GC–MS Combined with Fast GC E-Nose for the Analysis of Volatile Components of Chamomile (Matricaria chamomilla L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instruments and Reagents
2.2. Chamomile Samples
2.3. Determination of Volatile Oil Content
2.4. Volatile Oil GC–MS Analysis
2.5. Ultra-Fast Gas Chromatography Electronic Nose Analysis
2.5.1. Single-Factor Investigation
2.5.2. Sample Analysis
2.5.3. Methodological Investigation
2.6. Qualitative and Quantitative Analysis
2.7. Chemometric Analysis
3. Results
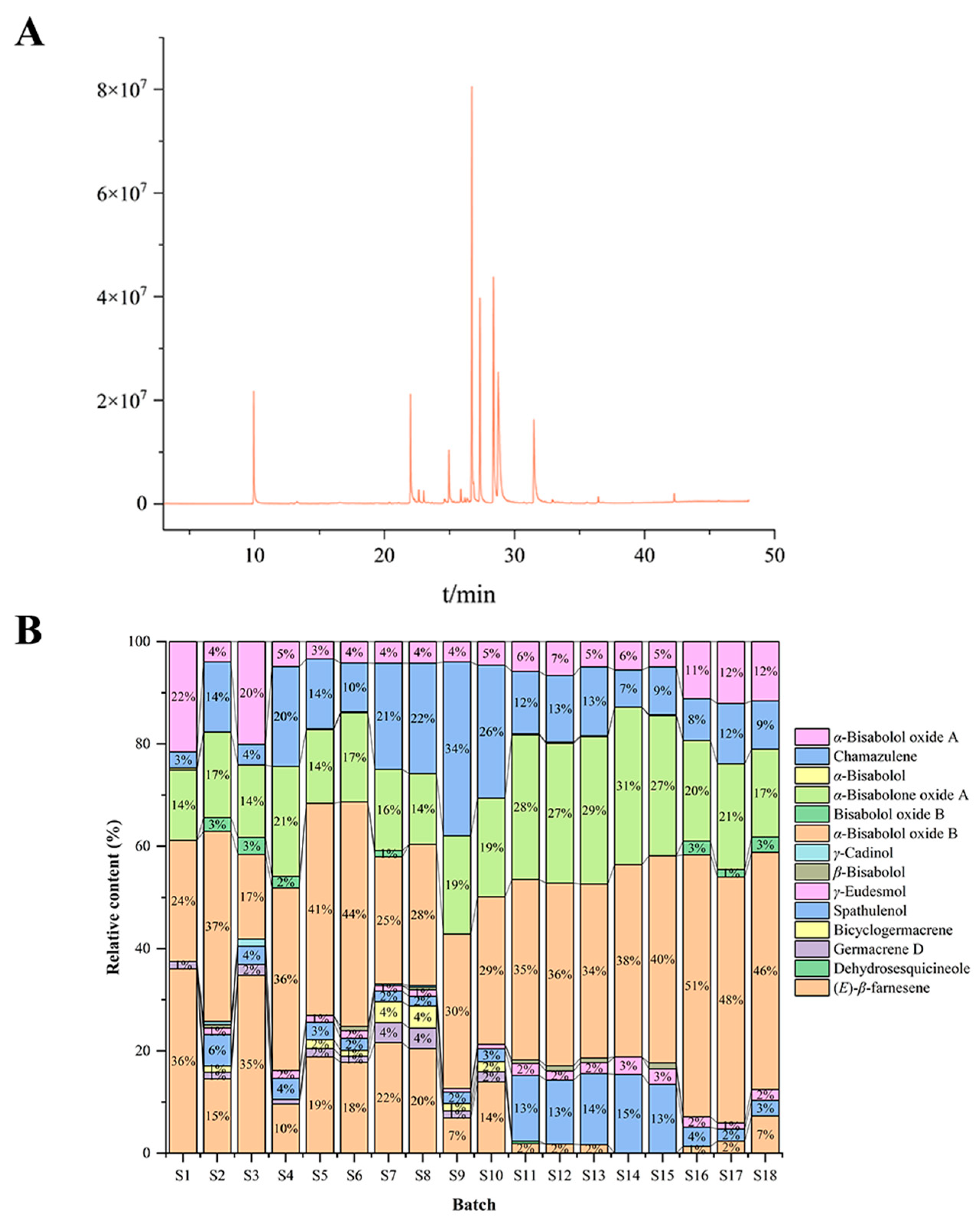
3.1. Chamomile Volatile Oil
3.2. GC–MS Identification of Chamomile Volatile Oil Components
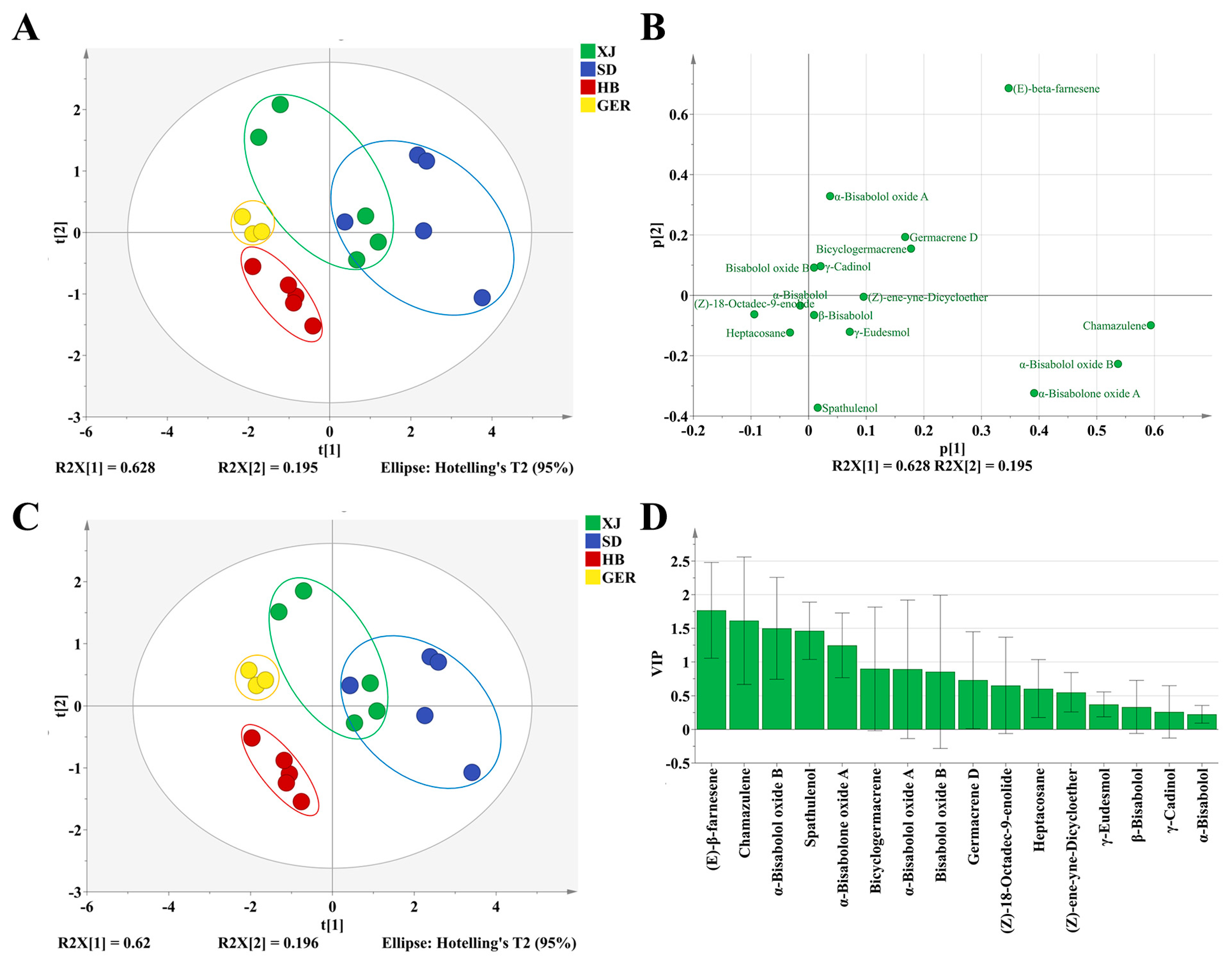
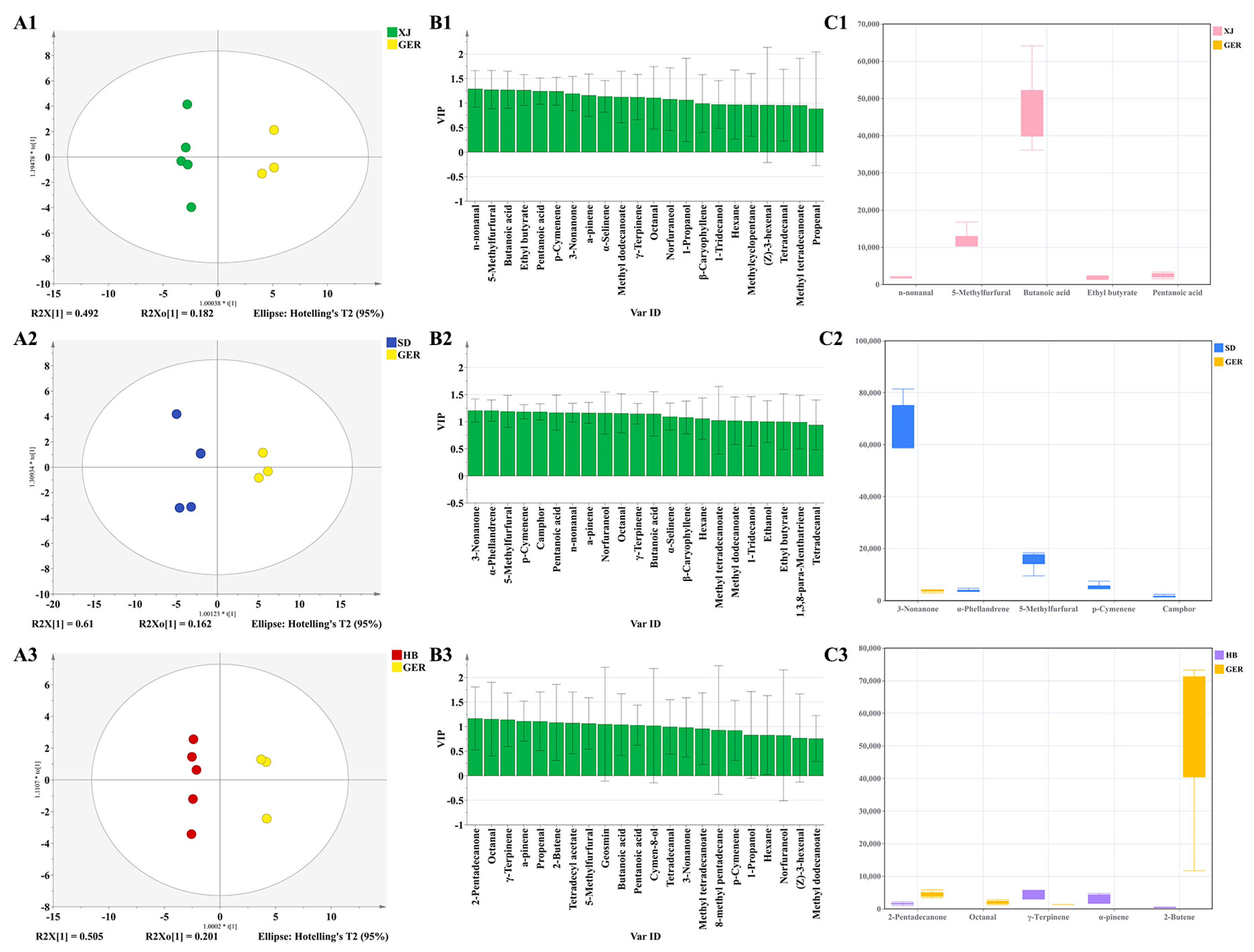
3.3. Discrimination of Chamomile by GC–MS
3.3.1. PCA (Principal Component Analysis)
3.3.2. PLS–DA (Partial Least Squares Discriminant Analysis)
3.4. Identification of Chamomile Using the Rapid Gas Chromatography Electronic Nose Method
3.4.1. Rapid Gas Chromatography Electronic Nose Determination Results
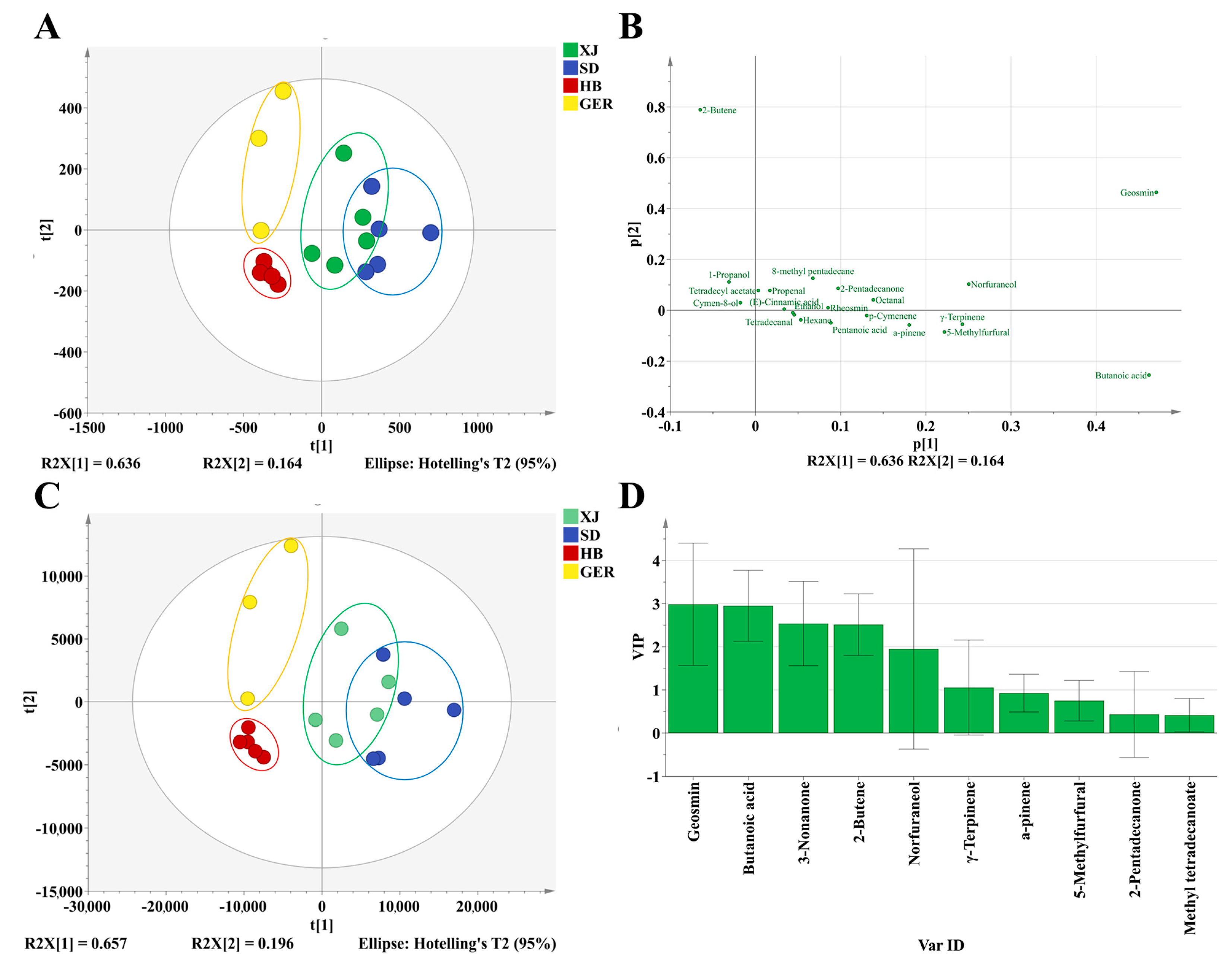
3.4.2. PCA
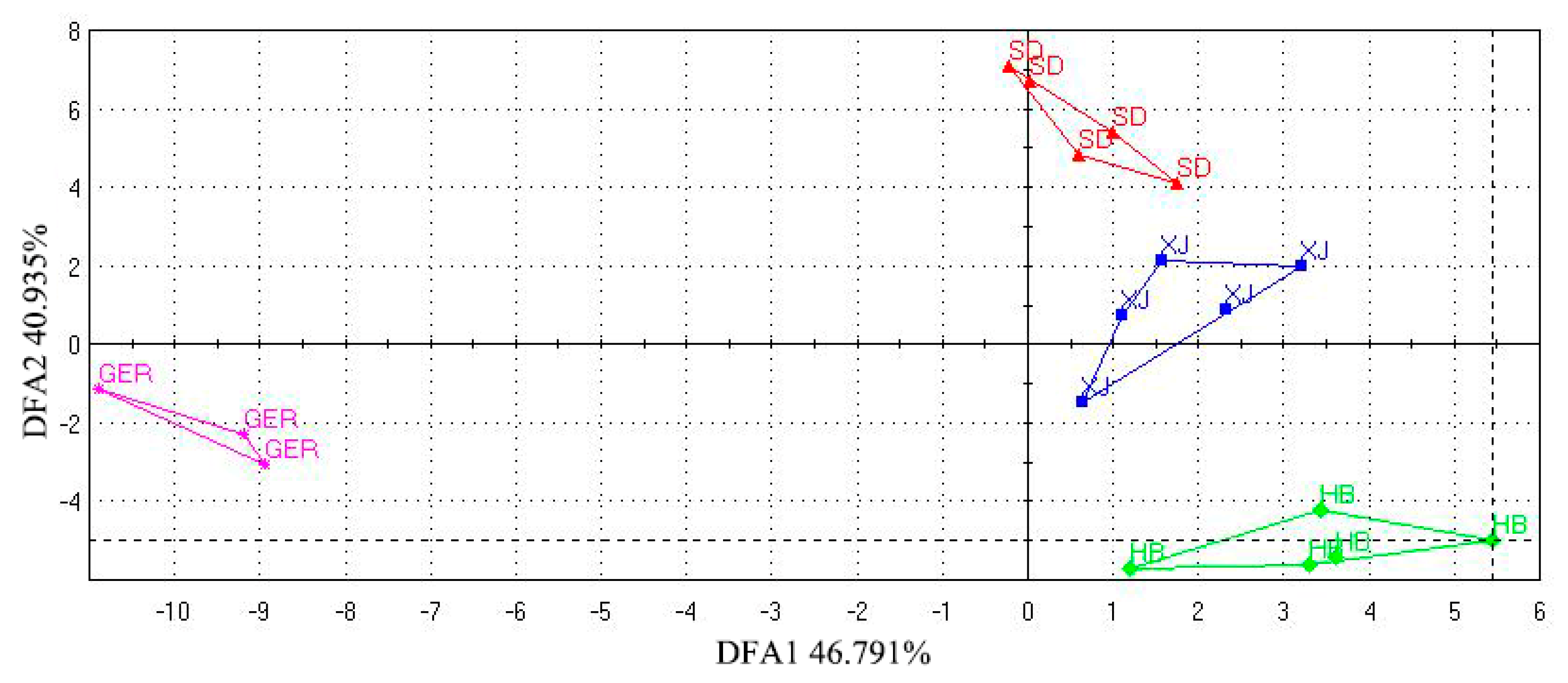
3.4.3. Establishment of the DFA Discrimination Model
3.4.4. Study on Odor Biomarkers of Chamomile from Different Areas
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El, M.; Borjac, M. Matricaria chamomilla: A valuable insight into recent advances in medicinal uses and pharmacological activities. Phytochem. Rev. 2022, 21, 1913–1940. [Google Scholar]
- Sah, A.; Naseef, P. A Comprehensive Study of Therapeutic Applications of Chamomile. Pharmaceuticals 2022, 15, 1284. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.; Shankar, E. Chamomile: A herbal medicine of the past with bright future. Mol. Med. Rep. 2010, 3, 895–901. [Google Scholar] [PubMed]
- Rafieiolhossaini, M.; Adams, A. Fast quality assessment of German chamomile (Matricaria chamomilla L.) by headspace solid-phase microextraction: Influence of flower development stage. Nat. Prod. Commun. 2012, 7, 97–100. [Google Scholar] [CrossRef]
- Dai, L.; Li, Y. Chamomile: A Review of Its Traditional Uses, Chemical Constituents, Pharmacological Activities and Quality Control Studies. Molecules 2022, 28, 133. [Google Scholar] [CrossRef] [PubMed]
- Shakya, P.; Thakur, R. GGE biplot and regression based multi-environment investigations for higher yield and essential oil content in German chamomile (Matricaria chamomilla L.). Ind. Crops Prod. 2023, 193, 116145. [Google Scholar] [CrossRef]
- Fernandes, J.; Reboredo, H. Elemental Composition of Commercial Herbal Tea Plants and Respective Infusions. Plants 2022, 11, 1412. [Google Scholar] [CrossRef] [PubMed]
- Raal, A.; Orav, A. Content of essential oil, terpenoids and polyphenols in commercial chamomile (Chamomilla recutita L. Rauschert) teas from different countries. Food Chem. 2012, 131, 632–638. [Google Scholar] [CrossRef]
- De, P.; Ercolano, G. Chamomile essential oils exert anti-inflammatory effects involving human and murine macrophages: Evidence to support a therapeutic action. J. Ethnopharmacol. 2023, 311, 116391. [Google Scholar]
- Can, D.; Demir, U. Psychopharmacological profile of Chamomile (Matricaria recutita L.) essential oil in mice. Phytomedicine 2012, 19, 306–310. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, Q. Phyllotaxis development: A lesson from the Asteraceae family. Trends Plant Sci. 2021, 26, 873–875. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Ou, X. Study on the key volatile compounds and aroma quality of jasmine tea with different scenting technology. Food Chem. 2022, 385, 132718. [Google Scholar] [CrossRef] [PubMed]
- Baranauskiene, R.; Venskutonis, R. Valorisation of Roman chamomile (Chamaemelum nobile L.) herb by comprehensive evaluation of hydrodistilled aroma and residual non-volatile fractions. Food Res. Int. 2022, 160, 111715. [Google Scholar] [CrossRef] [PubMed]
- Melucci, D.; Bendini, A. Rapid direct analysis to discriminate geographic origin of extra virgin olive oils by flash gas chromatography electronic nose and chemometrics. Food Chem. 2016, 204, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.X.; Zhang, X.; Guo, S.; Yan, H.; Wang, J.M.; Zhou, J.Q.; Yang, J.; Duan, J.A. Headspace GC/MS and fast GC e-nose combined with chemometric analysis to identify the varieties and geographical origins of ginger (Zingiber officinale Roscoe). Food Chem. 2022, 396, 133672. [Google Scholar] [CrossRef] [PubMed]
- Orav, A.; Raal, A. Content and composition of the essential oil of Chamomilla recutita (L.) Rauschert from some European countries. Nat. Prod. Res. 2010, 24, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Mahanta, P.; Bora, K. Thermolabile essential oils, aromas and flavours: Degradation pathways, effect of thermal processing and alteration of sensory quality. Food Res. Int. 2021, 145, 110404. [Google Scholar] [CrossRef]
- Formisano, C.; Delfine, S. Correlation among environmental factors, chemical composition and antioxidative properties of essential oil and extracts of chamomile (Matricaria chamomilla L.) collected in Molise (South-central Italy). Ind. Crops Prod. 2015, 63, 256–263. [Google Scholar] [CrossRef]
- Satyal, P.; Shrestha, S.; Setzer, W.N. Composition and Bioactivities of an (E)-beta-Farnesene Chemotype of Chamomile (Matricaria chamomilla) Essential Oil from Nepal. Nat. Prod. Commun. 2015, 10, 1453–1457. [Google Scholar]
- Piri, E.; Mahmoodi Sourestani, M.; Khaleghi, E.; Mottaghipisheh, J.; Zomborszki, Z.P.; Hohmann, J.; Csupor, D. Chemo-Diversity and Antiradical Potential of Twelve Matricaria chamomilla L. Populations from Iran: Proof of Ecological Effects. Molecules 2019, 24, 1315. [Google Scholar] [CrossRef]
- Dai, Y.; Yu, R. Flavorand taste change rules of Sophora Flavescentis Radix processed using ancient classical method:an exploration based on intelligent sensory analysis. Zhongguo Zhong Yao Za Zhi 2021, 46, 6410–6416. [Google Scholar] [PubMed]
- Biancolillo, A.; Aloia, R. Organosulfur volatile profiles in Italian red garlic (Allium sativum L.) varieties investigated by HS-SPME/GC-MS and chemometrics. Food Control 2022, 131, 108477. [Google Scholar] [CrossRef]
- Li, M.X.; Qin, Y.W.; Li, Y.; Zhang, J.B.; De, J.; Qu, L.Y.; Gong, J.W.; Jia, A.M.; Mao, C.Q.; Lu, T.L. Rapid identification and differential markers of Curcumae Radix decoction pieces of different sources based on Heracles Neo ultra-fast gas phase electronic nose. China J. Chin. Mater. Medica 2023, 48, 1518–1525. [Google Scholar]
- Alhasan, S.; Khadim, A. Effect of Applying Different Levels of Nitrogen Fertilizer on Growth and Essential Oil of Chamomile (Matricaria chamomilla L.). IOP Conf. Ser. Earth Environ. Sci. 2022, 1060, 012102. [Google Scholar] [CrossRef]
- Zengin, G.; Mollica, A. A Comparative Study of Chamomile Essential Oils and Lipophilic Extracts Obtained by Conventional and Greener Extraction Techniques: Chemometric Approach to Chemical Composition and Biological Activity. Separations 2023, 10, 18. [Google Scholar] [CrossRef]
- Zhou, Y.; He, L.; Wang, W.; Wei, G.; Ma, L.; Liu, H.; Yao, L. Artemisia sieversiana Ehrhart ex Willd. Essential Oil and Its Main Component, Chamazulene: Their Photoprotective Effect against UVB-Induced Cellular Damage and Potential as Novel Natural Sunscreen Additives. ACS Sustain. Chem. Eng. 2023, 11, 17675–17686. [Google Scholar] [CrossRef]
- Shaaban, M.; El-Hagrassi, A.M.; Osman, A.F.; Soltan, M.M. Bioactive compounds from Matricaria chamomilla: Structure identification, in vitro antiproliferative, antimigratory, antiangiogenic, and antiadenoviral activities. Z. Naturforschung C J. Biosci. 2022, 77, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Singh, O.; Khanam, Z. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn. Rev. 2011, 5, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Mckay, L.; Blumberg, B. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phytother. Res. 2006, 20, 519–530. [Google Scholar] [CrossRef]
- Chauhan, R.; Singh, S. A Comprehensive Review on Biology, Genetic Improvement, Agro and Process Technology of German Chamomile (Matricaria chamomilla L.). Plants 2021, 11, 29. [Google Scholar] [CrossRef]
- Zhao, Y. Study on the Chemical Composition and Quality Standards of Uighur Medicine Chamomile. Ph.D. Thesis, China Academy of Chinese Medical Sciences, Beijing, China, 2018; p. 72. [Google Scholar]
- Flemming, M.; Kraus, B. Revisited anti-inflammatory activity of matricine in vitro: Comparison with chamazulene. Fitoterapia 2015, 106, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.; Goeters, S. Chamazulene carboxylic acid and matricin: A natural profen and its natural prodrug, identified through similarity to synthetic drug substances. J. Nat. Prod. 2006, 69, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Qu, K. Analysis of thermal oxidation of different multi-element oleogels based on carnauba wax, sitosterol/lecithin, and ethyl cellulose by classical oxidation determination method combined with the electronic nose. Food Chem. 2023, 405, 134970. [Google Scholar] [CrossRef] [PubMed]
- Flambeau, J.; Lee, W. Discrimination and geographical origin prediction of washed specialty Bourbon coffee from different coffee growing areas in Rwanda by using electronic nose and electronic tongue. Food Sci. Biotechnol. 2017, 26, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, J.; Fan, X.; Zhu, G.; Lu, T.; Xue, R. Discrimination between raw and ginger juice processed Magnoliae officinalis cortex based on HPLC and Heracles NEO ultra-fast gas phase electronic nose. Phytochem. Anal. 2022, 33, 722–734. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X. Species discrimination among three kinds of puffer fish using an electronic nose combined with olfactory sensory evaluation. Sensors 2012, 12, 12562–12571. [Google Scholar] [CrossRef]
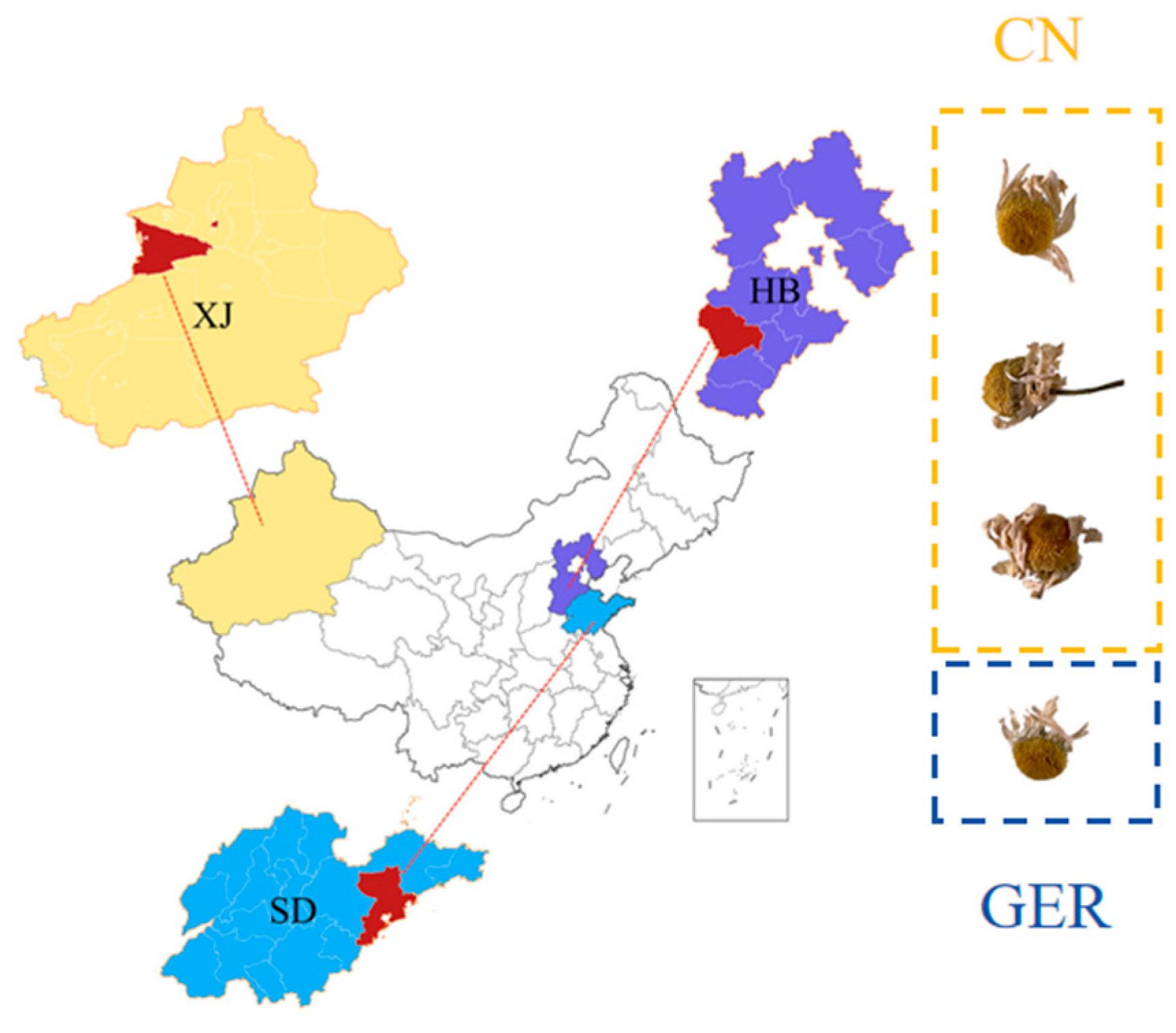

| No. | Sources | No. | Sources | No. | Sources |
|---|---|---|---|---|---|
| S1 | XJ | S7 | SD | S13 | HB |
| S2 | XJ | S8 | SD | S14 | HB |
| S3 | XJ | S9 | SD | S15 | HB |
| S4 | XJ | S10 | SD | S16 | GER |
| S5 | XJ | S11 | HB | S17 | GER |
| S6 | SD | S12 | HB | S18 | GER |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Jiang, Z.; Dang, J.; Li, D.; Yu, D.; Qu, C.; Wu, Q. GC–MS Combined with Fast GC E-Nose for the Analysis of Volatile Components of Chamomile (Matricaria chamomilla L.). Foods 2024, 13, 1865. https://doi.org/10.3390/foods13121865
Lu J, Jiang Z, Dang J, Li D, Yu D, Qu C, Wu Q. GC–MS Combined with Fast GC E-Nose for the Analysis of Volatile Components of Chamomile (Matricaria chamomilla L.). Foods. 2024; 13(12):1865. https://doi.org/10.3390/foods13121865
Chicago/Turabian StyleLu, Jiayu, Zheng Jiang, Jingjie Dang, Dishuai Li, Daixin Yu, Cheng Qu, and Qinan Wu. 2024. "GC–MS Combined with Fast GC E-Nose for the Analysis of Volatile Components of Chamomile (Matricaria chamomilla L.)" Foods 13, no. 12: 1865. https://doi.org/10.3390/foods13121865






