Investigating the Effects of Distillation System, Geographical Origin, and Aging Time on Aroma Characteristics in Brandy Using an Untargeted Metabonomic Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
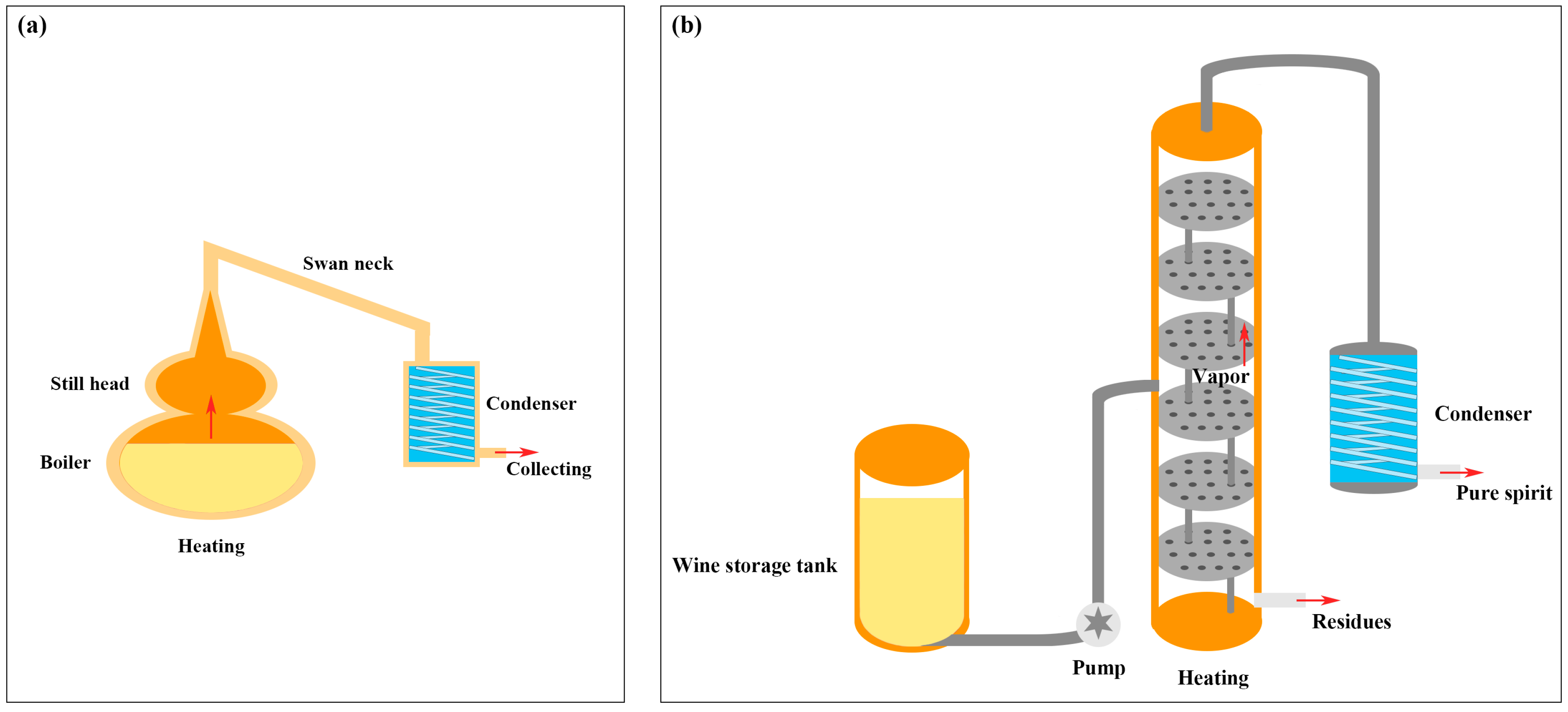
2.2. Brandy Samples
2.3. HS-SPME-GC-MS Analysis
2.4. Quantification of Volatile Compounds
2.5. Data Pre-Processing and Statistical Analysis
2.5.1. XCMS Online
2.5.2. Statistical Analysis
3. Results and Discussion
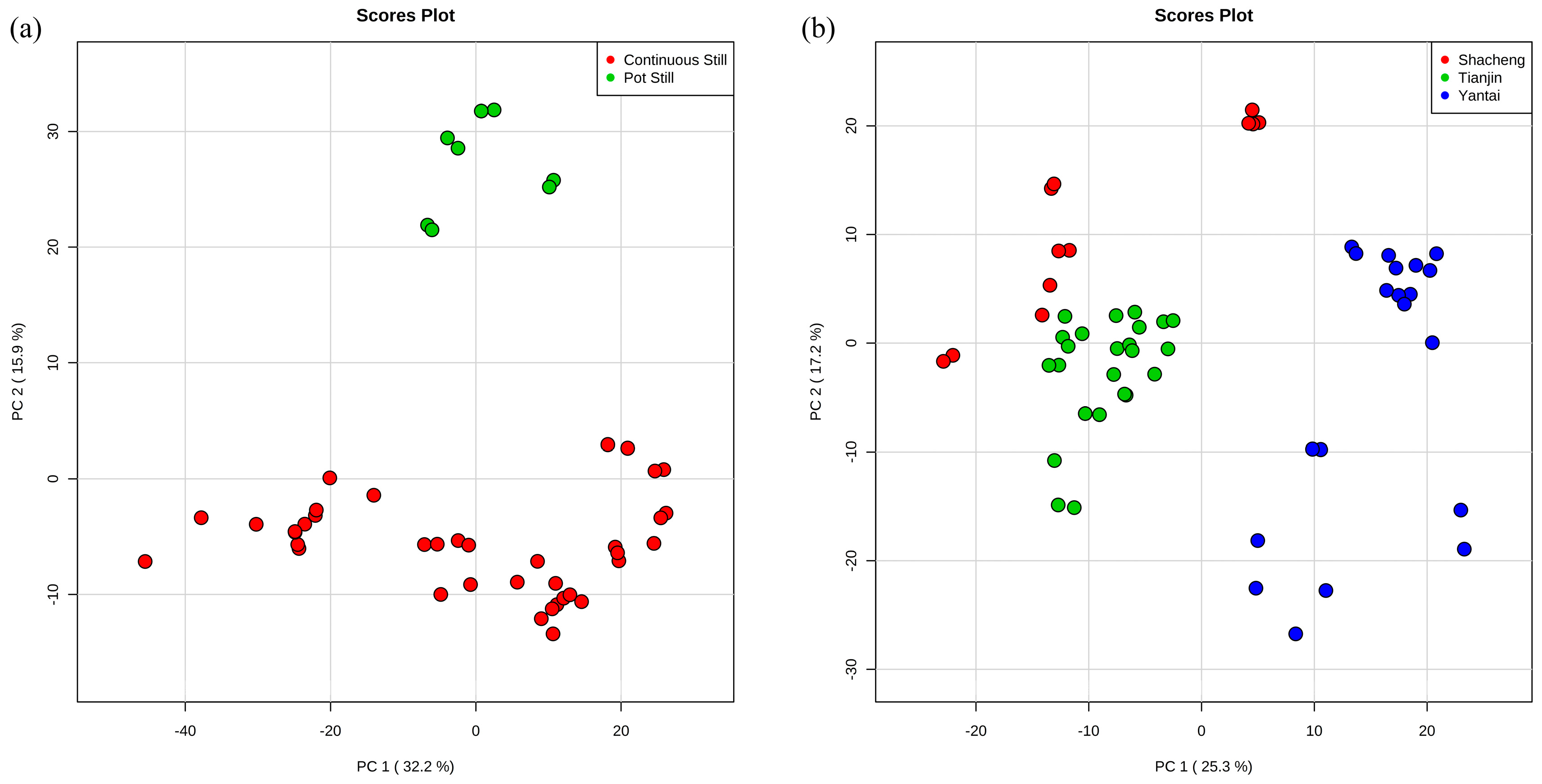
3.1. Exploratory Data Analysis by Principal Component Analysis (PCA)
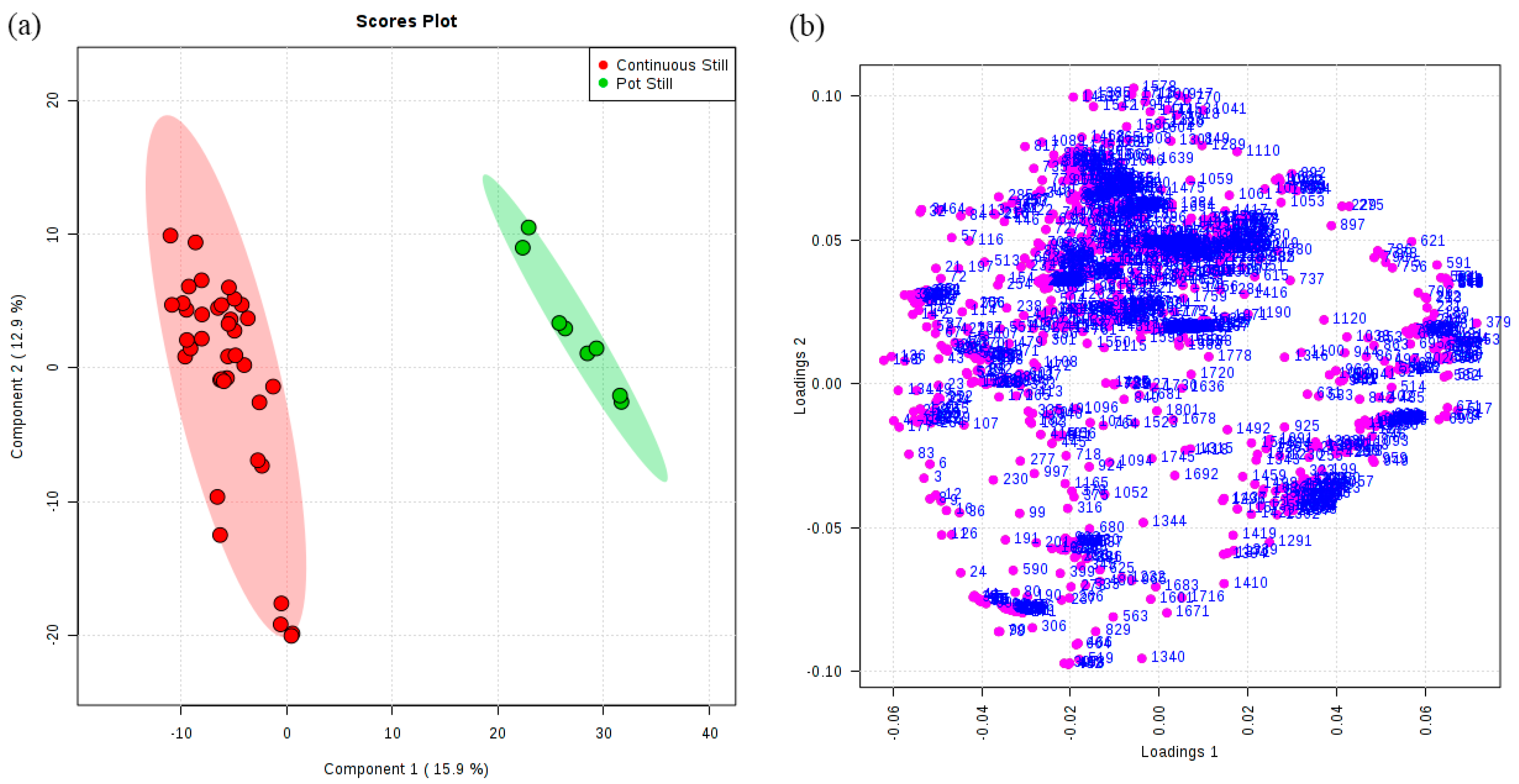
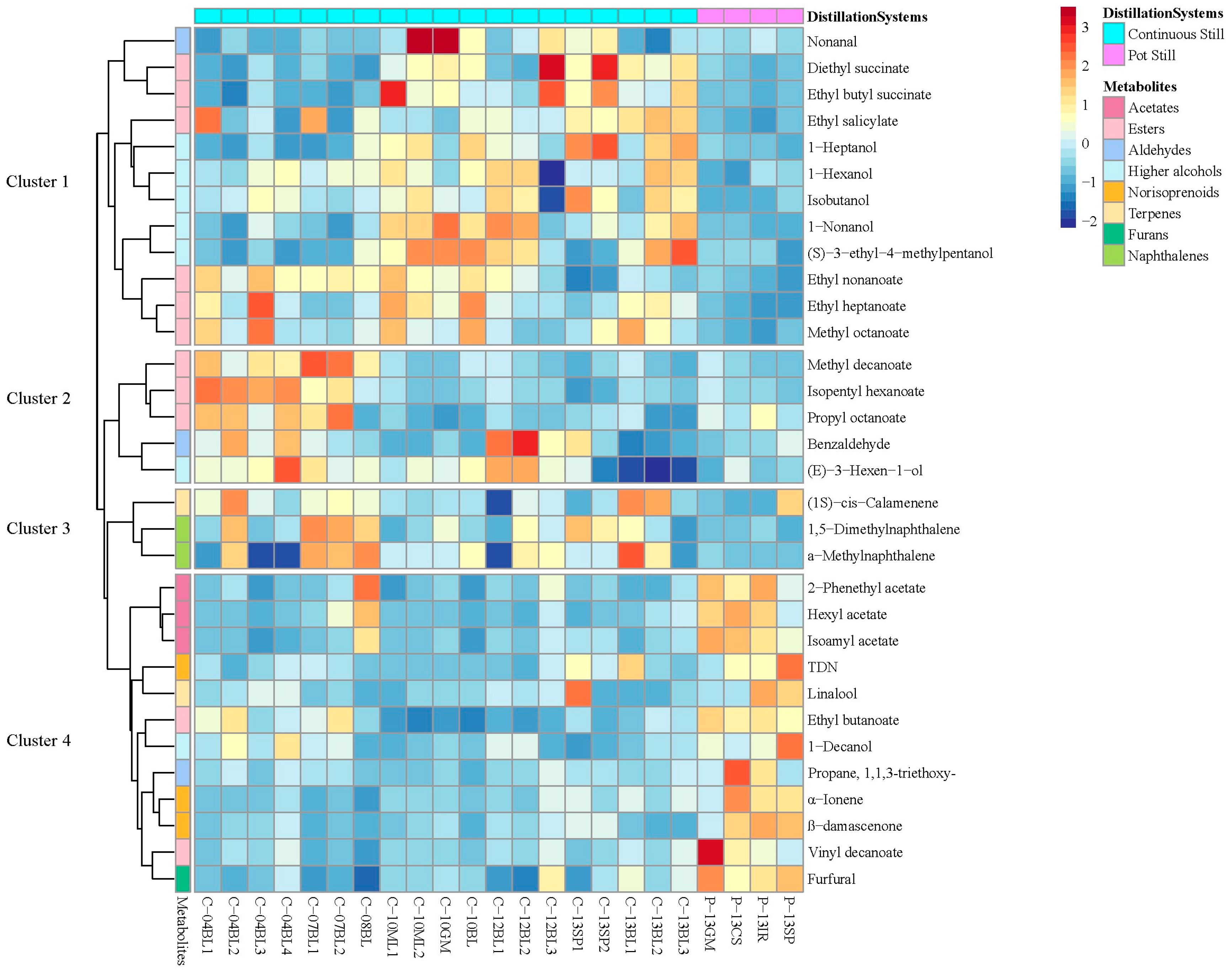
3.2. Classification of Original Brandies According to Distillation Systems
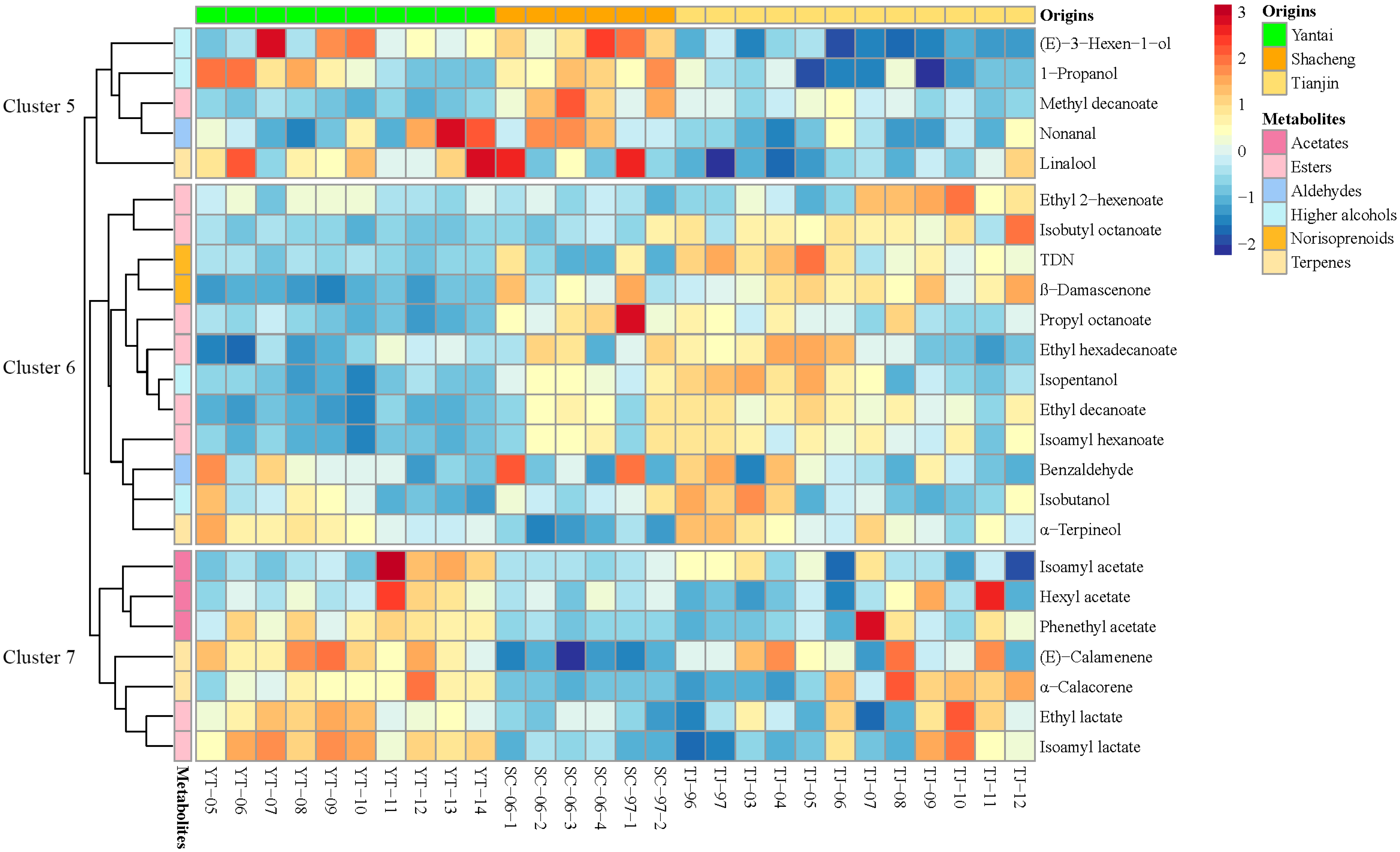
3.3. Classification of Brandies According to Geographical Origin
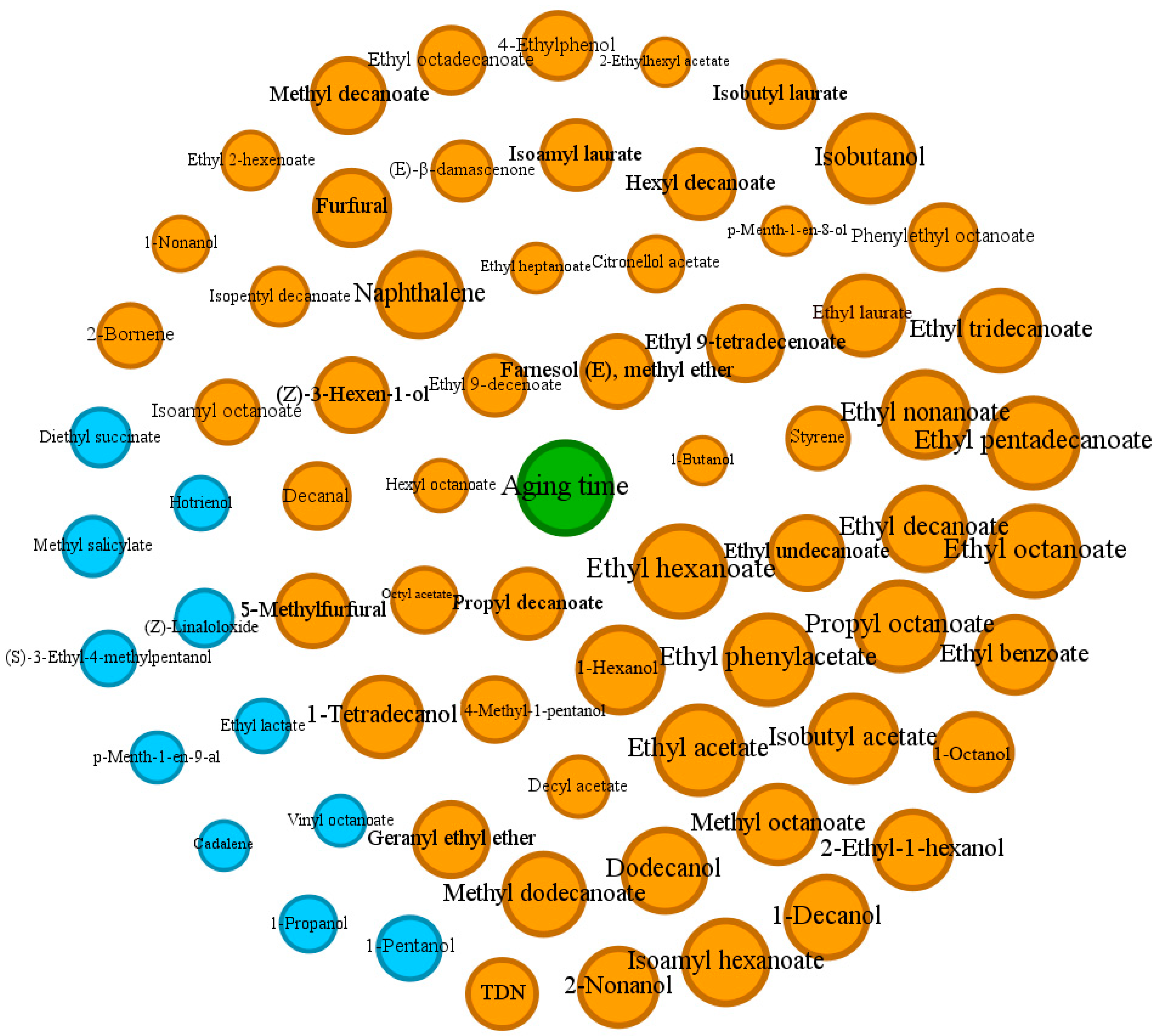
3.4. Network Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arrieta-Garay, Y.; Lopez-Vazquez, C.; Blanco, P.; Perez-Correa, J.R.; Orriols, I.; Lopez, F. Kiwi spirits with stronger floral and fruity characters were obtained with a packed column distillation system. J. Inst. Brew. 2014, 120, 111–118. [Google Scholar] [CrossRef]
- Tsakiris, A.; Kallithraka, S.; Kourkoutas, Y. Grape brandy production, composition and sensory evaluation. J. Sci. Food Agric. 2014, 94, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Awad, P.; Athès, V.; Decloux, M.E.; Ferrari, G.; Snakkers, G.; Raguenaud, P.; Giampaoli, P. Evolution of volatile compounds during the distillation of cognac spirit. J. Agric. Food Chem. 2017, 65, 7736–7748. [Google Scholar] [CrossRef] [PubMed]
- Canas, S.; Danalache, F.; Anjos, O.; Fernandes, T.A.; Caldeira, I.; Santos, N.; Fargeton, L.; Boissier, B.; Catarino, S. Behaviour of low molecular weight compounds, iron and copper of wine spirit aged with chestnut staves under different levels of micro-oxygenation. Molecules 2020, 25, 5266. [Google Scholar] [CrossRef] [PubMed]
- Leaute, R. Distillation in alambic. Am. J. Enol. Vitic. 1990, 41, 90–103. [Google Scholar] [CrossRef]
- Madrera, R.R.; Gomis, D.B.; Alonso, J.J.M. Influence of distillation system, oak wood type, and aging time on volatile compounds of cider brandy. J. Agric. Food Chem. 2003, 51, 5709–5714. [Google Scholar] [CrossRef] [PubMed]
- Maleta, V.N.; Kiss, A.A.; Taran, V.M.; Maleta, B.V. Understanding process intensification in cyclic distillation systems. Chem. Eng. Process. Process Intensif. 2011, 50, 655–664. [Google Scholar] [CrossRef]
- Arrieta-Garay, Y.; Garcia-Llobodanin, L.; Pérez-Correa, J.R.; Lopez-Vazquez, C.; Orriols, I.; López, F. Aromatically enhanced pear distillates from blanquilla and conference varieties using a packed column. J. Agric. Food Chem. 2013, 61, 4936–4942. [Google Scholar] [CrossRef]
- Malfondet, N.; Gourrat, K.; Brunerie, P.; Le Quéré, J.L. Aroma characterization of freshly-distilled french brandies; their specificity and variability within a limited geographic area. Flavour Fragr. J. 2016, 31, 361–376. [Google Scholar] [CrossRef]
- Šuklje, K.; Carlin, S.; Antalick, G.; Blackman, J.W.; Deloire, A.; Vrhovsek, U.; Schmidtke, L.M. Regional discrimination of Australian Shiraz wine volatome by two-dimensional gas chromatography coupled to time-of-flight mass spectrometry. J. Agric. Food Chem. 2019, 67, 10273–10284. [Google Scholar] [CrossRef]
- Biernacka, P.; Wardencki, W. Volatile Composition of raw spirits of different botanical origin. J. Inst. Brew. 2012, 118, 393–400. [Google Scholar] [CrossRef]
- Kyraleou, M.; Herb, D.; O’Reilly, G.; Conway, N.; Bryan, T.; Kilcawley, K.N. The impact of terroir on the flavour of single malt whisk(e)y new make spirit. Foods 2021, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- Lytra, G.; Miot-Sertier, C.; Moine, V.; Coulon, J.; Barbe, J.C. Influence of must yeast-assimilable nitrogen content on fruity aroma variation during malolactic fermentation in red wine. Food Res. Int. 2020, 135, 109294. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, I.; Clímaco, M.C.; De Sousa, R.B.; Belchior, A.P. Volatile composition of oak and chestnut woods used in brandy ageing: Modification induced by heat treatment. J. Food Eng. Belchior 2006, 76, 202–211. [Google Scholar] [CrossRef]
- Mangas, J.; Rodríguez, R.; Moreno, J.; Blanco, D. Volatiles in distillates of cider aged in American oak wood. J. Agric. Food Chem. 1996, 44, 268–273. [Google Scholar] [CrossRef]
- Nonato, E.A.; Carazza, F.; Silva, F.C.; Carvalho, C.R.; Cardeal, Z.d.L. A headspace solid-phase microextraction method for the determination of some secondary compounds of brazilian sugar cane spirits by gas chromatography. J. Agric. Food Chem. 2001, 49, 3533–3539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhu, Z.; Jia, W. Molecular mechanism associated with the use of magnetic fermentation in modulating the dietary lipid composition and nutritional quality of goat milk. Food Chem. 2022, 366, 130554. [Google Scholar] [CrossRef]
- Khakimov, B.; Bakhytkyzy, I.; Fauhl-Hassek, C.; Engelsen, S.B. Non-volatile molecular composition and discrimination of single grape white wines of Chardonnay, Riesling, Sauvignon Blanc and Silvaner using untargeted GC-MS analysis. Food Chem. 2022, 369, 130878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Pan, Q.; Yan, G.; Duan, C. Using headspace solid phase micro-extraction for analysis of aromatic compounds during alcoholic fermentation of red wine. Food Chem. 2011, 125, 743–749. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef]
- Awale, M.; Liu, C.; Kwasniewski, M.T. Workflow to investigate subtle differences in wine volatile metabolome induced by different root systems and irrigation regimes. Molecules 2021, 26, 6010. [Google Scholar] [CrossRef] [PubMed]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted metabolomics strategies—Challenges and emerging directions. J. Am. Soc. Mass Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef]
- Gong, M.; Zhou, Z.; Liu, S.; Zhu, S.; Li, G.; Zhong, F.; Mao, J. Formation pathways and precursors of furfural during Zhenjiang aromatic vinegar production. Food Chem. 2021, 354, 129503. [Google Scholar] [CrossRef]
- Black, C.A.; Parker, M.; Siebert, T.E.; Capone, D.L.; Francis, I.L. Terpenoids and their role in wine flavour: Recent advances. Aust. J. Grape Wine Res. 2015, 21, 582–600. [Google Scholar] [CrossRef]
- Marangon, C.M.; De Rosso, M.; Carraro, R.; Flamini, R. Changes in volatile compounds of grape pomace distillate (Italian grappa) during one-year ageing in oak and cherry barrels. Food Chem. 2021, 344, 128658. [Google Scholar] [CrossRef]
- Reche, R.V.; Neto, L.A.F.; Da Silva, A.A.; Galinaro, C.A.; De Osti, R.Z.; Franco, D.W. Influence of type of distillation apparatus on chemical profiles of Brazilian cachaças. J. Agric. Food Chem. 2007, 55, 6603–6608. [Google Scholar] [CrossRef]
- Ailer, Š.; Jakabová, S.; Benešová, L.; Ivanova-Petropulos, V. Wine faults: State of knowledge in reductive aromas, oxidation and atypical aging, prevention, and correction methods. Molecules 2022, 27, 3535. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; Rahimi, M.R.; Zivdar, M. Efficiencies of sieve tray distillation columns by CFD simulation. Chem. Eng. Technol. 2006, 29, 326–335. [Google Scholar] [CrossRef]
- Gadekar-Shinde, S.; Walekar, P.; Patil-Shinde, V.; Patil, S.S.; Deshannavar, U.B.; Shetti, N.P. Separation of C1-C6 alcohol/ester/water system in continuous reactive distillation unit a thermodynamic analysis. Mater. Sci. Energy Technol. 2023, 6, 48–64. [Google Scholar] [CrossRef]
- Vázquez, L.; Akoh, C.C. Fractionation of short and medium chain fatty acid ethyl esters from a blend of oils via ethanolysis and short-path distillation. J. Am. Oil Chem. Soc. 2010, 87, 917–928. [Google Scholar] [CrossRef]
- Matsui, K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, C.; Barbe, J.C.; Darriet, P.; Geffroy, O.; Gomès, E.; Guillaumie, S.; Thibon, C. Recent advancements in understanding the terroir effect on aromas in grapes and wines: This article is published in cooperation with the XIIIth International Terroir Congress November 17-18 2020, Adelaide, Australia. Guest editors: Cassandra Collins and Roberta De Bei. Oeno One 2020, 54, 985–1006. [Google Scholar]
- Zhang, P.; Fuentes, S.; Siebert, T.; Krstic, M.; Herderich, M.; Barlow, E.W.R.; Howell, K. Terpene evolution during the development of Vitis vinifera L. cv. Shiraz grapes. Food Chem. 2016, 204, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Brotchie, J.; Pang, M.; Marriott, P.J.; Howell, K.; Zhang, P. Free terpene evolution during the berry maturation of five Vitis vinifera L. cultivars. Food Chem. 2019, 299, 125101. [Google Scholar] [CrossRef]
- Nordström, K. Studies on the Formation of Volatile Esters in Fermentation with Brewer’s Yeast: Kungl; Tekniska Högskolan: Stockholm, Sweden, 1964. [Google Scholar]
- Ramey, D.D.; Ough, C.S. Volatile ester hydrolysis or formation storage of model solutions and wines. J. Agric. Food Chem. 1980, 28, 928–934. [Google Scholar] [CrossRef]
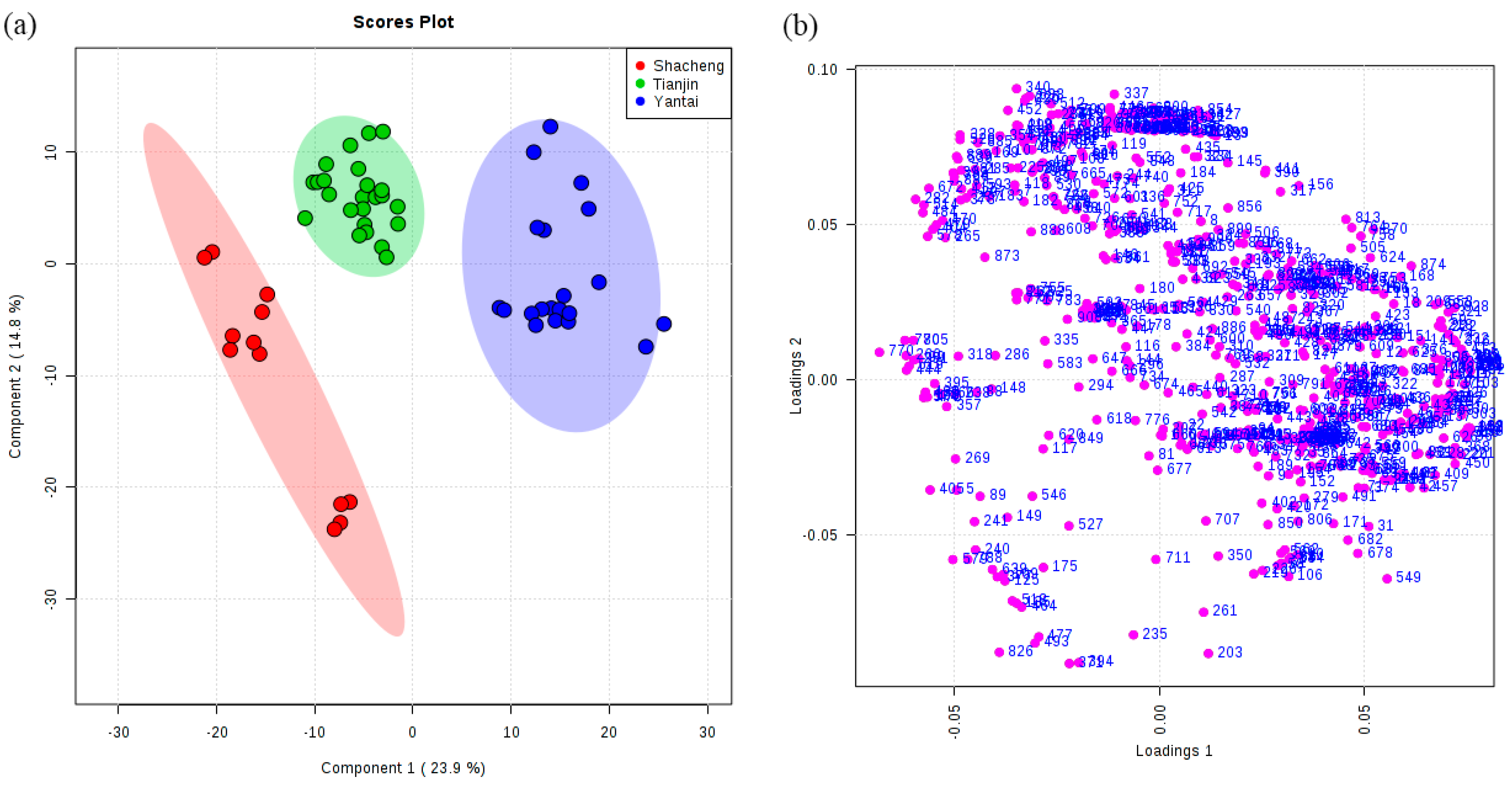
| Model | Class | Numbers | Correct Classifications (%) | R2 (%) | Q2 (%) | Selected Variables (VIP > 1) | GC-MS Peaks |
|---|---|---|---|---|---|---|---|
| (VIP > 1) | |||||||
| 1 | Continuous Still | 38 | 100 | 0.94 | 0.9 | 354 | 32 |
| Pot Still | 8 | 100 | |||||
| 2 | Shacheng | 12 | 100 | 0.85 | 0.82 | 235 | 24 |
| Tianjin | 24 | 100 | |||||
| Yantai | 20 | 100 |
| Compound | Feature ID | VIP | Significance | Pot Still | Continuous Still |
|---|---|---|---|---|---|
| (1S)-cis-Calamenene 3 | 379 | 2.469 | - | 0.002 ± 0.001 | 0.001 ± 0.001 |
| 1,5-Dimethylnaphthalene 3 | 540 | 2.3174 | ** | 0.000502 ± 0.000129 | 0.00121 ± 0.000535 |
| Furfural 1 | 189 | 2.2862 | *** | 0.584 ± 0.121 | 0.141 ± 0.054 |
| β-Damascenone 1 | 517 | 2.163 | *** | 5.126 ± 2.206 | 1.538 ± 0.55 |
| Isoamyl acetate 2 | 507 | 2.1451 | *** | 1992.545 ± 536.69 | 443.673 ± 309.236 |
| Propyl octanoate 2 | 756 | 1.9695 | - | 0.576 ± 0.18 | 0.558 ± 0.37 |
| Hexyl acetate 2 | 654 | 1.8633 | *** | 75.28 ± 34.752 | 8.898 ± 17.274 |
| Vinyl decanoate 2 | 786 | 1.8341 | *** | 42.054 ± 32.322 | 2.746 ± 3.368 |
| α-Ionene 2 | 785 | 1.7883 | *** | 0.001 ± 0.001 | 0 ± 0 |
| 2-Phenethyl acetate 2 | 483 | 1.7725 | *** | 57.768 ± 13.622 | 20.634 ± 11.516 |
| Isobutanol 2 | 167 | 1.7563 | *** | 31,002.732 ± 7642.192 | 48,972.568 ± 11,124.274 |
| 1-Hexanol 1 | 368 | 1.6953 | ** | 1787.951 ± 559.742 | 2611.528 ± 584.55 |
| 1-Heptanol 1 | 12 | 1.6619 | *** | 0.05 ± 0.018 | 0.388 ± 0.252 |
| Methyl octanoate 2 | 35 | 1.6531 | *** | 1.711 ± 0.397 | 3.768 ± 1.234 |
| Ethyl heptanoate 2 | 17 | 1.6307 | *** | 0.333 ± 0.024 | 1.178 ± 0.435 |
| Ethyl nonanoate 2 | 34 | 1.5589 | *** | 1.352 ± 0.151 | 2.903 ± 0.985 |
| Propane, 1,1,3-triethoxy- 3 | 972 | 1.5478 | *** | 0.535 ± 0.572 | 0.072 ± 0.064 |
| Ethyl butanoate 2 | 275 | 1.5156 | *** | 397.005 ± 25.995 | 239.21 ± 73.425 |
| Ethyl salicylate 2 | 107 | 1.5099 | *** | 1.065 ± 0.502 | 3.017 ± 1.2 |
| 1-Nonanol 1 | 43 | 1.4691 | *** | 0.296 ± 0.188 | 3.879 ± 1.979 |
| TDN 2 | 931 | 1.3725 | *** | 8.283 ± 5.478 | 3.508 ± 1.531 |
| Methyl decanoate 2 | 57 | 1.368 | - | 25.455 ± 2.78 | 35.997 ± 14.337 |
| α-Methylnaphthalene 3 | 88 | 1.3644 | ** | 0.00106 ± 0.000223 | 0.00225 ± 0.00104 |
| Ethyl butyl succinate 2 | 90 | 1.3313 | ** | 8.623 ± 7.038 | 56.841 ± 38.987 |
| (S)-3-Ethyl-4-methylpentanol 2 | 137 | 1.2702 | ** | 21.537 ± 15.378 | 105.636 ± 63.778 |
| Nonanal 1 | 111 | 1.2432 | - | 0.01 ± 0.005 | 0.021 ± 0.02 |
| Diethyl succinate 1 | 105 | 1.2407 | ** | 707.764 ± 754.868 | 6357.521 ± 4451.953 |
| Benzaldehyde 1 | 182 | 1.1356 | - | 65.154 ± 59.562 | 118.818 ± 80.712 |
| 1-Decanol 2 | 1025 | 1.1257 | *** | 10.803 ± 6.564 | 5.159 ± 2.351 |
| (E)-3-Hexen-1-ol 2 | 601 | 1.1189 | * | 44.742 ± 34.777 | 80.819 ± 41.385 |
| Linalool 1 | 1307 | 1.0883 | * | 17.954 ± 16.594 | 6.174 ± 6.813 |
| Isopentyl hexanoate 2 | 253 | 1.0096 | - | 6.391 ± 0.867 | 9.36 ± 4.226 |
| Compound | Feature ID | VIP Score | Yantai | Shacheng | Tianjin |
|---|---|---|---|---|---|
| (E)-Calamenene 3 | 828 | 2.1026 | 9.79 ± 1.79 a | 2.43 ± 0.73 b | 11.36 ± 3.5 a |
| α-Terpineol 1 | 321 | 2.039 | 5.69 ± 0.81 b | 2.94 ± 1.17 c | 8.12 ± 2.35 a |
| Isopentanol 1 | 292 | 2.0126 | 145,840.94 ± 11,667.12 b | 218,955.23 ± 34,414.72 a | 234,182.48 ± 45,982.5 a |
| Ethyl lactate 2 | 194 | 1.9334 | 25,559.98 ± 4127.49 a | 17,195.94 ± 6531.74 b | 28,964.51 ± 12,227.76 a |
| Isoamyl lactate 2 | 208 | 1.8654 | 4.11 ± 0.39 a | 2.73 ± 0.55 b | 3.96 ± 1.38 a |
| β-Damascenone 1 | 375 | 1.7845 | 5.15 ± 0.31 c | 6.99 ± 0.87 b | 7.8 ± 0.79 a |
| Benzaldehyde 1 | 626 | 1.7371 | 32.13 ± 10 b | 37.16 ± 22.16 ab | 46.43 ± 17.83 a |
| TDN 2 | 826 | 1.6852 | 12.35 ± 2.08 b | 15.63 ± 9.56 b | 34.92 ± 7.69 a |
| Methyl decanoate 2 | 444 | 1.6666 | 399.98 ± 1.52 c | 427.75 ± 15.68 a | 413.82 ± 5.52 b |
| Isobutanol 2 | 163 | 1.6226 | 89,967.2 ± 18,784.52 b | 116,229.41 ± 29,243.81 a | 127,043.67 ± 32,501.45 a |
| Isoamyl hexanoate 2 | 241 | 1.622 | 4.94 ± 1.11 b | 15.72 ± 7.68 a | 18.49 ± 5.11 a |
| Isobutyl octanoate 2 | 240 | 1.6134 | 1.47 ± 0.55 c | 4.68 ± 4.97 b | 9.9 ± 3.6 a |
| 1-Propanol 2 | 42 | 1.5665 | 17,093.15 ± 3865.7 b | 25,809.17 ± 7220.59 a | 16,193.9 ± 3949.2 b |
| α-Calacorene 3 | 377 | 1.5016 | 6.36 ± 2.69 b | 0.87 ± 0.45 c | 10.03 ± 6.76 a |
| Phenethyl acetate 2 | 624 | 1.4641 | 43.46 ± 16.3 a | 13.87 ± 4.32 b | 56.47 ± 52.28 a |
| Linalool 1 | 409 | 1.448 | 6.34 ± 1.65 | 6.62 ± 2.91 | 5.19 ± 1.93 |
| Propyl octanoate 2 | 780 | 1.4393 | 10.41 ± 5.37 b | 67.34 ± 57.43 a | 26.63 ± 13.74 b |
| (E)-3-Hexen-1-ol 2 | 353 | 1.3865 | 41.12 ± 63.89 b | 112.95 ± 69.26 a | 7.39 ± 20.1 b |
| Ethyl 2-hexenoate 2 | 581 | 1.3563 | 26.51 ± 1.7 b | 26.34 ± 3.5 b | 36.29 ± 8.34 a |
| Isoamyl acetate 2 | 150 | 1.2785 | 1048.62 ± 701.74 | 848.76 ± 268.75 | 1123.94 ± 490.86 |
| Ethyl decanoate 2 | 282 | 1.2544 | 1799.75 ± 503.38 c | 6517.48 ± 3360.27 b | 8082.6 ± 1807.78 a |
| Nonanal 1 | 304 | 1.1166 | 0.97 ± 1.12 ab | 1.59 ± 1.14 a | 0.9 ± 0.49 b |
| Ethyl hexadecanoate 2 | 526 | 1.0707 | 673.51 ± 275.44 b | 1244.49 ± 596.92 a | 1259.46 ± 373.08 a |
| Hexyl acetate 2 | 305 | 1.0102 | 55.82 ± 74.26 | 42.91 ± 34.8 | 77.56 ± 97.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, R.; Duan, C.; Lan, Y. Investigating the Effects of Distillation System, Geographical Origin, and Aging Time on Aroma Characteristics in Brandy Using an Untargeted Metabonomic Approach. Foods 2024, 13, 1922. https://doi.org/10.3390/foods13121922
Hu R, Duan C, Lan Y. Investigating the Effects of Distillation System, Geographical Origin, and Aging Time on Aroma Characteristics in Brandy Using an Untargeted Metabonomic Approach. Foods. 2024; 13(12):1922. https://doi.org/10.3390/foods13121922
Chicago/Turabian StyleHu, Ruiqi, Changqing Duan, and Yibin Lan. 2024. "Investigating the Effects of Distillation System, Geographical Origin, and Aging Time on Aroma Characteristics in Brandy Using an Untargeted Metabonomic Approach" Foods 13, no. 12: 1922. https://doi.org/10.3390/foods13121922





