Essential Oil Nanoemulsions—A New Strategy to Extend the Shelf Life of Smoothies
Abstract
:Highlights
- The use of essential oils in food is still limited.
- Emulsification reduces impact on sensory properties of essential oils.
- Essential oils are characterized by numerous health-promoting, antimicrobial and antioxidant properties.
- Essential oil nanoemulsions as promising ingredient for extending the shelf life of smoothies.
Abstract
1. Introduction
2. Essential Oils Are Effective against the Microflora Characteristic of Smoothies
2.1. Bacteria
Alicyclobacillus spp.
2.2. Yeasts
2.3. Molds
| Juice | Essential Oil | Obtained Results | References |
|---|---|---|---|
| Orange | sesame (Sesamum indicum) | Proved antibacterial activity against Staphylococcus aureus, prolonged shelf life of orange juice | [62] |
| sweet orange (Citrus sinensis) | Reduce the treatment time to cause the inactivation of up to 5 Log10 cycles of E. coli O157:H7 Sakai cells. The sensory analysis carried out showed that flavor, color, and odor were accepted by the panelists—the parameters were rated above 6 on the hedonic scale. | [63] | |
| Thymbra capitata L. | Growth inhibition of E. coli up to 20 days, L. monocytogenes, and S. aureus up to 15 days of storage at 5 °C | [64] | |
| cinnamon (Cinnamomum verum J.Presl) | Reduction of E. coli O157:H7 and Salmonella enteritidis by more than 6 log | [65] | |
| Apple | lemongrass (Cymbopogon citratus), mandarin (Citrus reticulata) | Reduction the E. coli population by more than 5 log-reduction after being in contact for 28 days | [66] |
| sweet orange (Citrus sinensis) | Reduction the treatment time to cause the inactivation of up to 5 Log10 cycles of E. coli O157:H7 Sakai cells | [53] | |
| cinnamon (Cinnamomum verum J.Presl) | Reduction of E. coli O157:H7 and Salmonella enteritidis by more than 6 log | [65] | |
| Strawberry | Solidago canadensis L. | Effective inhibition of the growth of Botrytis cinerea | [67] |
| cinnamon (Cinnamomum verum J.Presl) | Reduction of E. coli O157:H7 and Salmonella enteritidis by more than 6 log | [65] | |
| Pineapple | mint (Mentha piperita L.) | The reduction of C. albicans by more than 3 log CFU/mL, C. tropicalis by almost 2 log CFU/mL, P. anomala by almost 4 log, and S. cerevisiae by 4 log CFU/mL in 72 h. The attributes of appearance, odor, and viscosity were not affected (p > 0.05) by essential oil. Juice received hedonic scores varying between “like slightly” and “really enjoyed”. | [68] |
| lemongrass (Cymbopogon citratus D.C. Stapf.) | Reductions in counts of E. coli and L. monocytogenes ≥5 log cycles after 1 h and 45 min, respectively; for Salmonella enteritidis the same reduction was confirmed after 12 h | [69] | |
| Pear | cinnamon (Cinnamomum verum J.Presl) | Reduction of E. coli O157:H7 and Salmonella enteritidis by more than 6 log | [65] |
| Guava | mint (Mentha piperita L.) | Reduction of C. albicans by almost 3 log CFU/mL, C. tropicalis by 1 log CFU/mL, P. anomala, and S. cerevisiae by more than 6 log CFU/mL in 72 h. The attributes appearance, odor, and viscosity were not affected (p > 0.05) by essential oil. Juice received hedonic scores varying between “like slightly” and “really enjoyed”. | [68] |
| Lemon | lemon (Citrus limon L.) | Total inhibition of Alicyclobacillus acidoterrestris germination and outgrowth of spores under refrigerated storage over 11 d. | [50] |
| Mango | mint (Mentha piperita L.) | Reduction of C. albicans and C. tropicalis by almost 2 log CFU/mL, P. anomala by almost 4 log and S. cerevisiae by more than 2 log CFU/mL in 72 h. The attributes of appearance, odor, and viscosity were not affected (p > 0.05) by essential oil. Juice received hedonic scores varying between “like slightly” and “really enjoyed”. | [68] |
| Carrot | Thymbra capitata L. | E. coli O157:H7 count decreased after treatment | [70] |
Mycotoxins Can Come along with Molds
3. Essential Oils—Effective against Enzymatic Oxidation of Smoothies
3.1. Limitations on the Use of Essential Oils in Smoothies
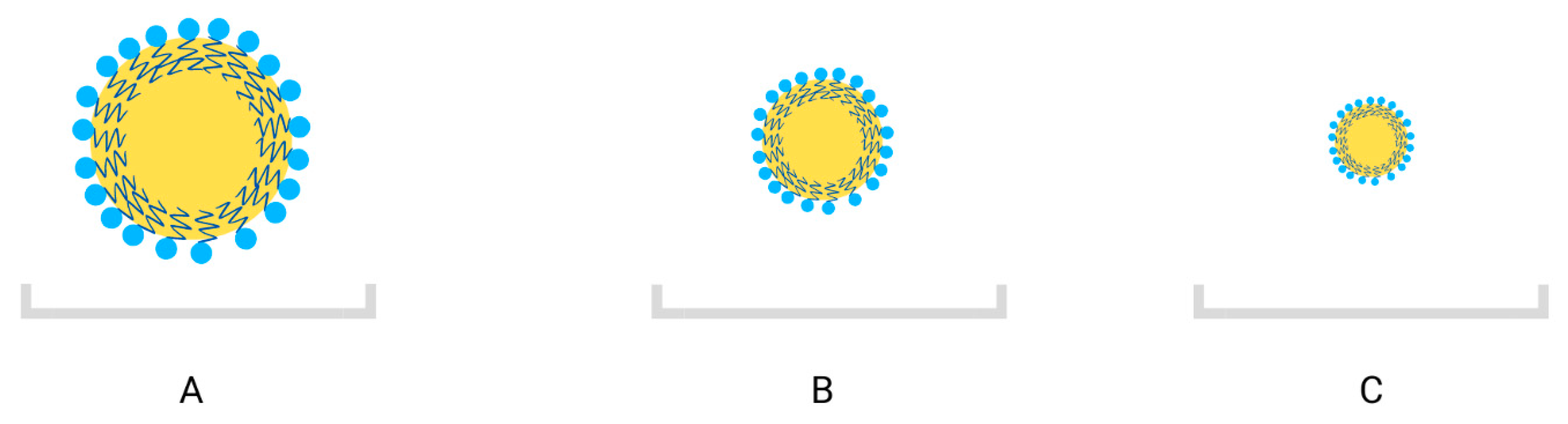
3.2. Essential Oil Nanoemulsions—Solution for Those Limitations
4. Comparison of Essential Oils and Essential Oil Nanoemulsions for Extending Smoothie Shelf Life
5. Future Perspectives, Challenges, and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Statista a. Available online: https://www.statista.com/statistics/425598/smoothies-consumption-frequency-germany/ (accessed on 24 May 2024).
- Statista b. Available online: https://www.statista.com/statistics/425632/consumption-of-smoothies-poland/ (accessed on 24 May 2024).
- Yi, J.; Kebede, B.; Grauwet, T.; Van Loey, A.; Hu, X.; Hendrick, M. A Multivariate Approach into Physicochemical, Biochemical and Aromatic Quality Changes of Puree Based on Hayward Kiwifruit during the Final Phase of Ripening. Postharvest Biol. Technol. 2016, 117, 206–216. [Google Scholar] [CrossRef]
- Yi, J.; Kebede, B.; Grauwet, T.; Van Loey, A.; Hu, X.; Hendrickx, M. Comparing the Impact of High-Pressure Processing and Thermal Processing on Quality of “Hayward” and “Jintao” Kiwifruit Puree: Untargeted Headspace Fingerprinting and Targeted Approaches. Food Bioprocess. Technol. 2016, 9, 2059–2069. [Google Scholar] [CrossRef]
- Xu, X.; Deng, J.; Luo, D.; Bao, Y.; Liao, X.; Gao, H.; Wu, J. Comparative Study of High Hydrostatic Pressure and High Temperature Short Time Processing on Quality of Clear and Cloudy Se-Enriched Kiwifruit Juices. Innov. Food Sci. Emerg. Technol. 2018, 49, 1–12. [Google Scholar] [CrossRef]
- Rollins, B.Y.; Stein, W.; Keller, K.L.; Savage, J.S. Preschoolers Will Drink Their GREENS! Children Accept, Like, and Drink Novel Smoothies Containing Dark Green Vegetables (DGVs). Appetite 2021, 162, 105148. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Ham, K.S.; Park, Y.K.; Leontowicz, H.; Leontowicz, M.; Namieśnik, J.; Katrich, E.; Gorinstein, S. The Effects of Treatment on Quality Parameters of Smoothie-Type “Hayward” Kiwi Fruit Beverages. Food Control 2016, 70, 221–228. [Google Scholar] [CrossRef]
- Tiwari, U. Production of Fruit-Based Smoothies. In Fruit Juices. Extraction, Composition, Quality and Analysis; Rajauria, G., Tiwari, B.K., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 261–278. [Google Scholar] [CrossRef]
- Mieszczakowska-Frąc, M. Przydatność Przetwórcza Jabłek Czerwonomiąższowych do Otrzymywania Wysokiej Jakości Produktów. Zesz. Nauk. Inst. Ogrodn. 2019, 1, 1–122. [Google Scholar]
- Fernandez, M.V.; Denoya, G.I.; Agüero, M.V.; Vaudagna, S.R.; Jagus, R.J. Quality preservation and safety ensurement of a vegetable smoothie by high-pressure processing. J. Food Process. Preserv. 2020, 44, e14326. [Google Scholar] [CrossRef]
- Guzik, P.; Szymkowiak, A.; Kulawik, P.; Zając, M. Consumer Attitudes towards Food Preservation Methods. Foods 2022, 11, 1349. [Google Scholar] [CrossRef] [PubMed]
- Deliza, R.; Ares, G. Consumer Perception of Novel Technologies. In Fruit Preservation: Novel and Conventional Technologies; Rosenthal, A., Deliza, R., Welti-Chanes, J., Barbosa-Canovas, G.V., Eds.; Springer: New York, NY, USA, 2018; pp. 1–20. [Google Scholar] [CrossRef]
- Sokołowska, B.; Skąpska, S.; Fonberg-Broczek, M.; Niezgoda, J.; Chotkiewicz, M.; Dekowska, A.; Rzoska, S.J. Factors Influencing the Inactivation of Alicyclobacillus Acidoterrestris Spores Exposed to High Hydrostatic Pressure in Apple Juice. High Press. Res. 2013, 33, 73–82. [Google Scholar] [CrossRef]
- Koutchma, T. HPP Microbial Effects in Foods. In Adapting High Hydrostatic Pressure (HPP) for Food Processing Operations; Koutchma, T., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 23–28. [Google Scholar]
- Szczepańska, J.; Skąpska, S.; Marszałek, K. Zastosowanie Wysokich Ciśnień do Utrwalania Soków NFC. Przemysł Ferment. Owocowo Warzywny 2017, 11, 48–52. [Google Scholar] [CrossRef]
- dos Santos Rocha, C.; Magnani, M.; de Paiva Anciens Ramos, G.L.; Bezerril, F.F.; Freitas, M.Q.; Cruz, A.G.; Pimentel, T.C. Emerging Technologies in Food Processing: Impacts on Sensory Characteristics and Consumer Perception. Curr. Opin. Food Sci. 2022, 47, 100892. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Falleh, H.; Benjemaa, M.B.; Saada, M.; Ksouri, R. Essential Oils: A Promising Eco-Friendly Food Preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef] [PubMed]
- Falleh, H.; Benjemaa, M.; Djeblai, K.; Abid, S.; Saada, M.; Ksouri, R. Application of the Mixture Design for Optimum Antimicrobial Activity: Combined Treatment of Syzygium Aromaticum, Cinnamomum Zeylanicum, Myrtus Communis, and Lavandula Stoechas Essential Oils against Escherichia coli. J. Food Process. Preserv. 2019, 43, e14257. [Google Scholar] [CrossRef]
- Kunicka-Styczyńska, A. Olejki Eteryczne Jako Alternatywa Dla Syntetycznych Konserwantów Żywności–Praca Przeglądowa. In Innowacyjne Rozwiązania w Technologii Żywności i Żywieniu Człowieka; Tarko, T., Drożdż, I., Najgebauer-Lejko, D., Duda-Chodak, A., Eds.; Oddział Małopolski Polskiego Towarzystwa Technologów Żywności: Kraków, Poland, 2016; pp. 175–184. [Google Scholar]
- Valderrama, F.; Ruiz, F. An Optimal Control Approach to Steam Distillation of Essential Oils from Aromatic Plants. Comput. Chem. Eng. 2018, 117, 25–31. [Google Scholar] [CrossRef]
- Najda, A. Roślinne Substancje Lotne–Olejki Eteryczne. Episteme 2015, 29, 65–77. [Google Scholar]
- Wróblewska-Łuczka, P. Prozdrowotne działanie olejków eterycznych cytrusów. Environ. Med. 2019, 22, 39–43. [Google Scholar]
- Vital, A.C.P.; Guerrero, A.; Kempinski, E.M.B.C.; de Oliveira Monteschio, J.; Sary, C.; Ramos, T.R.; del Mar Campo, M.; do Prado, I.N. Consumer Profile and Acceptability of Cooked Beef Steaks with Edible and Active Coating Containing Oregano and Rosemary Essential Oils. Meat Sci. 2018, 143, 153–158. [Google Scholar] [CrossRef]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial Herb and Spice Compounds in Food. Food Control. 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=182.20 (accessed on 25 May 2024).
- Mahato, D.K.; Kamle, M.; Sharma, B.; Pandhi, S.; Devi, S.; Dhawan, K.; Selvakumar, R.; Mishra, D.; Kumar, A.; Arora, S.; et al. Patulin in Food: A Mycotoxin Concern for Human Health and Its Management Strategies. Toxicon 2021, 198, 12–23. [Google Scholar] [CrossRef]
- McKay, A.M.; Linton, M.; Stirling, J.; Mackle, A.; Patterson, M.F. A Comparative Study of Changes in the Microbiota of Apple Juice Treated by High Hydrostatic Pressure (HHP) or High Pressure Homogenization (HPH). Food Microbiol. 2011, 28, 1426–1431. [Google Scholar] [CrossRef]
- McKnight, I.C.; Eiroa, M.N.U.; Sant’Ana, A.S.; Massaguer, P.R. Alicyclobacillus Acidoterrestris in Pasteurized Exotic Brazilian Fruit Juices: Isolation, Genotypic Characterization and Heat Resistance. Food Microbiol. 2010, 27, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Salomao, B.C.M. Pathogens and Spoilage Microorganisms in Fruit Juice: An Overview. In Fruit Juices: Extraction, Composition, Quality and Analysis; Rajauria, G., Tiwari, B.K., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 291–308. [Google Scholar] [CrossRef]
- Obasi, B.C.; Whong, C.M.Z.; Ameh, J.B.; Ella, E.E. Microbiological Quality Assessment of Commercially and Laboratory Prepared Orange Juice. J. Biotechnol. Res. 2019, 5, 19–27. [Google Scholar] [CrossRef]
- Leneveu-Jenvrin, C.; Aboudia, A.; Assemat, S.; Remize, F. A Three-Step Approach to Assess Efficacy of Alternative Chemical Treatments to Preserve Fresh Fruit Juices: Application to Pineapple (Ananas Comosus “Queen Victoria”). LWT 2022, 155, 112959. [Google Scholar] [CrossRef]
- Aganovic, K.; Grauwet, T.; Kebede, B.T.; Toepfl, S.; Heinz, V.; Hendrickx, M.; Van Loey, A. Impact of Different Large Scale Pasteurization Technologies and Refrigerated Storage on the Headspace Fingerprint of Tomato Juice. Innov. Food Sci. Emerg. Technol. 2014, 26, 431–444. [Google Scholar] [CrossRef]
- Obeng, F.A.; Gyasi, P.B.; Olu-Taiwo, M.; Ayeh-kumi, F.P. Microbial Assessment of Tomatoes (Lycopersicon Esculentum) Sold at Some Central Markets in Ghana. Food Microbiol. 2018, 2018, 6743826. [Google Scholar] [CrossRef]
- Timmermans, R.A.H.; Nederhoff, A.L.; Nierop Groot, M.N.; van Boekel, M.A.J.S.; Mastwijk, H.C. Effect of Electrical Field Strength Applied by PEF Processing and Storage Temperature on the Outgrowth of Yeasts and Moulds Naturally Present in a Fresh Fruit Smoothie. Int. J. Food Microbiol. 2016, 230, 21–30. [Google Scholar] [CrossRef]
- Danyluk, M.D.; Friedrich, L.M.; Jouquand, C.; Goodrich-Schneider, R.; Parish, M.E.; Rouseff, R. Prevalence, Concentration, Spoilage, and Mitigation of Alicyclobacillus Spp. in Tropical and Subtropical Fruit Juice Concentrates. Food Microbiol. 2011, 28, 472–477. [Google Scholar] [CrossRef]
- Skąpska, S.; Sokołowska, B.; Fonberg-Broczek, M.; Niezgoda, J.; Chotkiewicz, M.; Dekowska, A. Zastosowanie Pasteryzacji Wysokociśnieniowej do Inaktywacji Przetrwalników Alicyclobacillus Acidoterrestris w Soku Jabłkowym. ŻYWNOŚĆ Nauka Technol. Jakość 2012, 3, 187–196. [Google Scholar]
- Lee, S.; Kim, H.; Beuchat, L.R.; Kim, Y.; Ryu, J.H. Synergistic Antimicrobial Activity of Oregano and Thyme Thymol Essential Oils against Leuconostoc Citreum in a Laboratory Medium and Tomato Juice. Food Microbiol. 2020, 90, 103489. [Google Scholar] [CrossRef]
- Carvalho, F.; Coimbra, A.T.; Silva, L.; Duarte, A.P.; Ferreira, S. Melissa Officinalis Essential Oil as an Antimicrobial Agent against Listeria Monocytogenes in Watermelon Juice. Food Microbiol. 2023, 109, 104105. [Google Scholar] [CrossRef]
- de Souza Pedrosa, G.T.; de Souza, E.L.; de Melo, A.N.F.; da Cruz Almeida, E.T.; de Sousa Guedes, J.S.; de Carvalho, R.J.; Pagán, R.; Magnani, M. Physiological Alterations Involved in Inactivation of Autochthonous Spoilage Bacteria in Orange Juice Caused by Citrus Essential Oils and Mild Heat. Int. J. Food Microbiol. 2020, 334, 108837. [Google Scholar] [CrossRef]
- Lin, L.; Wang, X.; Cui, H. Synergistic Efficacy of Pulsed Magnetic Fields and Litseacubeba Essential Oil Treatment against Escherichia Coli O157:H7 in Vegetable Juices. Food Control. 2019, 2019, 106686. [Google Scholar] [CrossRef]
- Danyluk, M.D.; Goodrich-Schneider, R.M.; Schneider, K.R.; Harris, L.J.; Worobo, R.W. Outbreaks of Foodborne Disease Associated with Fruit and Vegetable Juices 1922–2010. FSHN Report 12-04. 2012. Available online: https://journals.flvc.org/edis/article/download/119658/117576/177053 (accessed on 5 April 2023).
- Sokołowska, B.; Niezgoda, J.; Dekowska, A.; Porębska, I.; Nasiłowska, J.; Waldon-Wiewióra, E.; Kowalska, M. Incidence of Alicyclobacillus Spp. in Polish Apple and Dark Berry Juice Concentrates and the Ability of Isolated A. Acidoterrestris Strains to Spoilage of These Juices. Postępy Nauk. I Technol. Przemysłu Rolno-Spożywczego 2016, 71, 5–20. [Google Scholar]
- Sokołowska, B.; Połaska, M.; Dekowska, A. Alicyclobacillus—Still Current Issues in the Beverage Industry. In Safety Issues in Beverage Production; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 105–146. [Google Scholar] [CrossRef]
- Feng, X.; He, C.; Jiao, L.; Liang, X.; Zhao, R.; Guo, Y. Analysis of Differential Expression Proteins Reveals the Key Pathway in Response to Heat Stress in Alicyclobacillus acidoterrestris DSM 3922 T. Food Microbiol. 2019, 80, 77–84. [Google Scholar] [CrossRef]
- Wang, Z.; Yue, T.; Yuan, Y.; Zhang, Y.; Gao, Z.; Cai, R. Targeting the Vanillic Acid Decarboxylase Gene for Alicyclobacillus acidoterrestris Quantification and Guaiacol Assessment in Apple Juices Using Real Time PCR. Int. J. Food Microbiol. 2021, 338, 109006. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Wang, W.; Pei, J.; Zhang, J.; Yue, T.; Youravong, W.; Li, Z. Bacteriocin Assisted Food Functional Membrane for Simultaneous Exclusion and Inactivation of Alicyclobacillus acidoterrestris in Apple Juice. J. Membr. Sci. 2021, 2, 118741. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Corbo, M.R.; Sinigaglia, M. Combining Eugenol and Cinnamaldehyde to Control the Growth of Alicyclobacillus acidoterrestris. Food Control 2010, 21, 172–177. [Google Scholar] [CrossRef]
- Huertas, J.P.; Esteban, M.D.; Antolinos, V.; Palop, A. Combined Effect of Natural Antimicrobials and Thermal Treatments on Alicyclobacillus acidoterrestris Spores. Food Control 2014, 35, 73–78. [Google Scholar] [CrossRef]
- Maldonado, M.C.; Aban, M.P.; Navarro, A.R. Chemicals and Lemon Essential Oil Effect on Alicyclobacillus acidoterrestris Viability. Braz. J. Microbiol. 2014, 44, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, A.; Campaniello, D.; Speranza, B.; Sinigaglia, M.; Corbo, M.R. Control of Alicyclobacillus acidoterrestris in Apple Juice by Citrus Extracts and a Mild Heat-Treatment. Food Control 2013, 31, 553–559. [Google Scholar] [CrossRef]
- Molva, C.; Baysal, A.H. Antimicrobial Activity of Grape Seed Extract on Alicyclobacillus acidoterrestris DSM 3922 Vegetative Cells and Spores in Apple Juice. LWT 2015, 60, 238–245. [Google Scholar] [CrossRef]
- Piskernik, S.; Klančnik, A.; Demšar, L.; Smole Možina, S.; Jeršek, B. Control of Alicyclobacillus spp. Vegetative Cells and Spores in Apple Juice with Rosemary Extracts. Food Control 2016, 60, 205–214. [Google Scholar] [CrossRef]
- Fleet, G.H. Yeast Spoilage of Foods and Beverages. The Yeasts 2011, 1, 53–63. [Google Scholar] [CrossRef]
- Silva, F.V. Resistant Moulds as Pasteurization Target for Cold Distributed High Pressure and Heat Assisted High Pressure Processed Fruit Products. J. Food Eng. 2020, 282, 109998. [Google Scholar] [CrossRef]
- Buerman, E.C.; Worobo, R.W.; Padilla-Zakour, O.I. High Pressure Processing of Heat and Pressure Resistant Fungi as Affected by pH, Water Activity, Sulfites, and Dimethyl Dicarbonate in a Diluted Apple Juice Concentrate. Food Control 2021, 120, 107551. [Google Scholar] [CrossRef]
- Rai, P.; Mehrotra, S.; Sharma, S.K. Challenges in Assessing the Quality of Fruit Juices: Intervening Role of Biosensors. Food Chem. 2022, 386, 132825. [Google Scholar] [CrossRef] [PubMed]
- Guerra, I.C.D.; de Oliveira, P.D.L.; de Souza Pontes, A.L.; Lúcio, A.S.S.C.; Tavares, J.F.; Barbosa-Filho, J.M.; Madruga, M.S.; de Souza, E.L. Coatings Comprising Chitosan and Mentha piperita L. or Mentha×villosa Huds Essential Oils to Prevent Common Postharvest Mold Infections and Maintain the Quality of Cherry Tomato Fruit. Int. J. Food Microbiol. 2015, 214, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-González, A.E.; Palou, E.; López-Malo, A. Antifungal Activity of Essential Oils of Clove (Syzygium aromaticum) and/or Mustard (Brassica nigra) in Vapor Phase against Gray Mold (Botrytis cinerea) in Strawberries. Innov. Food Sci. Emerg. Technol. 2015, 32, 181–185. [Google Scholar] [CrossRef]
- Sarkhosh, A.; Schaffer, B.; Vargas, A.I.; Palmateer, A.J.; Lopez, P.; Soleymani, A.; Farzaneh, M. Antifungal Activity of Five Plant-Extracted Essential Oils against Anthracnose in Papaya Fruit. Biol. Agric. Hortic. 2017, 34, 18–26. [Google Scholar] [CrossRef]
- da Silva, B.D.; Bernardes, P.C.; Pinheiro, P.F.; Fantuzzi, E.; Roberto, C.D. Chemical Composition, Extraction Sources and Action Mechanisms of Essential Oils: Natural Preservative and Limitations of Use in Meat Products. Meat Sci. 2021, 176, 108463. [Google Scholar] [CrossRef]
- Ghosh, V.; Mukherjee, A.; Chandrasekaran, N. Eugenol-Loaded Antimicrobial Nanoemulsion Preserves Fruit Juice against Microbial Spoilage. Colloids Surf. B Biointerfaces 2014, 114, 392–397. [Google Scholar] [CrossRef]
- Bento, R.; Pagán, E.; Berdejo, D.; de Carvalho, R.J.; García-Embid, S.; Maggi, F.; Magnani, M.; de Souza, L.E.; García-Gonzalo, D.; Pagán, R. Chitosan Nanoemulsions of Cold-Pressed Orange Essential Oil to Preserve Fruit Juices. Int. J. Food Microbiol. 2020, 331, 108786. [Google Scholar] [CrossRef]
- Charfi, S.; Boujida, N.; Abrini, J.; Senhaji, N.S. Study of Chemical Composition and Antibacterial Activity of Moroccan Thymbra capitata Essential Oil and Its Possible Use in Orange Juice Conservation. Mater. Today Proc. 2019, 13, 706–712. [Google Scholar] [CrossRef]
- Mosqueda-Melgar, J.; Raybaudi-Massilia, R.M.; Martín-Belloso, O. Non-Thermal Pasteurization of Fruit Juices by Combining High-Intensity Pulsed Electric Fields with Natural Antimicrobials. Innov. Food Sci. Emerg. Technol. 2008, 9, 328–340. [Google Scholar] [CrossRef]
- Molet-Rodríguez, A.; Turmo-Ibarz, A.; Salvia-Trujillo, L.; Martín-Belloso, O. Incorporation of Antimicrobial Nanoemulsions into Complex Foods: A Case Study in an Apple Juice-Based Beverage. LWT 2021, 141, 110926. [Google Scholar] [CrossRef]
- Liu, S.; Shao, X.; Wie, Y.; Li, Y.; Xu, F.; Wang, H. Solidago canadensis L. Essential Oil Vapor Effectively Inhibits Botrytis cinerea Growth and Preserves Postharvest Quality of Strawberry as a Food Model System. Front. Microbiol. 2016, 7, 01179. [Google Scholar] [CrossRef]
- da Cruz Almeida, E.T.; de Souza, G.T.; de Sousa Guedes, J.P.; Barbosa, I.M.; de Sousa, C.P.; Castellano, L.R.C.; Magnani, M.; de Souza, E.L. Mentha piperita L. Essential Oil Inactivates Spoilage Yeasts in Fruit Juices through the Perturbation of Different Physiological Functions in Yeast Cells. Food Microbiol. 2019, 82, 20–29. [Google Scholar] [CrossRef]
- Leite, C.J.B.; Sousa, J.P.; Medeiros, J.A.C.; Conceicao, M.L.; Falcao-Silva, V.S.; Souza, E.L. Inactivation of Escherichia coli, Listeria monocytogenes, and Salmonella enteritidis by Cymbopogon citratus D.C. Stapf. Essential oil in pineapple juice. J. Food Prot. 2016, 79, 213–219. [Google Scholar] [CrossRef]
- Charfi, S.; Boujida, N.; Bouyahya, A.; El-Shazly, M.; Khamlichi, A.; Abrini, J.; Senhaji, N.S. Mathematical Modeling of Escherichia coli O157:H7 Growth in Carrot Juice Influenced by Thymbra capitata Essential Oil, Heat Treatment, and Storage Temperature. Int. J. Food Microbiol. 2023, 386, 110044. [Google Scholar] [CrossRef]
- Mandappa, I.M.; Basavaraj, K.; Manonmani, H.K. Analysis of Mycotoxins in Fruit Juices. In Fruit Juices: Extraction, Composition, Quality and Analysis; Rajauria, G., Tiwari, B.K., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 763–777. [Google Scholar] [CrossRef]
- Quintela, S. Mycotoxins in Beverages: Occurrence, Regulation, Economic Impact and Cost-Effectiveness of Preventive and Removal Methods. In Safety Issues in Beverage Production; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 147–186. [Google Scholar] [CrossRef]
- Saleh, I.; Goktepe, I. The Characteristics, Occurrence, and Toxicological Effects of Patulin. Food Chem. Toxicol. 2019, 129, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.Z.; Malik, S.; Asi, M.R.; Selmat, J.; Malik, N. Natural Occurrence of Patulin in Different Fruits, Juices and Smoothies and Evaluation of Dietary Intake in Punjab, Pakistan. Food Control 2018, 84, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Prakash, B.; Singh, P.; Kedia, A.; Dubey, N.K. Assessment of Some Essential Oils as Food Preservatives Based on Antifungal, Antiaflatoxin, Antioxidant Activities and In Vivo Efficacy in Food System. Food Res. Int. 2012, 49, 201–208. [Google Scholar] [CrossRef]
- Prakash, B.; Kedia, A.; Mishra, P.K.; Dubey, N.K. Plant Essential Oils as Food Preservatives to Control Moulds, Mycotoxin Contamination and Oxidative Deterioration of Agri-Food Commodities–Potentials and Challenges. Food Control 2015, 47, 381–391. [Google Scholar] [CrossRef]
- Wan, J.; Zhong, S.; Schwarz, P.; Chen, B.; Rao, J. Enhancement of antifungal and mycotoxin inhibitory activities of food-grade thyme oil nanoemulsions with natural emulsifiers. Food Control 2019, 106, 106709. [Google Scholar] [CrossRef]
- Wan, J.; Zhong, S.; Schwarz, P.; Chen, B.; Rao, J. Physical properties, antifungal and mycotoxin inhibitory activities of five essential oil nanoemulsions: Impact of oil compositions and processing parameters. Food Chem. 2019, 291, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhong, S.; Schwarz, P.; Chen, B.; Rao, J. Antifungal activity, mycotoxin inhibitory efficacy, and mode of action of hop essential oil nanoemulsion against Fusarium graminearum. Food Chem. 2023, 400, 134016. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.; Sun, Y.; Liu, Y.; Li, R.; Chen, Y.; Zhou, T. Cinnamon Oil Inhibits Penicillium expansum Growth by Disturbing the Carbohydrate Metabolic Process. J. Fungi 2021, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Negi, P.S. Use of Natural Preservatives for Shelf Life Extension of Fruit Juices. In Fruit Juices; Rajauria, G., Tiwari, B.K., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 571–605. [Google Scholar] [CrossRef]
- Thada, R.; Chockalingam, S.; Dhandapani, R.K.; Panchamoorthy, R. Extraction and quantitation of coumarin from cinnamon and its effect on enzymatic browning in fresh apple juice: A bioinformatics approach to illuminate its antibrowning activity. J. Agric. Food Chem. 2013, 61, 5385–5390. [Google Scholar] [CrossRef]
- Tinello, F.; Mihaylova, D.; Lante, A. Effect of dipping pretreatment with unripe grape juice on dried “Golden Delicious” apple slices. Food Bioprocess Technol. 2018, 11, 2275–2285. [Google Scholar] [CrossRef]
- Ağçam, E.; Akyıldız, A.; Dündar, B. Thermal Pasteurization and Microbial Inactivation of Fruit Juices. In Fruit Juices; Rajauria, G., Tiwari, B.K., Eds.; Academic Pres: Cambridge, MA, USA, 2018; pp. 309–339. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, W.; Xiong, Z.; Zou, L.; Chen, J.; Liu, J.; Zhong, J. Different modes of inhibition for organic acids on polyphenoloxidase. Food Chem. 2016, 199, 439–446. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, W.; Stockmann, R.; Terefe, N.S. Effect of citric acid and high pressure thermal processing on enzyme activity and related quality attributes of pear puree. Innov. Food Sci. Emerg. Technol. 2018, 45, 196–207. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, L.; Miao, J.; Yu, W.; Zou, L.; Zhou, W.; Liu, C.; Liu, W. Effect of Cinnamon Essential Oil Nanoemulsion Combined with Ascorbic Acid on Enzymatic Browning of Cloudy Apple Juice. Food Bioprocess Technol. 2020, 13, 860–870. [Google Scholar] [CrossRef]
- Erol, N.D.; Erdem, O.A.; Yilmaz, S.T.; Kayalar, H.; Cakli, S. Effects of the BHA and basil essential oil on nutritional, chemical, and sensory characteristics of sunflower oil and sardine (Sardina pilchardus) fillets during repeated deep-frying. LWT 2022, 163, 113557. [Google Scholar] [CrossRef]
- Hussein, M.A.; Gobba, N.A.; El Bishbishy, M.H. Composition, in vitro antioxidant and antitumor properties of essential oil from the seeds of Moringa oleifera. Int. J. Pharma Sci. 2014, 4, 532–540. [Google Scholar]
- Khan, M.R.; Huang, C.; Zhao, H.; Huang, H.; Ren, L.; Faiq, M.; Hashmi, M.S.; Li, B.; Zheng, D.; Xu, Y.; et al. Antioxidant activity of thymol essential oil and inhibition of polyphenol oxidase enzyme: A case study on the enzymatic browning of harvested longan fruit. Chem. Biol. Technol. Agric. 2021, 8, 61. [Google Scholar] [CrossRef]
- Eissa, H.A.; Abd-Elfattah, S.M.; Abu-Seif, F.A. Anti-microbial, anti-browning and anti-mycotoxigenic activities of some essential oil extracts in apple juice. Pol. J. Food Nutr. Sci. 2008, 58, 425–432. [Google Scholar]
- Almas, I.; Innocent, E.; Machumi, F.; Kisinza, W. Chemical composition of essential oils from Eucalyptus globulus and Eucalyptus maculata grown in Tanzania. Sci. Afr. 2021, 12, e00758. [Google Scholar] [CrossRef]
- Sadeh, D.; Nitzan, N.; Chaimovitsh, D.; Shachter, A.; Ghanim, M.; Dudai, N. Interactive effects of genotype, seasonality and extraction method on chemical compositions and yield of essential oil from rosemary (Rosmarinus officinalis L.). Ind. Crops Prod. 2019, 138, 1–7. [Google Scholar] [CrossRef]
- Taban, A.; Saharkhiz, M.J.; Niakousari, M. Sweet bay (Laurus nobilis L.) essential oil and its chemical composition, antioxidant activity and leaf micromorphology under different extraction methods. Sustain. Chem. Pharm. 2018, 9, 12–18. [Google Scholar] [CrossRef]
- Lakušić, D.V.; Ristić, M.S.; Slavkovska, V.N.; Šinžar-Sekulić, J.B.; Lakušić, B.S. Environment-Related Variations of the Composition of the Essential Oils of Rosemary (Rosmarinus officinalis L.) in the Balkan Peninsula. Chem. Biodivers. 2012, 9, 1286–1302. [Google Scholar] [CrossRef]
- Narayanankutty, A.; Sasidharan, A.; Job, J.T.; Rajagopal, R.; Alfarhan, A.; Kim, Y.O.; Kim, H.J. Mango ginger (Curcuma amada Roxb.) rhizome essential oils as source of environmentally friendly biocides: Comparison of the chemical composition, antibacterial, insecticidal and larvicidal properties of essential oils extracted by different methods. Environ. Res. 2021, 202, 111718. [Google Scholar] [CrossRef]
- Mangalagiri, N.P.; Panditi, S.K.; Jeevigunta, N.L.L. Antimicrobial activity of essential plant oils and their major components. Heliyon 2021, 7, e06835. [Google Scholar] [CrossRef]
- Raspo, M.A.; Vignola, M.B.; Andreatta, A.E.; Juliani, H.R. Antioxidant and antimicrobial activities of citrus essential oils from Argentina and the United States. Food Biosci. 2020, 36, 100651. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Plaza-Diaz, J.; Gomez-Llorente, C.; Lucas Gómez, E.; Sabés-Alsina, M.; Gil, Á. In vitro examination of antibacterial and immunomodulatory activities of cinnamon, white thyme, and clove essential oils. J. Funct. Foods 2021, 81, 104436. [Google Scholar] [CrossRef]
- Falleh, H.; Benjemaa, M.; Neves, M.A.; Isoda, H.; Nakajima, M.; Ksouri, R. Formulation, physicochemical characterization, and anti-E. coli activity of food-grade nanoemulsions incorporating clove, cinnamon, and lavender essential oils. Food Chem. 2021, 359, 129963. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Kordala, N.; Bednarski, W. Nanoemulsje roślinnych olejków eterycznych oraz ich zastosowanie w utrwalaniu żywności. Zeszyty Naukowe Państwowej Wyższej Szkoły Zawodowej im. Witelona w Legn. 2017, 23, 21–32. [Google Scholar]
- Donsi, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Grau, M.A.; Soliva-Fortuny, R.; Martin-Belloso, O. Formulation of antimicrobial edible nanoemulsions with pseudo-ternary phase experimental design. Food Bioprocess Technol. 2014, 7, 3022–3032. [Google Scholar] [CrossRef]
- Teo, A.; Goh, K.K.T.; Wen, J.; Oey, I.; Ko, S.; Kwak, H.S.; Lee, S.J. Physicochemical properties of whey protein, lactoferrin and Tween 20 stabilised nanoemulsions: Effect of temperature, pH and salt. Food Chem. 2016, 197, 297–306. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Rao, J. Food-grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef]
- Donsi, F.; Annunziata, M.; Sessa, M.; Ferrari, G. Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT 2011, 44, 1908–1914. [Google Scholar] [CrossRef]
- Benjemaa, M.; Neves, M.A.; Falleh, H.; Isoda, H.; Ksouri, R.; Nakajima, M. Nanoencapsulation of Thymus capitatus essential oil: Formulation process, physical stability characterization and antibacterial efficiency monitoring. Ind. Crops Prod. 2018, 113, 414–421. [Google Scholar] [CrossRef]
- Chuesiang, P.; Siripatrawan, U.; Sanguandeekul, R.; Yang, J.S.; McClements, D.J.; McLandsborough, L. Antimicrobial activity and chemical stability of cinnamon oil in oil-in-water nanoemulsions fabricated using the phase inversion temperature method. LWT 2019, 110, 190–196. [Google Scholar] [CrossRef]
- Liew, S.N.; Utra, U.; Alias, A.K.; Tan, T.B.; Tan, C.P.; Yussof, N.S. Physical, morphological and antibacterial properties of lime essential oil nanoemulsions prepared via spontaneous emulsification method. LWT 2020, 128, 109388. [Google Scholar] [CrossRef]
- Pandey, A.K.; Chávez-González, M.L.; Silva, A.S.; Singh, P. Essential oils from the genus Thymus as antimicrobial food preservatives: Progress in their use as nanoemulsions-a new paradigm. Trends J. Food Sci. Technol. 2021, 111, 426–441. [Google Scholar] [CrossRef]
- Patrignani, F.; Siroli, L.; Braschi, G.; Lanciotti, R. Combined use of natural antimicrobial based nanoemulsions and ultra high pressure homogenization to increase safety and shelf-life of apple juice. Food Control. 2020, 111, 107051. [Google Scholar] [CrossRef]
- Jaworska, M.; Sikora, E.; Ogonowski, J. Factors influencing the percutaneous penetration of active ingerdients. Wiadomości Chem. 2011, 63, 3–4. [Google Scholar]
- Ashurst, P. The Stability and Shelf Life of Fruit Juices and Soft Drinks. In The Stability and Shelf Life of Food; Woodhead Publishing: Cambridge, UK, 2016; Volume 2, pp. 347–374. [Google Scholar] [CrossRef]
- Buvé, C.; Van Bedts, T.; Haenen, A.; Kebede, B.; Braekers, R.; Hendrickx, M.; Grauwet, T. Shelf-life dating of shelf-stable strawberry juice based on survival analysis of consumer acceptance information. J. Sci. Food Agric. 2018, 98, 3437–3445. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ranjan, S. An Introduction to Food Grade Nanoemulsions; Springer: Singapore, 2018; Volume 13, pp. 2–14. [Google Scholar]
- Anwer, M.K.; Jamil, S.; Ibnouf, E.O.; Shakeel, F. Enhanced antibacterial effects of clove essential oil by nanoemulsion. J. Oleo Sci. 2014, 63, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, R.; Ghaderi, L.; Rafati, H.; Aliahmadi, A.; McClements, D.J. Superior antibacterial activity of nanoemulsion of Thymus daenensis essential oil against E. coli. Food Chem. 2016, 194, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fisher, K.D.; Friest, M.A.; Gerard, G. Characterization and antifungal activity of lemongrass essential oil-loaded nanoemulsion stabilized by carboxylated cellulose nanofibrils and surfactant. Polymers 2023, 15, 3946. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.D.; Rosario, D.K.A.D.; Conte-Junior, C.A. Can droplet size influence antibacterial activity in ultrasound-prepared essential oil nanoemulsions? Crit. Rev. Food Sci. Nutr. 2023, 63, 12567–12577. [Google Scholar] [CrossRef]
- Özogul, Y.; El Abed, N.; Özogul, F. Antimicrobial effect of laurel essential oil nanoemulsion on food-borne pathogens and fish spoilage bacteria. Food Chem. 2022, 368, 130831. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Mereddy, R.; Li, L.; Sultanbawa, Y. Formulation, characterisation and antibacterial activity of lemon myrtle and anise myrtle essential oil in water nanoemulsion. Food Chem. 2018, 254, 173. [Google Scholar] [CrossRef] [PubMed]
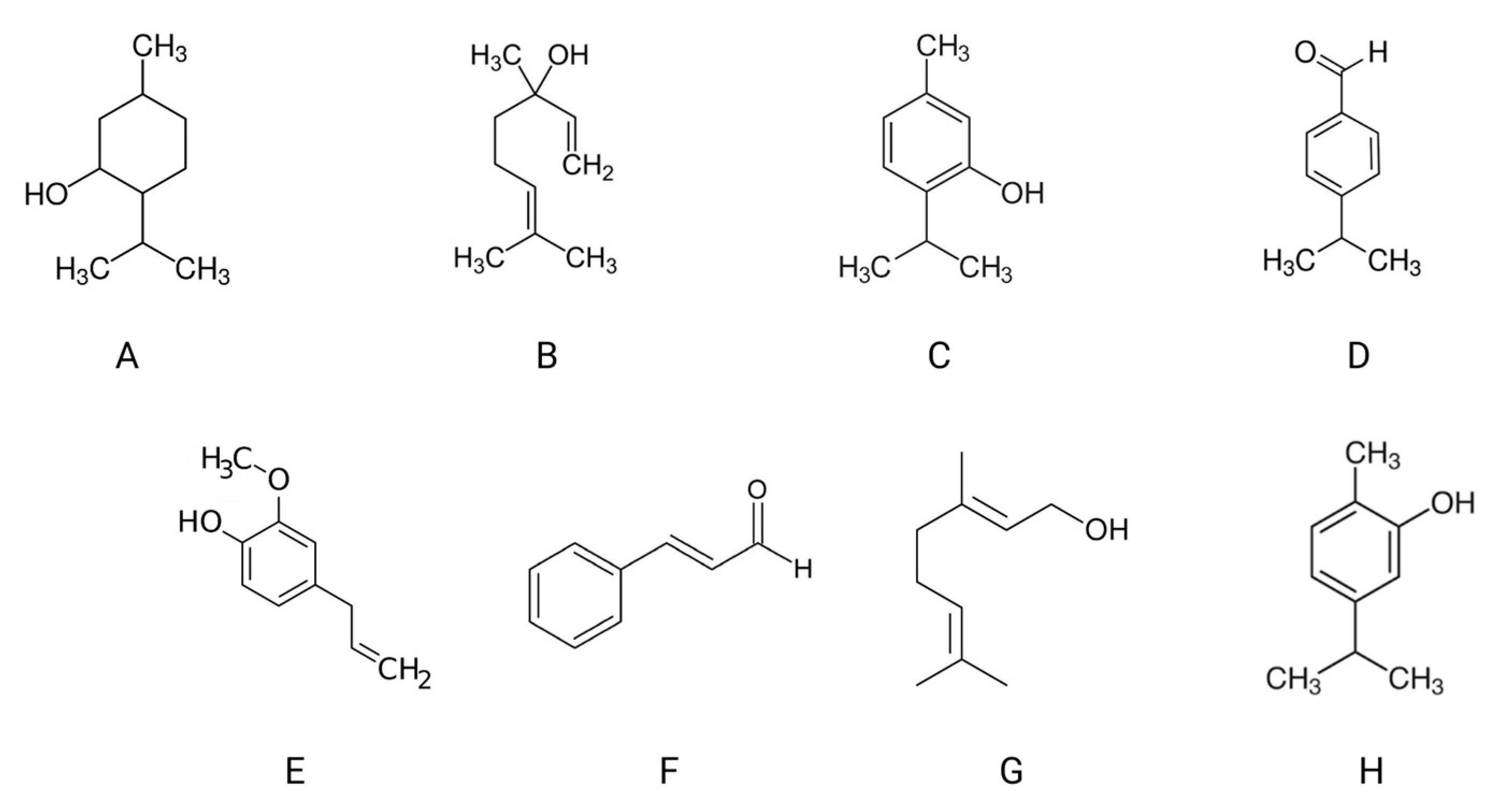
| Juice | Bacteria | Mold | Yeast | References |
|---|---|---|---|---|
| Apple | Alicyclobacillus acidoterrestris, Escherichia coli O157: H7, Bacillus spp., Streptomyces spp., Staphylococcus spp. | Penicillium expansum, Penicillium griseofulvum, Talaromyces spp., Byssochlamys spp. | Blastomyces sp., Rhodotorula rubra, Saccharomyces cerevisiae, Candida stellata, Candida glabrata, Pichia anomala | [27,28,29,30] |
| Orange | Alicyclobacillus acidoterrestris, Bacillus cereus, Bacillus megaterium, Pseudomonas aeruginosa, Escherichia coli | Aspergillus sp., Trichoderma sp., Penicillium islandicum, Geotrichum spp. | Saccharomyces cerevisiae, Rhodotorula mucilaginosa, Candida mesenteric, Blastomyces sp., Candida stellata, Candida glabrata, Pichia anomala | [29,30,31] |
| Pineapple | Alicyclobacillus acidoterrestris, Escherichia coli, Listeria monocytogenes, Salmonella enteritidis | Penicillium expansum, Penicillium griseofulvum, Penicillium citrinum, Talaromyces amestolkiae, Byssochlamys spp. | Saccharomyces cerevisiae, Rhodotorula mucilaginosa, Candida stellata, Candida glabrata, Pichia anomala | [27,29,30,32] |
| Tomato | Bacillus spp., Enterobacter spp., Klebsiella spp., Proteus mirabilis, Escherichia coli, Alicyclobacillus acidoterrestris | Aspergillus niger, Penicillium expansum, Penicillium griseofulvum | Saccharomyces cerevisiae, Pichia spp., Candida spp., Hanseniaspora valbyensis | [27,29,33,34,35] |
| Microorganism | Essential Oil | Inhibition Zones [mm] | MIC [%] | Essential Oil Nanoemulsion | Inhibition Zones [mm] | MIC [%] | Reference |
|---|---|---|---|---|---|---|---|
| Bacillus subtilis | cinnamon (Cinnamomum verum J.Presl) | 12.0 ± 0.6 | 0.130 | cinnamon (Cinnamomum verum J.Presl) | 18.0 ± 1.3 | 0.080 | [117] |
| Proteus vulgaris | 13.0 ± 0.8 | 0.130 | 20.0 ± 1.5 | 0.085 | |||
| Klebsiella pneumoniae | 11.0 ± 0.7 | 0.400 | 18.0 ± 1.4 | 0.250 | |||
| laurel (Laurus nobilis L.) | 17.25 ± 0.65 | 12.50 | laurel (Laurus nobilis L.) | 7.75 ± 0.65 | 25.00 | [121] | |
| Staphylococcus aureus | cinnamon (Cinnamomum verum J.Presl) | 10.0 ± 0.4 | 0.130 | cinnamon (Cinnamomum verum J.Presl) | 17.0 ± 1.2 | 0.075 | [117] |
| lemon myrtle (Backhousia citriodora F. Muell) | - | 0.156 | lemon myrtle (Backhousia citriodora F. Muell) | - | 0.062 | [122] | |
| laurel (Laurus nobilis L.) | 10.75 ± 0.25 | 12.50 | laurel (Laurus nobilis L.) | 15.00 ± 0.91 | >25.00 | [121] | |
| Pseudomonas aeruginosa | cinnamon (Cinnamomum verum J.Presl) | 9.0 ± 0.3 | 0.500 | cinnamon (Cinnamomum verum J.Presl) | 16.0 ± 1.0 | 0.300 | [117] |
| lemon myrtle (Backhousia citriodora F. Muell) | - | 0.156 | lemon myrtle (Backhousia citriodora F. Muell) | - | 0.031 | [122] | |
| Escherichia coli | lemon myrtle (Backhousia citriodora F. Muell) | - | 0.625 | lemon myrtle (Backhousia citriodora F. Muell) | - | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napiórkowska, A.; Khaneghah, A.M.; Kurek, M.A. Essential Oil Nanoemulsions—A New Strategy to Extend the Shelf Life of Smoothies. Foods 2024, 13, 1854. https://doi.org/10.3390/foods13121854
Napiórkowska A, Khaneghah AM, Kurek MA. Essential Oil Nanoemulsions—A New Strategy to Extend the Shelf Life of Smoothies. Foods. 2024; 13(12):1854. https://doi.org/10.3390/foods13121854
Chicago/Turabian StyleNapiórkowska, Alicja, Amin Mousavi Khaneghah, and Marcin Andrzej Kurek. 2024. "Essential Oil Nanoemulsions—A New Strategy to Extend the Shelf Life of Smoothies" Foods 13, no. 12: 1854. https://doi.org/10.3390/foods13121854








