Impact of Deformability and Rigidity of Starch Granules on Linear and Non-Linear Rheological Behavior of Waxy Rice Starch Gels and Applicability for Food End Uses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Particle Size Distribution
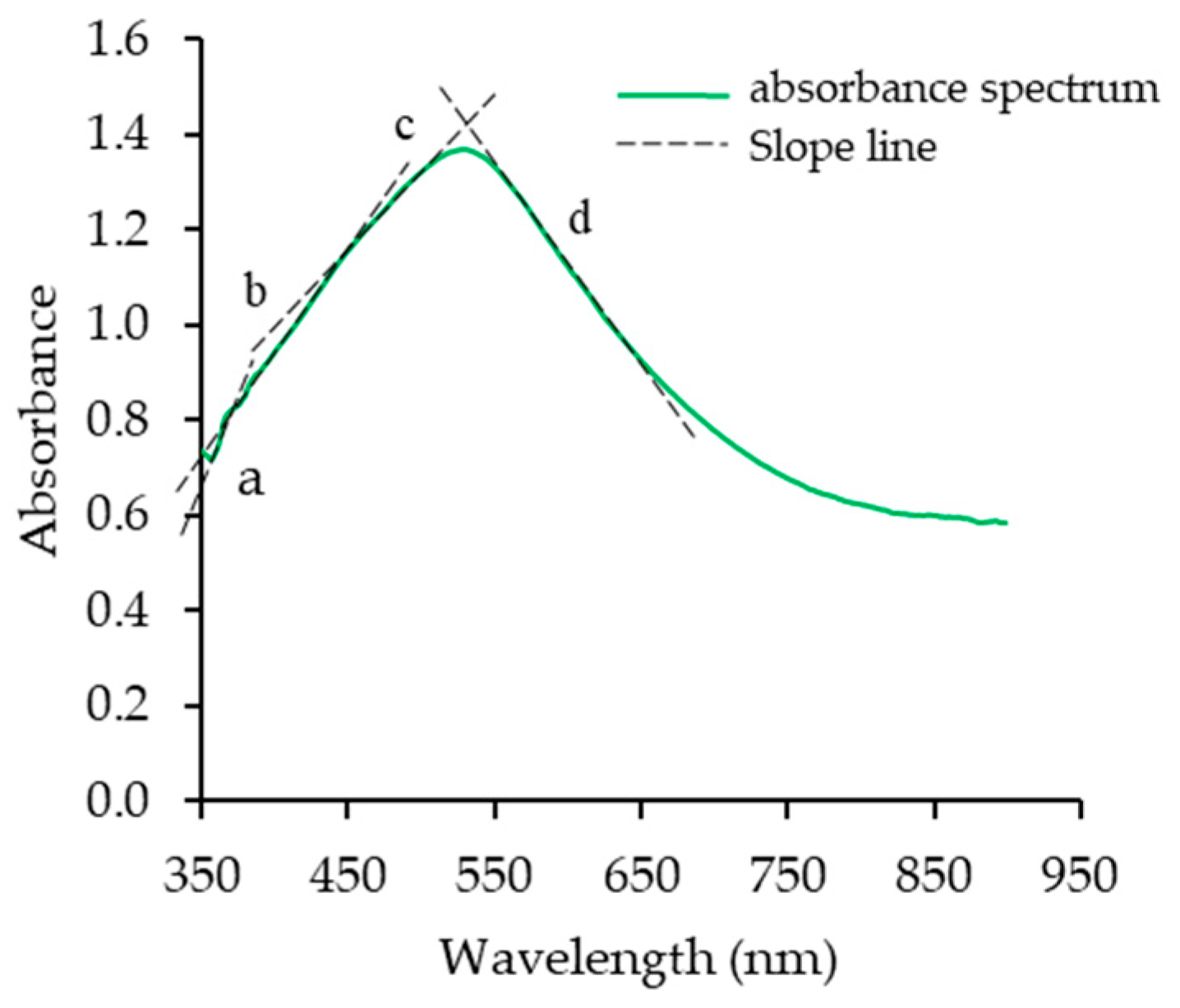
2.3. Chemical Compositions, Amylose Content, and Iodine Absorptivity Spectra
2.4. Analysis of Fine Structures
2.5. Physico-Chemical Properties of Starches
2.6. Linear and Non-Linear Rheological Properties
2.6.1. Preparation of Starch Suspensions
2.6.2. Small Amplitude Oscillatory Shear (SAOS)
2.6.3. Large Amplitude Oscillatory Shear (LAOS)
2.7. Pasting Properties
2.8. Microstructure of WRS, WRS Pastes, and WRS Gels
2.9. Statistical Analysis
3. Results and Discussion
3.1. Particle Size Distribution and Morphology of Native Waxy Rice Starches
3.2. Chemical Compositions and Molecular Characteristics
3.3. Swelling Characteristics and Thermal Properties of WRS
3.4. Rheological Properties of WRS and Granule Behavior
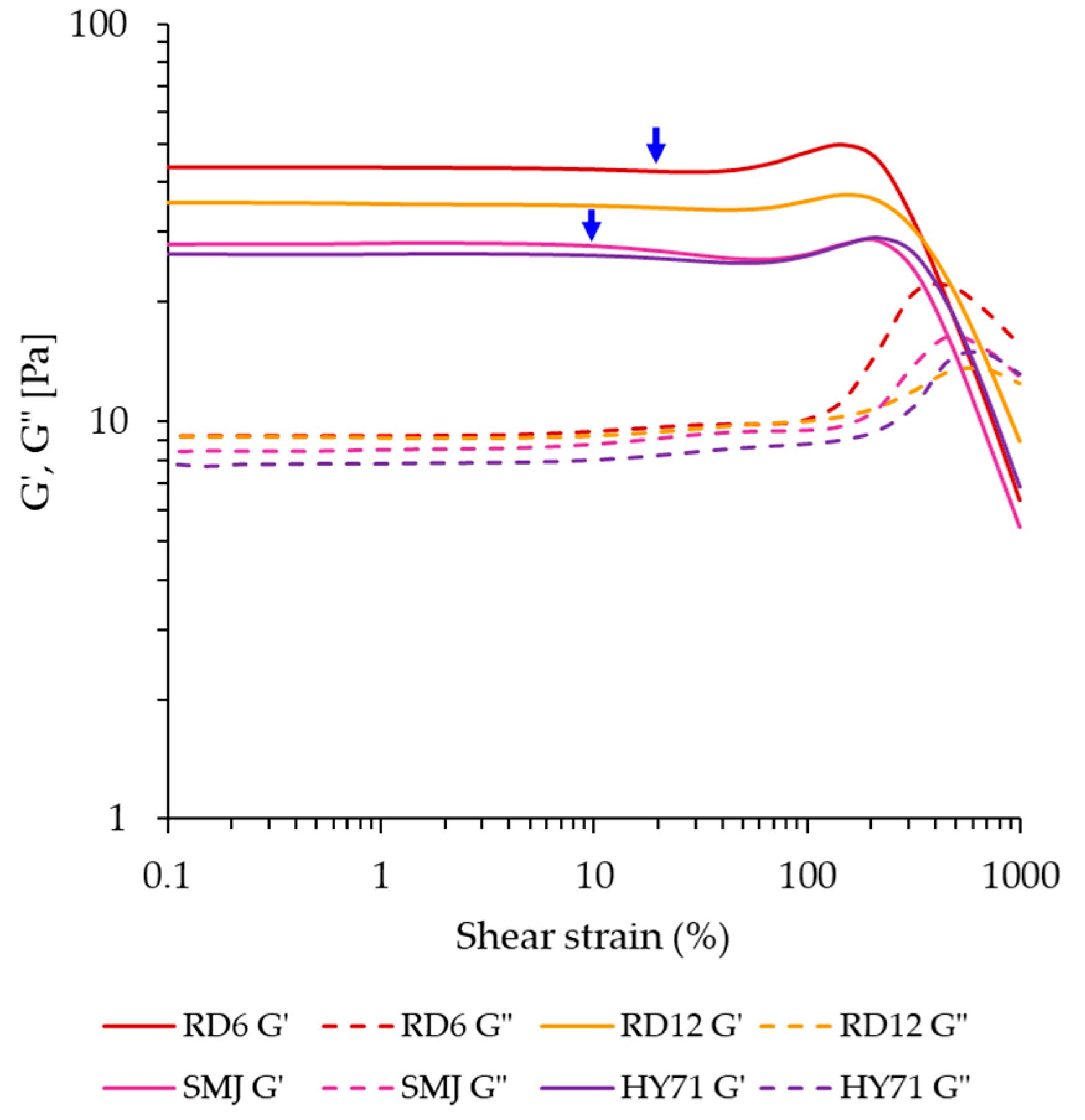
3.4.1. Small Amplitude Oscillatory Shear (SAOS)
3.4.2. Large Amplitude Oscillatory Shear (LAOS)
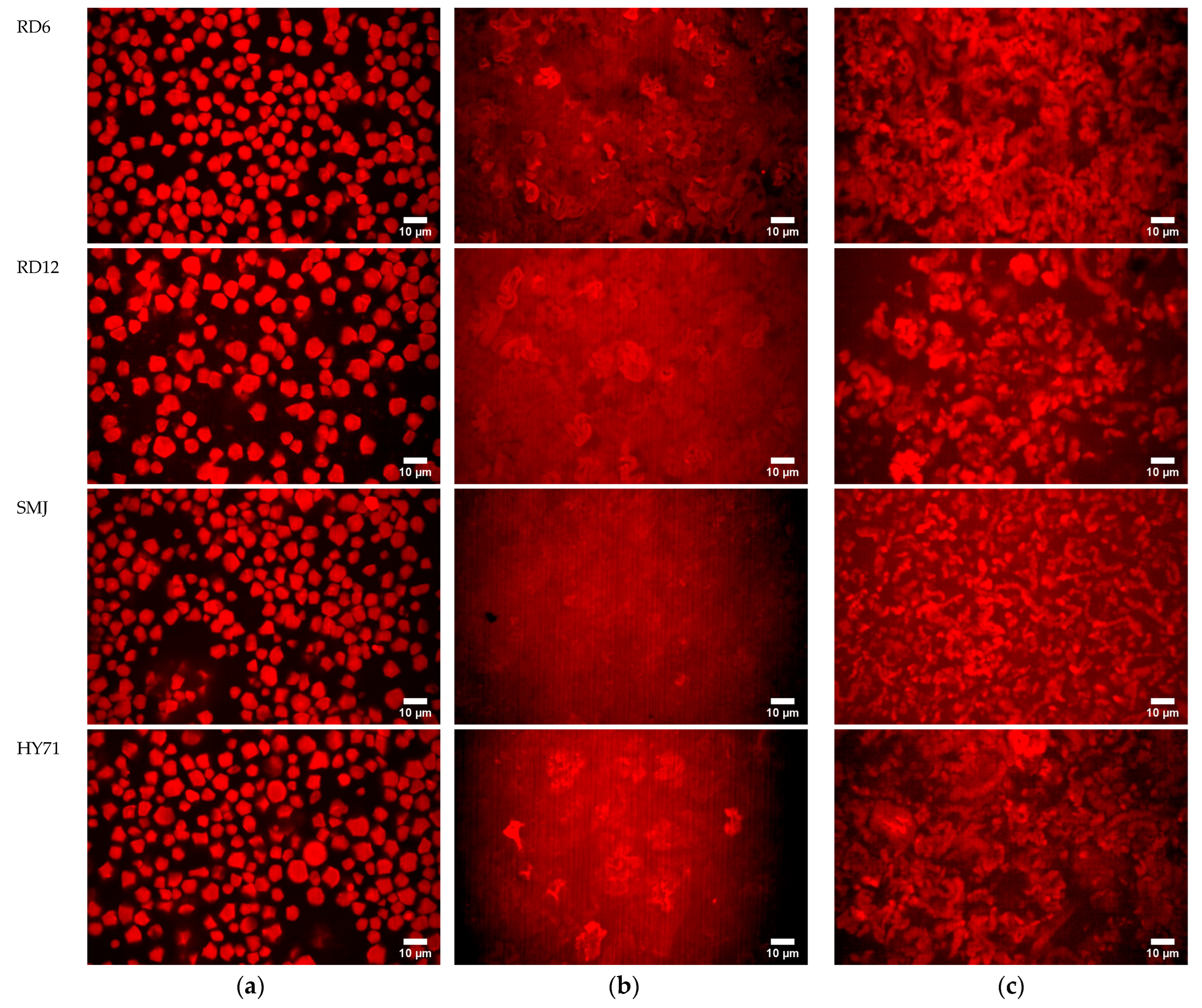
3.4.3. Microstructure of Granules and Pastes/Gels at Low Shear
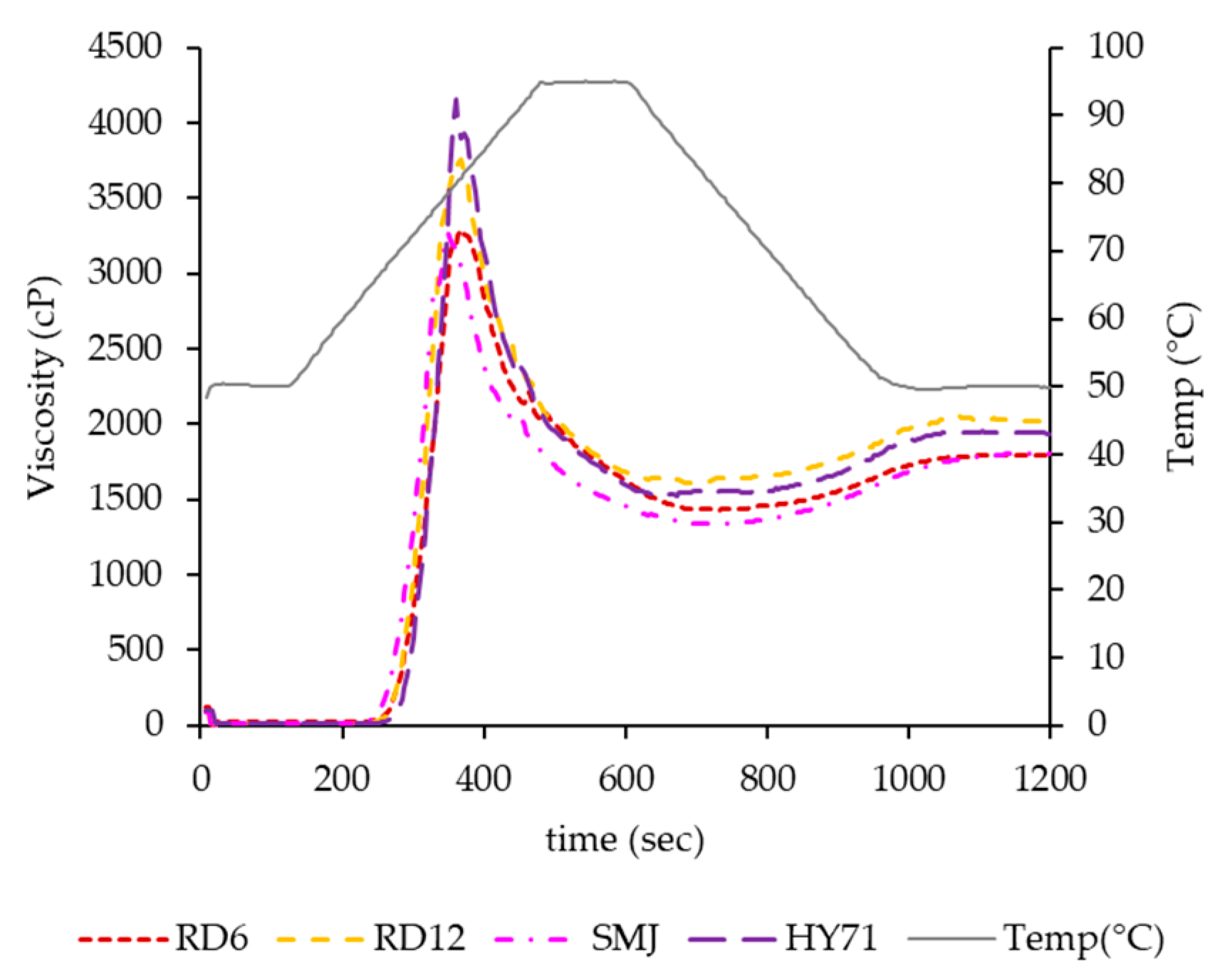
3.5. Pasting Properties
3.6. Findings of the Study and Evaluating the Applicability of Waxy Rice Starches for Food Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Šárka, E.; Dvořáček, V. New processing and applications of waxy starch (a review). J. Food Eng. 2017, 206, 77–87. [Google Scholar] [CrossRef]
- Amelia, I.; BeMiller, J.N. Preparation of Nonfragmented, Completely Amorphous, Pregelatinized Maize Starches and Determination of the Effects of Fragmentation on the Adhesiveness of Their Pastes. Starch-Stärke 2009, 61, 696–701. [Google Scholar] [CrossRef]
- Hsieh, C.-F.; Liu, W.; Whaley, J.K.; Shi, Y.-C. Structure and functional properties of waxy starches. Food Hydrocoll. 2019, 94, 238–254. [Google Scholar] [CrossRef]
- Bao, J.; Sun, M.; Zhu, L.; Corke, H. Analysis of quantitative trait loci for some starch properties of rice (Oryza sativa L.): Thermal properties, gel texture and swelling volume. J. Cereal Sci. 2004, 39, 379–385. [Google Scholar] [CrossRef]
- Kowittaya, C.; Lumdubwong, N. Molecular weight, chain profile of rice amylopectin and starch pasting properties. Carbohydr. Polym. 2014, 108, 216–223. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, Y.; Gu, Z.; Peng, Y.; Cheng, L.; Li, Z.; Li, C.; Chen, Z.; Hong, Y. Effects of different waxy rice varieties and their starch on the taste quality of zongzi. J. Cereal Sci. 2022, 108, 103571. [Google Scholar] [CrossRef]
- Singh, H.; Lin, J.-H.; Huang, W.-H.; Chang, Y.-H. Influence of amylopectin structure on rheological and retrogradation properties of waxy rice starches. J. Cereal Sci. 2012, 56, 367–373. [Google Scholar] [CrossRef]
- Chung, J.H.; Han, J.A.; Yoo, B.; Seib, P.A.; Lim, S.T. Effects of molecular size and chain profile of waxy cereal amylopectins on paste rheology during retrogradation. Carbohydr. Polym. 2008, 71, 365–371. [Google Scholar] [CrossRef]
- Precha-Atsawanan, S.; Uttapap, D.; Sagis, L.M.C. Linear and nonlinear rheological behavior of native and debranched waxy rice starch gels. Food Hydrocoll. 2018, 85, 1–9. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Ai, Y. Gelation mechanisms of granular and non-granular starches with variations in molecular structures. Food Hydrocoll. 2022, 129, 107658. [Google Scholar] [CrossRef]
- Eliasson, A.C. Viscoelastic behaviour during the gelatinization of starch I. Comparison of wheat, maize, potato and waxy-barley starches. J. Texture Stud. 1986, 17, 253–265. [Google Scholar] [CrossRef]
- Eliasson, A.C. Starch: Physicochemical and functional aspects. In Carbohydrates in Food, 3rd ed.; Eliasson, A.C., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 479–578. [Google Scholar]
- Tester, R.; Morrison, W.R. Swelling and gelatinization of cereal starches. II, Waxy rice starches. Cereal Chem. 1990, 67, 558–563. [Google Scholar]
- Rodríguez-Hernández, A.I.; Durand, S.; Garnier, C.; Tecante, A.; Doublier, J.L. Rheology-structure properties of waxy maize starch–gellan mixtures. Food Hydrocoll. 2006, 20, 1223–1230. [Google Scholar] [CrossRef]
- Steeneken, P. Rheological properties of aqueous suspensions of swollen starch granules. Carbohydr. Polym. 1989, 11, 23–42. [Google Scholar] [CrossRef]
- Hermansson, A.M.; Svegmark, K. Developments in the understanding of starch functionality. Trends Food Sci. Technol. 1996, 7, 345–353. [Google Scholar] [CrossRef]
- Boonkor, P.; Sagis, L.M.C.; Lumdubwong, N. Pasting and Rheological Properties of Starch Paste/Gels in a Sugar-Acid System. Foods 2022, 11, 4060. [Google Scholar] [CrossRef] [PubMed]
- Thitisaksakul, M.; Sangwongchai, W.; Mungmonsin, U.; Promrit, P.; Krusong, K.; Wanichthanarak, K.; Tananuwong, K. Granule morphological and structural variability of Thai certified glutinous rice starches in relation to thermal, pasting, and digestible properties. Cereal Chem. 2021, 98, 492–506. [Google Scholar] [CrossRef]
- Saleeto, S.; Jumpaget, R.; Gasemrat, C.; Ruangsook, S.; Wongkasem, O.; Youngsuk, L.; Youngsuk, P.; Hormsombut, W.; Chaowna, A.; Siangsai, S.; et al. RD12 (Nong Khai 80) Rice Variety. Thai Rice Res. J. 2009, 3, 5–20. [Google Scholar]
- Lumdubwong, N.; Seib, P.A. Rice Starch Isolation by Alkaline Protease Digestion of Wet-milled Rice Flour. J. Cereal Sci. 2000, 31, 63–74. [Google Scholar] [CrossRef]
- AACC. Approved Methods of the American Association of Cereal Chemists, 10th ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 2000. [Google Scholar]
- AOAC. Official Methods of Analysis, 15th ed.; The Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Chrastil, J. Improved colorimetric determination of amylose in starches or flours. Carbohydr. Res. 1987, 159, 154–158. [Google Scholar] [CrossRef]
- Knutson, C.A. Evaluation of variations in amylose–iodine absorbance spectra. Carbohydr. Polym. 2000, 42, 65–72. [Google Scholar] [CrossRef]
- Takeda, Y.; Hizukuri, S.; Juliano, B.O. Structures of rice amylopectins with low and high affinities for iodine. Carbohydr. Res. 1987, 168, 79–88. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Zhu, Q.; Bertoft, E. Enzymic analysis of the structure of oxidized potato starches. Int. J. Biol. Macromol. 1997, 21, 131–135. [Google Scholar] [CrossRef]
- Nelson, N. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 1944, 153, 375–380. [Google Scholar] [CrossRef]
- Zhu, F.; Bertoft, E.; Källman, A.; Myers, A.M.; Seetharaman, K. Molecular structure of starches from maize mutants deficient in starch synthase III. J. Agric. Food. Chem. 2013, 61, 9899–9907. [Google Scholar] [CrossRef]
- Jane, J.; Chen, J.F. Effect of amylose molecular size and amylopectin branch chain length on paste properties of starch. Cereal Chem. 1992, 69, 60–65. [Google Scholar]
- Hizukuri, S.; Takeda, Y.; Yasuda, M.; Suzuki, A. Multi-branched nature of amylose and the action of debranching enzymes. Carbohydr. Res. 1981, 94, 205–213. [Google Scholar] [CrossRef]
- Crosbie, G.B. The relationship between starch swelling properties, paste viscosity and boiled noodle quality in wheat flours. J. Cereal Sci. 1991, 13, 145–150. [Google Scholar] [CrossRef]
- Vandeputte, G.E.; Derycke, V.; Geeroms, J.; Delcour, J.A. Rice starches. II. Structural aspects provide insight into swelling and pasting properties. J. Cereal Sci. 2003, 38, 53–59. [Google Scholar] [CrossRef]
- Lii, C.Y.; Tsai, M.L.; Tseng, K.H. Effect of amylose content on the rheological property of rice starch. Cereal Chem. 1996, 73, 415–420. [Google Scholar]
- Ewoldt, R.H.; Hosoi, A.E.; McKinley, G.H. New measures for characterizing nonlinear viscoelasticity in large amplitude oscillatory shear. J. Rheol. 2008, 52, 1427–1458. [Google Scholar] [CrossRef]
- Fatokun, O.T. Micrometrics and Morphological Properties of Starch. In Chemical Properties of Starch; Martins, E., Ed.; IntechOpen: Rijeka, Croatia, 2019; p. 143. [Google Scholar]
- Chang, H.; Zhang, J.; Xia, J.; Kang, C.; Yan, Y. Influence of waxy proteins on wheat resistant starch formation, molecular structure and physicochemical properties. Food Chem. 2022, 376, 131944. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, G.E.; Vermeylen, R.; Geeroms, J.; Delcour, J.A. Rice starches. I. Structural aspects provide insight into crystallinity characteristics and gelatinisation behaviour of granular starch. J. Cereal Sci. 2003, 38, 43–52. [Google Scholar] [CrossRef]
- Jia, R.; Cui, C.; Gao, L.; Qin, Y.; Ji, N.; Dai, L.; Wang, Y.; Xiong, L.; Shi, R.; Sun, Q. A review of starch swelling behavior: Its mechanism, determination methods, influencing factors, and influence on food quality. Carbohydr. Polym. 2023, 321, 121260. [Google Scholar] [CrossRef] [PubMed]
- Gomand, S.V.; Lamberts, L.; Visser, R.G.F.; Delcour, J.A. Physicochemical properties of potato and cassava starches and their mutants in relation to their structural properties. Food Hydrocoll. 2010, 24, 424–433. [Google Scholar] [CrossRef]
- Bagley, E.B.; Christianson, D.D. Swelling capacity of starch and its relationship to suspension viscosity-effect of cooking time, temperature and concentration. J. Texture Stud. 1982, 13, 115–126. [Google Scholar] [CrossRef]
- Wong, S.-S.; Wicklund, R.; Bridges, J.; Whaley, J.; Koh, Y.B. Starch swelling behavior and texture development in stirred yogurt. Food Hydrocoll. 2020, 98, 105274. [Google Scholar] [CrossRef]
- Li, J.-Y.; Yeh, A.-I. Relationships between thermal, rheological characteristics and swelling power for various starches. J. Food Eng. 2001, 50, 141–148. [Google Scholar] [CrossRef]
- Keetels, C.J.A.M.; van Vliet, T.; Walstra, P. Gelation and retrogradation of concentrated starch systems: 1 Gelation. Food Hydrocoll. 1996, 10, 343–353. [Google Scholar] [CrossRef]
- Singh Sodhi, N.; Singh, N. Morphological, thermal and rheological properties of starches separated from rice cultivars grown in India. Food Chem. 2003, 80, 99–108. [Google Scholar] [CrossRef]
- Keetels, C.J.A.M.; Vliet, T.v.; Walstra, P. Gelation and retrogradation of concentrated starch systems: 2. Retrogradation. Food Hydrocoll. 1996, 10, 355–362. [Google Scholar] [CrossRef]
- Winter, H.H. Glass Transition as the Rheological Inverse of Gelation. Macromolecules 2013, 46, 2425–2432. [Google Scholar] [CrossRef]
- Hyun, K.; Kim, S.H.; Ahn, K.H.; Lee, S.J. Large amplitude oscillatory shear as a way to classify the complex fluids. J. Non-Newtonian Fluid Mech. 2002, 107, 51–65. [Google Scholar] [CrossRef]
- Hyun, K.; Wilhelm, M.; Klein, C.O.; Cho, K.S.; Nam, J.G.; Ahn, K.H.; Lee, S.J.; Ewoldt, R.H.; McKinley, G.H. A review of nonlinear oscillatory shear tests: Analysis and application of large amplitude oscillatory shear (LAOS). Prog. Polym. Sci. 2011, 36, 1697–1753. [Google Scholar] [CrossRef]
- Schirmer, M.; Höchstötter, A.; Jekle, M.; Arendt, E.; Becker, T. Physicochemical and morphological characterization of different starches with variable amylose/amylopectin ratio. Food Hydrocoll. 2013, 32, 52–63. [Google Scholar] [CrossRef]
- Singh, S.; Singh, N.; Isono, N.; Noda, T. Relationship of granule size distribution and amylopectin structure with pasting, thermal, and retrogradation properties in wheat starch. J. Agric. Food. Chem. 2010, 58, 1180–1188. [Google Scholar] [CrossRef]
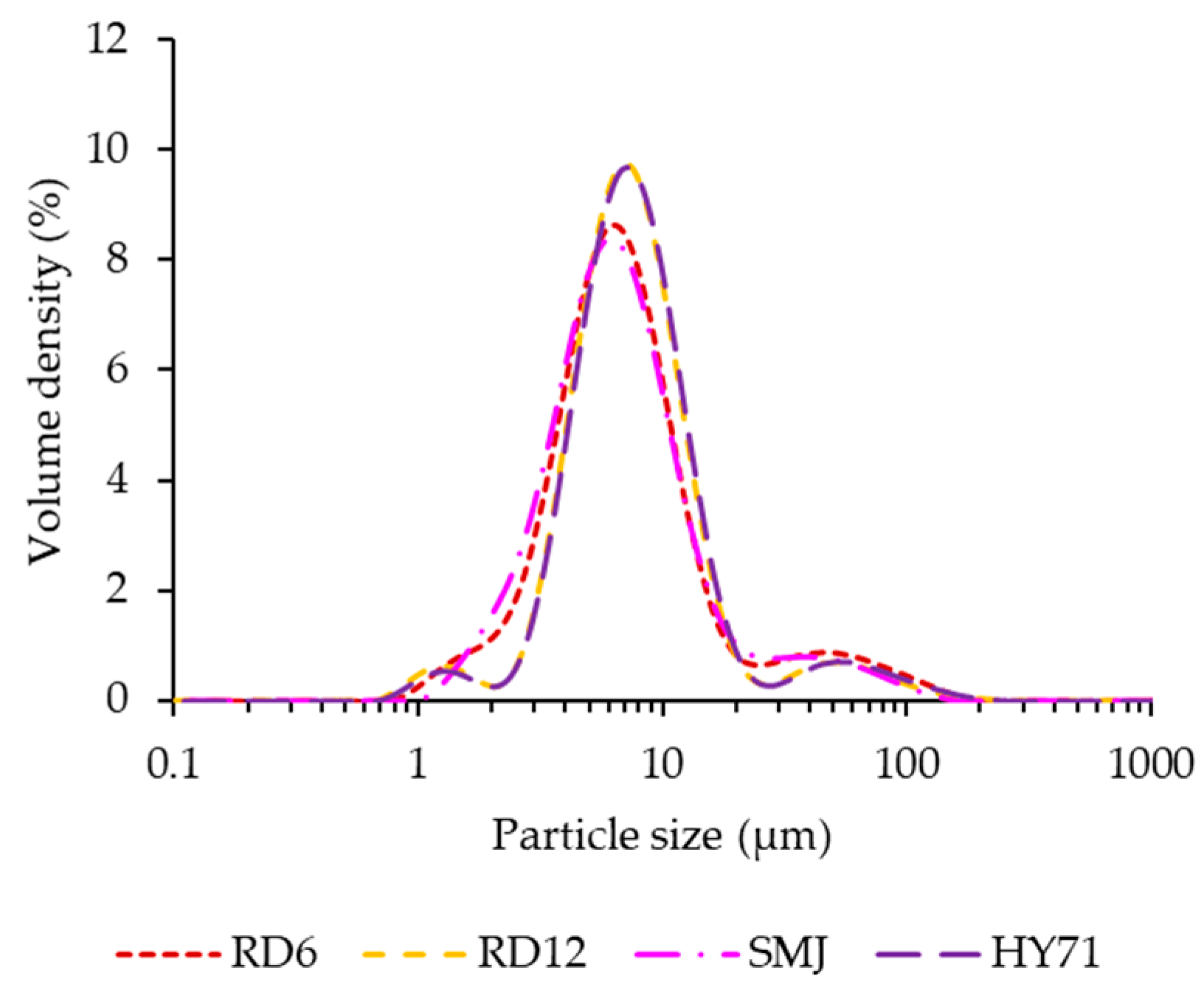



| Cultivar | Granule Size (μm) | D[3,2] | PDI | ||
|---|---|---|---|---|---|
| D10 | D50 | D90 | |||
| RD6 | 3.20 ± 0.01 c | 7.07 ± 0.04 b | 23.00 ± 1.41 a | 5.82 ± 0.04 c | 2.80 ± 0.19 a |
| RD12 | 3.96 ± 0.02 b | 7.76 ± 0.16 a | 17.10 ± 1.70 b | 6.34 ± 0.09 b | 1.69 ± 0.18 b |
| SMJ | 3.07 ± 0.01 d | 6.84 ± 0.16 b | 19.35 ± 1.77 ab | 5.76 ± 0.08 c | 2.38 ± 0.20 a |
| HY71 | 4.07 ± 0.01 a | 7.95 ± 0.08 a | 17.75 ± 1.20 b | 6.59 ± 0.05 a | 1.72 ± 0.13 b |
| Cultivar | Moisture Content (%) | Protein (%) | Fat (%) | Ash (%) | AM (%) |
|---|---|---|---|---|---|
| RD6 | 12.20 ± 0.01 c | 0.08 ± 0.00 a | 0.02 ± 0.01 a | 0.01 ± 0.00 c | 1.47 ± 0.51 a |
| RD12 | 13.51 ± 0.11 a | 0.08 ± 0.00 a | 0.04 ± 0.02 a | 0.03 ± 0.00 a | 0.71 ± 0.05 a |
| SMJ | 12.28 ± 0.04 c | 0.08 ± 0.00 a | 0.01 ± 0.00 a | 0.02 ± 0.00 b | 1.03 ± 0.05 a |
| HY71 | 12.60 ± 0.06 b | 0.08 ± 0.00 a | 0.03 ± 0.01 a | 0.02 ± 0.00 b | 1.26 ± 0.92 a |
| Cultivar | Absorbance Profiles | [η] | Branch Chain Profiles | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| λ-max (nm) | Absorptivity | Ratio Slope c/d | %β | (AGU) | (AGU) | (AGU) | ||||
| RD6 | 528.5 ± 0.7 b | 1.37 ± 0.12 a | 0.745 ± 0.010 b | 102.73 ± 2.99 b | 2209 ± 189 b | 54.16 ± 0.29 b | 136 ± 2 b | 20.19 ± 0.92 a | 12.93 ± 0.06 d | 6.25 ± 0.06 a |
| RD12 | 530.0 ± 0.0 a | 1.13 ± 0.04 a | 0.744 ± 0.025 b | 112.33 ± 2.04 a | 2758 ± 212 ab | 58.03 ± 0.64 a | 190 ± 19 a | 19.46 ± 1.79 a | 13.29 ± 0.86 c | 5.17 ± 0.62 c |
| SMJ | 528.0 ± 0.0 b | 1.24 ± 0.28 a | 0.696 ± 0.009 c | 105.64 ± 0.83 ab | 2759 ± 279 ab | 58.35 ± 0.61 a | 154 ± 10 ab | 20.44 ± 0.97 a | 13.93 ± 0.47 b | 5.51 ± 0.35 b |
| HY71 | 530.0 ± 0.0 a | 1.35 ± 0.73 a | 0.802 ± 0.008 a | 107.62 ± 3.30 ab | 3362 ± 364 a | 59.01 ± 0.08 a | 173 ± 22 ab | 20.98 ± 1.53 a | 14.38 ± 0.74 a | 5.60 ± 0.51 b |
| Cultivar | Swelling Properties | Gelatinization Properties | ||||||
|---|---|---|---|---|---|---|---|---|
| SP | WSI (%) | ϕ10% | To (°C) | Tp (°C) | Tc (°C) | Tc–To (°C) | ΔH (J/g) | |
| RD6 | 54.76 ± 1.70 bc | 21.44 ± 1.60 b | 4.30 ± 0.11 ab | 65.97 ± 0.33 b | 74.58 ± 0.09 ab | 86.21 ± 1.51 a | 20.24 ± 1.84 a | 6.28 ± 2.05 a |
| RD12 | 52.92 ± 1.26 c | 23.72 ± 3.60 ab | 4.03 ± 0.09 c | 66.68 ± 0.33 ab | 74.85 ± 0.43 a | 86.39 ± 0.12 a | 19.71 ± 0.21 a | 6.91 ± 0.92 a |
| SMJ | 57.12 ± 1.14 b | 29.10 ± 0.01 a | 4.05 ± 0.08 bc | 64.84 ± 0.29 b | 73.73 ± 0.33 b | 84.61 ± 0.45 a | 19.77 ± 0.16 a | 7.16 ± 0.13 a |
| HY71 | 60.71 ± 0.26 a | 28.02 ± 1.70 a | 4.37 ± 0.08 a | 67.15 ± 0.23 a | 75.49 ± 0.46 a | 86.74 ± 0.68 a | 19.60 ± 0.45 b | 6.83 ± 0.51 a |
| Parameters | RD6 | RD12 | SMJ | HY71 |
|---|---|---|---|---|
| Temperature sweep test | ||||
| G′max (Pa) | 93.14 ± 17.52 b | 138.45 ± 4.96 a | 44.64 ± 18.39 c | 67.28 ± 13.42 bc |
| G″max (Pa) | 18.14 ± 4.51 b | 34.30 ± 0.53 a | 11.84 ± 4.43 b | 17.43 ± 3.29 b |
| To G′ (°C) | 72.72 ± 0.78 b | 72.31 ± 0.04 b | 71.74 ± 0.81 b | 75.27 ± 0.14 a |
| TG′max (°C) | 79.81 ± 0.78 a | 76.85 ± 0.76 a | 80.83 ± 3.46 a | 79.82 ± 0.86 a |
| tan δG′max | 0.18 ± 0.01 b | 0.24 ± 0.01 a | 0.25 ± 0.00 a | 0.25 ± 0.01 a |
| G′25 (Pa) | 44.14 ± 0.48 a | 35.88 ± 0.69 b | 26.13 ± 2.79 c | 25.89 ± 1.10 c |
| G″25 (Pa) | 9.78 ± 0.18 a | 10.07 ± 0.16 a | 8.76 ± 1.19 a | 8.52 ± 0.20 a |
| tan δ25 | 0.22 ± 0.01 c | 0.28 ± 0.00 b | 0.34 ± 0.01 a | 0.33 ± 0.01 a |
| Amplitude sweep test | ||||
| G′LVE (Pa) | 43.56 ± 1.67 a | 35.20 ± 1.10 b | 28.19 ± 2.81 c | 26.39 ± 0.19 c |
| G″LVE (Pa) | 9.25 ± 0.38 a | 9.13 ± 0.21 a | 8.49 ± 0.89 a | 7.86 ± 0.06 a |
| tanδ1% | 0.21 ± 0.02 c | 0.26 ± 0.00 b | 0.30 ± 0.00 a | 0.30 ± 0.00 a |
| G″max (Pa) | 22.31 ± 0.89 a | 13.64 ± 0.63 b | 16.35 ± 2.41 b | 14.83 ± 1.52 b |
| Payne height | 1.41 ± 0.00 a | 0.49 ± 0.03 c | 0.92 ± 0.08 b | 0.89 ± 0.18 b |
| Cultivar | PT (°C) | Peak Time (min) | Viscosity (cP) | Heating Slope | BD Slope | ||||
|---|---|---|---|---|---|---|---|---|---|
| PV | HV | BD | FV | SB | |||||
| RD6 | 67.48 ± 0.04 b | 6.27 ± 0.19 a | 3454 ± 228 bc | 1511 ± 113 a | 1943 ± 115 c | 1879 ± 127 a | 368 ± 13 b | 37.9 ± 1.4 c | −5.83 ± 0.15 c |
| RD12 | 67.43 ± 0.04 b | 6.13 ± 0.00 a | 3753 ± 8 b | 1582 ± 35 a | 2171 ± 27 b | 1973 ± 64 a | 391 ± 30 b | 45.5 ± 0.0 b | −7.13 ± 0.06 b |
| SMJ | 65.90 ± 0.07 c | 5.80 ± 0.00 b | 3230 ± 53 c | 1330 ± 11 b | 1900 ± 42 c | 1793 ± 23 a | 463 ± 13 a | 39.7 ± 0.3 c | −5.93 ± 0.12 c |
| HY71 | 68.88 ± 0.04 a | 6.00 ± 0.00 ab | 4155 ± 4 a | 1524 ± 7 a | 2631 ± 11 a | 1948 ± 12 a | 424 ± 19 ab | 57.5 ± 0.4 a | −9.59 ± 0.20 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lowithun, N.; Sagis, L.M.C.; Lumdubwong, N. Impact of Deformability and Rigidity of Starch Granules on Linear and Non-Linear Rheological Behavior of Waxy Rice Starch Gels and Applicability for Food End Uses. Foods 2024, 13, 1864. https://doi.org/10.3390/foods13121864
Lowithun N, Sagis LMC, Lumdubwong N. Impact of Deformability and Rigidity of Starch Granules on Linear and Non-Linear Rheological Behavior of Waxy Rice Starch Gels and Applicability for Food End Uses. Foods. 2024; 13(12):1864. https://doi.org/10.3390/foods13121864
Chicago/Turabian StyleLowithun, Ngamjit, Leonard M. C. Sagis, and Namfone Lumdubwong. 2024. "Impact of Deformability and Rigidity of Starch Granules on Linear and Non-Linear Rheological Behavior of Waxy Rice Starch Gels and Applicability for Food End Uses" Foods 13, no. 12: 1864. https://doi.org/10.3390/foods13121864
APA StyleLowithun, N., Sagis, L. M. C., & Lumdubwong, N. (2024). Impact of Deformability and Rigidity of Starch Granules on Linear and Non-Linear Rheological Behavior of Waxy Rice Starch Gels and Applicability for Food End Uses. Foods, 13(12), 1864. https://doi.org/10.3390/foods13121864







