Preparation and Immobilization Mechanism on a Novel Composite Carrier PDA-CF/PUF to Improve Cells Immobilization and Xylitol Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Strains and Medium
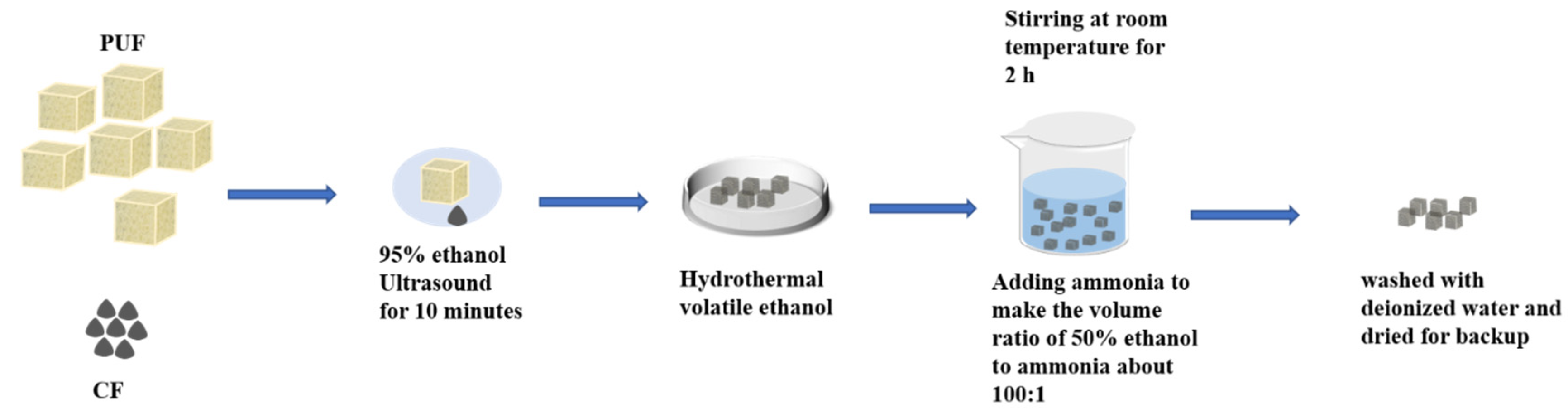
2.2. Preparation of Carrier
2.3. Structural and Performance Characterization of Composite Carriers
2.4. Biocompatibility Analysis of Composite Carrier
2.4.1. Hydrophilicity Detection of Carrier
2.4.2. Cell Viability by MTT Assay
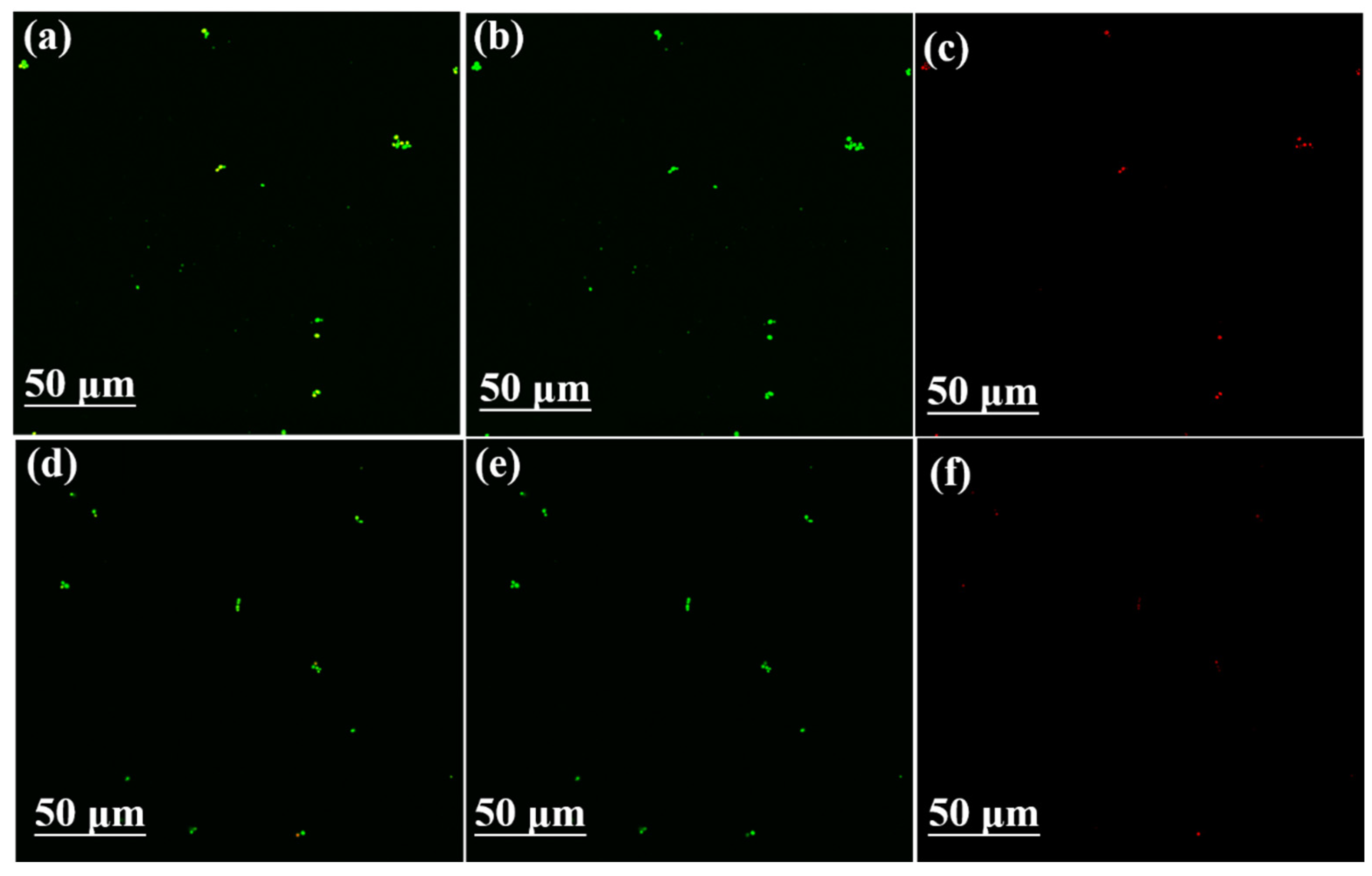
2.4.3. Cell Viability by Confocal Laser Scanning Microscope (CLSM)
2.4.4. ROS Levels by Flow Cytometry
2.4.5. Mitochondrial Membrane Potential by Flow Cytometry
2.5. Effects of Composite Carrier on Biomass and Cell Immobilization Efficiency
2.6. Effects of Composite Carrier on Multi-Batch Fermentation of Immobilized Cells
2.7. Effects of Immobilization on Expression Levels of Genes Related to Xylitol Synthesis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effect of Surface Morphology Detection of Composite Carrier on Cells Immobilization
3.2. Influence of Hydrophilicity and Chemical Structure of Composite Carrier on Cells Immobilization
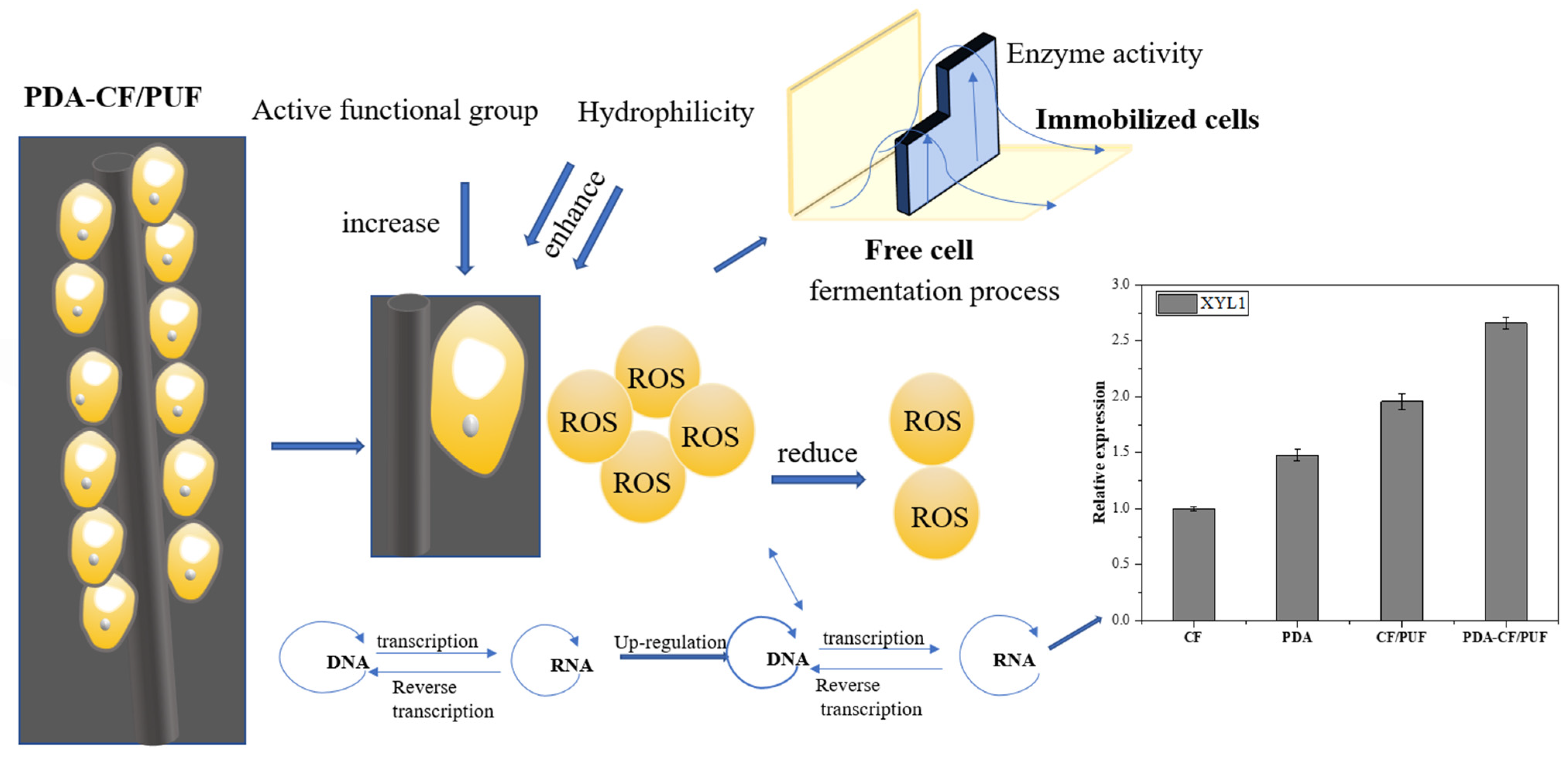
3.3. Effect of PDA-CF/PUF Composite Carrier on Biocompatibility of Immobilized Cells and Expression of Genes Related to Xylitol Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahuja, V.; Macho, M.; Ewe, D.; Singh, M.; Saha, S.; Saurav, K. Biological and Pharmacological Potential of Xylitol: A Molecular Insight of Unique Metabolism. Foods 2020, 9, 1592. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, V.; Sharma, A.; Rathour, R.K.; Sharma, V.; Rana, N.; Bhatt, A.K. In-Vitro and In-Silico Characterization of Xylose Reductase from Emericella nidulans. Curr. Chem. Biol. 2019, 13, 159–170. [Google Scholar] [CrossRef]
- Ur-Rehman, S.; Mushtaq, Z.; Zahoor, T.; Jamil, A.; Murtaza, M.A. Xylitol: A review on bioproduction, application, health benefits, and related safety issues. Crit. Rev. Food. Sci. Nutr. 2015, 55, 1514–1528. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, J.; Zhang, H.; Zheng, C.; Wei, R.; Gao, Y.; Yang, L. Efficient nitrate removal by Pseudomonas mendocina GL6 immobilized on biochar. Bioresour. Technol. 2021, 320, 124324. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, X.; Ai, S.; Huang, Y.; Yang, X.; Mei, Y.; Zhang, K.; Wang, H. The effective astaxanthin productivities of immobilized Haematococcus pluvialis with bacterial cellulose. Bioresour. Technol. 2022, 344, 126317. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jia, F.; Wu, D.; Wei, Q.; Liang, Y.; Hu, U.; Li, R.; Yu, G.; Yuan, Q. In-situ growth of graphene on carbon fibers for enhanced cell immobilization and xylitol fermentation. Appl. Surf. Sci. 2020, 527, 146793. [Google Scholar] [CrossRef]
- Wang, L.; Yin, Y.; Zhang, S.; Wu, D.; Lv, Y.; Hu, Y.; Wei, Q.; Yuan, Q.; Wang, J. A rapid microwave-assisted phosphoric-acid treatment on carbon fiber surface for enhanced cell immobilization in xylitol fermentation. Colloid Surf. B Biointerfaces 2019, 175, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, N.; Guo, Z.; Wu, D.; Chen, W.; Chang, Z.; Yuan, Q.; Hui, M.; Wang, J. Nitric Acid-Treated Carbon Fibers with Enhanced Hydrophilicity for Candida tropicalis Immobilization in Xylitol Fermentation. Materials 2016, 17, 206. [Google Scholar] [CrossRef]
- Chen, D.Z.; Fang, J.Y.; Shao, Q.; Ye, J.X.; Ouyang, D.J.; Chen, J.M. Biodegradation of tetrahydrofuran by Pseudomonas oleovorans DT4 immobilized in calcium alginate beads impregnated with activated carbon fiber: Mass transfer effect and continuous treatment. Bioresour. Technol. 2013, 139, 87–93. [Google Scholar] [CrossRef]
- Oda, M.; Kaieda, M.; Hama, S.; Yamaji, H.; Kondo, A.; Izumoto, E.; Fukuda, H. Facilitatory effect of immobilized lipase-producing Rhizopus oryzae cells on acyl migration in biodiesel-fuel production. Biochem. Eng. J. 2005, 23, 45–51. [Google Scholar] [CrossRef]
- Nyari, N.; Paulazzi, A.; Zamadei, R.; Steffens, C.; Zabot, G.L.; Tres, M.V.; Zeni, J.; Venquiaruto, L.; Dallago, R.M. Synthesis of isoamyl acetate by ultrasonic system using candida antarctica lipase B immobilized in polyurethane. J. Food Process Eng. 2018, 41, 12812. [Google Scholar] [CrossRef]
- Wang, L.; Wu, D.; Tang, P.; Fan, X.; Yuan, Q. Xylitol production from corncob hydrolysate using polyurethane foam with immobilized Candida tropicalis. Carbohydr. Polym. 2012, 90, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Khan, A.; Huang, H.; Tian, Y.; Yu, X.; Xu, Q.; Mou, L.; Lv, J.; Zhang, P.; Liu, P.; et al. Using nano-attapulgite clay compounded hydrophilic urethane foams (AT/HUFs) as biofilm support enhances oil-refinery wastewater treatment in a biofilm membrane bioreactor. Sci. Total Environ. 2019, 646, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gong, L.; Mao, Q.; Han, P.; Lu, X.; Qu, J. Laccase immobilization and surface modification of activated carbon fibers by bio-inspired poly-dopamine. RSC Adv. 2018, 8, 14414–14421. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, X.; Yu, J.; Wang, Y.; Zhu, J.; Hu, Z. Surface modification of UHMWPE/fabric composite membrane via self-polymerized polydopamine followed by mPEG-NH2 immobilization. J. Appl. Polym. Sci. 2018, 135, 46428. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Hong, S.; Na, Y.S.; Choi, S.; Song, I.T.; Kim, W.Y.; Lee, H. Non-Covalent Self-Assembly and Covalent Polymerization Co-Contribute to Polydopamine Formation. Adv. Funct. Mater. 2012, 22, 4711–4717. [Google Scholar] [CrossRef]
- Kopeć, K.; Wojasiński, M.; Ciach, T. Superhydrophilic Polyurethane/Polydopamine Nanofibrous Materials Enhancing Cell Adhesion for Application in Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 6798. [Google Scholar] [CrossRef]
- Alfieri, M.L.; Weil, T.; Ng, D.Y.W.; Ball, V. Polydopamine at biological interfaces. Adv. Colloid Interface 2022, 305, 102689. [Google Scholar] [CrossRef]
- Wang, J.; Ren, K.; Chang, H.; Jia, F.; Li, B.; Ji, Y.; Ji, J. Direct Adhesion of Endothelial Cells to Bioinspired Poly(dopamine) Coating Through Endogenous Fibronectin and Integrin α5β1. Macromol. Biosci. 2013, 13, 483–493. [Google Scholar] [CrossRef]
- Firoozi, N.; Kang, Y. Immobilization of FGF on Poly(xylitol dodecanedioic Acid) Polymer for Tissue Regeneration. Sci. Rep. 2020, 10, 10419. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Kim, K.Y.; Wook, H.J.; Park, S.Y.; Lee, K.D.; Lee, D.Y.; Lee, H. Attenuation of thein vivo toxicity of biomaterials by polydopamine surface modification. Nanomedicine 2011, 6, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Chi, H.; Yang, X.; Peng, J.; Wang, W.; Tang, D. Polydopamine-assisted shape memory of polyurethane nanofibers with light-induced tunable responsiveness and improved cell adhesiveness. Colloid Surf. A Physicochem. Eng. Asp. 2021, 627, 127100. [Google Scholar] [CrossRef]
- Sumitra, D.; Rene, L.C.; Sriramulu, R.Y.R. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Xiong, J.; Xiang, G. Enzyme immobilization on functionalized monolithic CNTs-Ni foam composite for highly active and stable biocatalysis in organic solvent. Mol. Catal. 2020, 483, 110714. [Google Scholar] [CrossRef]
- Yushkova, E.D.; Nazarova, E.A.; Matyuhina, A.V.; Noskova, A.O.; Shavronskaya, D.O.; Vinogradov, V.V.; Skvortsova, N.N.; Krivoshapkina, E.F. Application of Immobilized Enzymes in Food Industry. J. Agric. Food Chem. 2019, 67, 11553–11567. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, Z.; Yuan, Q. Xylose enhances furfural tolerance in Candida tropicalis by improving NADH recycle. Chem. Eng. Sci. 2017, 158, 37–40. [Google Scholar] [CrossRef]
- Wang, L.; Shen, Y.; Zhang, Y.; Wei, Q.; Liang, Y.; Tian, H.; Wu, D.; Yuan, X.; Yuan, Q.; Wang, J. A novel surface treatment of carbon fiber with Fenton reagent oxidization for improved cells immobilization and xylitol fermentation. Microporous Mesoporous Mater. 2021, 325, 111318. [Google Scholar] [CrossRef]
- Shahid, M.; Zaidi, A.; Ehtram, A.; Khan, M.S. In vitro investigation to explore the toxicity of different groups of pesticides for an agronomically important rhizosphere isolate Azotobacter vinelandii. Pestic. Biochem. Phys. 2019, 157, 33–44. [Google Scholar] [CrossRef]
- Wang, L.; Sha, Y.; Wu, D.; Wei, Q.; Chen, D.; Yang, S.; Jia, F.; Yuan, Q.; Han, X.; Wang, J. Surfactant induces ROS-mediated cell membrane permeabilization for the enhancement of mannatide production. Process Biochem. 2020, 91, 172–180. [Google Scholar] [CrossRef]
- Wang, Z.; Su, G.; Zhang, Z.; Dong, H.; Wang, Y.; Zhao, H.; Zhao, Y.; Sun, Q. 25-Hydroxyl-protopanaxatriol protects against H2O2-induced H9c2 cardiomyocytes injury via PI3K/Akt pathway and apoptotic protein down-regulation. Biomed. Pharmacother. 2018, 99, 33–42. [Google Scholar] [CrossRef]
- Cavero-Olguin, V.H.; Hatti-Kaul, R.; Cardenas-Alegria, O.V.; Gutierrez-Valverde, M.; Alfaro-Flores, A.; Romero-Calle, D.X.; Alvarez-Aliaga, M.T. Stress induced biofilm formation in Propionibacterium acidipropionici and use in propionic acid production. World J. Microbiol. Biotechnol. 2019, 35, 101. [Google Scholar] [CrossRef]
- Kang, S.M.; Rho, J.; Choi, I.S.; Messersmith, P.B.; Lee, H. Norepinephrine: Material-independent, multifunctional surface modification reagent. J. Am. Chem. Soc. 2009, 131, 13224–13225. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Edmondson, S. Polydopamine-melanin initiators for Surface-initiated ATRP. Polymer 2011, 52, 2141–2149. [Google Scholar] [CrossRef]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile Conjugation of Biomolecules onto Surfaces via Mussel Adhesive Protein Inspired Coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef]
- Bernsmann, F.; Frisch, B.; Ringwald, C.; Ball, V. Protein adsorption on dopamine–melanin films: Role of electrostatic interactions inferred from ζ-potential measurements versus chemisorption. J. Colloid Interface Sci. 2009, 344, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, Z.; Pu, X.; Chen, X.; Yin, G.; Wang, Y.; Miao, D.; Fan, J.; Mu, J. Polydopamine carboxylic graphene oxide-composited polypyrene films for promoting adhesion and alignment of Schwann cells. Colloids Surf. B Biointerfaces 2020, 191, 110972. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sha, Y.; Fan, Y.; Liang, Y.; Wu, D.; Wang, Q.; Zhang, X.; Gao, R.; Yuan, Q.; Wang, J. Electrospun nanofibers enhance trehalose synthesis by regulating gene expression for Micrococcus luteus fermentation. Colloids Surf. B Biointerfaces 2021, 202, 111714. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, W.; Fan, S.; Qiu, B.; Wang, Y.; Xiao, Z.; Tang, X.; Wang, W.; Jian, S.; Qin, Y. Coproduction of hydrogen and butanol by Clostridium acetobutylicum with the biofilm immobilized on porous particulate carriers. Int. J. Hydrog. Energy 2019, 44, 11617–11624. [Google Scholar] [CrossRef]
- Song, B.; Wang, T.; Sun, H.; Liu, H.; Mai, X.; Wang, X.; Wang, L.; Wang, N.; Huang, Y.; Guo, Z. Graphitic carbon nitride (g-C3N4) interracially strengthened carbon fiber epoxy composites. Compos. Sci. Technol. 2018, 167, 515–521. [Google Scholar] [CrossRef]
- Ren, L.; Tang, Z.; Du, J.; Chen, L.; Qiang, T. Recyclable polyurethane foam loaded with carboxymethyl chitosan for adsorption of methylene blue. J. Hazard. Mater. 2021, 4, 17126130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, L.; Su, Y.; Yu, H.; Liu, H.; Qian, S.; Zheng, W.; Zhao, Y. Zr-MOFs loaded on polyurethane foam by polydopamine for enhanced dye adsorption. J. Environ. Sci. 2021, 101, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Mustafov, S.D.; Sen, F.; Seydibeyoglu, M.O. Preparation and characterization of diatomite and hydroxyapatite reinforced porous polyurethane foam bio composites. Sci. Rep. 2020, 10, 13308. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tong, R.; Wang, Z.; Xia, H. Polydopamine Particles Filled Shape Memory Polyurethane Composites with Fast Near-infrared Light Responsibility. ChemPhysChem 2018, 19, 2052–2057. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Oshinaga, K.; Bu, X.R.; Zhang, M. Low temperature fabrication & photo catalytical activity of carbon fiber-supported TiO2 with different phase compositions. J. Hazard. Mater. 2015, 290, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, K.K.; Cazetta, A.L.; de Souza, P.S.C.; Spessato, L.; Silva, T.L.; Almeida, V.C. Adsorption of caffeine on mesoporous activated carbon fibers prepared from pineapple plant leaves. Ecotoxicol. Environ. Saf. 2018, 147, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Leng, W.; Li, J.; Cai, Z. Synthesis and Characterization of Cellulose Nanofibril-Reinforced Polyurethane Foam. Polymers 2017, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Wang, D.; Ke, J.; Ma, L.; Zhou, J.; Shao, H.; Zhu, H.; Liu, L.; Zhang, Y.U.; Peng, F.; et al. Improved in vitro angiogenic behavior of human umbilical vein endothelial cells with oxidized polydopamine coating. Colloids Surf. B Biointerfaces 2020, 194, 111176. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, Y.; Liao, Y.; Tian, M.; Zou, H.; Zhang, L. Fabrication of silver-coated silica microspheres through mussel-inspired surface functionalization. J. Colloid Interface Sci. 2011, 358, 567–574. [Google Scholar] [CrossRef]
- Davidsen, M.B.; Teixeira, J.F.L.; Dehli, J.; Karlsson, C.; Kraft, D.; Souza, P.P.C.; Foss, M. Post-treatments of polydopamine coatings influence cellular response. Colloid Surf. B Biointerfaces 2021, 207, 111972. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Wang, J.; Liu, C.; Liu, W.; Jian, X. Improving Hydrophilicity and Inducing Bone-Like Apatite Formation on PPBES by Polydopamine Coating for Biomedical Application. Molecules 2018, 23, 1643. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, H.; Liu, Z.; Wang, Y.; Lin, D.; Chen, L.; Dai, J.; Lin, K.; Shen, S.G. Polydopamine nanoparticles as dual-task platform for osteoarthritis therapy: A scavenger for reactive oxygen species and regulator for cellular powerhouses. Chem. Eng. J. 2021, 417, 129284. [Google Scholar] [CrossRef]
- Chaudhary, N.; Joshi, N.; Doloi, R.; Shivashankar, A.; Thorat, R.; Dalal, S.N. Plakophilin3 loss leads to an increase in autophagy and radio-resistance. Biochem. Bioph. Res. Commun. 2022, 620, 1–7. [Google Scholar] [CrossRef]
- Chen, B.; Wu, S.; Ye, Q. Fabrication and characterization of biodegradable KH560 crosslinked chitin hydrogels with high toughness and good biocompatibility. Carbohyd. Polym. 2021, 259, 117707. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, L.; Zhang, S.; He, R.; Wu, Y.; Chen, G.; Fu, Z. Cadmium exposure to murine macrophages decreases their inflammatory responses and increases their oxidative stress. Chemosphere 2016, 144, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, X.; Zhu, Y.; He, Y.; Xue, H.; Ding, J. Is polydopamine beneficial for cells on the modified surface? Regen. Biomater. 2022, 9, 928–941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, X.; Yao, Y.; Xu, Q.; Shi, H.; Wang, K.; Feng, W.; Shen, Y. Coexpression of VHb and MceG genes in Mycobacterium sp. Strain LZ2 enhances androstenone production via immobilized repeated batch fermentation. Bioresour. Technol. 2021, 342, 125965. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Xing, X.; Xie, Y.; Sun, Y.; Bian, S.; Liu, L.; Chen, G.; Wang, X.; Yu, X.; Su, Y. Evaluation of Preparation and Detoxification of Hemicellulose Hydrolysate for Improved Xylitol Production from Quinoa Straw. Int. J. Mol. Sci. 2022, 24, 516. [Google Scholar] [CrossRef]
- Eryasar-Orer, K.; Karasu-Yalcin, S. Optimization of activated charcoal detoxification and concentration of chestnut shell hydrolysate for xylitol production. Biotechnol. Lett. 2021, 43, 1195–1209. [Google Scholar] [CrossRef]
- Yan, M.; Yu, X.; Zhou, G.; Sun, D.; Hu, Y.; Huang, C.; Zheng, Q.; Sun, N.; Wu, J.; Fu, Z.; et al. GhCDPK60 positively regulates drought stress tolerance in both transgenic Arabidopsis and cotton by regulating proline content and ROS level. Front. Plant Sci. 2022, 13, 1072584. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, W.; Liu, J.; Caiyin, Q.; Qiao, J. Immobilization of Streptomyces thermotolerans 11,432 on polyurethane foam to improve production of Acetylisovaleryltylosin. J. Ind. Microbiol. Biotechnol. 2015, 42, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Angelova, M.B.; Pashova, S.B.; Slokoska, L.S. Comparison of antioxidant enzyme biosynthesis by free and immobilized Aspergillus niger cells. Enzyme Microb. Tech. 2000, 26, 544–549. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Liu, J.; Shen, Y.; Yin, Y.; Ni, Z.; Xi, J.; Hu, Y.; Yuan, Q. Preparation and Immobilization Mechanism on a Novel Composite Carrier PDA-CF/PUF to Improve Cells Immobilization and Xylitol Production. Foods 2024, 13, 1911. https://doi.org/10.3390/foods13121911
Wang L, Liu J, Shen Y, Yin Y, Ni Z, Xi J, Hu Y, Yuan Q. Preparation and Immobilization Mechanism on a Novel Composite Carrier PDA-CF/PUF to Improve Cells Immobilization and Xylitol Production. Foods. 2024; 13(12):1911. https://doi.org/10.3390/foods13121911
Chicago/Turabian StyleWang, Le, Jianguang Liu, Yan Shen, Yanli Yin, Zifu Ni, Jun Xi, Yuansen Hu, and Qipeng Yuan. 2024. "Preparation and Immobilization Mechanism on a Novel Composite Carrier PDA-CF/PUF to Improve Cells Immobilization and Xylitol Production" Foods 13, no. 12: 1911. https://doi.org/10.3390/foods13121911



