Screening Probiotics for Anti-Helicobacter pylori and Investigating the Effect of Probiotics on Patients with Helicobacter pylori Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Cell Culture
2.3. Screening for Strains with H. pylori Inhibition Ability
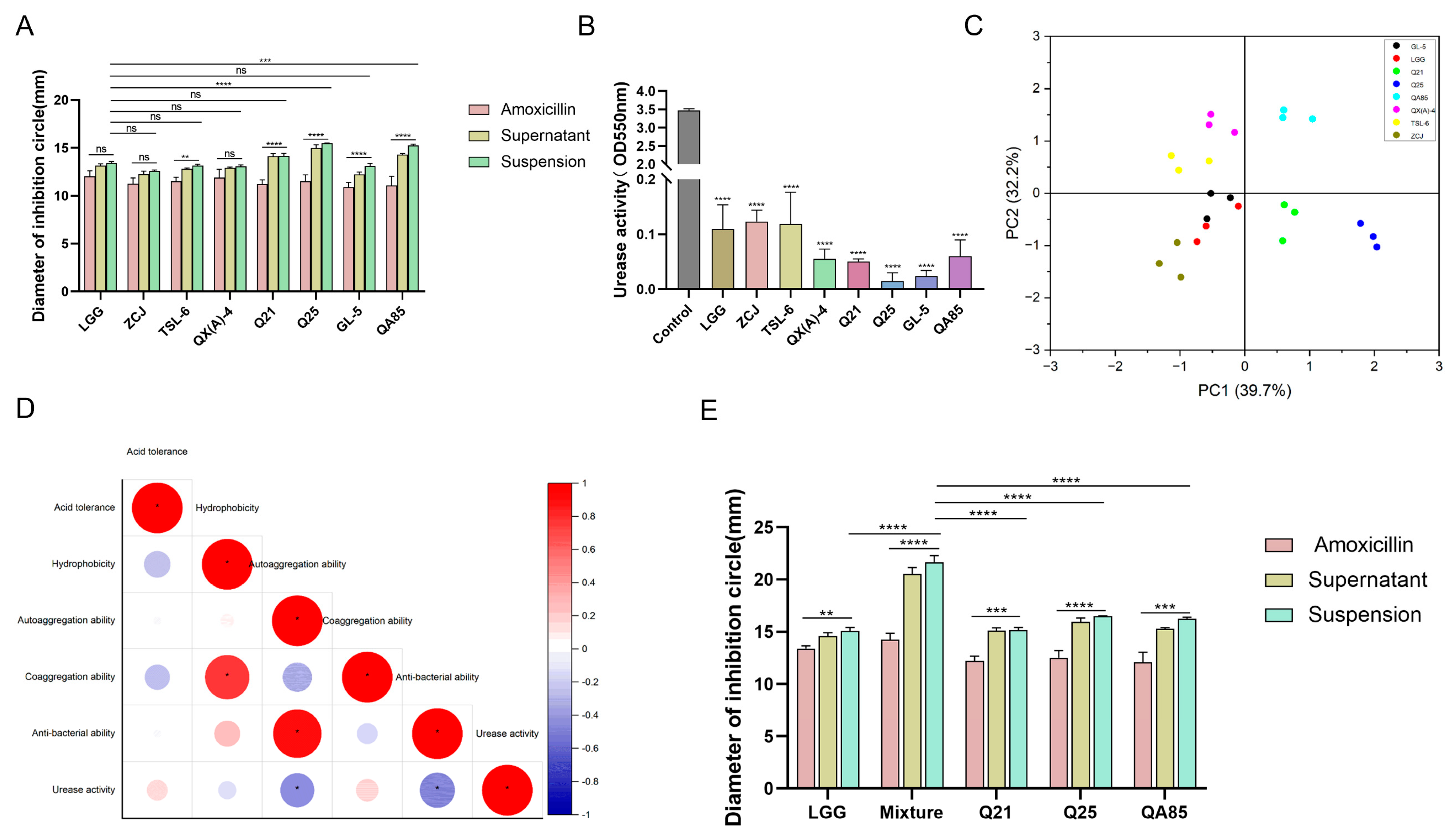
2.3.1. Acid Tolerance of Lactobacillus
2.3.2. Hydrophobic Properties of Lactobacillus
2.3.3. Aggregation Properties of Lactobacillus
2.3.4. Anti-H. pylori Activity of Lactobacillus
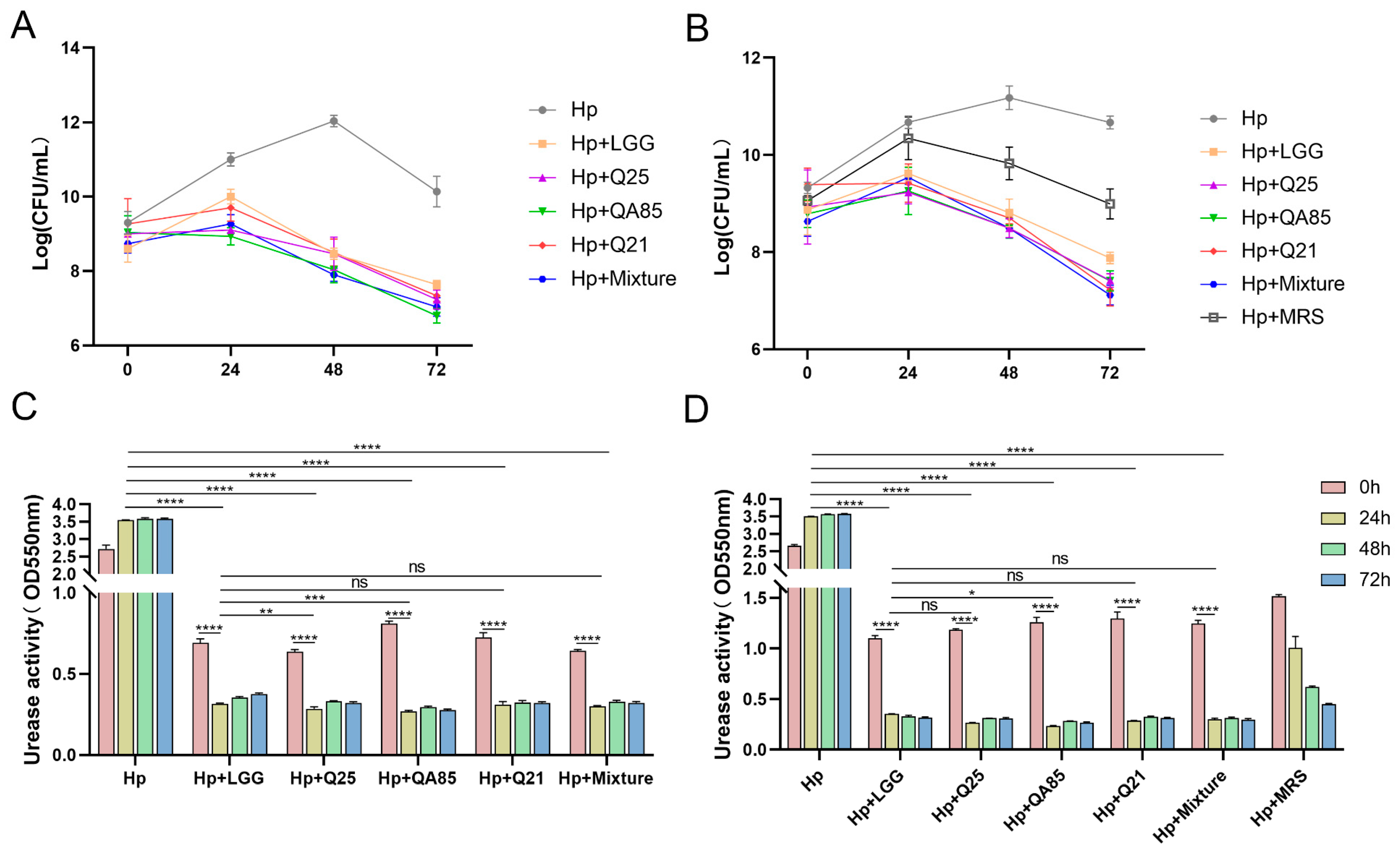
2.3.5. Urease Activity Assay
2.4. Co-Culture Assay of Lactobacillus with H. pylori
2.5. Anti-H. pylori Effects of Lactobacillus at the Cell Level
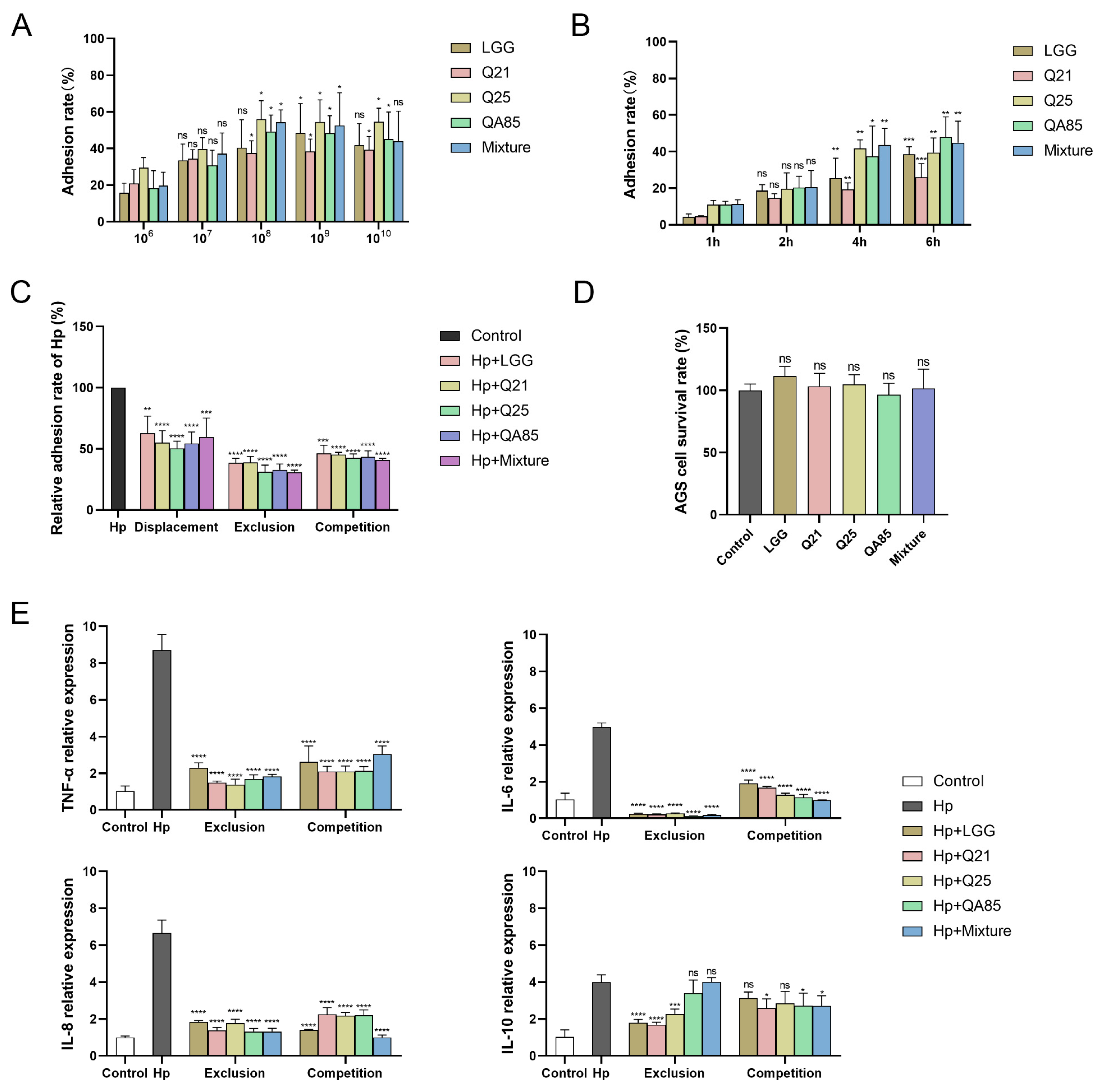
2.5.1. Adhesion Assay of Lactobacillus to AGS Cells
2.5.2. Inhibition Assay of H. pylori Adhesion to AGS Cells by Lactobacillus
2.5.3. Total RNA Extraction and Reverse-Transcription Quantitative PCR (RT-qPCR)
2.5.4. Cytotoxicity Assay on AGS Cells
2.6. Antibiotic Susceptibility Assay
2.7. Effects of Lactobacillus on Patients with H. pylori Infection
2.7.1. Participating Volunteers
2.7.2. Study Design
2.7.3. Volunteer Basic Information Statistics and 14C-UBT Detection
2.7.4. Gastrointestinal Symptom Assessment
2.7.5. Measurement of Cytokines
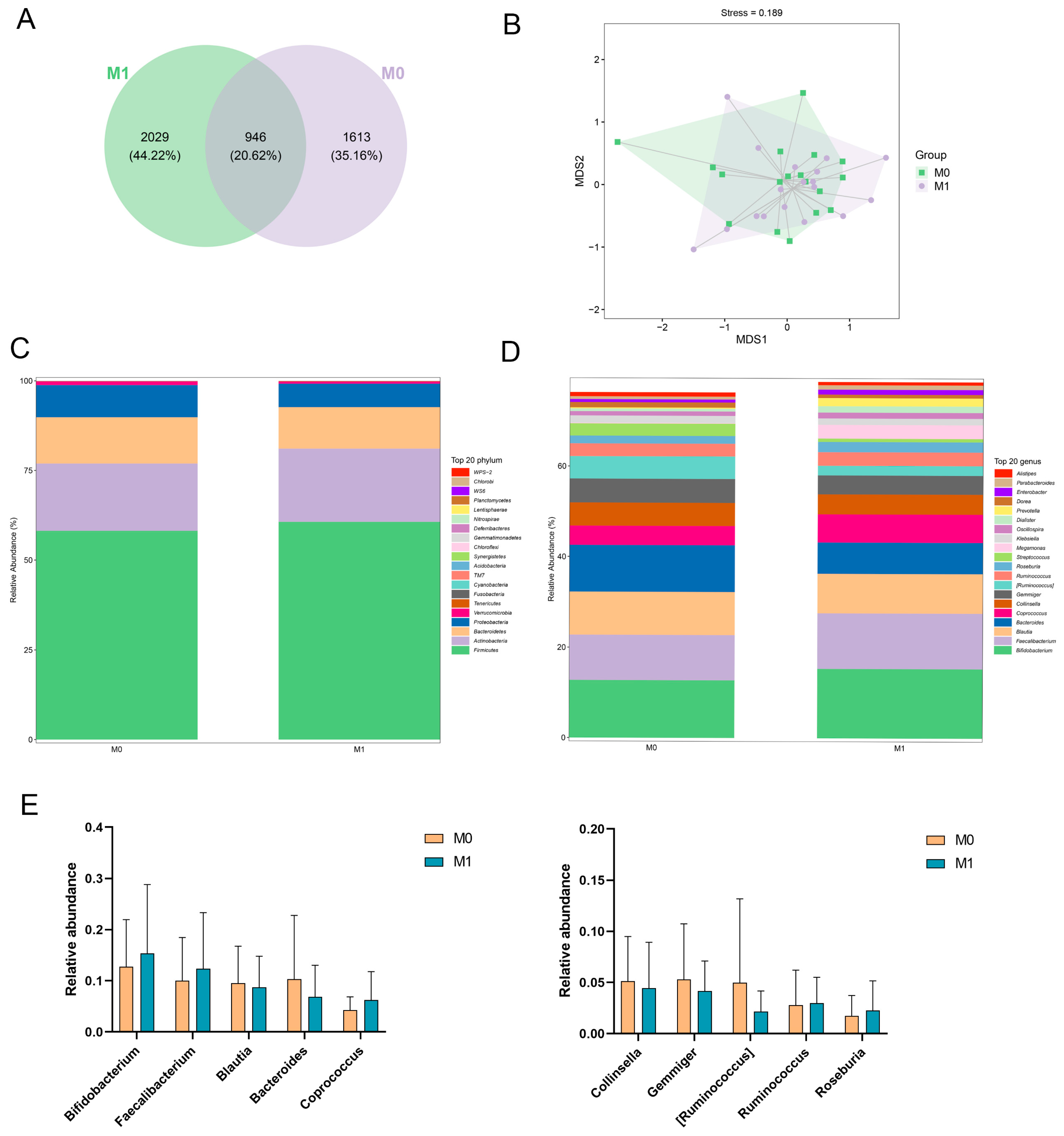
2.7.6. Analysis of Gut Microbiota
2.7.7. Metabolomics Testing and Analysis
2.8. Statistical Analysis
3. Results
3.1. Three Strains of Lactobacillus with Strongly Inhibitory Effect on H. pylori
3.2. Co-Culture Validation Results of Lactobacillus and H. pylori
3.3. Adhesion of Lactobacillus to AGS Cells and Inhibition of H. pylori Adhesion
3.4. Antibiotic Susceptibility Analysis of Lactobacillus
3.5. The Effect Analysis of Lactobacillus on Patients with H. pylori Infection
3.5.1. Trial Profile and Volunteer Characteristics
3.5.2. Effect of Lactobacillus on H. pylori Load and Gastrointestinal Symptoms in Volunteers
3.5.3. Effect of Lactobacillus on Immunity-Related Indicators in Volunteers
3.5.4. Gut Microbiota Analysis
3.5.5. Differential Metabolite Analysis and Related Metabolic Pathway Prediction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Benajah, D.A.; Lahbabi, M.; Alaoui, S.; El Rhazi, K.; El Abkari, M.; Nejjari, C.; Amarti, A.; Bennani, B.; Mahmoud, M.; Ibrahimi, S.A. Prevalence of Helicobacter pylori and Its Recurrence after Successful Eradication in a Developing Nation (Morocco). Clin. Res. Hepatol. Gastroenterol. 2013, 37, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Porras, C.; Nodora, J.; Sexton, R.; Ferreccio, C.; Jimenez, S.; Dominguez, R.L.; Cook, P.; Anderson, G.; Morgan, D.R.; Baker, L.H.; et al. Epidemiology of Helicobacter pylori Infection in Six Latin American Countries (SWOG Trial S0701). Cancer Causes Control 2013, 24, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Adeloye, D.; Luk, T.T.; Huang, L.; He, Y.; Xu, Y.; Ye, X.; Yi, Q.; Song, P.; Rudan, I.; et al. The Global Prevalence of and Factors Associated with Helicobacter pylori Infection in Children: A Systematic Review and Meta-Analysis. Lancet Child. Adolesc. Health 2022, 6, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Link, A.; Selgrad, M. Helicobacter pylori: Perspectives and Time Trends. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-Analysis in World Health Organization Regions. Gastroenterology 2018, 155, 1372–1382.e17. [Google Scholar] [CrossRef] [PubMed]
- Pelepenko, L.E.; Janini, A.C.P.; Gomes, B.P.F.A.; de-Jesus-Soares, A.; Marciano, M.A. Effects of Bismuth Exposure on the Human Kidney-A Systematic Review. Antibiotics 2022, 11, 1741. [Google Scholar] [CrossRef] [PubMed]
- Gebeyehu, E.; Nigatu, D.; Engidawork, E. Helicobacter pylori Eradication Rate of Standard Triple Therapy and Factors Affecting Eradication Rate at Bahir Dar City Administration, Northwest Ethiopia: A Prospective Follow up Study. PLoS ONE 2019, 14, e0217645. [Google Scholar] [CrossRef]
- Phumkhachorn, P.; Rattanachaikunsopon, P. Probiotics: Sources, Selection and Health Benefits. Res. J. Biotech. 2023, 18, 102–113. [Google Scholar] [CrossRef]
- Wilkins, T.; Sequoia, J. Probiotics for Gastrointestinal Conditions: A Summary of the Evidence. Am. Fam. Physician 2017, 96, 170–178. [Google Scholar]
- Sang, L.-X. Remission Induction and Maintenance Effect of Probiotics on Ulcerative Colitis: A Meta-Analysis. WJG 2010, 16, 1908. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, P.; Zhang, X. Probiotics Regulate Gut Microbiota: An Effective Method to Improve Immunity. Molecules 2021, 26, 6076. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Xie, Y.; Zhu, Y.; Zhuang, K.; Huo, L.; Yu, Y.; Guo, Q.; Shu, X.; Xiong, Z.; Zhang, Z.; et al. Probiotics Modulate Gastrointestinal Microbiota after Helicobacter pylori Eradication: A Multicenter Randomized Double-Blind Placebo-Controlled Trial. Front. Immunol. 2022, 13, 1033063. [Google Scholar] [CrossRef] [PubMed]
- Pernica, J.M.; Steenhoff, A.P.; Mokomane, M.; Moorad, B.; Lechiile, K.; Smieja, M.; Mazhani, L.; Cheng, J.; Kelly, M.S.; Loeb, M.; et al. Rapid Enteric Testing to Permit Targeted Antimicrobial Therapy, with and without Lactobacillus reuteri Probiotics, for Paediatric Acute Diarrhoeal Disease in Botswana: A Pilot, Randomized, Factorial, Controlled Trial. PLoS ONE 2017, 12, e0185177. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-Y.; Shih, H.-C.; Yu, M.-C.; Lee, M.-Y.; Chang, Y.-L.; Lai, Y.-Y.; Lee, Y.-C.; Kuan, Y.-H.; Lin, C.-C. Evaluation of the Potential Inhibitory Activity of a Combination of L. acidophilus, L. rhamnosus and L. sporogenes on Helicobacter pylori: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Chin. J. Integr. Med. 2017, 23, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Y. Effect of Lactobacillus acidophilus and Bifidobacterium bifidum Supplementation to Standard Triple Therapy on Helicobacter pylori Eradication and Dynamic Changes in Intestinal Flora. World J. Microbiol. Biotechnol. 2014, 30, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Myllyluoma, E.; Veijola, L.; Ahlroos, T.; Tynkkynen, S.; Kankuri, E.; Vapaatalo, H.; Rautelin, H.; Korpela, R. Probiotic Supplementation Improves Tolerance to Helicobacter pylori Eradication Therapy--a Placebo-Controlled, Double-Blind Randomized Pilot Study. Aliment. Pharmacol. Ther. 2005, 21, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Sgouras, D.; Maragkoudakis, P.; Petraki, K.; Martinez-Gonzalez, B.; Eriotou, E.; Michopoulos, S.; Kalantzopoulos, G.; Tsakalidou, E.; Mentis, A. In Vitro and in Vivo Inhibition of Helicobacter pylori by Lactobacillus casei Strain Shirota. Appl. Environ. Microbiol. 2004, 70, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Sahagún-Flores, J.E.; López-Peña, L.S.; de la Cruz-Ramírez Jaimes, J.; García-Bravo, M.S.; Peregrina-Gómez, R.; de Alba-García, J.E.G. Eradication of Helicobacter pylori: Triple treatment scheme plus Lactobacillus vs. triple treatment alone. Cir. Y Cir. 2007, 75, 333–336. [Google Scholar]
- Deguchi, R.; Nakaminami, H.; Rimbara, E.; Noguchi, N.; Sasatsu, M.; Suzuki, T.; Matsushima, M.; Koike, J.; Igarashi, M.; Ozawa, H.; et al. Effect of Pretreatment with Lactobacillus gasseri OLL2716 on First-line Helicobacter pylori Eradication Therapy. J. Gastro Hepatol. 2012, 27, 888–892. [Google Scholar] [CrossRef]
- Ismail, N.I.; Nawawi, K.N.M.; Hsin, D.C.C.; Hao, K.W.; Mahmood, N.R.K.N.; Chearn, G.L.C.; Wong, Z.; Tamil, A.M.; Joseph, H.; Raja Ali, R.A. Probiotic Containing Lactobacillus reuteri DSM 17648 as an Adjunct Treatment for Helicobacter pylori Infection: A Randomized, Double-blind, Placebo-controlled Trial. Helicobacter 2023, 28, e13017. [Google Scholar] [CrossRef] [PubMed]
- Del Piano, M.; Pagliarulo, M.; Tari, R.; Carmagnola, S.; Balzarini, M.; Lorenzini, P.; Pane, M. Correlation between Chronic Treatment with Proton Pump Inhibitors and Bacterial Overgrowth in the Stomach: Any Possible Beneficial Role for Selected Lactobacilli? J. Clin. Gastroenterol. 2014, 48 (Suppl. S1), S40–S46. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xu, Y.; Chen, Z.; Chen, Y.; Wei, F.; Xia, C.; Zhou, Q.; Li, P.; Gu, Q. Lactiplantibacillus plantarum ZJ316 Reduces Helicobacter pylori Adhesion and Inflammation by Inhibiting the Expression of Adhesin and Urease Genes. Mol. Nutr. Food Res. 2023, 67, 2300241. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Xiao, S.; Li, S.; Suo, B.; Wang, Y.; Meng, L.; Liu, Z.; Yin, Z.; Xue, Y.; Zhou, L. The Impact of Helicobacter pylori Infection, Eradication Therapy, and Probiotics Intervention on Gastric Microbiota in Young Adults. Helicobacter 2021, 26, e12848. [Google Scholar] [CrossRef] [PubMed]
- Yakovenko, E.P.; Strokova, T.V.; Iakovenko, A.V.; Ivanov, A.N.; Soluyanova, I.P.; Vasilyev, N.N. A Prospective Randomized Comparative Study of the Efficacy and Safety of a Two-Week Bismuth-Based Quadrotherapy of Helicobacter pylori Infection with the Inclusion of the Probiotic Containing Bifidobacterium longum BB-46 and Enterococcus faecium ENCfa-68. Ter. Arkhiv. 2021, 93, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-Y.; Zhou, L.; Liu, D.-Y.; Yao, X.-J.; Li, Y. What Roles Do Probiotics Play in the Eradication of Helicobacter pylori? Current Knowledge and Ongoing Research. Gastroenterol. Res. Pract. 2018, 2018, 9379480. [Google Scholar] [CrossRef] [PubMed]
- Buckley, M.; Lacey, S.; Doolan, A.; Goodbody, E.; Seamans, K. The Effect of Lactobacillus reuteri Supplementation in Helicobacter pylori Infection: A Placebo-Controlled, Single-Blind Study. BMC Nutr. 2018, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, J. Intelligent Algorithm-Based Gastrointestinal X-Ray Examination in Evaluating the Therapeutic Effect of Probiotics Combined with Triple Therapy on Children with Helicobacter Infection. Contrast Media Mol. Imaging 2022, 2022, 8464361. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, R.; Ni, P.; Chen, S.; Duan, G. Efficacy of Lactobacillus-Supplemented Triple Therapy for H. Pylori Eradication: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2019, 14, e0223309. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, C.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Meta-Analysis of the Efficacy of Probiotic-Supplemented Therapy on the Eradication of H. Pylori and Incidence of Therapy-Associated Side Effects. Microb. Pathog. 2020, 147, 104403. [Google Scholar] [CrossRef]
- Holz, C.; Busjahn, A.; Mehling, H.; Arya, S.; Boettner, M.; Habibi, H.; Lang, C. Significant Reduction in Helicobacter pylori Load in Humans with Non-Viable Lactobacillus reuteri DSM17648: A Pilot Study. Probiotics Antimicro. Prot. 2015, 7, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.; Kim, B.-S.; Kim, J.W.; Kim, J.S.; Koh, S.-J.; Kim, B.G.; Lee, K.L.; Chun, J. The Effect of Probiotics on Gut Microbiota during the Helicobacter pylori Eradication: Randomized Controlled Trial. Helicobacter 2016, 21, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, N.B.; Bryrup, T.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Pedersen, O. Alterations in Fecal Microbiota Composition by Probiotic Supplementation in Healthy Adults: A Systematic Review of Randomized Controlled Trials. Genome Med. 2016, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Yu-Li, W.; Xiao-Qian, C.; Ping, Z. Isolation and Initiative Identification of Microorganism from Traditional Fermentative Food-Jiangshui. Food Sci. 2007, 28, 219. [Google Scholar]
- Zhou, X.; Pan, Y.; Wang, Y.; Li, W. In Vitro Assessment of Gastrointestinal Viability of Two Photosynthetic Bacteria, Rhodopseudomonas Palustris and Rhodobacter Sphaeroides. J. Zhejiang Univ. Sci. B 2007, 8, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Kos, B.; Susković, J.; Vuković, S.; Simpraga, M.; Frece, J.; Matosić, S. Adhesion and Aggregation Ability of Probiotic Strain Lactobacillus acidophilus M92. J. Appl. Microbiol. 2003, 94, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Meriluoto, J.; Salminen, S. Adhesion and Aggregation Properties of Probiotic and Pathogen Strains. Eur. Food Res. Technol. 2008, 226, 1065–1073. [Google Scholar] [CrossRef]
- Ryan, K.A.; Daly, P.; Li, Y.; Hooton, C.; O’Toole, P.W. Strain-Specific Inhibition of Helicobacter pylori by Lactobacillus salivarius and Other Lactobacilli. J. Antimicrob. Chemother. 2008, 61, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Thuy, T.T.D.; Kuo, P.-Y.; Lin, S.-M.; Kao, C.-Y. Anti-Helicobacter pylori Activity of Potential Probiotic Lactiplantibacillus pentosus SLC13. BMC Microbiol. 2022, 22, 277. [Google Scholar] [CrossRef]
- Jing, D.; Jin, J.; Mei, Z.; Zhu, Q.; Lu, Y.; Wang, X. Effects of Helicobacter pylori Infection and Interleukin 6 on the Expression of ITIH4 in Human Gastric Cancer Cells. Transl. Cancer Res. 2020, 9, 4656–4665. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, F.; Wan, C.; Xiong, Y.; Shah, N.P.; Wei, H.; Tao, X. Evaluation of Probiotic Properties of Lactobacillus plantarum WLPL04 Isolated from Human Breast Milk. J. Dairy. Sci. 2016, 99, 1736–1746. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Xu, H.; Aguilar, Z.P.; Peng, S.; Dong, S.; Wang, B.; Li, P.; Chen, T.; Xu, F.; Wei, H. Safety Assessment and Probiotic Evaluation of Enterococcus Faecium YF5 Isolated from Sourdough. J. Food Sci. 2013, 78, M587–M593. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liang, L.; Lv, P.; Liu, L.; Wang, S.; Wang, Z.; Chen, Y. Effects of Non-Viable Lactobacillus reuteri Combining with 14-Day Standard Triple Therapy on Helicobacter pylori Eradication: A Randomized Double-Blind Placebo-Controlled Trial. Helicobacter 2021, 26, e12856. [Google Scholar] [CrossRef]
- Crowe, S.E. Helicobacter pylori Infection. N. Engl. J. Med. 2019, 380, 1158–1165. [Google Scholar] [CrossRef]
- Svedlund, J.; Sjödin, I.; Dotevall, G. GSRS—A Clinical Rating Scale for Gastrointestinal Symptoms in Patients with Irritable Bowel Syndrome and Peptic Ulcer Disease. Dig. Dis. Sci. 1988, 33, 129–134. [Google Scholar] [CrossRef]
- Kulich, K.R.; Madisch, A.; Pacini, F.; Piqué, J.M.; Regula, J.; Van Rensburg, C.J.; Ujszászy, L.; Carlsson, J.; Halling, K.; Wiklund, I.K. Reliability and Validity of the Gastrointestinal Symptom Rating Scale (GSRS) and Quality of Life in Reflux and Dyspepsia (QOLRAD) Questionnaire in Dyspepsia: A Six-Country Study. Health Qual. Life Outcomes 2008, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, W.; Lee, A.; He, J.; Huang, B.; Zheng, W.; Su, T.; Lai, S.; Long, Y.; Chu, H.; et al. The Impact of Helicobacter pylori Infection, Eradication Therapy and Probiotic Supplementation on Gut Microenvironment Homeostasis: An Open-Label, Randomized Clinical Trial. EBioMedicine 2018, 35, 87–96. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; Delgado, S.; Ruiz, L.; Ruas-Madiedo, P.; Sánchez, B.; Margolles, A. Bifidobacteria and Their Health-Promoting Effects. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Tshibangu-Kabamba, E.; Yamaoka, Y. Helicobacter pylori Infection and Antibiotic Resistance—From Biology to Clinical Implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 613–629. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Liu, F. Probiotics as an Adjuvant Treatment in Helicobacter pylori Eradication Therapy. J. Dig. Dis. 2017, 18, 195–202. [Google Scholar] [CrossRef]
- Qureshi, N.; Li, P.; Gu, Q. Probiotic Therapy in Helicobacter pylori Infection: A Potential Strategy against a Serious Pathogen? Appl. Microbiol. Biotechnol. 2019, 103, 1573–1588. [Google Scholar] [CrossRef]
- Sophatha, B.; Teanpaisan, R. Factors Relating to Adhesion and Aggregation of Lactobacillus paracasei and Lactobacillus rhamnosus Strains. Microbiology 2021, 90, 793–800. [Google Scholar] [CrossRef]
- Zuo, F.; Appaswamy, A.; Gebremariam, H.G.; Jonsson, A.-B. Role of Sortase A in Lactobacillus gasseri Kx110A1 Adhesion to Gastric Epithelial Cells and Competitive Exclusion of Helicobacter pylori. Front. Microbiol. 2019, 10, 2770. [Google Scholar] [CrossRef]
- Ekmekci, H.; Aslim, B.; Ozturk, S. Characterization of Vaginal Lactobacilli Coaggregation Ability with Escherichia coli. Microbiol. Immunol. 2009, 53, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.-X.; Fang, H.-Y.; Yang, H.-B.; Tien, N.-Y.; Wang, M.-C.; Wu, J.-J. Lactobacillus pentosus Strain LPS16 Produces Lactic Acid, Inhibiting Multidrug-Resistant Helicobacter pylori. J. Microbiol. Immunol. Infect. 2016, 49, 168–174. [Google Scholar] [CrossRef]
- Kim, T.-S.; Hur, J.-W.; Yu, M.-A.; Cheigh, C.-I.; Kim, K.-N.; Hwang, J.-K.; Pyun, Y.-R. Antagonism of Helicobacter pylori by Bacteriocins of Lactic Acid Bacteria. J. Food Prot. 2003, 66, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Alakomi, H.L.; Skyttä, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I.M. Lactic Acid Permeabilizes Gram-Negative Bacteria by Disrupting the Outer Membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005. [Google Scholar] [CrossRef]
- Bansil, R.; Celli, J.P.; Hardcastle, J.M.; Turner, B.S. The Influence of Mucus Microstructure and Rheology in Helicobacter pylori Infection. Front. Immunol. 2013, 4, 310. [Google Scholar] [CrossRef]
- Gotteland, M.; Cruchet, S.; Verbeke, S. Effect of Lactobacillus Ingestion on the Gastrointestinal Mucosal Barrier Alterations Induced by Indometacin in Humans. Aliment. Pharmacol. Ther. 2001, 15, 11–17. [Google Scholar] [CrossRef]
- Do, A.D.; Chang, C.-C.; Su, C.-H.; Hsu, Y.-M. Lactobacillus rhamnosus JB3 Inhibits Helicobacter pylori Infection through Multiple Molecular Actions. Helicobacter 2021, 26, e12806. [Google Scholar] [CrossRef]
- de Klerk, N.; Maudsdotter, L.; Gebreegziabher, H.; Saroj, S.D.; Eriksson, B.; Eriksson, O.S.; Roos, S.; Lindén, S.; Sjölinder, H.; Jonsson, A.-B. Lactobacilli Reduce Helicobacter pylori Attachment to Host Gastric Epithelial Cells by Inhibiting Adhesion Gene Expression. Infect. Immun. 2016, 84, 1526–1535. [Google Scholar] [CrossRef]
- Yang, H.; Hu, B. Immunological Perspective: Helicobacter pylori Infection and Gastritis. Mediat. Inflamm. 2022, 2022, 2944156. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, Z.; Alebouyeh, M.; Rezaei Tavirani, M.; Azimirad, M.; Yadegar, A. Helicobacter pylori CagA Induced Interleukin-8 Secretion in Gastric Epithelial Cells. Gastroenterol. Hepatol. Bed Bench 2016, 9, S42–S46. [Google Scholar] [PubMed]
- Imanishi, J.; Kita, M.; Yamaoka, Y.; Sawai, N.; Tanahashi, T.; Kodama, T. Role of cytokines in the pathogenesis of gastrointestinal diseases associated with Helicobacter pylori infection. C R Seances Soc. Biol. Fil. 1998, 192, 991–996. [Google Scholar] [PubMed]
- Noach, L.A.; Bosma, N.B.; Jansen, J.; Hoek, F.J.; van Deventer, S.J.; Tytgat, G.N. Mucosal Tumor Necrosis Factor-Alpha, Interleukin-1 Beta, and Interleukin-8 Production in Patients with Helicobacter pylori Infection. Scand. J. Gastroenterol. 1994, 29, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Castillo, V.; Marcial, G.; Albarracín, L.; Tomokiyo, M.; Clua, P.; Takahashi, H.; Kitazawa, H.; Garcia-Cancino, A.; Villena, J. The Exopolysaccharide of Lactobacillus fermentum UCO-979C Is Partially Involved in Its Immunomodulatory Effect and Its Ability to Improve the Resistance against Helicobacter pylori Infection. Microorganisms 2020, 8, 479. [Google Scholar] [CrossRef]
- Gill, H.S. Probiotics to Enhance Anti-Infective Defences in the Gastrointestinal Tract. Best. Pract. Res. Clin. Gastroenterol. 2003, 17, 755–773. [Google Scholar] [CrossRef]
- Haller, D.; Bode, C.; Hammes, W.P.; Pfeifer, A.M.; Schiffrin, E.J.; Blum, S. Non-Pathogenic Bacteria Elicit a Differential Cytokine Response by Intestinal Epithelial Cell/Leucocyte Co-Cultures. Gut 2000, 47, 79–87. [Google Scholar] [CrossRef]
- Patel, A.; Shah, N.; Prajapati, J.B. Clinical Application of Probiotics in the Treatment of Helicobacter pylori Infection—A Brief Review. J. Microbiol. Immunol. Infect. 2014, 47, 429–437. [Google Scholar] [CrossRef]
- Chen, M.-J.; Chen, C.-C.; Huang, Y.-C.; Tseng, C.-C.; Hsu, J.-T.; Lin, Y.-F.; Fang, Y.-J.; Wu, M.-S.; Liou, J.-M. Taiwan Gastrointestinal Disease, Helicobacter Consortium The Efficacy of Lactobacillus acidophilus and Rhamnosus in the Reduction of Bacterial Load of Helicobacter pylori and Modification of Gut Microbiota-a Double-Blind, Placebo-Controlled, Randomized Trial. Helicobacter 2021, 26, e12857. [Google Scholar] [CrossRef]
- Saracino, I.M.; Pavoni, M.; Saccomanno, L.; Fiorini, G.; Pesci, V.; Foschi, C.; Piccirilli, G.; Bernardini, G.; Holton, J.; Figura, N.; et al. Antimicrobial Efficacy of Five Probiotic Strains Against Helicobacter pylori. Antibiotics 2020, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Emara, M.H.; Elhawari, S.A.; Yousef, S.; Radwan, M.I.; Abdel-Aziz, H.R. Emerging Role of Probiotics in the Management of Helicobacter pylori Infection: Histopathologic Perspectives. Helicobacter 2016, 21, 3–10. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Chuang, C.-C.; Yang, H.-B.; Lu, C.-C.; Sheu, B.-S. Lactobacillus acidophilus Ameliorates H. Pylori-Induced Gastric Inflammation by Inactivating the Smad7 and NFκB Pathways. BMC Microbiol. 2012, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Chotivitayatarakorn, P.; Mahachai, V.; Vilaichone, R.-K. Effectiveness of 7-Day and 14-Day Moxifloxacin-Dexlansoprazole Based Triple Therapy and Probiotic Supplement for Helicobacter pylori Eradication in Thai Patients with Non-Ulcer Dyspepsia: A Double-Blind Randomized Placebo-Controlled Study. Asian Pac. J. Cancer Prev. 2017, 18, 2839–2843. [Google Scholar] [CrossRef] [PubMed]
- Emara, M.H.; Mohamed, S.Y.; Abdel-Aziz, H.R. Lactobacillus reuteri in Management of Helicobacter pylori Infection in Dyspeptic Patients: A Double-Blind Placebo-Controlled Randomized Clinical Trial. Ther. Adv. Gastroenterol. 2014, 7, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Hauser, G.; Salkic, N.; Vukelic, K.; JajacKnez, A.; Stimac, D. Probiotics for Standard Triple Helicobacter pylori Eradication: A Randomized, Double-Blind, Placebo-Controlled Trial. Medicine 2015, 94, e685. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.A.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The Gut Microbiota and Host Health: A New Clinical Frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef]
- Langdon, A.; Crook, N.; Dantas, G. The Effects of Antibiotics on the Microbiome throughout Development and Alternative Approaches for Therapeutic Modulation. Genome Med. 2016, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.-S.; Tap, J.; Chambaud, I.; Cools-Portier, S.; Quinquis, L.; Bourlioux, P.; Marteau, P.; Guillemard, E.; Schrezenmeir, J.; Derrien, M. Safety and Functional Enrichment of Gut Microbiome in Healthy Subjects Consuming a Multi-Strain Fermented Milk Product: A Randomised Controlled Trial. Sci. Rep. 2020, 10, 15974. [Google Scholar] [CrossRef]
- Park, Y.E.; Kim, M.S.; Shim, K.W.; Kim, Y.-I.; Chu, J.; Kim, B.-K.; Choi, I.S.; Kim, J.Y. Effects of Lactobacillus plantarum Q180 on Postprandial Lipid Levels and Intestinal Environment: A Double-Blind, Randomized, Placebo-Controlled, Parallel Trial. Nutrients 2020, 12, 255. [Google Scholar] [CrossRef]
- Rahayu, E.S.; Mariyatun, M.; Putri Manurung, N.E.; Hasan, P.N.; Therdtatha, P.; Mishima, R.; Komalasari, H.; Mahfuzah, N.A.; Pamungkaningtyas, F.H.; Yoga, W.K.; et al. Effect of Probiotic Lactobacillus plantarum Dad-13 Powder Consumption on the Gut Microbiota and Intestinal Health of Overweight Adults. World J. Gastroenterol. 2021, 27, 107–128. [Google Scholar] [CrossRef]
- Ferrario, C.; Taverniti, V.; Milani, C.; Fiore, W.; Laureati, M.; De Noni, I.; Stuknyte, M.; Chouaia, B.; Riso, P.; Guglielmetti, S. Modulation of Fecal Clostridiales Bacteria and Butyrate by Probiotic Intervention with Lactobacillus paracasei DG Varies among Healthy Adults. J. Nutr. 2014, 144, 1787–1796. [Google Scholar] [CrossRef]
- Sasset, L.; Di Lorenzo, A. Sphingolipid Metabolism and Signaling in Endothelial Cell Functions. Adv. Exp. Med. Biol. 2022, 1372, 87–117. [Google Scholar] [CrossRef]
- Sakai, A.; Nishiumi, S.; Shiomi, Y.; Kobayashi, T.; Izumi, Y.; Kutsumi, H.; Hayakumo, T.; Azuma, T.; Yoshida, M. Metabolomic Analysis to Discover Candidate Therapeutic Agents against Acute Pancreatitis. Arch. Biochem. Biophys. 2012, 522, 107–120. [Google Scholar] [CrossRef]
- Huwiler, A.; Kolter, T.; Pfeilschifter, J.; Sandhoff, K. Physiology and Pathophysiology of Sphingolipid Metabolism and Signaling. Biochim. Biophys. Acta 2000, 1485, 63–99. [Google Scholar] [CrossRef]


| Target Gene | Primer Sequence (5′ to 3′) | Tm | Size of Amplicon (bp) |
|---|---|---|---|
| TNF-α | F:TTTGATCCCTGACATCTGGA | 55.83 | 112 |
| R:GGCCTAAGGTCCACTTGTGT | 59.60 | ||
| IL-6 | F:GACAGCCACTCACCTCTTCA | 59.32 | 457 |
| R:CGCAGAATGAGATGAGTTGT | 55.87 | ||
| IL-8 | F:ACTGAGAGTGATTGAGAGTGGAC | 59.49 | 112 |
| R:AACCCTCTGCACCCAGTTTTC | 60.75 | ||
| IL-10 | F:AGGGAGGATGAGTGATTTGC | 57.27 | 783 |
| R:AACTGGGAGGAACACTGACC | 59.23 | ||
| β-actin | F:GACCTCTATGCCAACACAGT | 57.23 | 139 |
| R:AGTACTTGCGCTCAGGAGGA | 60.61 |
| Class | Antibiotic | Content | Sensitivity | |||
|---|---|---|---|---|---|---|
| LGG | Q21 | Q25 | QA85 | |||
| Penicillins | Penicillin | 10 U | R | R | R | S |
| Oxacillin | 1 μg | R | R | R | S | |
| Ampicillin | 10 μg | S | S | S | S | |
| Aminoglycoside | Gentamicin | 10 μg | I | R | R | R |
| Streptomycin | 10 μg | R | R | R | R | |
| Kanamycin | 30 μg | R | R | R | R | |
| Tetracyclines | Tetracycline | 30 μg | S | I | S | I |
| Doxycycline | 30 μg | S | S | S | S | |
| Cephalosporins | Imipenem | 10 μg | S | S | S | S |
| Ceftazidime | 30 μg | S | R | R | R | |
| Cefotaxime | 30 μg | I | R | R | R | |
| Cefuroxime | 30 μg | S | S | I | S | |
| Macrolide | Erythromycin | 15 μg | I | I | I | I |
| Quinolones | Ciprofloxacin | 5 μg | R | R | R | R |
| Norfloxacin | 10 μg | R | R | R | R | |
| Levofloxacin | 5 μg | R | R | R | R | |
| Folate metabolism pathway inhibitors | Sulfafurazole | 300 μg | R | R | R | R |
| Trimethoprim-sulfamethoxazole | 23.75/1.25 μg | S | S | S | S | |
| Glycopeptide | Vancomycin | 30 μg | R | R | R | R |
| Chloramphenicol | Chloramphenicol | 30 μg | S | S | S | S |
| Rifamycins | Rifampicin | 5 μg | S | R | I | I |
| Symptoms | Before (M0) | After (M1) |
|---|---|---|
| n (%) | n (%) | |
| Epigastric pain | 13 (13.5) | 13 (13.5) |
| Chest pain | 16 (16.2) | 8 (8.1) |
| Acid regurgitation | 21 (21.6) | 13 (13.5) |
| Nausea | 10 (10.8) | 10 (10.8) |
| Bowel sound | 21 (21.6) | 18 (18.9) |
| Abdominal distension | 59 (59.5) | 32 (32.4) |
| Throat discomfort | 27 (27.0) | 18 (18.9) |
| Halitosis | 35 (35.1) | 27 (27.0) |
| Constipation | 37 (37.8) | 35 (35.1) |
| Diarrhea | 43 (43.2) | 21 (21.6) |
| Group | Richness Index | Diversity Index | ||
|---|---|---|---|---|
| Observed Species | Chao1 | Simpson | Shannon | |
| M0 | 262.69 ± 80.34 | 275.99 ± 82.32 | 0.93 ± 0.04 | 5.12 ± 0.74 |
| M1 | 291.82 ± 119.62 | 305.04 ± 120.59 | 0.93 ± 0.05 | 5.31 ± 0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Lin, Y.; Ma, Y.; Li, J.; Li, J.; Huo, Z.; Yang, P.; Zhang, C. Screening Probiotics for Anti-Helicobacter pylori and Investigating the Effect of Probiotics on Patients with Helicobacter pylori Infection. Foods 2024, 13, 1851. https://doi.org/10.3390/foods13121851
Yang H, Lin Y, Ma Y, Li J, Li J, Huo Z, Yang P, Zhang C. Screening Probiotics for Anti-Helicobacter pylori and Investigating the Effect of Probiotics on Patients with Helicobacter pylori Infection. Foods. 2024; 13(12):1851. https://doi.org/10.3390/foods13121851
Chicago/Turabian StyleYang, Hui, Yang Lin, Yuchan Ma, Jiaru Li, Junxiang Li, Zeqi Huo, Pingrong Yang, and Chunjiang Zhang. 2024. "Screening Probiotics for Anti-Helicobacter pylori and Investigating the Effect of Probiotics on Patients with Helicobacter pylori Infection" Foods 13, no. 12: 1851. https://doi.org/10.3390/foods13121851
APA StyleYang, H., Lin, Y., Ma, Y., Li, J., Li, J., Huo, Z., Yang, P., & Zhang, C. (2024). Screening Probiotics for Anti-Helicobacter pylori and Investigating the Effect of Probiotics on Patients with Helicobacter pylori Infection. Foods, 13(12), 1851. https://doi.org/10.3390/foods13121851






