Quality Control and Safety Assessment of Online-Purchased Food Supplements Containing Red Yeast Rice (RYR)
Abstract
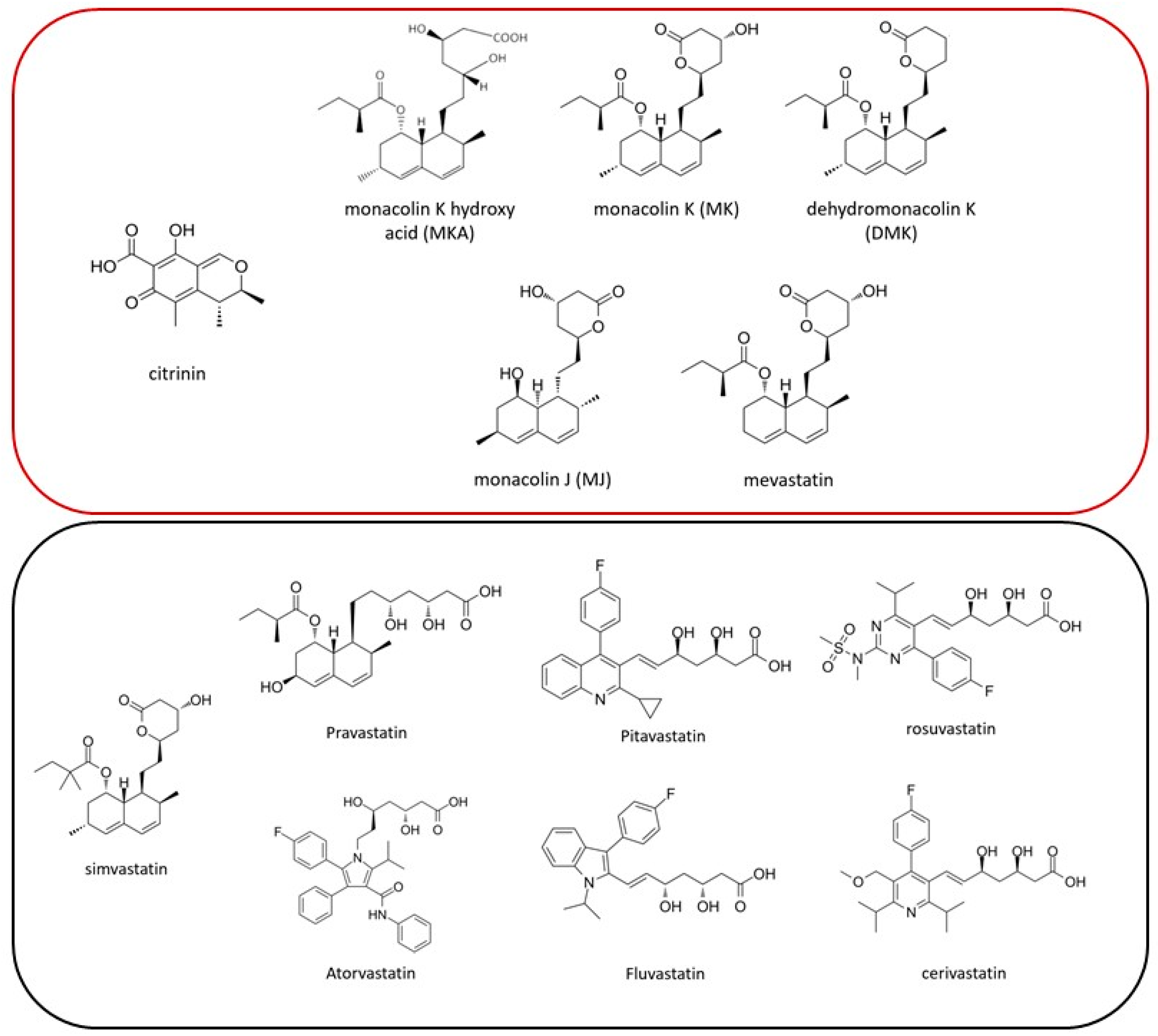
1. Introduction
2. Materials and Methods
2.1. Sample Set
2.2. Solvents, Reagents, and Standard Solutions
2.3. Targeted Screening Methodology for Natural Statins (Monacolins) and Synthetic Statins
2.4. Quantification of Citrinin and Monacolin K
2.5. Elemental Analyzer–Isotope Ratio Mass Spectrometry (EA-IRMS)
2.6. Bioburden Determination and Identification of the Encountered Micro-Organisms
2.7. Mycotoxin Determination
3. Results and Discussion
3.1. Food Supplement Labeling
3.2. Screening for Synthetic Statins
3.3. Citrinin, Total Monacolin K (MKtotal) Content, and Possible Adulteration
3.4. Bioburden, Identification of Micro-Organisms, and Mycotoxin Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Heart Federation. Available online: https://world-heart-federation.org/what-we-do/cholesterol/ (accessed on 21 March 2024).
- Mihaylova, B.; Emberson, J.; Blackwell, L.; Keech, A.; Simes, J.; Barnes, E.H.; Voysey, M.; Gray, A.; Collins, R.; Baigent, C. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet 2012, 380, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.G.; Ference, B.A.; Im, K.; Wiviott, S.D.; Giugliano, R.P.; Grundy, S.M.; Braunwald, E.; Sabatine, M.S. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA 2016, 316, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Y.; Li, H.; Tang, J.J.; Wang, J.; Luo, J.; Liu, B.; Wang, J.K.; Shi, X.J.; Cui, H.W.; Tang, J.; et al. Discovery of a potent HMG-CoA reductase degrader that eliminates statin-induced reductase accumulation and lowers cholesterol. Nat. Commun. 2018, 9, 5138. [Google Scholar] [CrossRef] [PubMed]
- García Rodríguez, L.A.; Cea Soriano, L.; de Abajo, F.J.; Valent, F.; Hallas, J.; Gil, M.; Cattaruzzi, C.; Rodriguez-Martin, S.; Vora, P.; Soriano-Gabarró, M.; et al. Trends in the use of oral anticoagulants, antiplatelets and statins in four European countries: A population-based study. Eur. J. Clin. Pharmacol. 2022, 78, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.Y.; Cooperman, T.; Obermeyer, W.; Becker, D.J. Marked variability of monacolin levels in commercial red yeast rice products: Buyer beware! Arch. Intern. Med. 2010, 170, 1722–1727. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Scientific opinion on the safety of monacolins in red yeast rice. EFSA J. 2018, 16, e05368. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Qi, F.; Wu, J.; Yin, G.; Hua, J.; Zhang, Q.; Qin, L. Red Yeast Rice: A Systematic Review of the Traditional Uses, Chemistry, Pharmacology, and Quality Control of an Important Chinese Folk Medicine. Front. Pharmacol. 2019, 10, 1449. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Luo, J.; Ma, Z.; Sun, Q.; Wu, C.; Li, X. Quality and Authenticity Control of Functional Red Yeast Rice—A Review. Molecules 2019, 24, 1944. [Google Scholar] [CrossRef]
- Fukami, H.; Higa, Y.; Hisano, T.; Asano, K.; Hirata, T.; Nishibe, S. A Review of Red Yeast Rice, a Traditional Fermented Food in Japan and East Asia: Its Characteristic Ingredients and Application in the Maintenance and Improvement of Health in Lipid Metabolism and the Circulatory System. Molecules. 2021, 26, 1619. [Google Scholar] [CrossRef]
- Banach, M.; Bruckert, E.; Descamps, O.S.; Ellegård, L.; Ezhov, M.; Föger, B.; Fras, Z.; Kovanen, P.T.; Latkovskis, G.; März, W.; et al. The role of red yeast rice (RYR) supplementation in plasma cholesterol control: A review and expert opinion. Atheroscler. Suppl. 2019, 39, e1–e8. [Google Scholar] [CrossRef]
- Beltrán, D.; Frutos-Lisón, M.D.; Espín, J.C.; García-Villalba, R. Re-examining the role of the gut microbiota in the conversion of the lipid-lowering statin monacolin K (lovastatin) into its active β-hydroxy acid metabolite. Food Funct. 2019, 10, 1787–1791. [Google Scholar] [CrossRef]
- Benjian, C.; Xiaodan, H.; Huiting, P.; Yishi, L.I.; Yongtao, C.; Huanlin, W.U.; Danping, X.U. Effectiveness and safety of red yeast rice predominated by monacolin K β-hydroxy acid form for hyperlipidemia treatment and management. J. Tradit. Chin. Med. 2022, 42, 264–271. [Google Scholar] [CrossRef]
- Smiley, I.I.I.W.H.; Khan, B.V.; Sperling, L.S. Management of the statin-intolerant patient. Curr. Treat. Options Cardiovasc. Med. 2009, 11, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.C.; Watts, G.F.; Eckel, R.H. Statin Toxicity-Mechanistic Insights and Clinical Implications. Circ. Res. 2019, 124, 328–350. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Jebari, S.; Larrea-Sebal, A.; Uribe, K.B.; Siddiqi, H.; Ostolaza, H.; Benito-Vicente, A.; Martín, C. Statin Treatment-Induced Development of Type 2 Diabetes: From Clinical Evidence to Mechanistic Insights. Int. J. Mol. Sci. 2020, 21, 4725. [Google Scholar] [CrossRef] [PubMed]
- Jahangir, E.; Fazio, S.; Sampson, U.K. Incident diabetes and statins: The blemish of an undisputed heavy weight champion? Br. J. Clin. Pharmacol. 2013, 75, 955–958. [Google Scholar] [CrossRef][Green Version]
- Cham, S.; Koslik, H.J.; Golomb, B.A. Mood, Personality, and Behavior Changes During Treatment with Statins: A Case Series. Drug Saf. Case Rep. 2016, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Childress, L.; Gay, A.; Zargar, A.; Ito, M.K. Review of red yeast rice content and current Food and Drug Administration oversight. J. Clin. Lipidol. 2013, 7, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Buzzelli, L.; Segreti, A.; Di Gioia, D.; Lemme, E.; Squeo, M.R.; Nenna, A.; Di Gioia, G. Alternative lipid lowering strategies: State-of-the-art review of red yeast rice. Fitoterapia 2024, 172, 105719. [Google Scholar] [CrossRef]
- European commission. COMMISSION REGULATION (EU) 2022/860, amending Annex III to Regulation (EC) No 1925/2006 of the European Parliament and of the Council as regards monacolins from red yeast rice. Off. J. Eur. Union 2022, 151, 37–41. [Google Scholar]
- Righetti, L.; Dall’Asta, C.; Bruni, R. Risk Assessment of RYR Food Supplements: Perception vs. Reality. Front Nutr. 2021, 8, 792529. [Google Scholar] [CrossRef]
- Silva, L.J.G.; Pereira, A.M.P.T.; Pena, A.; Lino, C.M. Citrinin in Foods and Supplements: A Review of Occurrence and Analytical Methodologies. Foods 2020, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- López, P.; de Nijs, M.; Spanjer, M.; Pietri, A.; Bertuzzi, T.; Starski, A.; Postupolski, J.; Castellari, M.; Hortós, M. Generation of occurrence data on citrinin in food. EFSA Support. Publ. 2017, 14, 1177E. [Google Scholar] [CrossRef]
- European Commission. COMMISSION REGULATION (EC) 1881/2006, setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- European Commission. COMMISSION REGULATION (EU) 2019/1901, amending Regulation (EC) No 1881/2006 as regards maximum levels of citrinin in food supplements based on rice fermented with red yeast Monascus purpureus. Off. J. Eur. Union 2019, 62, 2–5. [Google Scholar]
- Samsudin, N.I.P.; Abdullah, N. A preliminary survey on the occurrence of mycotoxigenic fungi and mycotoxins contaminating red rice at consumer level in Selangor, Malaysia. Mycotoxin Res. 2013, 29, 89–96. [Google Scholar] [CrossRef]
- ISO/IEC 17025:2017; General Requirements for the Competence of Testing and Calibration Laboratories. ISO: Geneva, Switzerland, 2017.
- Feinberg, M. Validation of analytical methods based on accuracy profiles. J. Chromatogr. A. 2007, 1158, 174–183. [Google Scholar] [CrossRef]
- Nannoni, G.; Alì, A.; Di Pierro, F. Development of a new highly standardized and granulated extract from Monascus purpureus with a high content of monacolin K and KA and free of inactive secondary monacolins and citrinin. Nutrafoods 2015, 14, 197–205. [Google Scholar] [CrossRef]
- Perini, M.; Carbone, G.; Camin, F. Stable isotope ratio analysis for authentication of red yeast rice. Talanta 2017, 174, 228–233. [Google Scholar] [CrossRef]
- United States Pharmacopoeia; United States Pharmacopeial Convention, Inc.: North Bethesda, MD, USA, 2024.
- European Pharmacopoeia 11.0; Council of Europe: Strasbourg, France, 2023.
- Ratajczak, M.; Kaminska, D.; Światły-Błaszkiewicz, A.; Matysiak, J. Quality of Dietary Supplements Containing Plant-Derived Ingredients Reconsidered by Microbiological Approach. Int. J. Environ. Res. Public Health 2020, 17, 6837. [Google Scholar] [CrossRef]
- International Conference on Harmonisation (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use—Validation of Analytical Procedures. Available online: https://database.ich.org/sites/default/files/ICH_Q2%28R2%29_Guideline_2023_1130.pdf (accessed on 21 May 2024).
- Hoffman, D.; Kringle, R. A Total Error Approach for the Validation of Quantitative Analytical Methods. Pharm. Res. 2007, 24, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Hubert, P.; Nguyen-Huu, J.J.; Boulanger, B.; Chapuzet, E.; Chiap, P.; Cohen, N.; Compagnon, P.A.; Dewé, W.; Feinberg, M.; Lallier, M.; et al. Harmonization of strategies for the validation of quantitative analytical procedures. A SFSTP proposal—Part II. J. Pharm. Biomed. Anal. 2007, 45, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Vanhee, C.; Barhdadi, S.; Kamugisha, A.; Van Mulders, T.; Vanbrusselen, K.; Willocx, M.; Deconinck, E. The Development and Validation of a Targeted LC-HRAM-MS/MS Methodology to Separate and Quantify p-Synephrine and m-Synephrine in Dietary Supplements and Herbal Preparations. Separations 2023, 10, 444. [Google Scholar] [CrossRef]
- Cohen, P.A.; Jacobs, B.; Van Hoorde, K.; Vanhee, C. Accuracy of Labeling of Galantamine Generic Drugs and Dietary Supplements. JAMA 2024, 331, 974–976. [Google Scholar] [CrossRef] [PubMed]
- Mandel, J. The Statistical Analysis of Experimental Data; Interscience Publ. J. Wiley & Sons: New York, NY, USA, 1964. [Google Scholar]
- Vanhee, C.; Jacobs, B.; Kamugisha, A.; Canfyn, M.; Van Der Meersch, H.; Ceyssens, B.; Deconinck, E.; Van Hoorde, K.; Willocx, M. Substandard and falsified ivermectin tablets obtained for self-medication during the COVID-19 pandemic as a source of potential harm. Drug Test. Anal. 2023. early view. [Google Scholar] [CrossRef]
- Vanhee, C.; Jacobs, B.; Mori, M.; Kamugisha, A.; Debehault, L.; Canfyn, M.; Ceyssens, B.; Van Der Meersch, H.; van Hoorde, K.; Deconinck, E.; et al. Uncovering the Quality Deficiencies with Potentially Harmful Effects in Substandard and Falsified PDE-5 Inhibitors Seized by Belgian Controlling Agencies. Forensic Sci. 2023, 3, 426–451. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Tangni, E.K.; Huybrechts, B.; Masquelier, J.; Van Hoeck, E. Organisation of Multi-Mycotoxin Proficiency Tests: Evaluation of the Performances of the Laboratories Using the Triple A Rating Approach. Toxins 2021, 13, 591. [Google Scholar] [CrossRef]
- European Commission. Regulation (EU) No. 1169/2011 on Consumer Information. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32011R1169 (accessed on 23 May 2024).
- European Commission. Directive 2011/91/EU of the European Parliament and of the Council of 13 December 2011 on Indications or Marks Identifying the Lot to Which a Foodstuff belongs (Codification) Text with EEA Relevance. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32011L0091 (accessed on 23 May 2024).
- Avula, B.; Cohen, P.A.; Wang, Y.H.; Sagi, S.; Feng, W.; Wang, M.; Zweigenbaum, J.; Shuangcheng, M.; Khan, I.A. Chemical profiling and quantification of monacolins and citrinin in red yeast rice commercial raw materials and dietary supplements using liquid chromatography-accurate QToF mass spectrometry: Chemometrics application. J. Pharm. Biomed. Anal. 2014, 100, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Hachem, R.; Assemat, G.; Balayssac, S.; Martins-Froment, N.; Gilard, V.; Martino, R.; Malet-Martino, M. Comparative Chemical Profiling and Monacolins Quantification in Red Yeast Rice Dietary Supplements by 1H-NMR and UHPLC-DAD-MS. Molecules 2020, 25, 317. [Google Scholar] [CrossRef]
- Xie, X.; Tang, Y. Efficient synthesis of simvastatin by use of whole-cell biocatalysis. Appl. Environ. Microbiol. 2007, 73, 2054–2060. [Google Scholar] [CrossRef]
- Kamle, M.; Mahato, D.K.; Gupta, A.; Pandhi, S.; Sharma, N.; Sharma, B.; Mishra, S.; Arora, S.; Selvakumar, R.; Saurabh, V.; et al. Citrinin Mycotoxin Contamination in Food and Feed: Impact on Agriculture, Human Health, and Detection and Management Strategies. Toxins 2022, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- European Commission. COMMISSION REGULATION (EU) No 212/2014, amending Regulation (EC) No 1881/2006 as regards maximum levels of the contaminant citrinin in food supplements based on rice fermented with red yeast Monascus purpureus. Off. J. Eur. Union 2014, 67, 3–4. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to monacolin K from red yeast rice and maintenance of normal blood LDL-cholesterol concentrations (ID 1648, 1700) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2304. [Google Scholar] [CrossRef]
- Tobert, J. Lovastatin and beyond: The history of the HMG-CoA reductase inhibitors. Nat. Rev. Drug Discov. 2003, 2, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Hannon, K.M.; Sabala, J.D.; Mantha, M.; Lorenz, L.M.; Roetting Ii, J.P.; Perini, M.; Pianezze, S.; Kubachka, K.M. Using stable carbon isotope ratio analysis to detect adulteration in red yeast rice dietary supplements. Talanta 2024, 266, 125076. [Google Scholar] [CrossRef] [PubMed]
- Opuni, K.F.M.; Kretchy, J.P.; Agyabeng, K.; Boadu, J.A.; Adanu, T.; Ankamah, S.; Appiah, A.; Amoah, G.B.; Baidoo, M.; Kretchy, I.A. Contamination of herbal medicinal products in low-and-middle-income countries: A systematic review. Heliyon 2023, 9, e19370. [Google Scholar] [CrossRef]
- Fiedler, G.; Schneider, C.; Igbinosa, E.O.; Kabisch, J.; Brinks, E.; Becker, B.; Stoll, D.A.; Cho, G.S.; Huch, M.; Franz, C.M.A.P. Antibiotics resistance and toxin profiles of Bacillus cereus-group isolates from fresh vegetables from German retail markets. BMC Microbiol. 2019, 19, 250. [Google Scholar] [CrossRef]
- Fraccalvieri, R.; Bianco, A.; Difato, L.M.; Capozzi, L.; Del Sambro, L.; Simone, D.; Catanzariti, R.; Caruso, M.; Galante, D.; Normanno, G.; et al. Toxigenic Genes, Pathogenic Potential and Antimicrobial Resistance of Bacillus cereus Group Isolated from Ice Cream and Characterized by Whole Genome Sequencing. Foods 2022, 11, 2480. [Google Scholar] [CrossRef]
- Gupta, R.S.; Patel, S.; Saini, N.; Chen, S. Robust demarcation of 17 distinct Bacillus species clades, proposed as novel Bacillaceae genera, by phylogenomics and comparative genomic analyses: Description of Robertmurraya kyonggiensis sp. nov. and proposal for an emended genus Bacillus limiting it only to the members of the Subtilis and Cereus clades of species. Int. J. Syst. Evol. Microbiol. 2020, 70, 5753–5798. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Gushiken, A.C. Roseomonas Species Bacteremia with Associated Endocarditis and Possible CNS Septic Embolic Phenomenon. Cureus 2023, 15, e40318. [Google Scholar] [CrossRef]
- Ramos, G.L.D.P.A.; Vigoder, H.C.; dos Santos Nascimento, J. Kocuria spp. in Foods: Biotechnological Uses and Risks for Food Safety. Appl. Food Biotechnol. 2021, 8, 79–88. [Google Scholar] [CrossRef]
- Carvalheira, A.; Silva, J.; Teixeira, P. Acinetobacter spp. in food and drinking water—A review. Food Microbiol. 2021, 95, 103675. [Google Scholar] [CrossRef] [PubMed]
- Lampe, J.W. Spicing up a vegetarian diet: Chemopreventive effects of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 579S–583S. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority); Arcella, D.; Gergelova, P.; Innocenti, M.L.; Steinkellner, H. Scientific report on human and animal dietary exposure to T-2 and HT-2 toxin. EFSA J. 2017, 15, 4972. [Google Scholar] [CrossRef] [PubMed]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B.; et al. Scientific Opinion on the risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15, 4718. [Google Scholar] [CrossRef]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain); Knutsen, H.-K.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Scientific opinion on the appropriateness to set a group health-based guidance value for fumonisins and their modified forms. EFSA J. 2018, 16, 5172. [Google Scholar] [CrossRef]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Scientific opinion on the appropriateness to set a group health-based guidance value for zearalenone and its modified forms. EFSA J. 2016, 14, 4425. [Google Scholar] [CrossRef]
| Retention Time (min) | Precursor Ion (m/z) | Fragment Ions (m/z) and Their Relative Intensities | Limit of Quantification (LLOQ) | Linear Range (LLOQ-ULOQ) | |
|---|---|---|---|---|---|
| citrinine | 3.4 | 251.091 [M + H]+ | 91.054 (100), 119.085 (50) | 5 ng/mL (20 ng/g food supplement) | 5–1000 ng/mL |
| SIL-citrinine | 3.4 | 264.1339 [M + H]+ | 98.0781 (100), 128.1159 (70) | ||
| MKA | 4.0 | 405.262 [M - H2O + H]+ | 199.148 (100), 173.132 (80) | 100 ng/mL (500 ng/g food supplement) | 100–5000 ng/mL |
| D3-MKA | 4.0 | 408.282 [M - H2O + H]+ | 199.148 (100), 173.132 (90) | ||
| MK | 4.5 | 405.262 [M - H2O + H]+ | 199.148 (100), 173.132 (75) | 50 ng/mL (250 ng/g food supplement) | 50–2000 ng/mL |
| D3-MK | 4.5 | 408.282 [M - H2O + H]+ | 199.148 (90), 173.132 (100) |
| Concentration Level | Recovery (%) | |||
|---|---|---|---|---|
| Citrinine | MKA | MK | ||
| Matrix 1 | LLOQ | 93.2 | 104.4 | 96.5 |
| ULOQ | 102.8 | 104.7 | 96.7 | |
| Matrix 2 | LLOQ | 98.3 | 96.3 | 100.5 |
| ULOQ | 97.5 | 97.9 | 98.4 | |
| Matrix 3 | LLOQ | 92.6 | 91.5 | 98.5 |
| ULOQ | 97.2 | 105.6 | 94.8 | |
| Matrix 4 | LLOQ | n.a. | 94.5 | 103.7 |
| ULOQ | n.a. | 98.6 | 95.6 | |
| Sample N° | Lot Number and Expiration Date Present | Warning on the Label a | Ingredients or Other API Declared on the Label | Maximum Serving Size According to Labeled Directions | Labeled Amount (mg) per Maximum Serving Size | Synthetic Statins Found in the Sample c | Amount of Citrinin Found (Mean ± MU, ng/g) | Measured Amount (mean ± MU, mg) per Maximum Serving MKtotal | |
|---|---|---|---|---|---|---|---|---|---|
| Total Monacolin | MKtotal b | ||||||||
| 1 | yes | n.a. | n.d. | 2 capsules | absent | absent | n.d. | 46.2 ± 0.3 | n.d. |
| 2 | yes | yes | Coenzyme Q10 and phytosterols | 3 capsules | n.d. | 2.8 | n.d. | n.d. | 2.36 ± 0.1 |
| 3 | yes | no | Leucine | 1 capsule | n.d. | 2.95 | n.d. | n.d. | 1.79 ± 0.02 |
| 4 | yes | yes | Coenzyme Q10, vitamin E, vitamin C, niacin, plant extracts (sage, garlic, Astragalus, sugar cane) | 1 capsule | n.d. | 2.54 | n.d. | n.d. | 1.19 ± 0.03 |
| 5 | yes | yes | Coenzyme Q10, vitamin B6, plant extracts (Silybum and Filipendula) | 1 capsule | n.d. | 2.9 | n.d. | n.d. | 1.54 ± 0.03 |
| 6 | yes | no | n.d. | 1 capsule | n.d. | 2.9 | n.d. | n.d. | 2.78 ± 0.12 |
| 7 | yes | yes | Leucine | 1 capsule | n.d. | 2.95 | n.d. | n.d. | 2.57 ± 0.04 |
| 8 | yes | yes | n.d. | 1 capsule | n.d. | 1.0 | n.d. | 40.5 ± 0.6 | 1.15 ± 0.04 |
| 9 | yes | yes | Coenzyme Q10, vitamin B3 | 1 tablet | n.d. | 2.9 | n.d. | n.d. | 2.15 ± 0.06 |
| 10 | yes | n.a. | n.d. | 2 tablets | n.d. | n.d. | n.d. | 58.7 ± 0.3 | n.d. |
| 11 | yes | n.a. | Coenzyme Q10 | 2 soft gels | n.d. | n.d. | n.d. | <20 | n.d. |
| 12 | yes | yes | Vitamin B1 | 1 capsule | n.d. | 2.9 | n.d. | n.d. | 1.13 ± 0.02 |
| 13 | no | no | n.d. | 2 capsules | n.d. | n.d. | n.d. | n.d. | n.d. |
| 14 | yes | no | Coenzyme Q10 | 2 capsules | 2.4 | n.d. | n.d. | <20 | 3.51 ± 0.06 |
| 15 | yes | no | n.d. | 2 capsules | n.d. | absent | n.d. | 23.8 ± 0.1 | n.d. |
| 16 | yes | no | n.d. | 2 capsules | n.d. | absent | n.d. | 24.0 ± 0.04 | n.d. |
| 17 | yes | yes | n.d. | 1 capsule | 2.9 | n.d. | n.d. | <20 | 2.66 ± 0.02 |
| 18 | yes | yes | n.d. | 1 tablet | 2.99 | n.d. | n.d. | <20 | 4.25 ± 0.24 |
| 19 | yes | yes | Folic acid, coenzyme Q10, berberine | 1 tablet | 2.8 | n.d. | n.d. | n.d. | 1.84 ± 0.05 |
| 20 | yes | yes | Coenzyme Q10, folic acid, vitamin B12, plant extract (Coriandrum sativum) | 1 tablet | 2.9 | n.d. | n.d. | 41.9 ± 0.2 | 1.97 ± 0.05 |
| 21 | yes | yes | Coenzyme Q10, different plant extracts (Allium sativum and Olea europeae) | 2 liquid capsules | 2.99 | n.d. | n.d. | 21.4 ± 0.1 | 1.52 ± 0.02 |
| 22 | yes | yes | Coenzyme Q10, different plant extracts (Allium sativum and Olea europeae) | 2 capsules | 2.99 | n.d. | n.d. | n.d. | 1.31 ± 0.05 |
| 23 | yes | yes | n.d. | 1 capsule | 2.9 | n.d. | n.d. | n.d. | 1.41 ± 0.04 |
| 24 | yes | yes | Coenzyme Q10 and berberine | 2 capsules | 2.99 | n.d. | n.d. | n.d. | 3.19 ± 0.2 |
| 25 | yes | yes | Vitamin B1, plant extracts (Cynara scolymus and Olea europeae) | 1 tablet | 2.9 | n.d. | n.d. | n.d. | 1.30 ± 0.15 |
| 26 | yes | yes | Plant extracts (Cynara scolymus and Olea europeae) | 1 tablet | 2.95 | n.d. | n.d. | n.d. | 1.25 ± 0.03 |
| 27 | yes | yes | Coenzyme Q10, cinnamon and green tea extract | ½ tablet | 2.885 | 1.9 | n.d. | 33.6 ± 0.2 | 0.81 ± 0.01 |
| 28 | yes | yes | Vitamin B3, vitamin E, and coenzyme Q10 | 1 tablet | n.d. | 2.99 | n.d. | n.d. | 1.58 ± 0.03 |
| 29 | yes | yes | n.d. | 1 tablet | 2.5 | n.d. | n.d. | 38.7 ± 0.1 | 2.31 ± 0.07 |
| 30 | yes | yes | Vitamin B1 | 1 tablet | 2.9 | n.d. | n.d. | <20 | 1.83 ± 0.07 |
| 31 | yes | yes | Ascorbic acid, vitamin B1, different plant extracts (sage, Coriandrum sativum, and Tinospora cordifolia) | 2 capsules | n.d. | 1.48 | n.d. | n.d | 6.13 ± 0.12 |
| 32 | yes | yes | n.d. | 1 capsule | 2.95 | n.d. | n.d. | n.d | 2.71 ± 0.04 |
| 33 | yes | yes | Plant extract (Allium sativum and Cynara scolymus) | 1 capsule | 2.99 | n.d. | n.d. | n.d. | 0.99 ± 0.03 |
| 34 | yes | yes | n.d. | 1 capsule | n.d. | 2.95 | n.d. | <20 | 2.53 ± 0.09 |
| 35 | yes | yes | n.d. | 1 capsule | n.d. | 2.9 | n.d. | <20 | 2.34 ± 0.04 |
| Sample N° | Amount Found per Maximum Serving Size (mean ± MU, mg) | Ratio MKA/MKtotal | δ13 C Values of MK (‰) | ||
|---|---|---|---|---|---|
| MKA | MK | MKtotal | |||
| 2 | 0.70 (±0.03) | 1.66 (±0.02) | 2.36 (±0.09) | 0.30 | n.a. |
| 3 | 1.35 (±0.03) | 0.44 (±0.02) | 1.79 (±0.03) | 0.75 | n.a. |
| 4 | 0.15 (±0.01) | 1.04 (±0.03) | 1.19 (±0.03) | 0.13 | −17.6 |
| 5 | 0.70 (±0.03) | 0.84 (±0.01) | 1.54 (±0.03) | 0.45 | n.a. |
| 6 | 2.17 (±0.12) | 0.61 (±0.003) | 2.78 (±0.12) | 0.78 | n.a. |
| 7 | 1.95 (±0.03) | 0.61 (±0.03) | 2.56 (±0.04) | 0.76 | n.a. |
| 8 | 0.18 (±0.01) | 0.96 (±0.03) | 1.15 (±0.04) | 0.16 | −30.1 |
| 9 | 0.88 (±0.02) | 1.26 (±0.03) | 2.15 (±0.06) | 0.41 | n.a. |
| 12 | 0.56 (±0.03) | 0.58 (±0.01) | 1.13 (±0.02) | 0.49 | n.a. |
| 14 | 0.99 (±0.02) | 2.52 (±0.05) | 3.51 (±0.06) | 0.28 | −28.5 |
| 17 | 0.63 (±0.01) | 2.02 (±0.02) | 2.66 (±0.02) | 0.24 | −28.1 |
| 18 | 3.67 (±0.25) | 0.58 (±0.01) | 4.25 (±0.24) | 0.86 | n.a. |
| 19 | 1.23 (±0.03) | 0.61 (±0.02) | 1.84 (±0.05) | 0.67 | n.a. |
| 20 | 0.23 (±0.01) | 1.73 (±0.05) | 1.97 (±0.05) | 0.12 | −29.6 |
| 21 | 0.41 (±0.02) | 1.11 (±0.03) | 1.52 (±0.02) | 0.27 | n.d. |
| 22 | 0.43 (±0.03) | 0.88 (±0.01) | 1.31 (±0.05) | 0.33 | n.a. |
| 23 | 0.42 (±0.04) | 0.99 (±0.02) | 1.41 (±0.04) | 0.30 | n.a. |
| 24 | 1.15 (±0.05) | 2.03 (±0.03) | 3.19 (±0.20) | 0.36 | n.a. |
| 25 | 0.51 (±0.01) | 0.79 (±0.02) | 1.3 (±0.02) | 0.39 | n.a. |
| 26 | 0.35 (±0.03) | 0.91 (±0.04) | 1.25 (±0.03) | 0.28 | −22.6 |
| 27 | 0.44 (±0.02) | 0.37 (±0.01) | 0.81 (±0.01) | 0.54 | n.a. |
| 28 | 1.04 (±0.05) | 0.54 (±0.02) | 1.58 (±0.03) | 0.66 | n.a. |
| 29 | 0.72 (±0.03) | 1.58 (±0.06) | 2.3 (±0.07) | 0.31 | n.a. |
| 30 | 0.93 (±0.01) | 0.90 (±0.02) | 1.83 (±0.07) | 0.51 | n.a. |
| 31 | 5.97 (±0.06) | 0.16 (±0.004) | 6.13 (±0.12) | 0.97 | n.a. |
| 32 | 0.39 (±0.01) | 2.31 (±0.05) | 2.7 (±0.04) | 0.14 | −30.0 |
| 33 | 0.22 (±0.02) | 0.77 (±0.04) | 0.99 (±0.03) | 0.22 | −25.0 |
| 34 | 0.56 (±0.03) | 1.96 (±0.12) | 2.52 (±0.09) | 0.22 | −30.4 |
| 35 | 0.56 (±0.03) | 1.78 (±0.06) | 2.34 (±0.04) | 0.24 | −30.2 |
| Sample N° | TAMC | Growth on Bile Acid Medium (CFU/g or mL) | TYMC | ||
|---|---|---|---|---|---|
| CFU/g or mL | Identified Organism | CFU/g or mL | Identified Organism | ||
| 1 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 2 | 2.5 × 103 | Bacillus velenzis | n.d. | n.d. | n.d. |
| 3 | n.d. | n.d. | n.d. | 50 | Penicillium olsonii |
| 4 | 2 × 103 | Bacillus velenzis and Bacillus spp. | n.d. | n.d. | n.d. |
| 5 | 2.5 × 102 | Bacillus velenzis | n.d. | 50 | Aspergillus niger |
| 6 | 1 × 103 | Bacillus spp. | n.d. | n.d. | n.d. |
| 7 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 8 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 9 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 10 | 2 × 102 | Bacillus subtilis | n.d. | n.d. | n.d. |
| 11 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 12 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 13 | 1.5 × 102 | Roseomonas mucosa | n.d. | n.d. | n.d. |
| 14 | 3.5 × 104 | Bacillus subtilis | n.d. | n.d. | n.d. |
| 15 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 16 | 3 × 102 | Kocuria rhizophila | n.d. | n.d. | n.d. |
| 17 | 1.5 × 103 | Bacillus spp. and Staphylococcus capitis | n.d. | n.d. | n.d. |
| 18 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 19 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 20 | 4 × 102 | Bacillus cereus | n.d. | n.d. | n.d. |
| 21 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 22 | 2 × 102 | Bacillus amyloliquifaciens | n.d. | n.d. | n.d. |
| 23 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 24 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 25 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 26 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 27 | 50 | Niallia taxi | n.d. | n.d. | n.d. |
| 28 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 29 | 2.5 × 103 | Acinetobacter johnsonii | 2.5 × 103 | 150 | Cladosporium uwebraunianum |
| 30 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 31 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 32 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 33 | n.d. | n.d. | n.d. | 75 | Penicillium rubens |
| 34 | 1 × 102 | Bacillus spp. | n.d. | n.d. | n.d. |
| 35 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Sample N° | Mycotoxin Concentration (ng/g) | |||||
|---|---|---|---|---|---|---|
| AF | OTA | T2 + HT2 | DON | FB1–3 | ZEN | |
| 1 | n.d. | n.d. | n.d. | n.d. | n.d. | 96.70 |
| 5 | n.d. | 5.82 | 724.09 | 288.25 | <10 | n.d. |
| 6 | n.d. | n.d. | n.d. | 412.35 | n.d. | n.d. |
| 7 | n.d. | n.d. | n.d. | 28.05 | <10 | n.d. |
| 10 | n.d. | n.d. | n.d. | n.d. | n.d. | 29.06 |
| 11 | n.d. | n.d. | n.d. | n.d. | n.d. | 25.90 |
| 13 | n.d. | n.d. | n.d. | n.d. | n.d. | 16.95 |
| 14 | <1 | n.d. | n.d. | n.d. | 17.72 | n.d. |
| 16 | n.d. | n.d. | n.d. | n.d. | n.d. | 65.19 |
| 21 | n.d. | n.d. | n.d. | n.d. | 32.23 | n.d. |
| 33 | n.d. | n.d. | n.d. | 51.57 | 17.75 | n.d. |
| 35 | <1 | n.d. | n.d. | n.d. | 17.41 | n.d. |
| Max. limit (ng/g) | 4 a | 15 b | - | - | - | - |
| TDI (ng/kg bw) | - | - | 20 | 1000 | 2000 | 250 |
| ADI (ng) | - | - | 1200 | 60,000 | 120,000 | 15,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanhee, C.; Jacobs, B.; Canfyn, M.; Malysheva, S.V.; Willocx, M.; Masquelier, J.; Van Hoorde, K. Quality Control and Safety Assessment of Online-Purchased Food Supplements Containing Red Yeast Rice (RYR). Foods 2024, 13, 1919. https://doi.org/10.3390/foods13121919
Vanhee C, Jacobs B, Canfyn M, Malysheva SV, Willocx M, Masquelier J, Van Hoorde K. Quality Control and Safety Assessment of Online-Purchased Food Supplements Containing Red Yeast Rice (RYR). Foods. 2024; 13(12):1919. https://doi.org/10.3390/foods13121919
Chicago/Turabian StyleVanhee, Celine, Bram Jacobs, Michael Canfyn, Svetlana V. Malysheva, Marie Willocx, Julien Masquelier, and Koenraad Van Hoorde. 2024. "Quality Control and Safety Assessment of Online-Purchased Food Supplements Containing Red Yeast Rice (RYR)" Foods 13, no. 12: 1919. https://doi.org/10.3390/foods13121919
APA StyleVanhee, C., Jacobs, B., Canfyn, M., Malysheva, S. V., Willocx, M., Masquelier, J., & Van Hoorde, K. (2024). Quality Control and Safety Assessment of Online-Purchased Food Supplements Containing Red Yeast Rice (RYR). Foods, 13(12), 1919. https://doi.org/10.3390/foods13121919







