Regional Variability in Sugar and Amino Acid Content of U.S. Soybeans and the Impact of Autoclaving on Reducing Sugars and Free Lysine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Sample Preparation
2.3. Analysis of Sugar Component
2.4. Analysis of Total Amino Acids
2.5. Analysis of Free Amino Acid and Sugars
2.6. Analysis of Protein Content
2.7. Analysis of Oil Content
2.8. Statistical Analysis
3. Results and Discussion
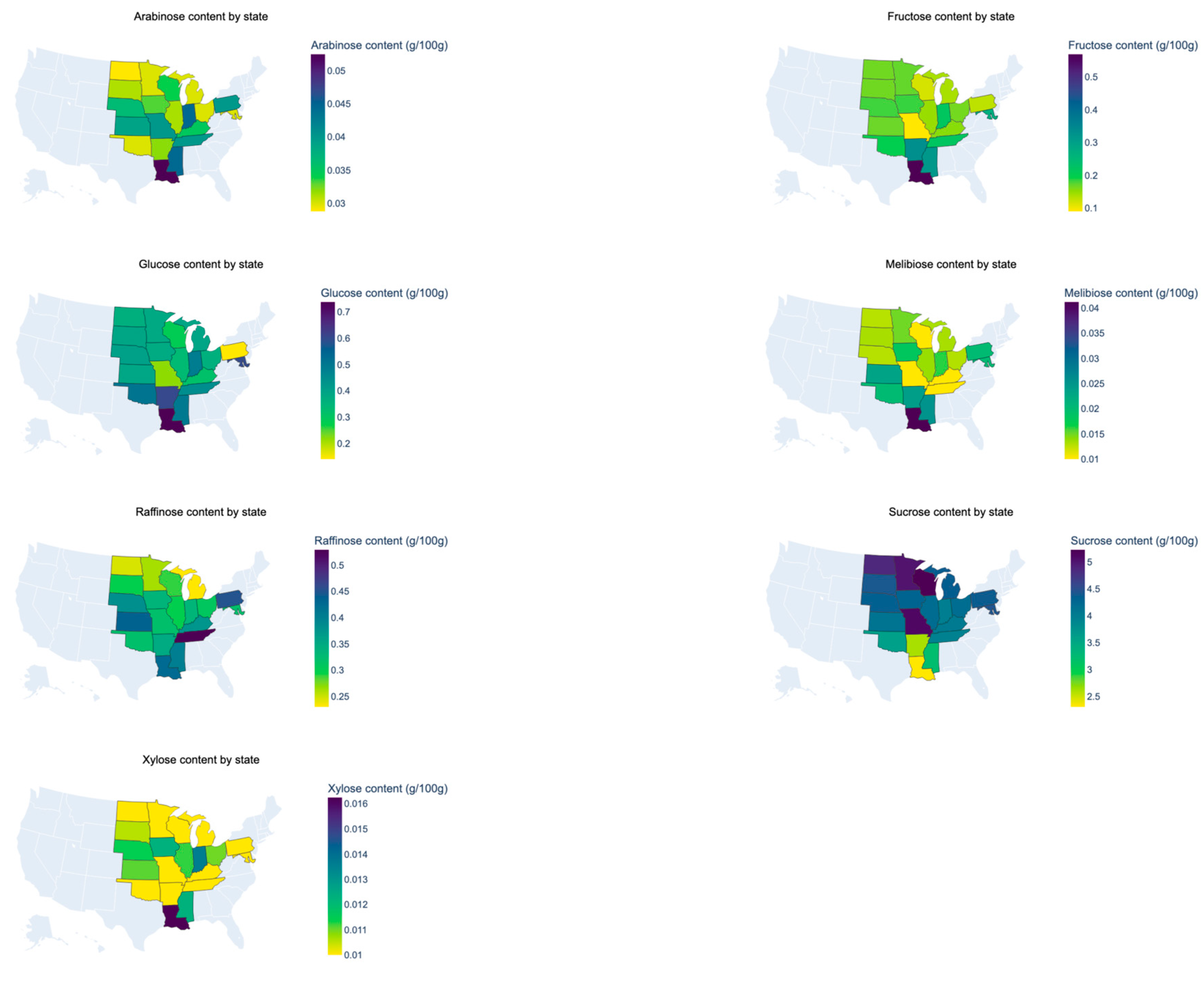
3.1. Sugar Composition of Soybeans
3.2. Total Amino Acid Composition of Soybeans
3.3. Protein Content of Soybeans
3.4. Oil Content of Soybeans
3.5. Seed Size of Soybeans
3.6. Effect of Thermal Processing on Lysine and Sugar Content of Soybeans
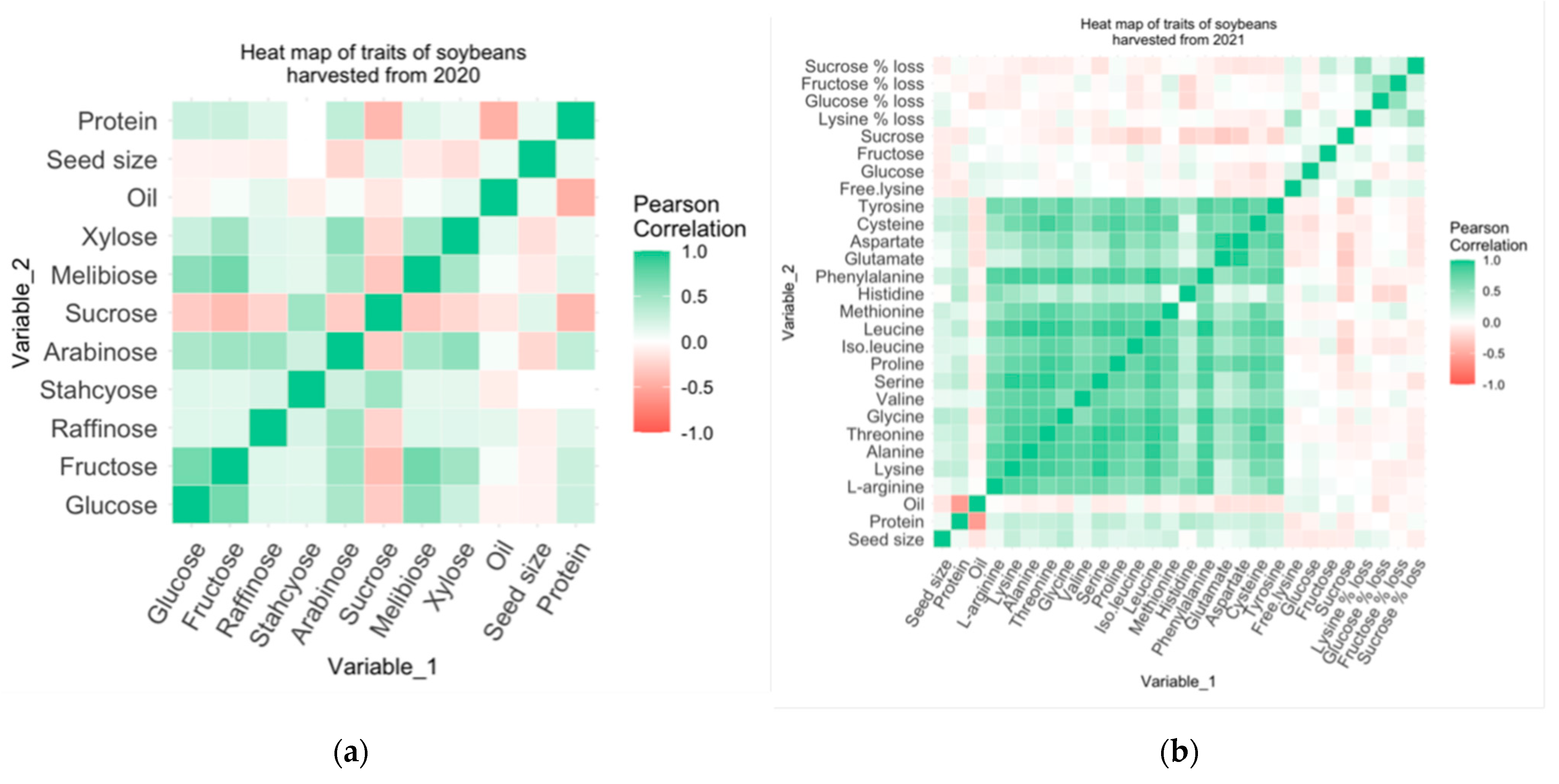
3.7. Correlation between Traits of Soybeans
3.8. Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, K. Soybeans: Chemistry, Technology, and Utilization; Springer: Boston, MA, USA, 1997. [Google Scholar]
- Maeda, M.H.; Toda, K.; Kaga, A. Novel Soybean Variety Lacking Raffinose Synthase 2 Activity. ACS Omega 2024, 9, 2134–2144. [Google Scholar] [CrossRef] [PubMed]
- Coon, C.N.; Leske, K.L.; Akavanichan, O.; Cheng, T.K. Effect of Oligosaccharide-Free Soybean Meal on True Metabolizable Energy and Fiber Digestion in Adult Roosters. Poult. Sci. 1990, 69, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, J.; Yang, G. Effects of Soybean Oligosaccharide, Stachyose, and Raffinose on Growth Performance and Cecal Microbiota in Broiler Chickens. Anim. Sci. J. 2021, 92, e13668. [Google Scholar] [CrossRef] [PubMed]
- Valentine, M.F.; De Tar, J.R.; Mookkan, M.; Firman, J.D.; Zhang, Z.J. Silencing of Soybean Raffinose Synthase Gene Reduced Raffinose Family Oligosaccharides and Increased True Metabolizable Energy of Poultry Feed. Front. Plant Sci. 2017, 8, 692. [Google Scholar] [CrossRef] [PubMed]
- Hou, A.; Chen, P.; Alloatti, J.; Li, D.; Mozzoni, L.; Zhang, B.; Shi, A. Genetic Variability of Seed Sugar Content in Worldwide Soybean Germplasm Collections. Crop Sci. 2009, 49, 903–912. [Google Scholar] [CrossRef]
- Jiang, G.-L.; Chen, P.; Zhang, J.; Florez-Palacios, L.; Zeng, A.; Wang, X.; Bowen, R.A.; Miller, A.; Berry, H. Genetic Analysis of Sugar Composition and Its Relationship with Protein, Oil, and Fiber in Soybean. Crop Sci. 2018, 58, 2413–2421. [Google Scholar] [CrossRef]
- Kumar, V.; Rani, A.; Goyal, L.; Dixit, A.K.; Manjaya, J.G.; Dev, J.; Swamy, M. Sucrose and Raffinose Family Oligosaccharides (RFOs) in Soybean Seeds as Influenced by Genotype and Growing Location. J. Agric. Food Chem. 2010, 58, 5081–5085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hou, Y.; Bazer, F.W.; He, W.; Posey, E.A.; Wu, G. Amino Acids in Swine Nutrition and Production. In Amino Acids in Nutrition and Health; Wu, G., Ed.; Springer International Publishing: Cham, Switzerland, 2021; Volume 1285, pp. 81–107. [Google Scholar] [CrossRef]
- Liao, S.F.; Wang, T.; Regmi, N. Lysine Nutrition in Swine and the Related Monogastric Animals: Muscle Protein Biosynthesis and Beyond. SpringerPlus 2015, 4, 147. [Google Scholar] [CrossRef]
- Karr-Lilienthal, L.K.; Grieshop, C.M.; Spears, J.K.; Fahey, G.C. Amino Acid, Carbohydrate, and Fat Composition of Soybean Meals Prepared at 55 Commercial U.S. Soybean Processing Plants. J. Agric. Food Chem. 2005, 53, 2146–2150. [Google Scholar] [CrossRef]
- Grieshop, C.M.; Kadzere, C.T.; Clapper, G.M.; Flickinger, E.A.; Bauer, L.L.; Frazier, R.L.; Fahey, G.C., Jr. Chemical and Nutritional Characteristics of United States Soybeans and Soybean Meals. J. Agric. Food Chem. 2003, 51, 7684–7691. [Google Scholar] [CrossRef]
- De Borja Reis, A.F.; Tamagno, S.; Moro Rosso, L.H.; Ortez, O.A.; Naeve, S.; Ciampitti, I.A. Historical Trend on Seed Amino Acid Concentration Does Not Follow Protein Changes in Soybeans. Sci. Rep. 2020, 10, 17707. [Google Scholar] [CrossRef] [PubMed]
- Erickson, D.R. (Ed.) Practical Handbook of Soybean Processing and Utilization; AOCS Press: St. Louis, MO, USA, 1995. [Google Scholar]
- Liener, I.E. Implications of Antinutritional Components in Soybean Foods. Crit. Rev. Food Sci. Nutr. 1994, 34, 31–67. [Google Scholar] [CrossRef] [PubMed]
- Ruan, D.; Cheng, F.; Wang, H. The Maillard Reaction in Food Chemistry: Current Technology and Applications, 1st ed.; Chemistry of Foods Series; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Zhang, Q.; Ames, J.M.; Smith, R.D.; Baynes, J.W.; Metz, T.O. A Perspective on the Maillard Reaction and the Analysis of Protein Glycation by Mass Spectrometry: Probing the Pathogenesis of Chronic Disease. J. Proteome Res. 2009, 8, 754–769. [Google Scholar] [CrossRef] [PubMed]
- Troise, A.D.; Wiltafsky, M.; Fogliano, V.; Vitaglione, P. The Quantification of Free Amadori Compounds and Amino Acids Allows to Model the Bound Maillard Reaction Products Formation in Soybean Products. Food Chem. 2018, 247, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Žilić, S.; Mogol, B.A.; Akıllıoğlu, G.; Serpen, A.; Delić, N.; Gökmen, V. Effects of Extrusion, Infrared and Microwave Processing on Maillard Reaction Products and Phenolic Compounds in Soybean. J. Sci. Food Agric. 2014, 94, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Parsons, C.M.; Hashimoto, K.; Wedekind, K.J.; Han, Y.; Baker, D.H. Effect of Overprocessing on Availability of Amino Acids and Energy in Soybean Meal. Poult. Sci. 1992, 71, 133–140. [Google Scholar] [CrossRef]
- Giannoccaro, E.; Wang, Y.-J.; Chen, P. Comparison of Two HPLC Systems and an Enzymatic Method for Quantification of Soybean Sugars. Food Chem. 2008, 106, 324–330. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific Inc. Determination of Protein Concentration Using AAA-Direct (Application Note 163). Available online: https://assets.thermofisher.com/TFS-Assets/CMD/Application-Notes/AN-163-LC-Protein-AAA-Direct-LPN1634-EN.pdf (accessed on 14 June 2024).
- American Oil Chemists’ Society. Official Method Ba 4e-93: Generic Combustion Method for Crude Protein; AOCS Press: Champaign, IL, USA, 2017. [Google Scholar]
- American Oil Chemists’ Society. Official Method Ac 3-44: Oil in Soybeans; AOCS Press: Champaign, IL, USA, 2017. [Google Scholar]
- La, T.; Large, E.; Taliercio, E.; Song, Q.; Gillman, J.D.; Xu, D.; Nguyen, H.T.; Shannon, G.; Scaboo, A. Characterization of Select Wild Soybean Accessions in the USDA Germplasm Collection for Seed Composition and Agronomic Traits. Crop Sci. 2019, 59, 233–251. [Google Scholar] [CrossRef]
- Wolf, R.B.; Cavins, J.F.; Kleiman, R.; Black, L.T. Effect of Temperature on Soybean Seed Constituents: Oil, Protein, Moisture, Fatty Acids, Amino Acids, and Sugars. J. Am. Oil Chem. Soc. 1982, 59, 230–232. [Google Scholar] [CrossRef]
- Xu, J.; Misra, G.; Sreenivasulu, N.; Henry, A. What Happens at Night? Physiological Mechanisms Related to Maintaining Grain Yield under High Night Temperature in Rice. Plant Cell Environ. 2021, 44, 2245–2261. [Google Scholar] [CrossRef]
- Yang, L.; Song, W.; Xu, C.; Sapey, E.; Jiang, D.; Wu, C. Effects of High Night Temperature on Soybean Yield and Compositions. Front. Plant Sci. 2023, 14, 1065604. [Google Scholar] [CrossRef] [PubMed]
- Loka, D.A.; Oosterhuis, D.M. Effect of High Night Temperatures on Cotton Respiration, ATP Levels, and Carbohydrate Content. Environ. Exp. Bot. 2010, 68, 258–263. [Google Scholar] [CrossRef]
- Assefa, Y.; Bajjalieh, N.; Archontoulis, S.; Casteel, S.; Davidson, D.; Kovács, P.; Naeve, S.; Ciampitti, I.A. Spatial Characterization of Soybean Yield and Quality (Amino Acids, Oil, and Protein) for United States. Sci. Rep. 2018, 8, 14653. [Google Scholar] [CrossRef]
- Morell, M.; Copeland, L. Enzymes of Sucrose Breakdown in Soybean Nodules: Alkaline Invertase. Plant Physiol. 1984, 74, 1030–1034. [Google Scholar] [CrossRef]
- Koch, K. Sucrose Metabolism: Regulatory Mechanisms and Pivotal Roles in Sugar Sensing and Plant Development. Curr. Opin. Plant Biol. 2004, 7, 235–246. [Google Scholar] [CrossRef]
- Singh, R.; Singh, A.; Sachan, S. Enzymes Used in the Food Industry: Friends or Foes? In Enzymes in Food Biotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 827–843. [Google Scholar]
- Hymowitz, T.; Collins, F.I. Variability of Sugar Content in Seed of Glycine max (L.) Merrill and G. Soja Sieb. and Zucc. Agron. J. 1974, 66, 239–240. [Google Scholar] [CrossRef]
- Hartwig, E.E.; Kuo, T.M.; Kenty, M.M. Seed Protein and Its Relationship to Soluble Sugars in Soybean. Crop Sci. 1997, 37, 770–773. [Google Scholar] [CrossRef]
- Wilson, R.F. Seed Composition. In Agronomy Monographs; Shibles, R.M., Harper, J.E., Wilson, R.F., Shoemaker, R.C., Eds.; American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America: Madison, WI, USA, 2004; pp. 621–677. [Google Scholar]
- Dills, W.L. Protein Fructosylation: Fructose and the Maillard Reaction. Am. J. Clin. Nutr. 1993, 58, 779S–787S. [Google Scholar] [CrossRef]
- Duan, Z.; Li, Q.; Wang, H.; He, X.; Zhang, M. Genetic Regulatory Networks of Soybean Seed Size, Oil and Protein Contents. Front. Plant Sci. 2023, 14, 1160418. [Google Scholar] [CrossRef]

| Time (Min) | 250 mM NaOH (%) | 1 M C2H3NaO2 (%) | H2O (%) | Curve |
|---|---|---|---|---|
| Initial | 76 | 24 | 0 | |
| 0.0 | 76 | 24 | 0 | |
| 2.0 | 76 | 24 | 0 | |
| 8.0 | 64 | 36 | 0 | 8 |
| 11.0 | 64 | 36 | 0 | |
| 18.0 | 40 | 20 | 40 | 8 |
| 21.0 | 4 | 16 | 40 | 5 |
| 23.0 | 14 | 16 | 70 | 8 |
| 42.0 | 14 | 16 | 70 | |
| 42.1 | 20 | 80 | 0 | 5 |
| 44.1 | 20 | 80 | 0 | |
| 44.2 | 76 | 24 | 0 | 5 |
| 75.0 | 76 | 24 | 0 |
| Parameter | Average | Parameter | Average | Parameter | Average |
|---|---|---|---|---|---|
| Glucose a | 0.43 ± 0.22 | Alanine b | 2.74 ± 0.88 | Phenylalanine b | 2.64 ± 0.76 |
| Fructose a | 0.21 ± 0.17 | Threonine b | 2.71 ± 0.75 | Glutamate b | 4.90 ± 1.65 |
| Sucrose a | 4.45 ± 0.96 | Glycine b | 2.29 ± 0.71 | Aspartate b | 4.27 ± 1.36 |
| Raffinose a | 0.34 ± 0.10 | Valine b | 3.49 ± 1.22 | Cysteine b | 0.90 ± 0.27 |
| Stachyose a | 1.34 ± 0.19 | Serine b | 3.81 ± 1.14 | Tyrosine b | 1.71 ± 0.56 |
| Arabinose a | 0.04 ± 0.01 | Proline b | 4.61 ± 1.39 | Free lysine b | 0.03 ± 0.04 |
| Xylose a | 0.01 ± 0.00 | Iso-leucine b | 3.83 ± 1.16 | Free glucose b | 1.92 ± 2.27 |
| Melibiose a | 0.02 ± 0.01 | Leucine b | 5.29 ± 1.54 | Free fructose b | 1.86 ± 3.24 |
| L-arginine b | 9.82 ± 3.31 | Methionine b | 1.55 ± 0.47 | Free sucrose b | 8.33 ± 2.14 |
| Lysine b | 4.75 ± 1.83 | Histidine b | 2.29 1.32 |
| State | Region | n | Glucose | Fructose | Sucrose | Raffinose | Stachyose | Arabinose | Xylose | Melibiose | Oil | Protein | Seed Size |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (g 100 g−1) | (g 100 seeds −1) | ||||||||||||
| AR | MDS | 5 | 0.64 ab | 0.35 ab | 2.75 de | 0.37 abcdef | 1.19 | 0.03 abd | 0.01 ab | 0.02 ab | 23.19 abc | 40.74 abc | 15.86 |
| IA | WCB | 35 | 0.40 a | 0.19 a | 4.52 ab | 0.35 abcde | 1.35 | 0.04 ab | 0.01 ab | 0.02 a | 23.49 a | 38.09 a | 15.29 |
| IL | ECB | 24 | 0.35 a | 0.16 a | 4.42 a | 0.31 acf | 1.3 | 0.03 a | 0.01 ab | 0.01 a | 23.11 ab | 37.64 a | 15.58 |
| IN | ECB | 11 | 0.52 ab | 0.23 a | 4.21 ac | 0.35 abcdef | 1.39 | 0.05 bcd | 0.01 ab | 0.02 a | 22.31 abc | 40.76 bc | 15.00 |
| KS | WCB | 9 | 0.41 a | 0.18 a | 4.35 abc | 0.46 b | 1.38 | 0.04 abcd | 0.01 ab | 0.02 ab | 21.73 abc | 41.30 abc | 15.85 |
| KY | MDS | 2 | 0.33 ab | 0.16 ab | 4.26 abcde | 0.4 abcdef | 1.38 | 0.04 abcd | 0.01 ab | 0.01 ab | 21.73 abc | 39.36 abc | 14.31 |
| LA | MDS | 8 | 0.78 b | 0.60 b | 2.43 d | 0.45 bd | 1.25 | 0.06 c | 0.02 a | 0.04 b | 23.30 abc | 41.14 b | 15.85 |
| MD | EC | 1 | 0.63 ab | 0.29 ab | 4.68 abcde | 0.34 abcdef | 1.37 | 0.04 abcd | 0.01 ab | 0.02 ab | 21.45 abc | 39.99 abc | 16.80 |
| MI | ECB | 3 | 0.41 ab | 0.15 a | 4.60 abce | 0.24 acef | 1.22 | 0.03 abd | 0.01 ab | 0.02 ab | 22.27 abc | 40.97 abc | 18.87 |
| MN | WCB | 16 | 0.40 a | 0.18 a | 5.29 b | 0.28 cf | 1.39 | 0.03 a | 0.01 ab | 0.02 a | 21.9 bc | 38.93 abc | 17.30 |
| MO | WCB | 1 | 0.24 ab | 0.10 ab | 5.39 abce | 0.34 abcdef | 1.41 | 0.05 abcd | 0.01 ab | 0.01 ab | 21.9 abc | 36.66 abc | 18.20 |
| MS | MS | 13 | 0.52 ab | 0.33 a | 3.38 cde | 0.42 bde | 1.35 | 0.05 cd | 0.01 ab | 0.03 ab | 23.30 ab | 39.88 abc | 16.55 |
| ND | WCB | 25 | 0.39 a | 0.17 a | 5.12 ab | 0.26 f | 1.36 | 0.03 a | 0.01 ab | 0.01 a | 21.35 c | 38.24 ac | 14.47 |
| NE | WCB | 14 | 0.43 a | 0.19 a | 4.58 ab | 0.4 abde | 1.29 | 0.04 cd | 0.01 ab | 0.02 a | 23.21 ab | 38.16 abc | 14.82 |
| OH | ECB | 10 | 0.38 a | 0.18 a | 4.42 abc | 0.33 acdef | 1.31 | 0.03 a | 0.01 ab | 0.01 a | 22.29 abc | 39.72 abc | 16.42 |
| OK | MDS | 1 | 0.53 ab | 0.21 ab | 3.80 abcde | 0.34 abcdef | 1.29 | 0.03 abcd | 0.01 ab | 0.02 ab | 24.64 abc | 36.3 abc | 13.70 |
| PA | EC | 1 | 0.15 ab | 0.14 ab | 4.61 abcde | 0.47 abcdef | 1.47 | 0.04 abcd | 0.02 ab | 0.02 ab | 22.88 abc | 39.55 abc | 16.8 |
| SD | WCB | 18 | 0.41 a | 0.18 a | 4.64 ab | 0.31 acf | 1.36 | 0.03 a | 0.01 b | 0.01 a | 22.32 abc | 39.12 abc | 15.46 |
| TN | MDS | 1 | 0.47 ab | 0.24 ab | 4.17 abcde | 0.56 abcde | 1.42 | 0.05 abcd | 0.02 ab | 0.02 ab | 23.33 abc | 37.25 abc | 13.70 |
| WI | WCB | 5 | 0.30 a | 0.13 a | 5.51 ab | 0.3 abcdef | 1.47 | 0.03 abcd | 0.01 ab | 0.01 a | 22.03 abc | 39.88 abc | 18.46 |
| Region | n | Glucose | Fructose | Sucrose | Raffinose | Stachyose | Arabinose | Xylose | Melibiose | Oil | Protein | Seed Size |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (g 100 g−1) | (g 100 seeds −1) | |||||||||||
| 2020 samples | ||||||||||||
| ECB | 53 | 0.39 b | 0.17 b | 4.49 a | 0.32 b | 1.33 | 0.04 b | 0.01 | 0.01 b | 22.64 | 39.08 ab | 16.08 |
| EC | 2 | 0.39 ab | 0.22 ab | 4.65 a | 0.41 ab | 1.42 | 0.04 ab | 0.02 | 0.02 ab | 22.17 | 39.77 ab | 16.80 |
| MDS | 30 | 0.60 a | 0.39 a | 3.12 b | 0.42 a | 1.30 | 0.05 a | 0.01 | 0.03 a | 23.24 | 40.24 a | 15.79 |
| WCB | 118 | 0.40 b | 0.18 b | 4.77 a | 0.33 b | 1.36 | 0.03 b | 0.01 | 0.02 b | 22.46 | 38.49 b | 15.31 |
| 2021 samples | ||||||||||||
| ECB | 11 | NA | NA | NA | NA | NA | NA | NA | NA | 20.71 a | 42.15 b | 17.65 b |
| WCB | 9 | NA | NA | NA | NA | NA | NA | NA | NA | 21.60 a | 37.50 a | 16.27 ab |
| EC | 1 | NA | NA | NA | NA | NA | NA | NA | NA | 23.48 ab | 40.34 ab | 16.80 ab |
| SE | 2 | NA | NA | NA | NA | NA | NA | NA | NA | 24.11 ab | 43.83 b | 13.50 a |
| MDS | 32 | NA | NA | NA | NA | NA | NA | NA | NA | 23.65 b | 42.93 b | 15.20 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murai, T.; Naeve, S.; Annor, G.A. Regional Variability in Sugar and Amino Acid Content of U.S. Soybeans and the Impact of Autoclaving on Reducing Sugars and Free Lysine. Foods 2024, 13, 1884. https://doi.org/10.3390/foods13121884
Murai T, Naeve S, Annor GA. Regional Variability in Sugar and Amino Acid Content of U.S. Soybeans and the Impact of Autoclaving on Reducing Sugars and Free Lysine. Foods. 2024; 13(12):1884. https://doi.org/10.3390/foods13121884
Chicago/Turabian StyleMurai, Takehiro, Seth Naeve, and George A. Annor. 2024. "Regional Variability in Sugar and Amino Acid Content of U.S. Soybeans and the Impact of Autoclaving on Reducing Sugars and Free Lysine" Foods 13, no. 12: 1884. https://doi.org/10.3390/foods13121884




