Nanocomposite Films of Babassu Coconut Mesocarp and Green ZnO Nanoparticles for Application in Antimicrobial Food Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanocomposite Films
2.3. Characterization of Nanocomposite Films
2.3.1. Instrumental Color Analysis
2.3.2. Film Thickness
2.3.3. Optical Properties
2.3.4. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.5. X-ray Diffraction (XRD)
2.3.6. Thermogravimetric Analysis (TGA)
2.3.7. Water Vapor Permeability (WVP)
2.3.8. Mechanical Properties
2.3.9. Scanning Electron Microscopy (SEM)
2.4. Antimicrobial Effect of Nanocomposite Films on Cooked Turkey Ham Samples
2.5. Statistical Analysis
3. Results and Discussion
3.1. Instrumental Color Analysis and Optical Properties
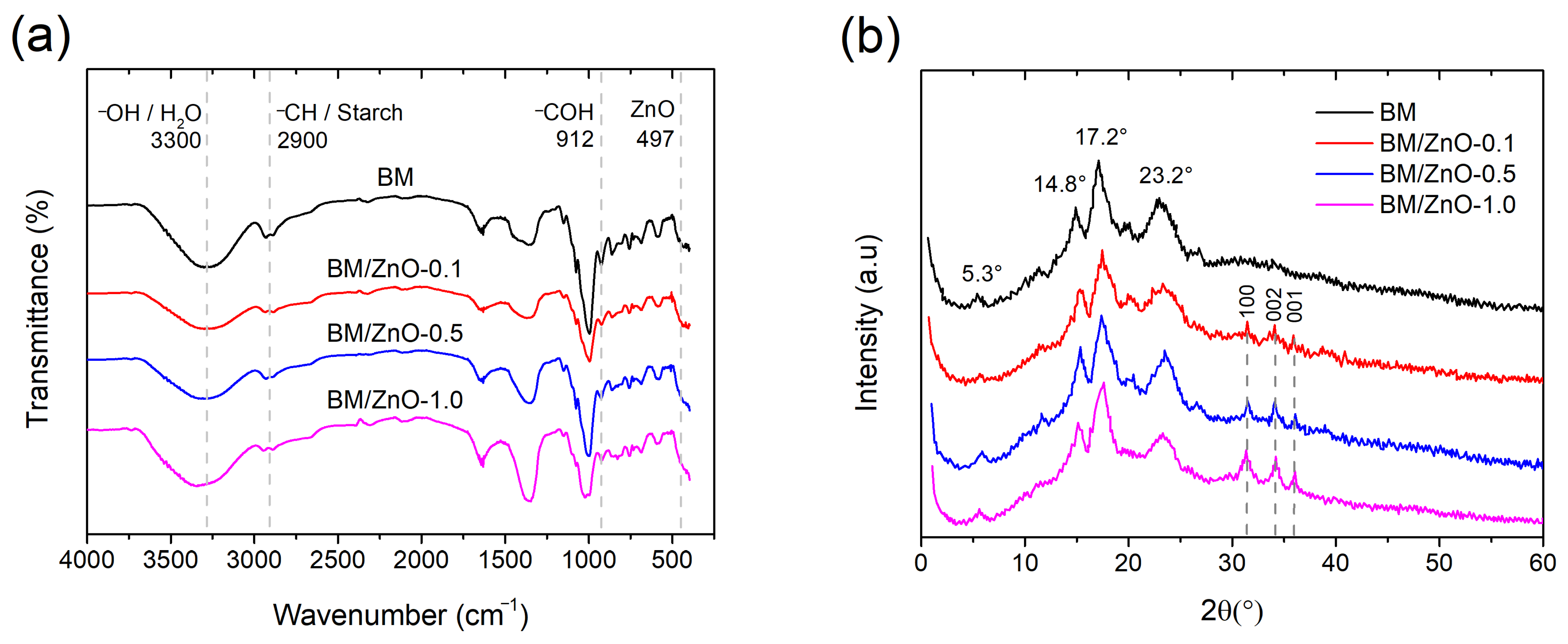
3.2. FTIR and XRD Analysis
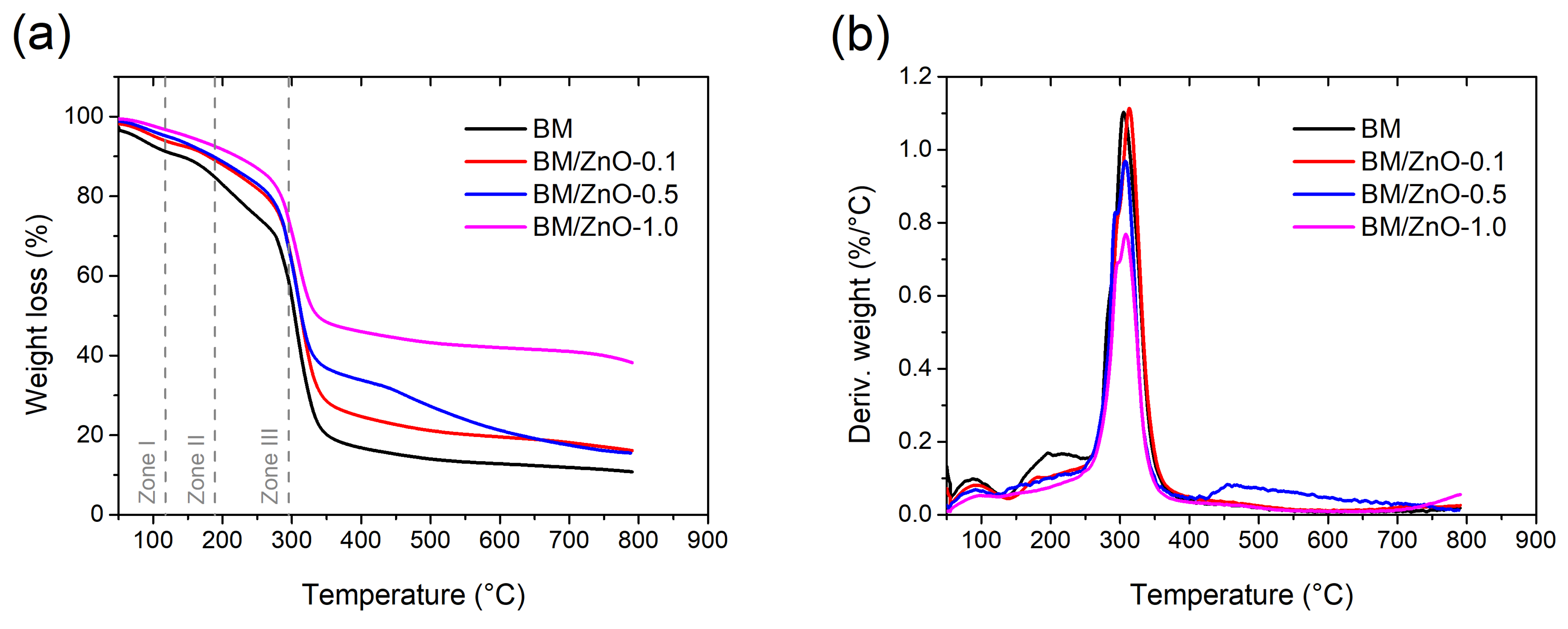
3.3. TGA Analysis
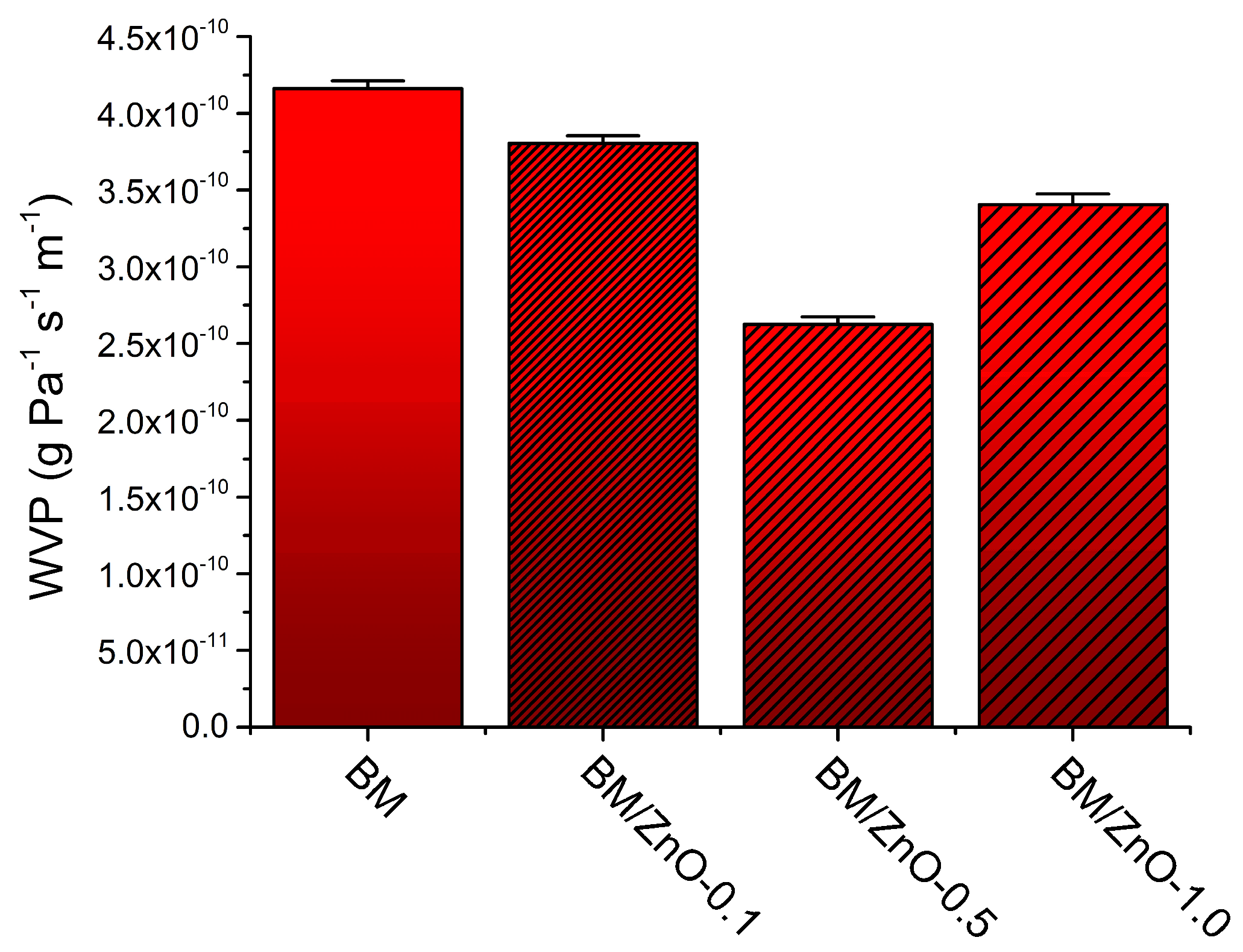
3.4. WVP
3.5. Mechanical Properties
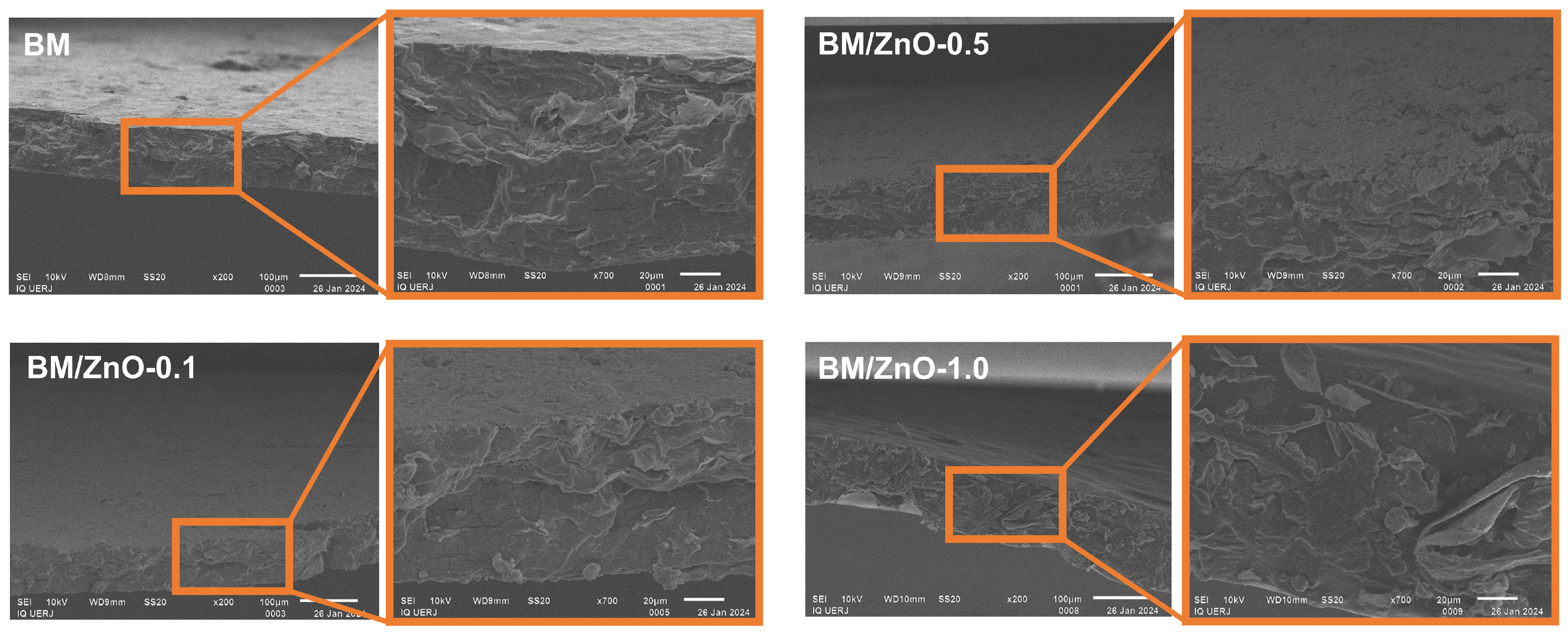
3.6. Morphological Analysis by SEM
3.7. Antimicrobial Effect of Nanocomposite Films on Cooked Turkey Ham Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roohi, S.P.; Bano, K.; Zaheer, M.R.; Kuddus, M. Biodegradable Smart Biopolymers for Food Packaging: Sustainable Approach Toward Green Environment. Bio-Based Mater. Food Packag. Green Sustain. Adv. Packag. Mater. 2018, 1, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Veerendra, G.T.N.; Babu, P.S.S.A.; Manoj, A.V.P.; Nagarjuna, K. Degradation of Plastics Waste and Its Effects on Biological Ecosystems: A Scientific Analysis and Comprehensive Review. Biomed. Mater. Devices 2023, 2, 70–112. [Google Scholar] [CrossRef]
- Zhao, X.; Cornish, K.; Vodovotz, Y. Narrowing the Gap for Bioplastic Use in Food Packaging: An Update. Environ. Sci. Technol. 2020, 54, 4712–4732. [Google Scholar] [CrossRef] [PubMed]
- Wrońska, N.; Katir, N.; Nowak-Lange, M.; El Kadib, A.; Lisowska, K. Biodegradable Chitosan-Based Films as an Alternative to Plastic Packaging. Foods 2023, 12, 3519. [Google Scholar] [CrossRef] [PubMed]
- Andretta, R.; Luchese, C.L.; Tessaro, I.C.; Spada, J.C. Development and Characterization of PH-Indicator Films Based on Cassava Starch and Blueberry Residue by Thermocompression. Food Hydrocoll. 2019, 93, 317–324. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; de Carvalho, A.P.A.; Conte-Junior, C.A. Recent Advances in Biobased and Biodegradable Polymer Nanocomposites, Nanoparticles, and Natural Antioxidants for Antibacterial and Antioxidant Food Packaging Applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3673–3716. [Google Scholar] [CrossRef] [PubMed]
- Saral Sarojini, K.; Indumathi, M.P.; Rajarajeswari, G.R. Mahua Oil-Based Polyurethane/Chitosan/Nano ZnO Composite Films for Biodegradable Food Packaging Applications. Int. J. Biol. Macromol. 2019, 124, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Matheus, J.R.V.; Nogueira, T.B.d.B.; Pereira, A.P.A.; Correia, T.R.; de Sousa, A.M.F.; Pastore, G.M.; Pelissari, F.M.; Miyahira, R.F.; Fai, A.E.C. Antibacterial Films Made with Persimmon (Diospyros kaki L.), Pectin, and Glycerol: An Experimental Design Approach. J. Food Sci. 2021, 86, 4539–4553. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Wang, W.; Kambiz, S.; Alahmed, A.; Simsek, S. Enhancing Mechanical Properties of Corn Bran Arabinoxylan Films for Sustainable Food Packaging. Foods 2024, 13, 1314. [Google Scholar] [CrossRef]
- Souza, M.H.S.L.; Monteiro, C.A.; Figueredo, P.M.S.; Nascimento, F.R.F.; Guerra, R.N.M. Ethnopharmacological Use of Babassu (Orbignya phalerata Mart) in Communities of Babassu Nut Breakers in Maranhão, Brazil. J. Ethnopharmacol. 2011, 133, 1–5. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; Silva, A.C.L.N.; Castro, N.R.; Pinto, C.d.S.C.; de Freitas, Z.M.F.; Ricci-Júnior, E.; Dos Santos, E.P.; Camara, A.L.; Costa, M.C.P.; Conte-Junior, C.A. Development and Characterization of Photoprotective Nanoemulsions Containing Babassu (Orbignya phalerata Mart.) Lipophilic Extract. Braz. J. Pharm. Sci. 2023, 59, e23011. [Google Scholar] [CrossRef]
- de Farias, G.B.; da Silva Rodrigues, J.L.; de Sousa Ribeiro, M.N.; Magalhães, L.O.; Ferreira, A.G.; da Paz Lima, M. Chemical Constituent Analysis of the Babassu (Orbignya phalerata Mart.) Mesocarp. Univ. Sci. 2019, 24, 323–335. [Google Scholar] [CrossRef]
- Lima, R.C.; de Carvalho, A.P.A.; Lelis, C.A.; Faria, D.J.; da Silva, B.D.; da Silva de Figueiredo, M.R.; Chaves, P.H.T.; de Almeida, A.E.C.C.; Conte-Junior, C.A. An Innovative Alternative to Reduce Sodium in Cheese: Babassu Coconut Byproduct Improving Quality and Shelf-Life of Reduced-sodium Minas Fresh Cheese. Innov. Food Sci. Emerg. Technol. 2024, 92, 103601. [Google Scholar] [CrossRef]
- Barroqueiro, T.L.S.; Maciel, M.C.G.; Vale, A.A.M.; Silva, M.C.P.; dos Santos Maia, A.C.; dos Santos, A.P.A.; do Nascimento, J.R.; do Nascimento, F.R.F.; Rocha, C.Q.; Fernandes, E.S.; et al. The Anti-Infective and Immunologic Effect of Babassu (Attalea speciosa, Mart. Ex Spreng) Reduces Mortality Induced by MRSA-Staphylococcus aureus. J. Ethnopharmacol. 2024, 320, 117363. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.C.; Lopes, I.A.; Da Silva, L.J.S.; Lima, M.F.; Barros Filho, A.K.D.; Villa-Vélez, H.A.; Santana, A.A. Physical Properties of Films Based on Pectin and Babassu Coconut Mesocarp. Int. J. Biol. Macromol. 2019, 130, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Alves Lopes, I.; Coelho Paixão, L.; Souza da Silva, L.J.; Almeida Rocha, A.; Allan, A.K.; Amorim Santana, A. Elaboration and Characterization of Biopolymer Films with Alginate and Babassu Coconut Mesocarp. Carbohydr. Polym. 2020, 234, 115747. [Google Scholar] [CrossRef]
- Saraiva Rodrigues, S.C.; da Silva, A.S.; de Carvalho, L.H.; Alves, T.S.; Barbosa, R. Morphological, Structural, Thermal Properties of a Native Starch Obtained from Babassu Mesocarp for Food Packaging Application. J. Mater. Res. Technol. 2020, 9, 15670–15678. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Tessaro, L.; Lucas, A.A.; Tapia-Blácido, D.R. Bioactive Films Based on Babassu Mesocarp Flour and Starch. Food Hydrocoll. 2017, 70, 383–391. [Google Scholar] [CrossRef]
- Tan, C.; Han, F.; Zhang, S.; Li, P.; Shang, N. Novel Bio-Based Materials and Applications in Antimicrobial Food Packaging: Recent Advances and Future Trends. Int. J. Mol. Sci. 2021, 22, 9663. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; da Silva, A.A.; da Silva, B.D.; Neto, L.T.; Tessaro, L.; Furtado, C.R.G.; de Sousa, A.M.F.; Carvalho, N.M.F.; Conte-Junior, C.A. Eco-Friendly Synthesis of ZnO Nanomaterial from Green Tea Extract: Photocatalytic, Antibacterial and Antioxidant Potential. Biomass Convers. Biorefinery 2023, 1, 1–15. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ahmed, L.; Anika, F.; Riya, A.A.; Kali, S.K.; Rauf, A.; Sharma, R. Bioinorganic Nanoparticles for the Remediation of Environmental Pollution: Critical Appraisal and Potential Avenues. Bioinorg. Chem. Appl. 2023, 2023, 2409642. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.; Namratha, K.; Thakur, M.S.; Byrappa, K. Biocompatibility Assessment and Photocatalytic Activity of Bio-Hydrothermal Synthesis of ZnO Nanoparticles by Thymus Vulgaris Leaf Extract. Mater. Res. Bull. 2019, 109, 49–59. [Google Scholar] [CrossRef]
- Agarwal, H.; Venkat Kumar, S.; Rajeshkumar, S. A Review on Green Synthesis of Zinc Oxide Nanoparticles—An Eco-Friendly Approach. Resour. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; da Silva, A.A.; da Silva, B.D.; Torres Neto, L.; Tessaro, L.; Lima, A.K.O.; Garcia, M.P.; Ribeiro, J.A.d.A.; Rodrigues, C.M.; de Sousa, A.M.F.; et al. Antioxidant, Antimicrobial, and Anticancer Potential of Green Synthesized ZnO Nanoparticles from Açaí (Euterpe oleracea Mart.) Berry Seed Residue Extract. Waste Biomass Valorization 2024, 1, 1–18. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, G.; Cao, L.; Wang, L. Accurately Intelligent Film Made from Sodium Carboxymethyl Starch/κ-Carrageenan Reinforced by Mulberry Anthocyanins as an Indicator. Food Hydrocoll. 2020, 108, 106012. [Google Scholar] [CrossRef]
- Kalia, A.; Kaur, M.; Shami, A.; Jawandha, S.K.; Alghuthaymi, M.A.; Thakur, A.; Abd-Elsalam, K.A. Nettle-Leaf Extract Derived ZnO/CuO Nanoparticle-Biopolymer-Based Antioxidant and Antimicrobial Nanocomposite Packaging Films and Their Impact on Extending the Post-Harvest Shelf Life of Guava Fruit. Biomol. 2021, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Raposo, A.K.d.S.; Paixão, L.C.; Rocha, A.A.; Lopes, I.A.; Santos, G.A.S.; Ribeiro, G.A.C.; de Menezes, A.S.; Barros Filho, A.K.D.; Santana, A.A. Characterization of Biodegradable Films Produced from Mixtures of Alginate, Starch and Babassu Fibers. J. Polym. Environ. 2021, 29, 1212–1226. [Google Scholar] [CrossRef]
- CLSI M100|Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute Document; CLSI: Wayne, PA, USA, 2006; Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 8 November 2023).
- Dutra da Silva, B.; Bernardes, P.C.; Pinheiro, P.F.; Di Giorgio Giannotti, J.; Roberto, C.D. Plectranthus amboinicus (Lour.) Spreng. Essential Oil as a Natural Alternative for the Conservation of Beef Patties Stored under Refrigeration. Food Biosci. 2022, 49, 101896. [Google Scholar] [CrossRef]
- Spampinato, G.; Candeliere, F.; Amaretti, A.; Licciardello, F.; Rossi, M.; Raimondi, S. Microbiota Survey of Sliced Cooked Ham During the Secondary Shelf Life. Front. Microbiol. 2022, 13, 842390. [Google Scholar] [CrossRef]
- Ramos-Escudero, F.; Gómez-Coca, R.B.; Muñoz, A.M.; Fuente-Carmelino, L.D.L.; Pérez-Camino, M.d.C. Oil From Three Aguaje Morphotypes (Mauritia flexuosa L.f.) Extracted by Supercritical Fluid With CO2: Chemical Composition and Chromatic Properties. Front. Sustain. Food Syst. 2022, 6, 843772. [Google Scholar] [CrossRef]
- Yi, F.; Chen, X.; Hou, F.; Song, L.; Zhan, S.; Wang, X.; Zhang, R.; Yang, Q.; Wang, X.; Liu, Z. Chitosan/Zein-Based Sustained-Release Composite Films: Fabrication, Physicochemical Properties and Release Kinetics of Tea Polyphenols from Polymer Matrix. Int. J. Biol. Macromol. 2024, 269, 131970. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Ho, T.C.; Park, J.S.; Han, J.M.; Surendhiran, D.; Lee, H.J.; Zhang, W.; Chun, B.S. Fabrication of Smart Film Based on Fish Gelatin Incorporating Phycoerythrin and Cellulose Nanofibrils to Monitor Fish Freshness. Food Biosci. 2024, 59, 104111. [Google Scholar] [CrossRef]
- Homthawornchoo, W.; Kaewprachu, P.; Pinijsuwan, S.; Romruen, O.; Rawdkuen, S. Enhancing the UV-Light Barrier, Thermal Stability, Tensile Strength, and Antimicrobial Properties of Rice Starch–Gelatin Composite Films through the Incorporation of Zinc Oxide Nanoparticles. Polymers 2022, 14, 2505. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.K.; Yadav, M.; Maurya, P.; Fatima, A.; Yadav, D. UV-Resistant Gellan Gum Film Reinforced with Chitosan Nanoparticle for Eco-Friendly Packaging. Emergent Mater. 2024, 1, 1–15. [Google Scholar] [CrossRef]
- Kumar, P.; Gautam, S.; Bansal, D.; Kaur, R. Starch-Based Antibacterial Food Packaging with ZnO Nanoparticle. J. Food Sci. Technol. 2024, 61, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Vieira, I.R.S.; Costa, L.d.F.d.O.; Miranda, G.d.S.; Nardecchia, S.; Monteiro, M.S.d.S.d.B.; Ricci-Júnior, E.; Delpech, M.C. Waterborne Poly(Urethane-Urea)s Nanocomposites Reinforced with Clay, Reduced Graphene Oxide and Respective Hybrids: Synthesis, Stability and Structural Characterization. J. Polym. Environ. 2020, 28, 74–90. [Google Scholar] [CrossRef]
- Teixeira, P.R.S.; Teixeira, A.S.D.N.M.; de Oliveira Farias, E.A.; da Silva, D.A.; Nunes, L.C.C.; da Silva Leite, C.M.; da Silva Filho, E.C.; Eiras, C. Chemically Modified Babassu Coconut (Orbignya Sp.) Biopolymer: Characterization and Development of a Thin Film for Its Application in Electrochemical Sensors. J. Polym. Res. 2018, 25, 1–11. [Google Scholar] [CrossRef]
- Rodrigues Sousa, H.; Sá Lima, I.; Matheus Lima Neris, L.; Santos Silva, A.; Maria Silva Santos Nascimento, A.; Pereira de Araújo, F.; Felippe Ratke, R.; Anteveli Osajima, J.; Loiola Edvan, R.; Kauany da Silva Azevedo, C.; et al. Innovative Hydrogels Made from Babassu Mesocarp for Technological Application in Agriculture. J. Mol. Liq. 2023, 376, 121463. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, D.; Cai, J. Robust Design of Starch Composite Nanofibrous Films for Active Food Packaging: Towards Improved Mechanical, Antioxidant, and Antibacterial Properties. Int. J. Biol. Macromol. 2024, 260, 129329. [Google Scholar] [CrossRef]
- Abdullah, A.H.D.; Putri, O.D.; Fikriyyah, A.K.; Nissa, R.C.; Hidayat, S.; Septiyanto, R.F.; Karina, M.; Satoto, R. Harnessing the Excellent Mechanical, Barrier and Antimicrobial Properties of Zinc Oxide (ZnO) to Improve the Performance of Starch-Based Bioplastic. Polym. Technol. Mater. 2020, 59, 1259–1267. [Google Scholar] [CrossRef]
- Lian, R.; Cao, J.; Jiang, X.; Rogachev, A.V. Physicochemical, Antibacterial Properties and Cytocompatibility of Starch/Chitosan Films Incorporated with Zinc Oxide Nanoparticles. Mater. Today Commun. 2021, 27, 102265. [Google Scholar] [CrossRef]
- Marra, A.; Silvestre, C.; Duraccio, D.; Cimmino, S. Polylactic Acid/Zinc Oxide Biocomposite Films for Food Packaging Application. Int. J. Biol. Macromol. 2016, 88, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Tang, P.; Li, G. Development of Composite Film Based on Collagen and Phenolic Acid-Grafted Chitosan for Food Packaging. Int. J. Biol. Macromol. 2023, 241, 124494. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Fan, F.; Chu, Z.; Fan, C.; Qin, Y. Barrier Properties and Characterizations of Poly(Lactic Acid)/ZnO Nanocomposites. Molecules 2020, 25, 1310. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M.S.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Hussain, A. Preparation and Characterization of PVA/Nanocellulose/Ag Nanocomposite Films for Antimicrobial Food Packaging. Carbohydr. Polym. 2018, 184, 453–464. [Google Scholar] [CrossRef]
- Vasile, C. Polymeric Nanocomposites and Nanocoatings for Food Packaging: A Review. Materials 2018, 11, 1834. [Google Scholar] [CrossRef]
- Hu, X.; Jia, X.; Zhi, C.; Jin, Z.; Miao, M. Improving the Properties of Starch-Based Antimicrobial Composite Films Using ZnO-Chitosan Nanoparticles. Carbohydr. Polym. 2019, 210, 204–209. [Google Scholar] [CrossRef]
- Cruzen, S.M.; Cetin-Karaca, H.; Tarté, R.; Sebranek, J.G.; Dickson, J.S. Survival of Clostridium perfringens, Staphylococcus aureus, Listeria monocytogenes and Salmonella enterica in Alternatively Cured Ham during Cooking and Process Deviations. LWT 2022, 163, 113347. [Google Scholar] [CrossRef]
- Lima, R.C.; de Carvalho, A.P.A.; da Silva, B.D.; Torres Neto, L.; de Figueiredo, M.R.d.S.; Chaves, P.H.T.; de Almeida, A.E.C.C.; Conte-Junior, C.A. Green Ultrasound-Assisted Extraction of Bioactive Compounds of Babassu (Attalea speciosa) Mesocarp: Effects of Solid-Liquid Ratio Extraction, Antioxidant Capacity, and Antimicrobial Activity. Appl. Food Res. 2023, 3, 100331. [Google Scholar] [CrossRef]
- Wang, C.; Chang, T.; Dong, S.; Zhang, D.; Ma, C.; Chen, S.; Li, H. Biopolymer Films Based on Chitosan/Potato Protein/Linseed Oil/ZnO NPs to Maintain the Storage Quality of Raw Meat. Food Chem. 2020, 332, 127375. [Google Scholar] [CrossRef]
- Yasmeen, F.; Karamat, H.; Rehman, R.; Akram, M.; Ghfar, A.A.; Abdelghani, H.T.M.; Dar, A.; Mitu, L. Fabrication and Testing of Edible Films Incorporated with ZnO Nanoparticles to Enhance the Shelf Life of Bread. Food Biosci. 2023, 56, 103111. [Google Scholar] [CrossRef]




| Samples | Instrumental Color Parameters | Opacity | ||||
|---|---|---|---|---|---|---|
| L* | a* | b* | C* | h° | ||
| BM | 56.61 ± 0.58 | 15.58 ± 0.08 | 39.90 ± 0.03 | 42.84 ± 0.06 | 68.67 ± 0.08 | 10.62 ± 0.02 |
| BM/ZnO-0.1 | 41.32 ± 0.69 | 24.03 ± 0.08 | 35.41 ± 0.91 | 42.79 ± 0.80 | 55.83 ± 0.60 | 18.65 ± 0.04 |
| BM/ZnO-0.5 | 40.08 ± 0.65 | 20.38 ± 0.07 | 29.76 ± 0.75 | 36.07 ± 0.67 | 55.58 ± 0.58 | 21.52 ± 0.01 |
| BM/ZnO-1.0 | 33.77 ± 0.98 | 12.29 ± 0.16 | 21.51 ± 1.13 | 24.77 ± 1.06 | 60.22 ± 0.98 | 12.01 ± 0.02 |
| Treatments | 0D | 2D | 6D | 7D |
|---|---|---|---|---|
| C | 4.93 ± 0.15 Aa | 4.85 ± 0.02 Aa | 4.68 ± 0.02 Aa | 4.58 ± 0.04 Aa |
| BM | 4.91 ± 0.14 Aa | 4.89 ± 0.16 Aa | 4.15 ± 0.06 Bb | 4.18 ± 0.03 Bb |
| BM/ZnO-0.5 | 4.90 ± 0.01 Aa | 4.60 ± 0.15 Aa | 3.64 ± 0.08 Cb | 3.84 ± 0.20 Bb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirres, A.C.d.M.; Vieira, I.R.S.; Tessaro, L.; da Silva, B.D.; de Andrade, J.C.; da Silva, A.A.; Carvalho, N.M.F.; de Sousa, A.M.F.; Conte-Junior, C.A. Nanocomposite Films of Babassu Coconut Mesocarp and Green ZnO Nanoparticles for Application in Antimicrobial Food Packaging. Foods 2024, 13, 1895. https://doi.org/10.3390/foods13121895
Mirres ACdM, Vieira IRS, Tessaro L, da Silva BD, de Andrade JC, da Silva AA, Carvalho NMF, de Sousa AMF, Conte-Junior CA. Nanocomposite Films of Babassu Coconut Mesocarp and Green ZnO Nanoparticles for Application in Antimicrobial Food Packaging. Foods. 2024; 13(12):1895. https://doi.org/10.3390/foods13121895
Chicago/Turabian StyleMirres, Ana Carolina de Morais, Italo Rennan Sousa Vieira, Leticia Tessaro, Bruno Dutra da Silva, Jelmir Craveiro de Andrade, Arianne Aparecida da Silva, Nakédia M. F. Carvalho, Ana Maria Furtado de Sousa, and Carlos Adam Conte-Junior. 2024. "Nanocomposite Films of Babassu Coconut Mesocarp and Green ZnO Nanoparticles for Application in Antimicrobial Food Packaging" Foods 13, no. 12: 1895. https://doi.org/10.3390/foods13121895










