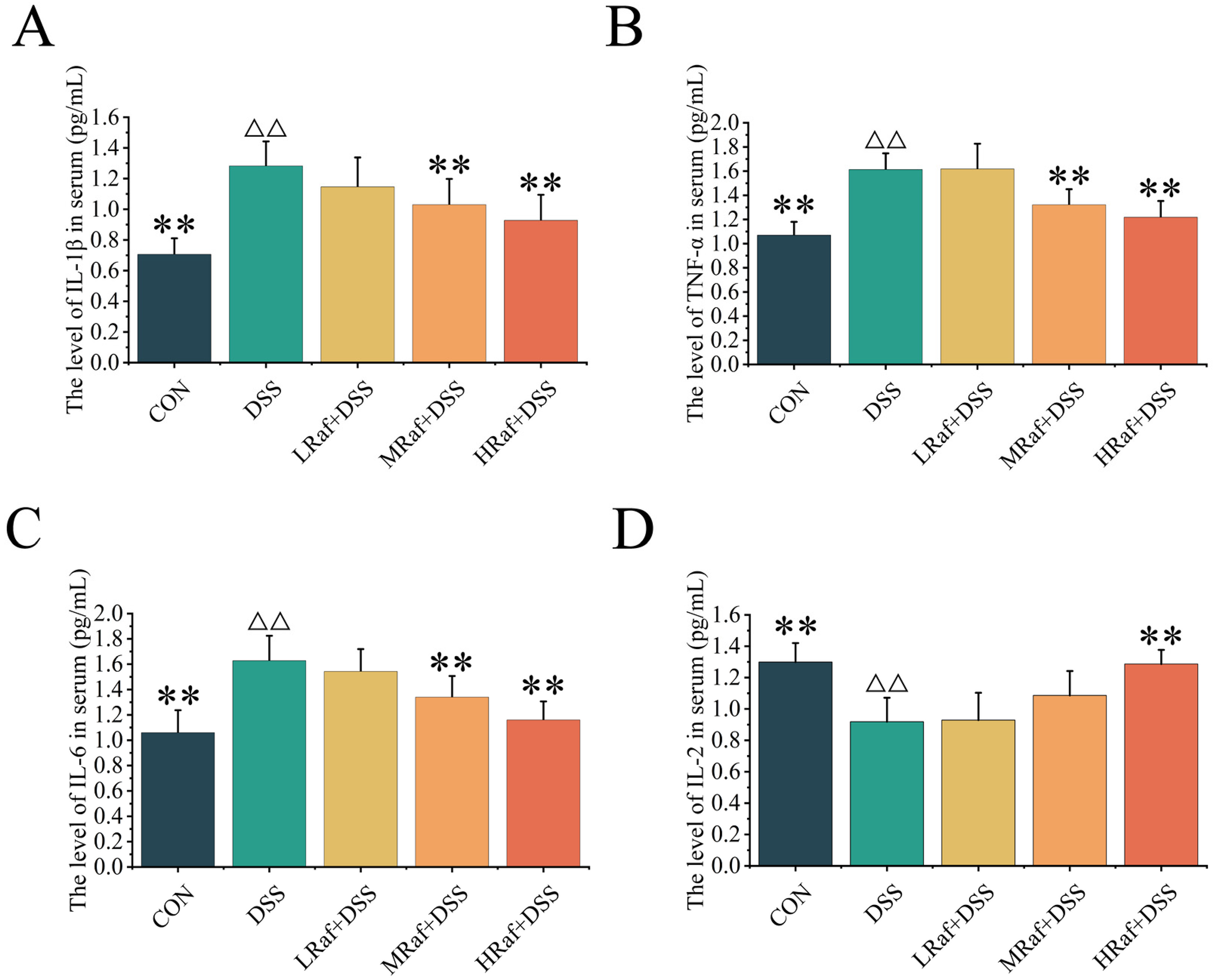
3.4. Effects of Raf on Inflammatory Factor Levels
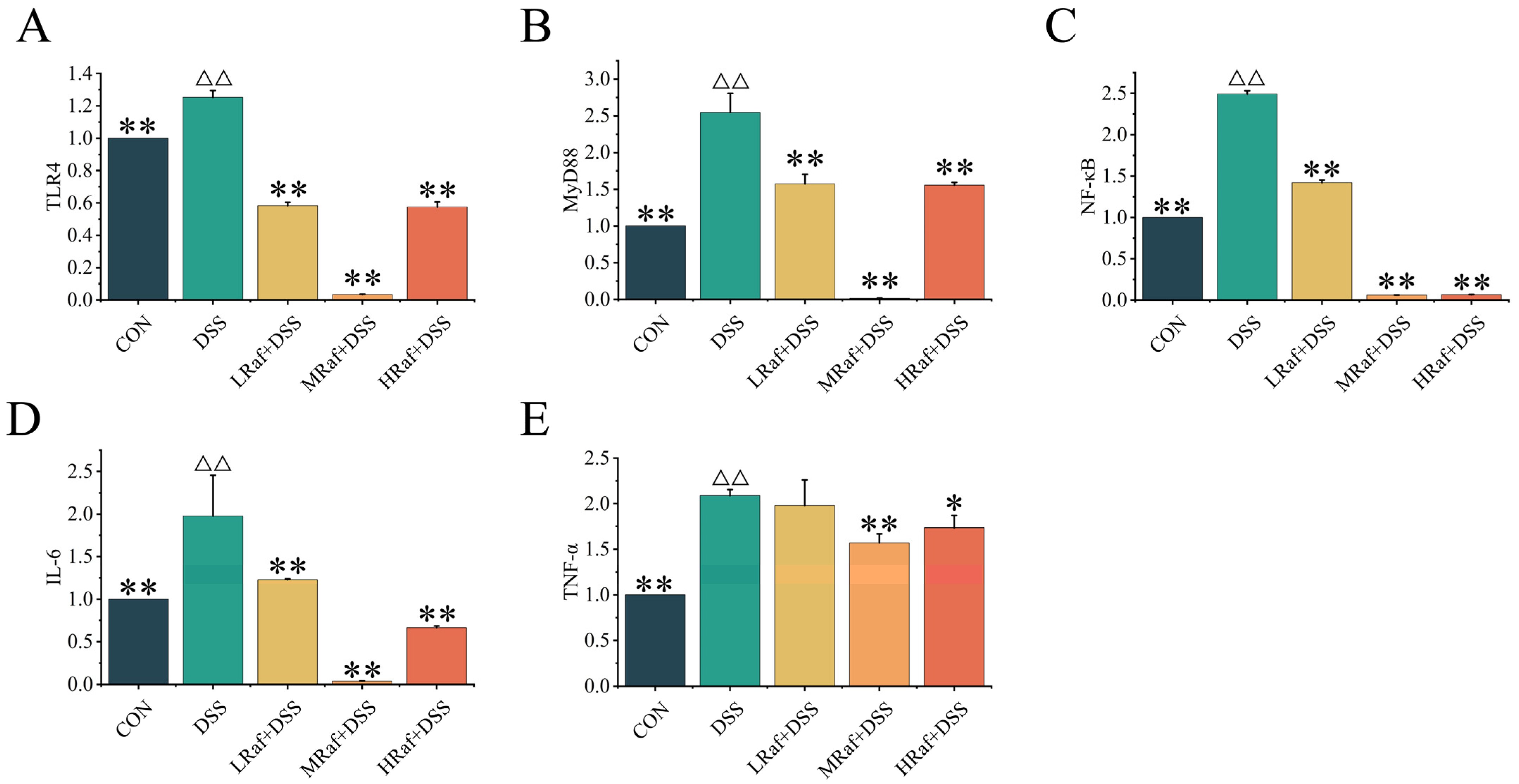
3.5. Effects of Raf on NF-κB Signaling Pathway-Related Gene Expression
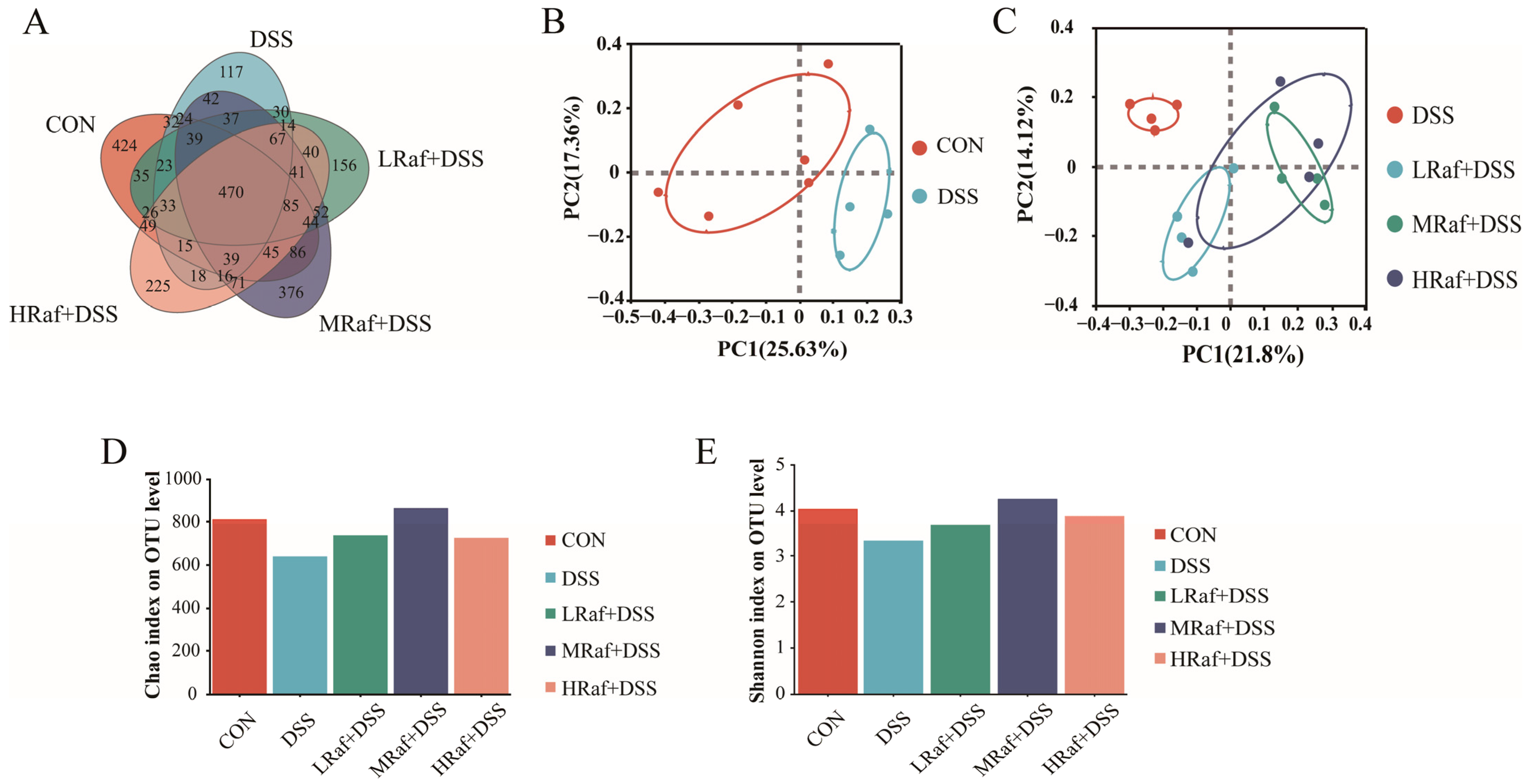
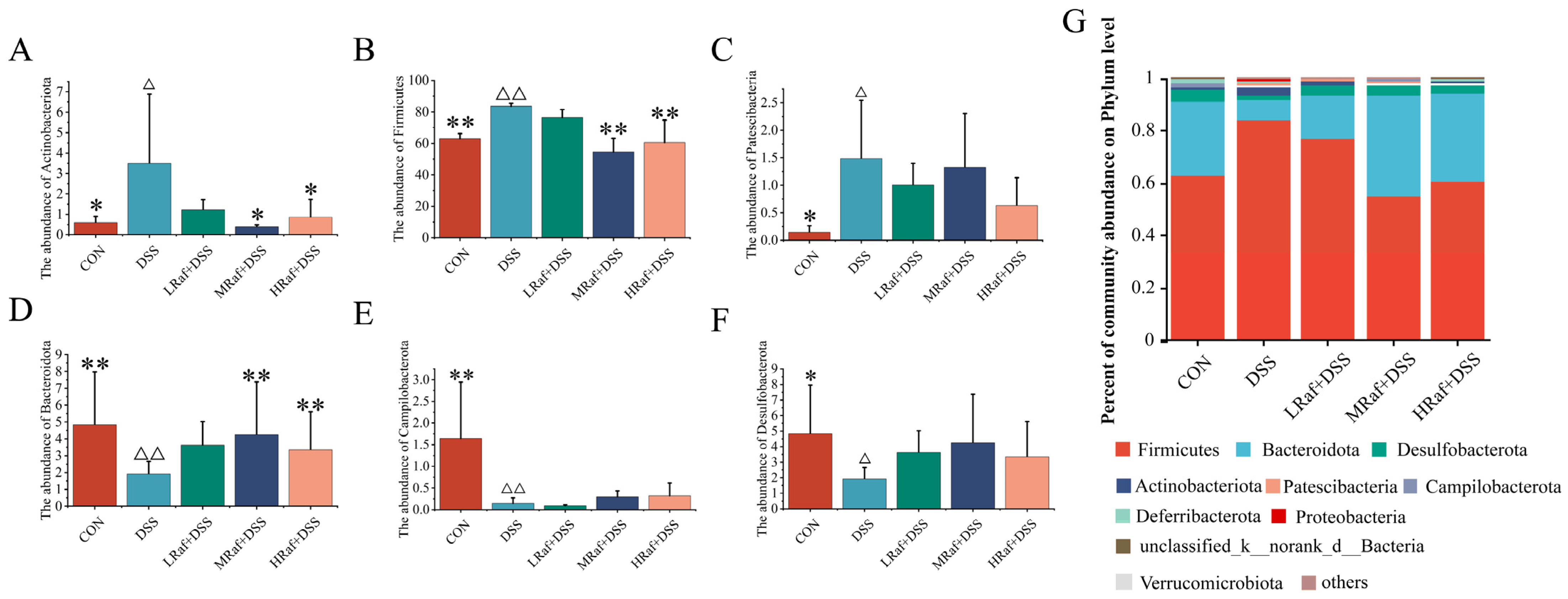
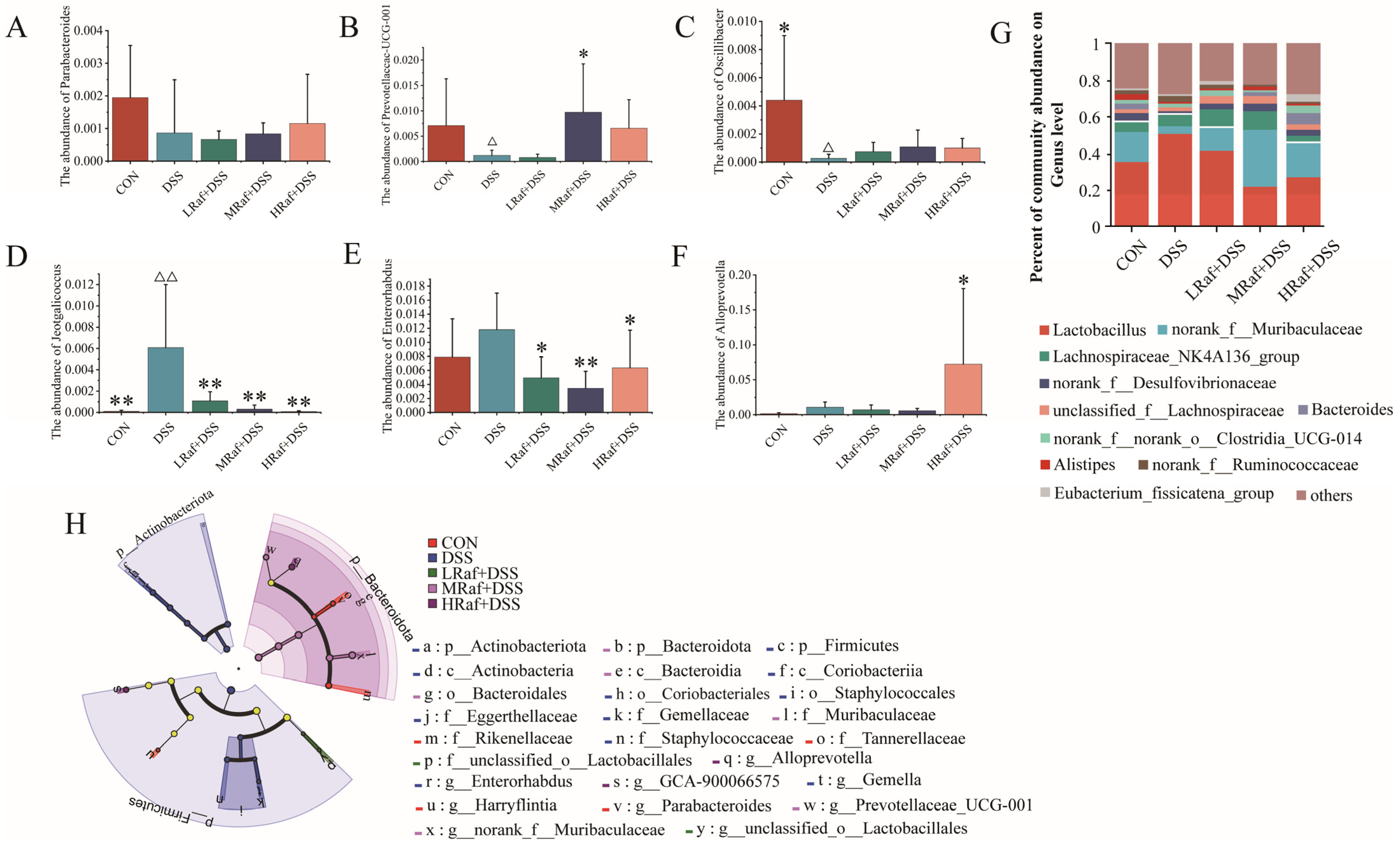
3.6. Intestinal Microbiota Analysis via High-Throughput Sequencing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abraham, C.; Cho, J.H. Inflammatory Bowel Disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef]
- Dulai, P.S.; Sandborn, W.J.; Gupta, S. Colorectal Cancer and Dysplasia in Inflammatory Bowel Disease: A Review of Disease Epidemiology, Pathophysiology, and Management. Cancer Prev. Res. 2016, 9, 887–894. [Google Scholar] [CrossRef]
- Windsor, J.W.; Kaplan, G.G. Evolving Epidemiology of IBD. Curr. Gastroenterol. Rep. 2019, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Coward, S.; Benchimol, E.I.; Clement, F.; Hazlewood, G.; Kuenzig, E.; McBrien, K.; Deardon, R.; Panaccione, R.; Seow, C.H.; Windsor, J.W.; et al. Sa1792—The Incidence of Inflammatory Bowel Disease: Analyzing Historical Trends to Predict the Future. Gastroenterology 2019, 156, S-403–S-404. [Google Scholar] [CrossRef]
- Cui, G.; Yuan, A. A Systematic Review of Epidemiology and Risk Factors Associated With Chinese Inflammatory Bowel Disease. Front. Med. 2018, 5, 183. [Google Scholar] [CrossRef] [PubMed]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef]
- Hodgson, H.J.F.; Mazlam, M.Z. Review article: Assessment ofdrug therapy in inflammatory bowel disease. Aliment. Pharmacol. Ther. 1991, 14, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Binienda, A.; Fichna, J.; Salaga, M. Recent advances in inflammatory bowel disease therapy. Eur. J. Pharm. Sci. 2020, 155, 105550. [Google Scholar] [CrossRef]
- Huang, H.; Fang, M.; Jostins, L.; Umicevic Mirkov, M.; Boucher, G.; Anderson, C.A.; Andersen, V.; Cleynen, I.; Cortes, A.; Crins, F.; et al. Fine-mapping inflammatory bowel disease loci to single-variant resolution. Nature 2017, 547, 173–178. [Google Scholar] [CrossRef]
- Kudelka, M.R.; Stowell, S.R.; Cummings, R.D.; Neish, A.S. Intestinal epithelial glycosylation in homeostasis and gut microbiota interactions in IBD. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 597–617. [Google Scholar] [CrossRef] [PubMed]
- Halfvarson, J.; Brislawn, C.J.; Lamendella, R.; Vazquez-Baeza, Y.; Walters, W.A.; Bramer, L.M.; D’Amato, M.; Bonfiglio, F.; McDonald, D.; Gonzalez, A.; et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat. Microbiol. 2017, 2, 17004. [Google Scholar] [CrossRef] [PubMed]
- de Jong, R.J.; Ohnmacht, C. Defining Dysbiosis in Inflammatory Bowel Disease. Immunity 2019, 50, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Celiberto, L.S.; Graef, F.A.; Healey, G.R.; Bosman, E.S.; Jacobson, K.; Sly, L.M.; Vallance, B.A. Inflammatory bowel disease and immunonutrition: Novel therapeutic approaches through modulation of diet and the gut microbiome. Immunology 2018, 155, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, S.; Iwama, A.; Dinoto, A.; Suksomcheep, A.; Maeta, K.; Kasai, T.; Hara, H.; Yokota, A. Synbiotic promotion of epithelial proliferation by orally ingested encapsulated Bifidobacterium breve and raffinose in the small intestine of rats. Mol. Nutr. Food Res. 2009, 53 (Suppl. 1), S62–S67. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, Y.; Sakanaka, M.; Fukuma, H.; Murayama, H.; Kano, Y.; Fukiya, S.; Yokota, A. Development of a double-crossover markerless gene deletion system in Bifidobacterium longum: Functional analysis of the alpha-galactosidase gene for raffinose assimilation. Appl. Environ. Microbiol. 2012, 78, 4984–4994. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Paknejad, H.; Hoseinifar, S.H.; Shabani, A.; Torfi Mozanzadeh, M. The effects of dietary raffinose on skin mucus immune parameters and protein profile, serum non-specific immune parameters and immune related genes expression in common carp (Cyprinus carpio L.). Aquaculture 2020, 520, 734525. [Google Scholar] [CrossRef]
- Amorim, C.; Silvério, S.C.; Cardoso, B.B.; Alves, J.I.; Pereira, M.A.; Rodrigues, L.R. In vitro fermentation of raffinose to unravel its potential as prebiotic ingredient. Lwt 2020, 126, 109322. [Google Scholar] [CrossRef]
- Altamimi, M.; Abdelhay, O.; Rastall, R.A. Effect of oligosaccharides on the adhesion of gut bacteria to human HT-29 cells. Anaerobe 2016, 39, 136–142. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, J.; Liu, H.; Yang, G. Soybean oligosaccharide, stachyose, and raffinose in broilers diets: Effects on odor compound concentration and microbiota in cecal digesta. Poult. Sci. 2020, 99, 3532–3539. [Google Scholar] [CrossRef]
- Xu, G.; Xing, W.; Li, T.; Ma, Z.; Liu, C.; Jiang, N.; Luo, L. Effects of dietary raffinose on growth, non-specific immunity, intestinal morphology and microbiome of juvenile hybrid sturgeon (Acipenser baeri Brandt female symbol × A. schrenckii Brandt male symbol). Fish Shellfish Immunol. 2018, 72, 237–246. [Google Scholar] [CrossRef]
- Berrocoso, J.D.; Kida, R.; Singh, A.K.; Kim, Y.S.; Jha, R. Effect of in ovo injection of raffinose on growth performance and gut health parameters of broiler chicken. Poult. Sci. 2017, 96, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Zhang, Y.; He, J.; Yu, J.; Mao, X.; Zheng, P.; Luo, Y.; Luo, J.; Huang, Z.; Yu, B.; et al. Effects of soybean raffinose on growth performance, digestibility, humoral immunity and intestinal morphology of growing pigs. Anim. Nutr. 2021, 7, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Tang, H.; Gu, J.; Li, D.; Cui, S.; Zhao, J.; Zhang, H.; Chen, W. In vitro fermentation of raffinose by the human gut bacteria. Food Funct. 2018, 9, 5824–5831. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Wang, J.-Y.; Xiong, F.; Wu, B.-H.; Luo, M.-H.; Yu, Z.-C.; Liu, T.-T.; Li, D.-F.; Tang, Q.; Li, Y.-X.; et al. Curcumin ameliorates DSS-induced colitis in mice by regulating the Treg/Th17 signaling pathway. Mol. Med. Rep. 2020, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Jiamei Ma, L.Y. Casticin prevents DSS induced ulcerative colitis in mice through inhibitions of NF-κB pathway and ROS signaling. Phytother. Res. 2018, 32, 1770–1783. [Google Scholar]
- Xia, Y.; Chen, Y.; Wang, G.; Yang, Y.; Song, X.; Xiong, Z.; Zhang, H.; Lai, P.; Wang, S.; Ai, L. Lactobacillus plantarum AR113 alleviates DSS-induced colitis by regulating the TLR4/MyD88/NF-κB pathway and gut microbiota composition. J. Funct. Foods 2020, 67, 103854. [Google Scholar] [CrossRef]
- Zhang, X.J.; Yuan, Z.W.; Qu, C.; Yu, X.T.; Huang, T.; Chen, P.V.; Su, Z.R.; Dou, Y.X.; Wu, J.Z.; Zeng, H.F.; et al. Palmatine ameliorated murine colitis by suppressing tryptophan metabolism and regulating gut microbiota. Pharmacol. Res. 2018, 137, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Bai, J.; Wang, B.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Suo, H.; Chen, W.; Zhai, Q. Stachyose modulates gut microbiota and alleviates DSS-induced ulcerative colitis in mice. Food Sci. Hum. Wellness 2023, 12, 2211–2220. [Google Scholar] [CrossRef]
- Ou, Y.; Guo, Y.; Chen, M.; Lu, X.; Guo, Z.; Zheng, B. Gut microbiome-serum metabolic profiles: Insight into the hypoglycemic effect of Porphyra haitanensis glycoprotein on hyperglycemic mice. Food Funct. 2023, 14, 7977–7991. [Google Scholar] [CrossRef]
- Bayer, A.L.; Pugliese, A.; Malek, T.R. The IL-2/IL-2R system: From basic science to therapeutic applications to enhance immune regulation. Immunol. Res. 2013, 57, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Ni, J.; Zhang, M.; Xu, Y.; Li, Y.; Karim, N.; Chen, W. Mulberry Anthocyanins Ameliorate DSS-Induced Ulcerative Colitis by Improving Intestinal Barrier Function and Modulating Gut Microbiota. Antioxidants 2022, 11, 1674. [Google Scholar] [CrossRef] [PubMed]
- Elson, C.O.; Cong, Y.; McCracken, V.J.; Dimmitt, R.A.; Lorenz, R.G.; Weaver, C.T. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol. Rev. 2005, 206, 260–276. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.T.; Tergaonkar, V. Roles of NF-kappaB in health and disease: Mechanisms and therapeutic potential. Clin. Sci. 2009, 116, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tan, L.; Xie, J.; Lai, Z.; Huang, Y.; Qu, C.; Luo, D.; Lin, Z.; Huang, P.; Su, Z.; et al. Characterization of brusatol self-microemulsifying drug delivery system and its therapeutic effect against dextran sodium sulfate-induced ulcerative colitis in mice. Drug Deliv. 2017, 24, 1667–1679. [Google Scholar] [CrossRef]
- Johnson, R.J.; Bakris, G.L.; Borghi, C.; Chonchol, M.B.; Feldman, D.; Lanaspa, M.A.; Merriman, T.R.; Moe, O.W.; Mount, D.B.; Sanchez Lozada, L.G.; et al. Hyperuricemia, Acute and Chronic Kidney Disease, Hypertension, and Cardiovascular Disease: Report of a Scientific Workshop Organized by the National Kidney Foundation. Am. J. Kidney Dis. 2018, 71, 851–865. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Kaplan, G.G. The Role of TNFα in Ulcerative Colitis. J. Clin. Pharmacol. 2013, 47, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Ye, D.; Boivin, M.; Guo, S.; Hashimi, M.; Ereifej, L.; Ma, T.Y. Interleukin-6 modulation of intestinal epithelial tight junction permeability is mediated by JNK pathway activation of claudin-2 gene. PLoS ONE 2014, 9, e85345. [Google Scholar] [CrossRef] [PubMed]
- Alfonso-Loeches, S.; Pascual-Lucas, M.; Blanco, A.M.; Sanchez-Vera, I.; Guerri, C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J. Neurosci. 2010, 30, 8285–8295. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Gill, R.; Chen, M.L.; Zhang, F.; Linhardt, R.J.; Dudeja, P.K.; Tobacman, J.K. Toll-like receptor 4 mediates induction of the Bcl10-NFkappaB-interleukin-8 inflammatory pathway by carrageenan in human intestinal epithelial cells. J. Biol. Chem. 2008, 283, 10550–10558. [Google Scholar] [CrossRef]
- Furuta, T.; Kikuchi, T.; Akira, S.; Watanabe, N.; Yoshikawa, Y. Roles of the small intestine for induction of toll-like receptor 4-mediated innate resistance in naturally acquired murine toxoplasmosis. Int. Immunol. 2006, 18, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.Z.; Meng, M.; Feng, C.C.; Wang, X.; Wang, C.L. A novel polysaccharide obtained from Craterellus cornucopioides enhances immunomodulatory activity in immunosuppressive mice models via regulation of the TLR4-NF-kappaB pathway. Food Funct. 2019, 10, 4792–4801. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Ahmad, A.; Di Paola, R.; Campolo, M.; Navarra, M.; Esposito, E.; Cuzzocrea, S. Role of Toll like receptor 4 signaling pathway in the secondary damage induced by experimental spinal cord injury. Immunobiology 2015, 220, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Laird, M.H.; Rhee, S.H.; Perkins, D.J.; Medvedev, A.E.; Piao, W.; Fenton, M.J.; Vogel, S.N. TLR4/MyD88/PI3K interactions regulate TLR4 signaling. J. Leukoc. Biol. 2009, 85, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A. IL-2: The first effective immunotherapy for human cancer. J. Immunol. 2014, 192, 5451–5458. [Google Scholar] [CrossRef]
- Sepehri, S.; Kotlowski, R.; Bernstein, C.N.; Krause, D.O. Microbial diversity of inflamed and noninflamed gut biopsy tissues in inflammatory bowel disease. Inflamm. Bowel Dis. 2007, 13, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Munyaka, P.M.; Rabbi, M.F.; Khafipour, E.; Ghia, J.E. Acute dextran sulfate sodium (DSS)-induced colitis promotes gut microbial dysbiosis in mice. J. Basic Microbiol. 2016, 56, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; Ke, X.; Hitchcock, D.; Jeanfavre, S.; Avila-Pacheco, J.; Nakata, T.; Arthur, T.D.; Fornelos, N.; Heim, C.; Franzosa, E.A.; et al. Bacteroides-Derived Sphingolipids Are Critical for Maintaining Intestinal Homeostasis and Symbiosis. Cell Host Microbe 2019, 25, 668–680.e667. [Google Scholar] [CrossRef]
- Eom, T.; Kim, Y.S.; Choi, C.H.; Sadowsky, M.J.; Unno, T. Current understanding of microbiota- and dietary-therapies for treating inflammatory bowel disease. J. Microbiol. 2018, 56, 189–198. [Google Scholar] [CrossRef]
- Zhu, H.-C.; Jia, X.-K.; Fan, Y.; Xu, S.-H.; Li, X.-Y.; Huang, M.-Q.; Lan, M.-L.; Xu, W.; Wu, S.-S. Alisol B 23-Acetate Ameliorates Azoxymethane/Dextran Sodium Sulfate-Induced Male Murine Colitis-Associated Colorectal Cancer via Modulating the Composition of Gut Microbiota and Improving Intestinal Barrier. Front. Cell. Infect. Microbiol. 2021, 11, 640225. [Google Scholar] [CrossRef]
- Litvak, Y.; Byndloss, M.X.; Tsolis, R.M.; Bäumler, A.J. Dysbiotic Proteobacteria expansion: A microbial signature of epithelial dysfunction. Curr. Opin. Microbiol. 2017, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD—What role do Proteobacteria play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Malek, T.R. The Biology of Interleukin-2. Annu. Rev. Immunol. 2008, 26, 453–479. [Google Scholar] [CrossRef] [PubMed]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wang, Y.; Zhang, S.; Zhang, X.; Du, Z.; Li, M.; Ding, K. Crataegus pinnatifida polysaccharide alleviates colitis via modulation of gut microbiota and SCFAs metabolism. Int. J. Biol. Macromol. 2021, 181, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria With Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, E.; Dziarski, R.; Park, S.Y.; Kashyap, D.R.; Dowd, S.E.; Gupta, D. Pglyrp-Regulated Gut Microflora Prevotella falsenii, Parabacteroides distasonis and Bacteroides eggerthii Enhance and Alistipes finegoldii Attenuates Colitis in Mice. PLoS ONE 2016, 11, e0146162. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Li, D.; Ma, C.; Feng, Y.; Hu, X.; Chen, F. Barley Leaf Insoluble Dietary Fiber Alleviated Dextran Sulfate Sodium-Induced Mice Colitis by Modulating Gut Microbiota. Nutrients 2021, 13, 846. [Google Scholar] [CrossRef]



| Gene Name | Forward(5′-3′) | Reverse(5′-3′) |
|---|---|---|
| β-actin | ATATCGCTGCGCTGGTCG | GATCTTCTCCATGTCGTCCC |
| TLR4 | GCACTGTTCTTCTCCTGCCT | AGAGGTGGTGTAAGCCATGC |
| MyD88 | GCATGGTGGTGGTTGTTTCTG | GAATCAGTCGCTTCTGTTGG |
| NF-κB | ACACTGGAAGCACGGATGAC | TGTCTGTGAGTTGCCGGTCT |
| TNF-α | CAAAATTCGAGTGACAAGCC | TTGTCCCTTGAAGAGAACCT |
| IL-6 | GGGACCCGAGTTACTACTTG | CTGGGCTCTGCTATCCAAGG |
| Group | Raf | Initial Weight (g) | Final Weight (g) | Daily Weight Gain (g) | Thymus Index (mg 10 g−1) | Spleen Index (mg 10 g−1) |
|---|---|---|---|---|---|---|
| CON | — | 20.83 ± 1.18 | 33.89 ± 0.87 ** | 0.55 ± 0.05 ** | 34.72 ± 2.68 ** | 33.95 ± 1.71 ** |
| DSS | — | 20.71 ± 0.99 | 26.48 ± 1.76 ΔΔ | 0.24 ± 0.09 ΔΔ | 18.45 ± 3.22 ΔΔ | 20.93 ± 4.19 ΔΔ |
| LRaf+DSS | 100 | 20.63 ± 1.25 | 29.81 ± 1.09 ** | 0.38 ± 0.06 ** | 21.87 ± 3.03 | 22.56 ± 3.73 |
| MRaf+DSS | 200 | 20.94 ± 0.84 | 30.74 ± 0.88 ** | 0.41 ± 0.06 ** | 26.48 ± 3.92 ** | 26.05 ± 4.02 ** |
| HRaf+DSS | 400 | 20.81 ± 0.79 | 32.19 ± 0.76 ** | 0.47 ± 0.07 ** | 29.91 ± 2.33 ** | 29.15 ± 1.95 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Xue, Y.; Cao, Z.; Guo, Y.; Pang, X.; Chen, C.; Zhang, W. Raffinose Ameliorates DSS-Induced Colitis in Mice by Modulating Gut Microbiota and Targeting the Inflammatory TLR4–MyD88–NF-κB Signaling Pathway. Foods 2024, 13, 1849. https://doi.org/10.3390/foods13121849
Zhang P, Xue Y, Cao Z, Guo Y, Pang X, Chen C, Zhang W. Raffinose Ameliorates DSS-Induced Colitis in Mice by Modulating Gut Microbiota and Targeting the Inflammatory TLR4–MyD88–NF-κB Signaling Pathway. Foods. 2024; 13(12):1849. https://doi.org/10.3390/foods13121849
Chicago/Turabian StyleZhang, Peng, Yuyang Xue, Zhengyu Cao, Yaya Guo, Xiaotong Pang, Cheng Chen, and Wenju Zhang. 2024. "Raffinose Ameliorates DSS-Induced Colitis in Mice by Modulating Gut Microbiota and Targeting the Inflammatory TLR4–MyD88–NF-κB Signaling Pathway" Foods 13, no. 12: 1849. https://doi.org/10.3390/foods13121849






