Distribution of Elements in Durum Wheat Seed and Milling Products: Discrimination between Cultivation Methods through Multivariate Data Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Samples
2.2. Milling
- Flour: ≤160 μm;
- Semolina: 160 < semolina ≤ 560 μm;
- Bran: >560 μm.
2.3. Mineralization
2.4. ICP Analysis
2.5. Processing of the Results: Methods and Softwares
3. Results and Discussion
3.1. Descriptive Statistics
3.2. Pattern Recognition
3.3. Classification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| All Soil Samples | Soils from Organic Cultivation | Soils from Conventional Cultivation | ||||||
|---|---|---|---|---|---|---|---|---|
| LOD | LOQ | Min–Max | Mean ± SD | Min–Max | Mean +SD | Mean +SD | ||
| Ag | 4 × 10−4 | 0.001 | LOD–LOD | LOD ± 0.000 | LOD–LOQ | LOD ± 0.000 | LOD–LOQ | LOD ± 0.000 |
| Al (**) | 0.001 | 0.003 | 1488.550–4140.450 | 2092.514 ± 670.361 | 1488.550–4140.450 | 2167.355 ± 451.637 | 1560.910–3189.510 | 1980.254 ± 466.345 |
| As | 0.001 | 0.004 | 1.000–3.343 | 2.322 ± 0.612 | 1.366–3.073 | 2.219 ± 0.679 | 1.000–3.343 | 2.476 ± 0.701 |
| B | 0.830 | 2.767 | 66.027–144.156 | 91.205 ± 18.454 | 66.027–125.625 | 86.689 ± 19.376 | 74.560–144.156 | 97.978 ± 18.515 |
| Ba (**) | 8 × 10−5 | 3×10−4 | 140.287–233.653 | 189.969 ± 21.746 | 140.287–228.053 | 183.784 ± 17.618 | 168.206–233.653 | 199.247 ± 17.175 |
| Be (**) | 3 × 10−5 | 1×10−4 | 1.249–2.093 | 1.690 ± 0.236 | 1.249–2.003 | 1.563 ± 0.167 | 1.585–2.093 | 1.879 ± 0.144 |
| Ca | 0.009 | 0.029 | 2231.142–2993.378 | 2436.184 ± 176.779 | 2231.142–2993.378 | 2441.475 ± 162.532 | 2261.407–2840.278 | 2428.246 ± 167.641 |
| Cd (**) | 2 × 10−4 | 6×10−4 | 0.132–0.304 | 0.223 ± 0.041 | 0.167–0.304 | 0.235 ± 0.048 | 0.132–0.291 | 0.205 ± 0.047 |
| Cr (**) | 4 × 10−4 | 0.001 | 53.245–96.209 | 76.454 ± 11.511 | 53.245–93.176 | 70.706 ± 7.699 | 70.934–96.209 | 85.076 ± 6.785 |
| Cu (**) | 4 × 10−4 | 0.001 | 26.933–48.164 | 35.780 ± 4.645 | 26.933–41.021 | 33.726 ± 4.346 | 31.228–48.164 | 38.860 ± 4.470 |
| Fe (**) | 2 × 10−4 | 6×10−4 | 13,698.050–21,144.190 | 17,352.365 ± 2128.555 | 13,698.050–20,820.220 | 16,409.707 ± 1624.874 | 16,299.890–21,144.190 | 18,766.351 ± 1596.296 |
| K | 2 × 10−4 | 8×10−4 | 755.832–1222.931 | 963.475 ± 85.755 | 755.832–1118.040 | 947.159 ± 102.945 | 774.873–1222.931 | 987.948 ± 105.488 |
| Mg | 0.001 | 0.005 | 1990.411–2677.165 | 2269.414 ± 175.033 | 1990.411–2612.228 | 2240.159 ± 165.078 | 2148.065–2677.165 | 2313.296 ± 170.404 |
| Mn (**) | 5 × 10−5 | 2×10−4 | 693.361–1277.959 | 931.030 ± 147.022 | 693.361–1277.959 | 885.505 ± 91.233 | 883.112–1147.556 | 999.318 ± 84.972 |
| Mo | 6×10−4 | 0.002 | 0.660–5.678 | 1.262 ± 1.012 | 0.660–5.678 | 1.212 ± 0.944 | 0.806–4.693 | 1.337 ± 0.968 |
| Na | 0.001 | 0.004 | 334.815–802.104 | 550.969 ± 95.093 | 456.723–712.040 | 543.160 ± 118.666 | 334.815–802.104 | 562.683 ± 121.713 |
| Ni (**) | 5 × 10−4 | 0.002 | 39.274–76.972 | 53.301 ± 10.004 | 39.274–76.972 | 49.046 ± 7.288 | 48.823–74.643 | 59.683 ± 6.668 |
| P | 0.004 | 0.012 | 98.932–871.575 | 680.919 ± 159.183 | 103.282–871.575 | 715.453 ± 163.216 | 98.932–786.637 | 629.120 ± 167.303 |
| Pb | 7 × 10−4 | 0.002 | 13.623–43.143 | 19.372 ± 4.417 | 13.623–43.143 | 19.043 ± 1.897 | 16.091–23.817 | 19.866 ± 1.959 |
| Sb | 0.003 | 0.010 | LOD–LOQ | LOD ± 0.001 | LOD–LOQ | LOD ± 0.001 | LOD–LOD | LOD ± 0.001 |
| Se | 0.003 | 0.010 | LOD–LOQ | LOD ± 0.001 | LOD–LOQ | LOD ± 0.001 | LOD–LOQ | LOD ± 0.001 |
| Si | 0.018 | 0.060 | 197.230–2826.853 | 963.128 ± 830.102 | 218.109–2588.145 | 927.417 ± 889.582 | 197.230–2826.853 | 1016.695 ± 902.656 |
| Sn | 0.007 | 0.023 | 0.962–655.190 | 34.511 ± 136.265 | 1.161–655.190 | 30.777 ± 140.891 | 0.962–584.401 | 40.113 ± 145.189 |
| Tl | 0.001 | 0.003 | LOD–1.239 | 0.494 ± 0.338 | LOD–1.239 | 0.469 ± 0.298 | LOD–0.890 | 0.530 ± 0.307 |
| V (**) | 2 × 10−4 | 5×10−4 | 42.138–76.322 | 58.657 ± 6.575 | 42.138–63.256 | 55.289 ± 5.513 | 55.762–76.322 | 63.710 ± 5.165 |
| Zn (**) | 7 × 10−5 | 2×10−4 | 64.296–96.585 | 80.211 ± 9.031 | 64.296–94.897 | 75.987 ± 6.488 | 75.810–96.585 | 86.545 ± 6.163 |
References
- Sissons, M.; Abecassis, J.; Marchylo, B.; Carcea, M. (Eds.) Durum Wheat Chemistry and Technology, 2nd ed.; AACC International: St. Paul, MN, USA, 2012; ISBN 978-1-891127-65-6. [Google Scholar]
- Bux, C.; Lombardi, M.; Varese, E.; Amicarelli, V. Economic and Environmental Assessment of Conventional versus Organic Durum Wheat Production in Southern Italy. Sustainability 2022, 14, 9143. [Google Scholar] [CrossRef]
- Fagnano, M.; Fiorentino, N.; D’Egidio, M.G.; Quaranta, F.; Ritieni, A.; Ferracane, R.; Raimondi, G. Durum Wheat in Conventional and Organic Farming: Yield Amount and Pasta Quality in Southern Italy. Sci. World J. 2012, 2012, 973058. [Google Scholar] [CrossRef] [PubMed]
- Ludajic, G.; Pezo, L.; Filipovic, J.; Filipovic, V.; Kosanic, N. Determination of Essential and Toxic Elements in Products of Milling Wheat. Hem. Ind. 2016, 70, 707–715. [Google Scholar] [CrossRef]
- Vergine, M.; Aprile, A.; Sabella, E.; Genga, A.; Siciliano, M.; Rampino, P.; Lenucci, M.S.; Luvisi, A.; Bellis, L. De Cadmium Concentration in Grains of Durum Wheat (Triticum turgidum L. subsp. Durum). J. Agric. Food Chem. 2017, 65, 6240–6246. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, Y.; Wang, L.; Huang, Q.; Yan, X.; Sun, Y.; Qin, X.; Liang, X. Variations in Cadmium and Lead Bioaccessibility in Wheat Cultivars and Their Correlations with Nutrient Components. J. Agric. Food Chem. 2024, 72, 1768–1778. [Google Scholar] [CrossRef] [PubMed]
- Kovarikova, M.; Tomaskova, I.; Soudek, P. Rare Earth Elements in Plants. Biol. Plant 2019, 63, 20–32. [Google Scholar] [CrossRef]
- Baize, D.; Bellanger, L.; Tomassone, R. Relationships between Concentrations of Trace Metals in Wheat Grains and Soil. Agron. Sustain. Dev. 2009, 29, 297–312. [Google Scholar] [CrossRef]
- Mermut, A.R.; Jain, J.C.; Song, L.; Kerrich, R.; Kozak, L.; Jana, S. Trace Element Concentrations of Selected Soils and Fertilizers in Saskatchewan, Canada. J. Environ. Qual. 1996, 25, 845–853. [Google Scholar] [CrossRef]
- Ma, C.; Liu, F.; Jin, K.; Hu, B.; Wei, M.; Zhao, J.; Zhang, H.; Zhang, K. Effects of Atmospheric Fallout on Lead Contamination of Wheat Tissues Based on Stable Isotope Ratios. Bull. Environ. Contam. Toxicol. 2019, 103, 676–682. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.-H. Heavy Metals in Food Crops: Health Risks, Fate, Mechanisms, and Management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Zhang, X.; Blennow, A.; Jekle, M.; Zörb, C. Climate–Nutrient–Crop Model: Novel Insights into Grain-Based Food Quality. J. Agric. Food Chem. 2023, 71, 10228–10237. [Google Scholar] [CrossRef] [PubMed]
- Dangour, A.D.; Dodhia, S.K.; Hayter, A.; Allen, E.; Lock, K.; Uauy, R. Nutritional Quality of Organic Foods: A Systematic Review. Am. J. Clin. Nutr. 2009, 90, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Zörb, C.; Niehaus, K.; Barsch, A.; Betsche, T.; Langenkämper, G. Levels of Compounds and Metabolites in Wheat Ears and Grains in Organic and Conventional Agriculture. J. Agric. Food Chem. 2009, 57, 9555–9562. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, S.A.; Seitz, B.; van der Heijden, M.G.A.; Schulin, R.; Tandy, S. Impact of Organic and Conventional Farming Systems on Wheat Grain Uptake and Soil Bioavailability of Zinc and Cadmium. Sci. Total Environ. 2018, 639, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Laursen, K.H.; Schjoerring, J.K.; Olesen, J.E.; Askegaard, M.; Halekoh, U.; Husted, S. Multielemental Fingerprinting as a Tool for Authentication of Organic Wheat, Barley, Faba Bean, and Potato. J. Agric. Food Chem. 2011, 59, 4385–4396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, Q.; Yan, J.; Tang, J.; Zhao, R.; Zhang, Y.; He, Z.; Zou, C.; Ortiz-Monasterio, I. Mineral Element Concentrations in Grains of Chinese Wheat Cultivars. Euphytica 2010, 174, 303–313. [Google Scholar] [CrossRef]
- Brizio, P.; Benedetto, A.; Squadrone, S.; Curcio, A.; Pellegrino, M.; Ferrero, M.; Abete, M.C. Heavy Metals and Essential Elements in Italian Cereals. Food Addit. Contam. Part B 2016, 9, 261–267. [Google Scholar] [CrossRef]
- Harmankaya, M.; Özcan, M.M.; Gezgin, S. Variation of Heavy Metal and Micro and Macro Element Concentrations of Bread and Durum Wheats and Their Relationship in Grain of Turkish Wheat Cultivars. Environ. Monit. Assess. 2012, 184, 5511–5521. [Google Scholar] [CrossRef]
- Menga, V.; Giovanniello, V.; Savino, M.; Gallo, A.; Colecchia, S.A.; De Simone, V.; Zingale, S.; Ficco, D.B.M. Comparative Analysis of Qualitative and Bioactive Compounds of Whole and Refined Flours in Durum Wheat Grains with Different Year of Release and Yield Potential. Plants 2023, 12, 1350. [Google Scholar] [CrossRef]
- Monti, C.; Cavanna, D.; Rodushkin, I.; Monti, A.; Leporati, A.; Suman, M. Determining the Geographical Origin of Durum Wheat Samples by Combining Strontium Isotope Ratio and Multielemental Analyses. Cereal Chem. 2023, 100, 522–531. [Google Scholar] [CrossRef]
- Bacher, F.; Aguzzoni, A.; Chizzali, S.; Pignotti, E.; Puntscher, H.; Zignale, P.; Voto, G.; Tagliavini, M.; Tirler, W.; Robatscher, P. Geographic Tracing of Cereals from South Tyrol (Italy) and Neighboring Regions via 87Sr/86Sr Isotope Analysis. Food Chem. 2023, 405, 134890. [Google Scholar] [CrossRef] [PubMed]
- Marini, F. (Ed.) Chemometrics in Food Chemistry, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 28, ISBN 978-0-444-59528-7. [Google Scholar]
- Cozzolino, D. An Overview of the Use of Infrared Spectroscopy and Chemometrics in Authenticity and Traceability of Cereals. Food Res. Int. 2014, 60, 262–265. [Google Scholar] [CrossRef]
- De Flaviis, R.; Sacchetti, G.; Mastrocola, D. Wheat Classification According to Its Origin by an Implemented Volatile Organic Compounds Analysis. Food Chem. 2021, 341, 128217. [Google Scholar] [CrossRef] [PubMed]
- Cervellieri, S.; Lippolis, V.; Mancini, E.; Pascale, M.; Logrieco, A.F.; De Girolamo, A. Mass Spectrometry-Based Electronic Nose to Authenticate 100% Italian Durum Wheat Pasta and Characterization of Volatile Compounds. Food Chem. 2022, 383, 132548. [Google Scholar] [CrossRef] [PubMed]
- De Flaviis, R.; Mutarutwa, D.; Sacchetti, G.; Mastrocola, D. Quantitatively Unravelling the Effect of Altitude of Cultivation on the Volatiles Fingerprint of Wheat by a Chemometric Approach. Food Chem. 2022, 370, 131296. [Google Scholar] [CrossRef] [PubMed]
- Giorgia Potortì, A.; Francesco Mottese, A.; Rita Fede, M.; Sabatino, G.; Dugo, G.; Lo Turco, V.; Costa, R.; Caridi, F.; Di Bella, M.; Di Bella, G. Multielement and Chemometric Analysis for the Traceability of the Pachino Protected Geographical Indication (PGI) Cherry Tomatoes. Food Chem. 2022, 386, 132746. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, L.; Fontanella, M.C.; Amalfitano, C.; Beone, G.M.; Adamo, P. Provenance Discrimination of Sorrento Lemon with Protected Geographical Indication (PGI) by Multi-Elemental Fingerprinting. Food Chem. 2021, 362, 130168. [Google Scholar] [CrossRef] [PubMed]
- Cera, M.C. (Ed.) Cereali in Coltivazione Biologica. Guida Pratica Alle Colture Autunno-Vernine; Il Sole 24 Ore Edagricole: Bologna, Italy, 2004; ISBN 978-8850649945. [Google Scholar]
- Ballabio, D.; Consonni, V. Classification Tools in Chemistry. Part 1: Linear Models. PLS-DA. Anal. Methods 2013, 5, 3790. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Principal Component Analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef]
- Stocchero, M.; De Nardi, M.; Scarpa, B. PLS for Classification. Chemom. Intell. Lab. Syst. 2021, 216, 104374. [Google Scholar] [CrossRef]
- Stone, M. Cross-Validatory Choice and Assessment of Statistical Predictions. J. R. Stat. Soc. Ser. B (Methodol.) 1974, 36, 111–133. [Google Scholar] [CrossRef]
- Pomerantsev, A.L.; Rodionova, O.Y. New Trends in Qualitative Analysis: Performance, Optimization, and Validation of Multi-Class and Soft Models. TrAC Trends Anal. Chem. 2021, 143, 116372. [Google Scholar] [CrossRef]
- Martens, H.; Martens, M. Modified Jack-Knife Estimation of Parameter Uncertainty in Bilinear Modelling by Partial Least Squares Regression (PLSR). Food Qual. Prefer. 2000, 11, 5–16. [Google Scholar] [CrossRef]
- Conti, M.E.; Cubadda, F.; Carcea, M. Trace Metals in Soft and Durum Wheat from Italy. Food Addit. Contam. 2000, 17, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, H.; Sager, M.; Oberforster, M.; Mechtler, K.; Stüger, H.P.; Baumgarten, A. Nutritionally Relevant Elements in Staple Foods: Influence of Arable Site versus Choice of Variety. Environ. Geochem. Health 2009, 31, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Cubadda, F.; Baldini, M.; Carcea, M.; Pasqui, L.A.; Raggi, A.; Stacchini, P. Influence of Laboratory Homogenization Procedures on Trace Element Content of Food Samples: An ICP-MS Study on Soft and Durum Wheat. Food Addit. Contam. 2001, 18, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Ballabio, D.; Grisoni, F.; Todeschini, R. Multivariate Comparison of Classification Performance Measures. Chemom. Intell. Lab. Syst. 2018, 174, 33–44. [Google Scholar] [CrossRef]
- Ficco, D.B.M.; Beleggia, R.; Pecorella, I.; Giovanniello, V.; Frenda, A.S.; Vita, P. De Relationship between Seed Morphological Traits and Ash and Mineral Distribution along the Kernel Using Debranning in Durum Wheats from Different Geographic Sites. Foods 2020, 9, 1523. [Google Scholar] [CrossRef]
- Foy, C.D. Physiological Effects of Hydrogen, Aluminum, and Manganese Toxicities in Acid Soil. In Agronomy Monographs; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2015; pp. 57–97. [Google Scholar]
- Khabaz-Saberi, H.; Rengel, Z. Aluminum, Manganese, and Iron Tolerance Improves Performance of Wheat Genotypes in Waterlogged Acidic Soils. J. Plant Nutr. Soil Sci. 2010, 173, 461–468. [Google Scholar] [CrossRef]
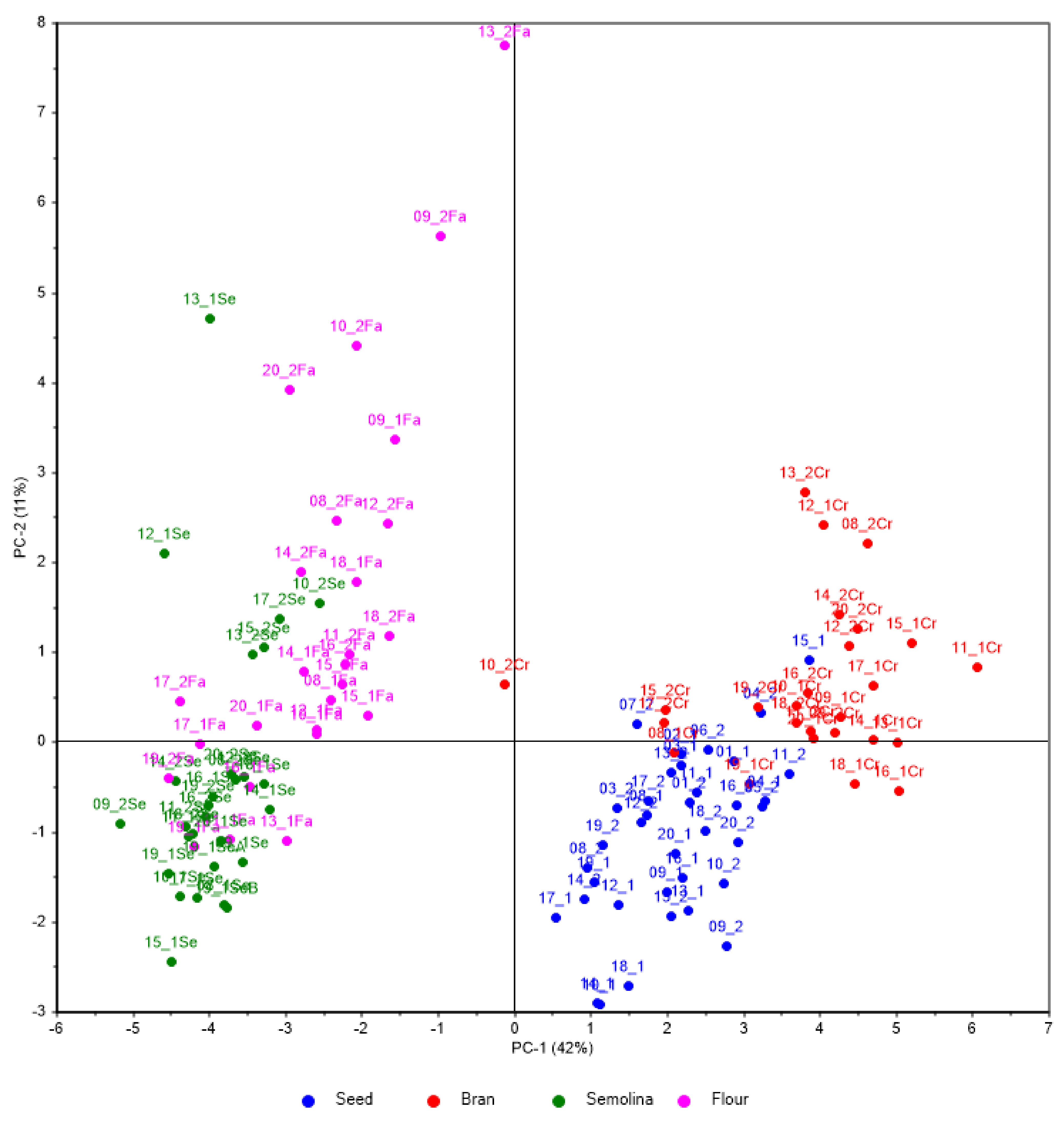
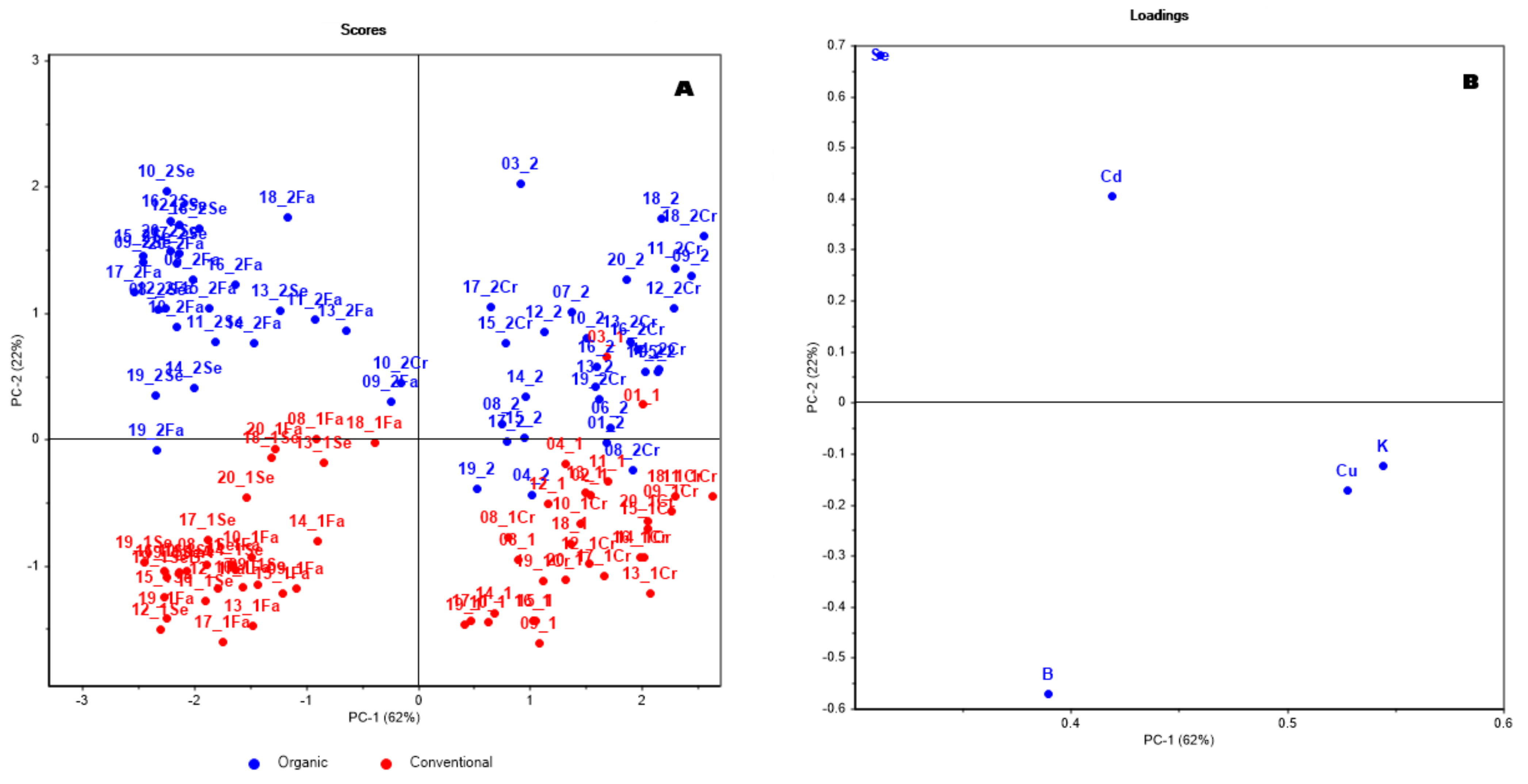
| Wheat Variety | Code of the Variety | Code of the Organic Samples | Code of the Conventional Samples | Seed | Bran | Semolina | Flour |
|---|---|---|---|---|---|---|---|
| Saragolla new | 01 | 01_2 | 01_1 | Yes | NO | NO | NO |
| San Carlo | 02 | NO | 02_1 | Yes | NO | NO | NO |
| Fuego grown in a large plot | 03 | 03_2 | 03_1 | Yes | NO | NO | NO |
| EVOLDUR evolutionary population harvest 2021 | 04 | 04_2 | NO | Yes | NO | NO | NO |
| Evoldur grown in a large plot | 04 | NO | 04_1 | Yes | NO | NO | NO |
| Senatore Cappelli | 05 | 05_2 | NO | Yes | NO | NO | NO |
| Saragolla old | 06 | 06_2 | NO | Yes | NO | NO | NO |
| Fuego grown in the edge near the railway | 07 | 07_2 | NO | Yes | NO | NO | NO |
| Antalis | 08 | 08_2 | 08_1 | Yes | Yes | Yes | Yes |
| Bering | 09 | 09_2 | 09_1 | Yes | Yes | Yes | Yes |
| Casteldoux | 10 | 10_2 | 10_1 | Yes | Yes | Yes | Yes |
| Claudio | 11 | 11_2 | 11_1 | Yes | Yes | Yes | Yes |
| Fuego | 12 | 12_2 | 12_1 | Yes | Yes | Yes | Yes |
| Idefix | 13 | 13_2 | 13_1 | Yes | Yes | Yes | Yes |
| Iride | 14 | 14_2 | 14_1 | Yes | Yes | Yes | Yes |
| Marakas | 15 | 15_2 | 15_1 | Yes | Yes | Yes | Yes |
| Marco Aurelio | 16 | 16_2 | 16_1 | Yes | Yes | Yes | Yes |
| Monastir | 17 | 17_2 | 17_1 | Yes | Yes | Yes | Yes |
| Platone | 18 | 18_2 | 18_1 | Yes | Yes | Yes | Yes |
| RGT Natur | 19 | 19_2 | 19_1 | Yes | Yes | Yes | Yes |
| Tito Flavio | 20 | 20_2 | 20_1 | Yes | Yes | Yes | Yes |
| Organic Seeds | Conventional Seeds | ||||
|---|---|---|---|---|---|
| Element | p-Value (t) | Mean ± SD | Min–Max | Mean ± SD | Min–Max |
| Ag | 0.161 (1.433) | 0.003 ± 0.002 | LOD–0.006 | 0.003 ± 0.002 | LOD–0.009 |
| Al | 0.375 (0.900) | 4.811 ± 1.522 | 1.788–6.583 | 4.216 ± 1.897 | 1.533–6.741 |
| As | 0.736 (0.340) | 0.073 ± 0.031 | 0.024–0.156 | 0.075 ± 0.043 | 0.020–0.218 |
| B (**) | 0.000 (3.876) | 2.107 ± 0.405 | 0.526–2.502 | 2.686 ± 0.453 | 1.999–3.185 |
| Ba | 0.464 (0.741) | 0.842 ± 0.357 | 0.358–1.631 | 0.756 ± 0.282 | 0.394–1.359 |
| Be | 0.160 (1.436) | LOD ± 0.001 | LOD–0.001 | 0.000 ± 0.000 | LOD–0.001 |
| Ca | 0.316 (1.017) | 482.951 ± 23.743 | 432.194–519.332 | 472.957 ± 35.970 | 392.386–531.502 |
| Cd (*) | 0.021 (2.420) | 0.029 ± 0.010 | 0.016–0.045 | 0.022 ± 0.007 | 0.013–0.036 |
| Co | 0.936 (0.081) | 0.005 ± 0.003 | LOD–0.011 | 0.005 ± 0.003 | LOQ–0.009 |
| Cr | 0.433 (0.793) | 0.084 ± 0.010 | 0.072–0.115 | 0.109 ± 0.131 | 0.064–0.615 |
| Cu (*) | 0.018 (2.495) | 4.091 ± 0.534 | 3.433–5.226 | 4.610 ± 0.537 | 3.851–5.814 |
| Fe (**) | 0.002 (3.277) | 28.111 ± 2.702 | 23.929–34.350 | 25.455 ± 2.115 | 21.324–29.221 |
| K | 0.289 (1.077) | 3203.997 ± 176.816 | 2933.319–3697.111 | 3287.373 ± 157.776 | 3046.468–3587.377 |
| Mg (*) | 0.025 (2.338) | 526.603 ± 18.206 | 481.189–558.077 | 513.229 ± 18.949 | 484.819–553.452 |
| Mn | 0.298 (1.056) | 35.561 ± 4.322 | 28.101–42.808 | 34.665 ± 4.715 | 28.301–41.289 |
| Mo | 0.385 (0.881) | 0.929 ± 0.215 | 0.566–1.327 | 1.001 ± 0.296 | 0.659–1.644 |
| Na | 0.518 (0.654) | 27.683 ± 7.748 | 18.349–48.180 | 25.580 ± 4.584 | 20.586–37.991 |
| Ni | 0.649 (0.459) | 0.177 ± 0.059 | 0.111–0.327 | 0.198 ± 0.110 | 0.105–0.580 |
| P | 0.542 (0.615) | 3373.664 ± 268.498 | 2984.810–3903.900 | 3341.108 ± 245.271 | 3001.920–3889.520 |
| Pb | 0.522 (0.646) | 0.057 ± 0.018 | 0.036–0.092 | 0.054 ± 0.025 | 0.026–0.128 |
| Sb | 0.606 (0.521) | LOQ ± 0.005 | LOD–0.019 | LOD ± 0.005 | LOD–0.020 |
| Se (**) | 0.000 (5.357) | 0.238 ± 0.036 | 0.151–0.290 | 0.178 ± 0.029 | 0.131–0.222 |
| Si | 0.927 (0.093) | 22.694 ± 5.045 | 13.869–32.932 | 22.010 ± 8.297 | 13.517–48.981 |
| Sn | 0.821 (0.228) | 0.005 ± 0.002 | 0.003–0.009 | 0.005 ± 0.003 | 0.002–0.011 |
| Ti | 0.830 (0.216) | 0.021 ± 0.013 | 0.007–0.064 | 0.020 ± 0.011 | 0.007–0.039 |
| Tl | 0.196 (1.320) | 0.004 ± 0.007 | LOD–0.022 | 0.010 ± 0.013 | LOD–0.043 |
| V | 0.439 (0.784) | 0.009 ± 0.002 | 0.006–0.011 | 0.010 ± 0.002 | 0.006–0.015 |
| Zn | 0.349 (0.950) | 30.130 ± 5.757 | 22.541–45.556 | 28.863 ± 3.997 | 24.495–40.231 |
| Organic Brans | Conventional Brans | ||||
|---|---|---|---|---|---|
| Element | p-Value (t) | Mean ± SD | Min–Max | Mean ± SD | Min–Max |
| Ag | 0.132 (1.559) | 0.005 ± 0.002 | LOQ–0.007 | 0.007 ± 0.003 | LOQ–0.013 |
| Al (*) | 0.040 (2.167) | 5.916 ± 2.512 | 3.784–12.263 | 4.270 ± 0.797 | 2.579–5.757 |
| As | 0.278 (1.111) | 0.070 ± 0.025 | 0.021–0.118 | 0.088 ± 0.052 | 0.045–0.236 |
| B (**) | 0.001 (3.781) | 1.504 ± 0.340 | 0.578–1.890 | 1.919 ± 0.139 | 1.561–2.092 |
| Ba | 0.588 (0.549) | 1.093 ± 0.379 | 0.603–1.953 | 1.181 ± 0.440 | 0.587–2.057 |
| Be | 0.337 (0.980) | 0.001 ± 0.000 | 0.001–0.001 | 0.001 ± 0.001 | 0.001–0.003 |
| Ca (*) | 0.045 (2.118) | 465.731 ± 23.447 | 412.787–490.929 | 486.383 ± 26.193 | 447.286–525.940 |
| Cd | 0.099 (1.714) | 0.031 ± 0.012 | 0.017–0.053 | 0.024 ± 0.007 | 0.017–0.035 |
| Co | 0.067 (1.915) | 0.004 ± 0.002 | LOD–0.008 | 0.006 ± 0.003 | LOQ–0.012 |
| Cr | 0.298 (1.063) | 0.104 ± 0.020 | 0.072–0.140 | 0.112 ± 0.015 | 0.087–0.137 |
| Cu (**) | 0.000 (4.295) | 4.925 ± 0.712 | 3.456–5.848 | 6.168 ± 0.763 | 5.078–7.457 |
| Fe | 0.996 (0.005) | 36.673 ± 6.217 | 25.335–48.132 | 36.684 ± 3.136 | 30.115–41.784 |
| K (**) | 0.006 (3.005) | 3702.171 ± 516.030 | 2561.379–4242.568 | 4206.877 ± 283.921 | 3511.909–4540.521 |
| Mg | 0.137 (1.538) | 558.948 ± 36.235 | 462.179–603.480 | 576.091 ± 15.827 | 547.554–603.324 |
| Mn | 0.063 (1.953) | 46.950 ± 9.574 | 24.625–61.671 | 54.534 ± 10.216 | 35.295–73.585 |
| Mo (**) | 0.001 (3.794) | 0.980 ± 0.166 | 0.733–1.258 | 1.264 ± 0.213 | 0.973–1.598 |
| Na (*) | 0.026 (2.378) | 35.491 ± 9.294 | 24.952–61.069 | 49.927 ± 19.203 | 34.075–97.760 |
| Ni | 0.574 (0.570) | 0.223 ± 0.090 | 0.086–0.419 | 0.241 ± 0.063 | 0.147–0.343 |
| P (**) | 0.010 (2.817) | 3974.312 ± 596.423 | 2544.200–4748.610 | 4520.654 ± 365.057 | 3664.670–4985.480 |
| Pb (*) | 0.012 (2.707) | 0.100 ± 0.027 | 0.055–0.135 | 0.077 ± 0.016 | 0.055–0.106 |
| Sb | 0.385 (0.885) | LOD ± 0.001 | LOD–LOD | LOD ± 0.001 | LOD–LOD |
| Se (**) | 0.000 (9.601) | 0.242 ± 0.024 | 0.188–0.275 | 0.166 ± 0.015 | 0.141–0.195 |
| Si | 0.790 (0.269) | 33.757 ± 12.361 | 14.746–56.431 | 35.167 ± 14.267 | 19.242–69.089 |
| Sn (*) | 0.027 (2.350) | 0.007 ± 0.003 | 0.004–0.016 | 0.005 ± 0.001 | 0.003–0.009 |
| Ti | 0.240 (1.204) | 0.061 ± 0.040 | 0.019–0.153 | 0.046 ± 0.021 | 0.023–0.099 |
| Tl | 0.969 (0.039) | 0.007 ± 0.010 | LOD–0.033 | 0.007 ± 0.007 | LOD–0.021 |
| V | 0.473 (0.730) | 0.009 ± 0.006 | LOQ–0.021 | 0.008 ± 0.006 | LOQ–0.018 |
| Zn | 0.079 (1.835) | 37.297 ± 6.372 | 22.804–45.948 | 41.169 ± 4.154 | 33.005–47.971 |
| Organic Semolina | Conventional Semolina | ||||
|---|---|---|---|---|---|
| Element | p-Value (t) | Mean ± SD | Min–Max | Mean ± SD | Min–Max |
| Ag | 0.074 (1.871) | LOQ ± 0.001 | LOD–0.005 | 0.003 ± 0.002 | LOD–0.007 |
| Al (*) | 0.023 (2.436) | 6.147 ± 2.871 | 3.461–13.392 | 3.662 ± 1.678 | 1.447–7.705 |
| As | 0.459 (0.753) | 0.070 ± 0.038 | 0.027–0.182 | 0.083 ± 0.081 | 0.024–0.363 |
| B (**) | 0.000 (6.983) | 0.288 ± 0.432 | LOD–0.951 | 1.346 ± 0.188 | 1.109–1.851 |
| Ba | 0.121 (1.606) | 0.482 ± 0.125 | 0.321–0.767 | 0.395 ± 0.109 | 0.279–0.635 |
| Be | 0.337 (0.980) | 0.001 ± 0.000 | 0.001–0.001 | 0.001 ± 0.000 | 0.001–0.002 |
| Ca | 0.524 (0.647) | 437.659 ± 28.502 | 404.677–486.215 | 430.917 ± 31.543 | 390.838–479.139 |
| Cd (*) | 0.024 (2.414) | 0.017 ± 0.006 | 0.010–0.031 | 0.012 ± 0.004 | 0.008–0.018 |
| Co | 0.386 (0.882) | LOD ± 0.002 | LOD–0.009 | LOD ± 0.000 | LOD–LOD |
| Cr | 0.309 (1.039) | 0.057 ± 0.051 | 0.034–0.226 | 0.042 ± 0.009 | 0.024–0.061 |
| Cu (**) | 0.008 (2.871) | 2.341 ± 0.226 | 2.034–2.745 | 2.614 ± 0.281 | 2.138–3.228 |
| Fe (**) | 0.007 (2.950) | 13.298 ± 2.761 | 9.983–19.124 | 10.612 ± 1.404 | 7.844–13.894 |
| K (**) | 0.004 (3.138) | 1657.293 ± 134.168 | 1377.052–1864.274 | 1808.033 ± 112.416 | 1623.165–2009.210 |
| Mg | 0.289 (1.085) | 349.486 ± 24.248 | 300.566–383.394 | 341.154 ± 15.061 | 314.100–365.416 |
| Mn | 0.216 (1.270) | 8.885 ± 0.868 | 7.173–10.532 | 9.391 ± 1.373 | 7.456–11.745 |
| Mo | 0.349 (0.955) | 0.810 ± 0.210 | 0.539–1.249 | 0.855 ± 0.131 | 0.702–1.101 |
| Na | 0.278 (1.111) | 21.091 ± 4.336 | 16.225–32.199 | 31.682 ± 23.065 | 15.214–95.377 |
| Ni | 0.654 (0.454) | 0.074 ± 0.102 | 0.027–0.411 | 0.088 ± 0.071 | 0.041–0.330 |
| P | 0.238 (1.209) | 1506.895 ± 135.215 | 1286.050–1710.380 | 1577.838 ± 123.078 | 1337.920–1710.160 |
| Pb | 0.556 (0.597) | 0.069 ± 0.014 | 0.054–0.102 | 0.077 ± 0.054 | 0.038–0.263 |
| Sb | 0.935 (0.083) | LOD ± 0.001 | LOD–LOD | LOD ± 0.002 | LOD–LOQ |
| Se (**) | 0.000 (6.063) | 0.191 ± 0.025 | 0.155–0.240 | 0.118 ± 0.032 | 0.077–0.212 |
| Si (**) | 0.003 (3.285) | 11.120 ± 3.979 | 6.314–19.054 | 7.135 ± 1.866 | 4.997–11.171 |
| Sn | 0.349 (0.954) | 0.007 ± 0.002 | 0.005–0.010 | 0.010 ± 0.014 | 0.004–0.059 |
| Ti | 0.665 (0.439) | 0.033 ± 0.018 | 0.016–0.077 | 0.028 ± 0.018 | 0.008–0.077 |
| Tl | 0.258 (1.158) | LOD ± 0.000 | LOD–LOD | LOQ ± 0.001 | LOD–0.003 |
| V | 0.279 (1.108) | 0.008 ± 0.006 | LOQ–0.020 | 0.046 ± 0.132 | LOQ–0.520 |
| Zn | 0.200 (1.319) | 10.907 ± 0.955 | 8.952–12.198 | 11.478 ± 1.072 | 9.035–12.972 |
| Organic Flours | Conventional Flours | ||||
|---|---|---|---|---|---|
| Element | p-Value (t) | Mean ± SD | Min–Max | Mean ± SD | Min–Max |
| Ag | 0.297 (1.067) | LOQ ± 0.002 | LOD–0.006 | LOQ ± 0.003 | LOD–0.007 |
| Al (*) | 0.023 (2.431) | 11.869 ± 10.517 | 5.225–44.802 | 4.233 ± 1.379 | 2.295–7.391 |
| As | 0.114 (1.639) | 0.063 ± 0.020 | 0.036–0.095 | 0.103 ± 0.084 | 0.036–0.305 |
| B (**) | 0.000 (6.543) | 0.400 ± 0.467 | LOD–1.106 | 1.464 ± 0.230 | 1.192–2.030 |
| Ba | 0.124 (1.592) | 0.530 ± 0.142 | 0.285–0.784 | 0.446 ± 0.126 | 0.266–0.641 |
| Be | 0.136 (1.544) | 0.002 ± 0.001 | 0.001–0.006 | 0.001 ± 0.000 | 0.001–0.001 |
| Ca | 0.242 (1.200) | 447.069 ± 28.188 | 391.647–486.933 | 434.249 ± 26.244 | 372.960–480.070 |
| Cd | 0.056 (2.006) | 0.017 ± 0.006 | 0.010–0.030 | 0.013 ± 0.004 | 0.008–0.020 |
| Co | 0.908 (0.117) | LOQ ± 0.003 | LOD–0.010 | LOD ± 0.001 | LOD–0.004 |
| Cr | 0.054 (2.029) | 0.117 ± 0.092 | 0.046–0.382 | 0.061 ± 0.029 | 0.037–0.149 |
| Cu | 0.245 (1.193) | 2.724 ± 0.509 | 2.000–3.861 | 2.924 ± 0.326 | 2.401–3.426 |
| Fe (*) | 0.031 (2.289) | 19.134 ± 8.469 | 11.067–45.423 | 13.320 ± 2.018 | 10.950–17.896 |
| K (*) | 0.038 (2.197) | 1910.892 ± 172.820 | 1664.408–2246.599 | 2051.040 ± 151.758 | 1832.890–2303.912 |
| Mg | 0.388 (0.878) | 390.328 ± 30.020 | 329.942–424.400 | 380.778 ± 25.209 | 343.350–426.213 |
| Mn | 0.879 (0.154) | 9.434 ± 1.645 | 6.734–11.814 | 9.343 ± 1.349 | 7.660–12.084 |
| Mo | 0.429 (0.804) | 1.105 ± 1.003 | 0.551–4.341 | 0.875 ± 0.144 | 0.666–1.180 |
| Na | 0.173 (1.405) | 28.049 ± 14.513 | 17.849–74.832 | 44.567 ± 39.018 | 17.774–141.929 |
| Ni | 0.523 (0.648) | 0.161 ± 0.234 | 0.039–0.920 | 0.117 ± 0.047 | 0.062–0.229 |
| P | 0.513 (0.665) | 1746.273 ± 213.606 | 1335.880–2115.440 | 1799.051 ± 190.710 | 1517.020–2159.440 |
| Pb | 0.250 (1.180) | 0.088 ± 0.025 | 0.062–0.148 | 0.074 ± 0.037 | 0.036–0.186 |
| Sb | 0.765 (0.303) | LOD ± 0.001 | LOD–LOD | LOD ± 0.001 | LOD–LOD |
| Se (**) | 0.000 (5.020) | 0.186 ± 0.023 | 0.158–0.230 | 0.127 ± 0.036 | 0.091–0.200 |
| Si (*) | 0.019 (2.525) | 28.860 ± 20.680 | 15.683–85.435 | 13.105 ± 4.473 | 10.116–27.071 |
| Sn | 0.285 (1.094) | 0.011 ± 0.003 | 0.005–0.016 | 0.015 ± 0.011 | 0.006–0.035 |
| Ti | 0.060 (1.975) | 0.104 ± 0.122 | 0.027–0.484 | 0.034 ± 0.018 | 0.018–0.080 |
| Tl | 0.342 (0.970) | 0.002 ± 0.005 | LOD–0.019 | LOD ± 0.000 | LOD–LOQ |
| V (*) | 0.017 (2.560) | 0.021 ± 0.018 | 0.003–0.075 | 0.082 ± 0.079 | 0.007–0.235 |
| Zn | 0.600 (0.531) | 12.892 ± 1.982 | 9.403–16.102 | 13.264 ± 1.569 | 11.396–15.950 |
| Product | Data Pre-Treatment | Selected Elements | Number of Latent Variables (Computed; Optimal) | R2 | Q2 | Precision (Conv; Org) | Accuracy | Pred. Precision (Conv; Org) | Pred. Accuracy |
|---|---|---|---|---|---|---|---|---|---|
| All samples together | Log10 transform, autoscaled | B, Cd, Cu, Fe, Se | 4; 4 | 0.78 | 0.76 | 0.98; 0.97 | 0.97 | 0.96; 0.95 | 0.96 |
| All samples together | autoscaled | B, Cd, Cu, Se, Si | 3; 2 | 0.74 | 0.72 | 0.93; 0.93 | 0.93 | 0.93; 0.92 | 0.92 |
| Seed | Log10 transform, autoscaled | B, Cd, Cu, Fe, Mg, Se | 2; 1 | 0.62 | 0.59 | 1;0.95 | 0.97 | 0.88; 0.89 | 0.89 |
| Seed | autoscaled | B, Cd, Cu, Fe, Mg, Se | 6; 1 | 0.63 | 0.59 | 1; 0.95 | 0.97 | 0.88; 0.85 | 0.86 |
| Bran | Log10 transform, autoscaled | B, Cd, Co, Cu, Fe, K, Na, Pb, Se, Sn | 3; 2 | 0.89 | 0.83 | 1; 1 | 1 | 1; 1 | 1 |
| Bran | autoscaled | B, Cd, Co, Cu, Fe, K, Mo, Na, P, Pb, Se, Si, Sn | 2; 2 | 0.91 | 0.86 | 1; 1 | 1 | 1; 1 | 1 |
| Semolina | Log10 transform, autoscaled | Ag, B, Cd, Cu, K, Se, Si | 2; 1 | 0.91 | 0.89 | 1; 1 | 1 | 1; 1 | 1 |
| Semolina | autoscaled | Ag, B, Cd, Cu, K, Se | 2; 1 | 0.89 | 0.87 | 1; 1 | 1 | 1; 1 | 1 |
| Flour | Log10 transform, autoscaled | B, K, Se | 3; 1 | 0.8 | 0.73 | 0.92; 0.92 | 0.92 | 0.85; 0.95 | 0.85 |
| Flour | Log10 transform, autoscaled | Al, B, Cd, Cr, Fe, K, Se, Si, V | 3; 2 | 0.84 | 0.71 | 1; 1 | 1 | 1; 0.93 | 0.96 |
| Flour | autoscaled | B, K, Se | 3; 1 | 0.81 | 0.75 | 0.92; 0.92 | 0.92 | 0.92; 0.92 | 0.92 |
| Flour | autoscaled | Al, B, Cd, Co, Cr, Cu, Fe, K, Na, Se, Si, V | 12; 1 | 0.79 | 0.75 | 1; 1 | 1 | 0.93; 1 | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fattobene, M.; Liu, F.; Conti, P.; Zamponi, S.; Governatori, C.; Nardi, S.; Russo, R.E.; Berrettoni, M. Distribution of Elements in Durum Wheat Seed and Milling Products: Discrimination between Cultivation Methods through Multivariate Data Analysis. Foods 2024, 13, 1924. https://doi.org/10.3390/foods13121924
Fattobene M, Liu F, Conti P, Zamponi S, Governatori C, Nardi S, Russo RE, Berrettoni M. Distribution of Elements in Durum Wheat Seed and Milling Products: Discrimination between Cultivation Methods through Multivariate Data Analysis. Foods. 2024; 13(12):1924. https://doi.org/10.3390/foods13121924
Chicago/Turabian StyleFattobene, Martina, Fuyong Liu, Paolo Conti, Silvia Zamponi, Catia Governatori, Sandro Nardi, Raffaele Emanuele Russo, and Mario Berrettoni. 2024. "Distribution of Elements in Durum Wheat Seed and Milling Products: Discrimination between Cultivation Methods through Multivariate Data Analysis" Foods 13, no. 12: 1924. https://doi.org/10.3390/foods13121924





