Analysis of the Volatile Components in Different Parts of Three Species of the Genus Amomum via Combined HS–SPME–GC–TOF–MS and Multivariate Statistical Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. CG–MS Analysis
2.3. Data Analysis
3. Results and Discussion
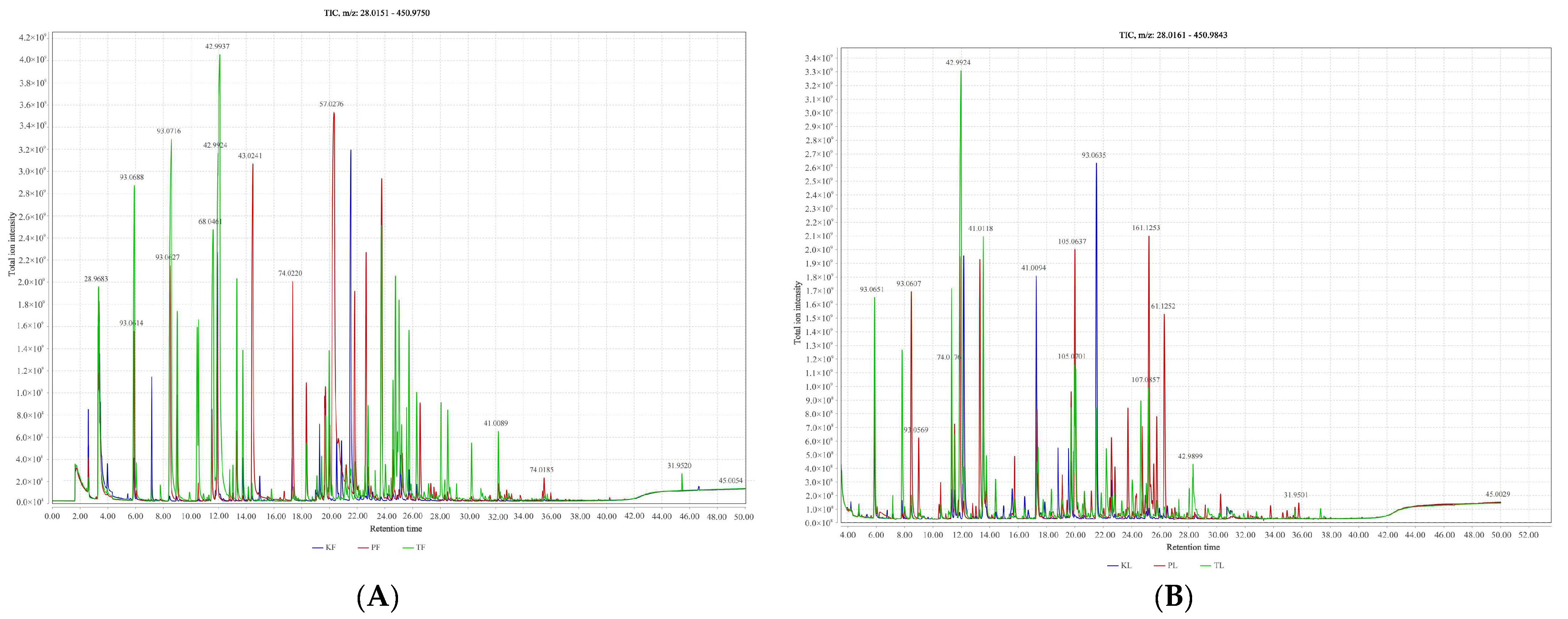
3.1. Identification and Quantitation of Volatiles from Three Genus Amomum Species by GC–MS
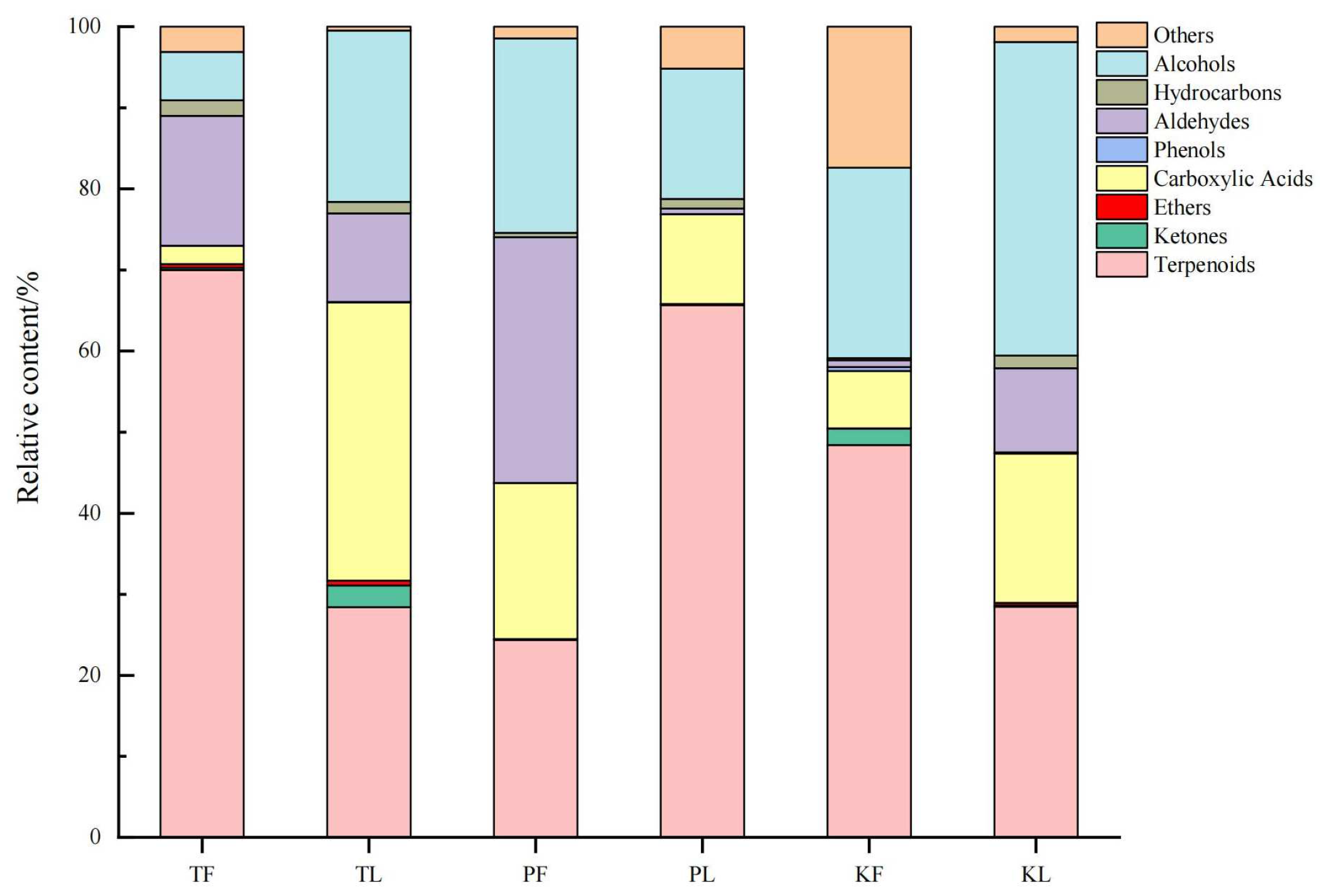
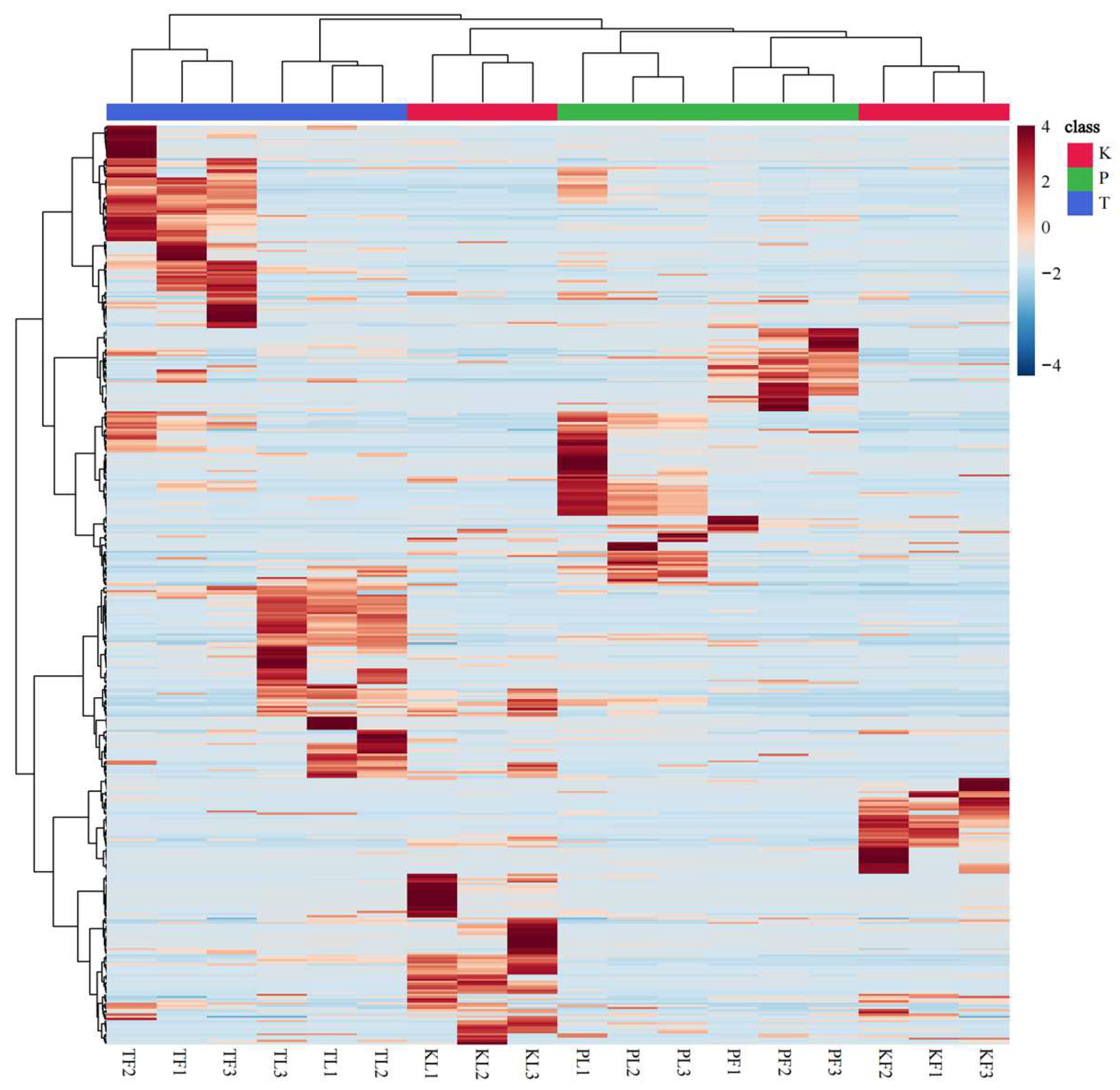
3.2. Comparative Analysis of Volatile Metabolites in Different Parts of Three Species of the Genus Amomum
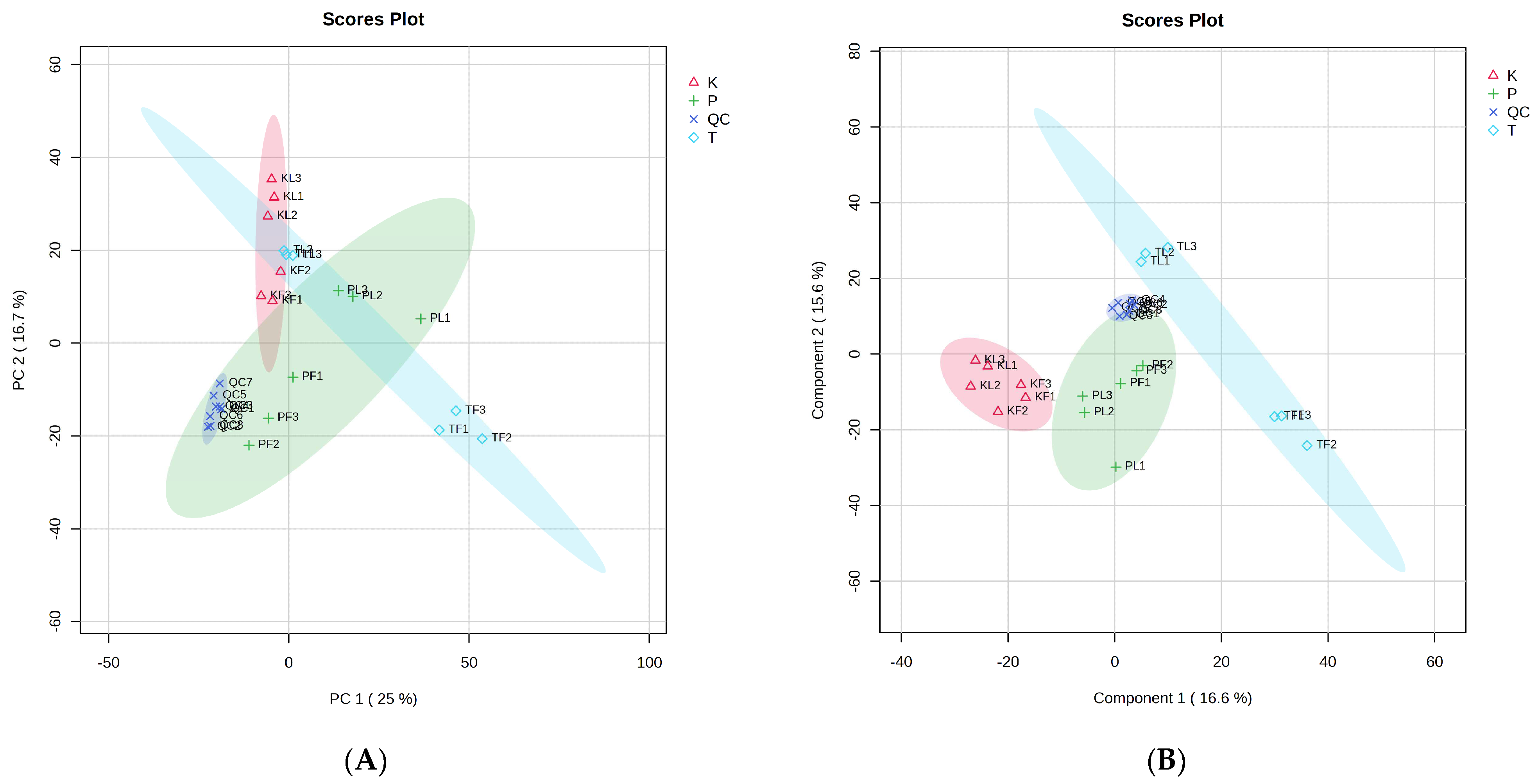
3.3. PCA Analysis of Volatile Components
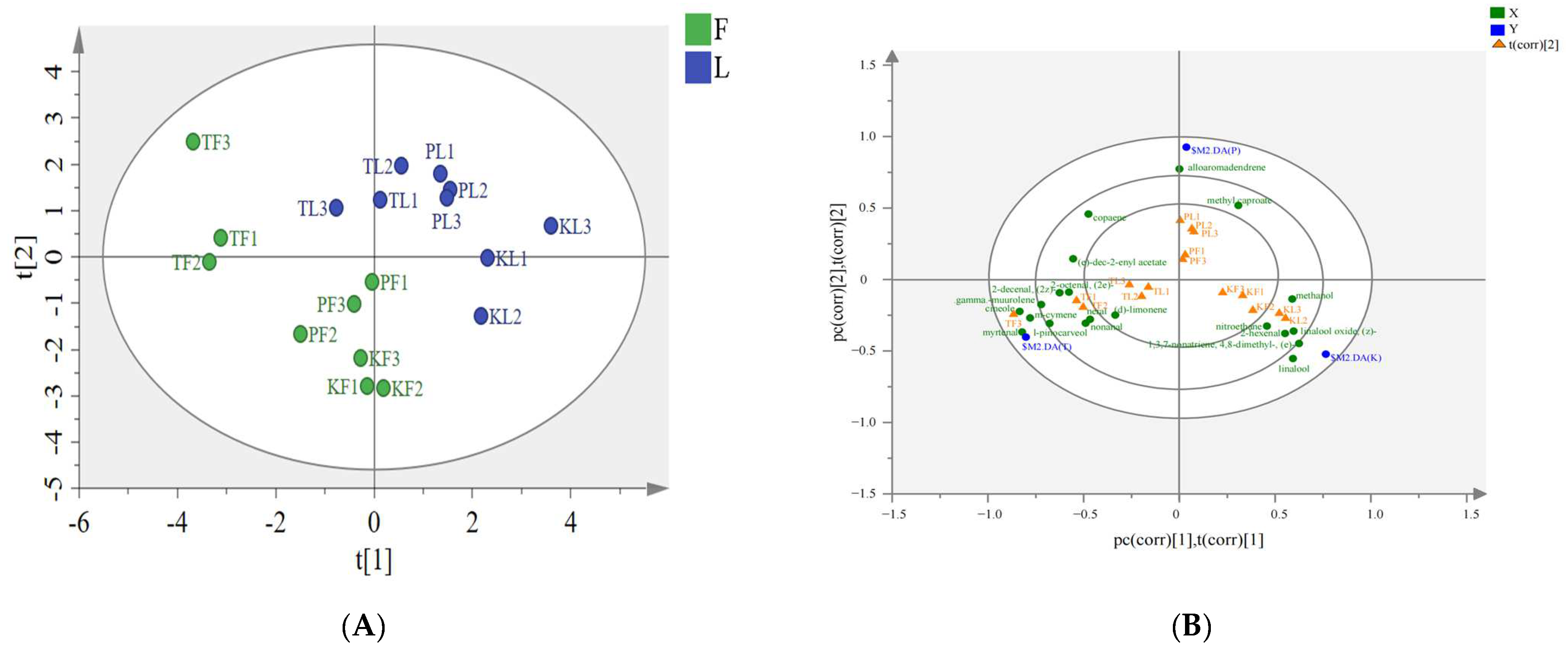
3.4. PLS–DA Analysis of Volatile Components
3.5. Analysis of Characteristic Compounds
| Compound Name | Class | Average Relative Content/% | p-Value | VIP | Odor Description | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TF | TL | PF | PL | KF | KL | ||||||
| Cineole | Terpenoids | 16.5 ± 3.64 Aa | 5.62 ± 3.24 Ab | 1.68 ± 0.39 Ba | 5.03 ± 4.2 Aa | 3.72 ± 0.89 Ba | 0.1 ± 03 Aa | 0.000 | 3.13 | Pungent, Cooling, Spicy | [45] |
| (E)-,4,8-Dimethyl-1,3,7-nonatriene | 0.02 ± 0 Ba | 0 ± 0 Ba | 0 ± 0 Ba | 0.01 ± 0 Ba | 0.59 ± 0.34 Aa | 0.35 ± 0.11 Aa | 0.000 | 3 | - | - | |
| .gamma.-Muurolene | 3.61 ± 1.2 Aa | 0.06 ± 0.05 Bb | 0.08 ± 0.01 Bb | 0.97 ± 0.44 Ab | 0 ± 0 Ba | 0.02 ± 0.01 Bb | 0.016 | 2.79 | - | - | |
| Copaene | 2.36 ± 0.88 Aa | 0.95 ± 0.37 Ba | 0.53 ± 0.2 Ba | 3.49 ± 1.59 Aa | 0.01 ± 0 Ba | 0.02 ± 0 Ba | 0.000 | 2.36 | - | - | |
| m-Cymene | 1.05 ± 0.8 Aa | 0.51 ± 0.08 Aa | 0 ± 0 Ba | 0.25 ± 0.22 ABa | 0 ± 0 Ba | 0.14 ± 0.12 Ba | 0.000 | 2.26 | - | - | |
| (D)-Limonene | 16.5 ± 5.64 Aa | 5.62 ± 3.24 Ab | 1.68 ± 0.39 Ba | 5.03 ± 4.2 Aa | 3.72 ± 0.89 Ba | 0.1 ± 0.03 Ab | 0.017 | 2.22 | Fresh, Citrus | [46] | |
| Myrtenal | 0.27 ± 0.12 Aa | 0.06 ± 0.01 Aa | 0.01 ± 0 Ba | 0 ± 0 Aa | 0 ± 0 Ba | 0 ± 0 Aa | 0.000 | 2.01 | Refreshing, Spicy-herbaceous odor | [47] | |
| Alloaromadendrene | 0.05 ± 0.03 Aa | 0 ± 0 Aa | 0 ± 0 Ba | 0.09 ± 0.01 Aa | 0 ± 0 Ba | 0 ± 0 Aa | 0.002 | 2.19 | - | - | |
| (Z)-2-Decenal | Aldehydes | 4.41 ± 3.7 Aa | 0 ± 0 Aa | 1.42 ± 1.16 Aa | 0 ± 0 Aa | 0 ± 0 Aa | 0 ± 0 Aa | 0.013 | 3.64 | - | - |
| Neral | 0.14 ± 0.05 Aa | 0.24 ± 0.14 Aa | 0 ± 0 Aa | 0 ± 0 Aa | 0.14 ± 0.08 Aa | 0 ± 0 Aa | 0.034 | 2.34 | Lemon-like | [44] | |
| (E)-2-Octenal | 1.46 ± 1.16 Aa | 0.17 ± 0.09 Aa | 0.72 ± 0.53 Aa | 0.03 ± 0.02 Ba | 0.01 ± 0 Aa | 0.01 ± 0 Aa | 0.008 | 3.07 | |||
| 2-Hexenal | 0 ± 0 Ba | 0.16 ± 0.01 Bb | 0 ± 0 Aa | 0.06 ± 0.04 Ba | 0 ± 0 Ba | 3.54 ± 1.6 Aa | 0.019 | 2.62 | Fatty, Green | [34] | |
| Nonanal | 0.14 ± 0.05 Aa | 0.24 ± 0.14 Aa | 0.24 ± 0.14 Aa | 0 ± 0 Aa | 0 ± 0 Ba | 0 ± 0 Aa | 0.002 | 2.19 | Orange-rose odor Floral, Waxy, Green | [48] | |
| I-Pinocarveol | Alcohols | 0.24 ± 0.15 Aa | 0.37 ± 0.09 Aa | 0.02 ± 0.01 Aa | 0 ± 0 Ba | 0 ± 0 Aa | 0.01 ± 0.1 Aa | 0.000 | 2.25 | - | - |
| cis-Linalool Oxide | 1.42 ± 0.2 Aa | 0.15 ± 0.07 Aa | 0.29 ± 0.05 Ba | 0 ± 0 Bb | 0.34 ± 0.18 Ba | 0.09 ± 0.2 Ba | 0.002 | 2.19 | - | - | |
| Methyl caproate | Esters | 0.03 ± 0.02 ABa | 0 ± 0 Ca | 0.06 ± 0.03 Aa | 1.01 ± 0.22 Ab | 0 ± 0 Ca | 0.5 ± 0.21 Ba | 0.004 | 2.29 | Pineapple, Ethereal | [49] |
| (E)-Dec-2-enyl acetate | 0.06 ± 0.05 Aa | 0.06 ± 0.02 Aa | 0.09 ± 0.05 Aa | 0 ± 0 Ba | 0 ± 0 Aa | 0 ± 0 Ba | 0.002 | 2.01 | - | - | |
| Nitroethane | Other | 0 ± 0 Aa | 0 ± 0 Aa | 0 ± 0 Aa | 0 ± 0 Aa | 4.65 ± 4.03 Aa | 0 ± 0 Aa | 0.019 | 3.23 | Mild, Fruity | - |
3.6. Correlation between Plant Parts and Chemical Components
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ji, K.L.; Fan, Y.Y.; Ge, Z.P.; Sheng, L.; Xu, Y.K.; Gan, L.S.; Li, J.Y.; Yue, J.M. Maximumins A-D, Rearranged Labdane-Type Diterpenoids with Four Different Carbon Skeletons from Amomum maximum. J. Org. Chem. 2019, 84, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Yue, X.; Wang, Y.; Yang, Y.; Sun, D.; Li, H.; Chen, L. Chemistry and bioactivity of plants from the genus Amomum. J. Ethnopharmacol. 2021, 281, 114563. [Google Scholar] [CrossRef] [PubMed]
- Subba, B.; Seling, T.R.; Kandel, R.C.; Phuyal, G.P. Assessment of Antimicrobial and Antioxidant Activities of Amomum subulatum Roxb. Of Nepal. Asian J. Pharm. Clin. Res. 2017, 10, 95. [Google Scholar] [CrossRef]
- Nurcholis, W.; Sya’bani Putri, D.N.; Husnawati, H.; Aisyah, S.I.; Priosoeryanto, B.P. Total flavonoid content and antioxidant activity of ethanol and ethyl acetate extracts from accessions of Amomum compactum fruits. Ann. Agric. Sci. 2021, 66, 58–62. [Google Scholar] [CrossRef]
- Kim, J.G.; Jang, H.; Le, T.P.L.; Hong, H.R.; Lee, M.K.; Hong, J.T.; Lee, D.; Hwang, B.Y. Pyranoflavanones and Pyranochalcones from the Fruits of Amomum tsao-ko. J. Nat. Prod. 2019, 82, 1886–1892. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.C.; Subedi, L.; Cho, K.H.; Kim, S.Y.; Park, H.J.; Kim, K.H. Bioactive compounds from the seeds of Amomum tsaoko Crevost et Lemaire, a Chinese spice as inhibitors of sphingosine kinases, SPHK1/2. RSC Adv. 2019, 9, 33957–33968. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.G.; Je, I.G.; Kim, G.J.; Choi, H.; Kim, S.H.; Kim, J.A.; Lee, S.H. Anti-allergic Inflammatory Activities of Compounds of Amomi Fructus. Nat. Prod. Commun. 2015, 10, 631–632. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.Y.; Je, I.G.; Kim, M.J.; Kang, B.C.; Choi, Y.A.; Baek, M.C.; Lee, B.; Choi, J.K.; Park, H.R.; Shin, T.Y.; et al. 2-Hydroxy-3-methoxybenzoic acid attenuates mast cell-mediated allergic reaction in mice via modulation of the FcepsilonRI signaling pathway. Acta Pharmacol Sin. 2017, 38, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xue, Y.; Chen, D.; Wang, Z. Amomum tsao-ko Crevost & Lemarié: A comprehensive review on traditional uses, botany, phytochemistry, and pharmacology. Phytochem. Rev. 2022, 21, 1487–1521. [Google Scholar]
- Li, Y.; Lin, Y.; Shin, S.; Yu, L.; Yi, W. Studies on the Antioxidant Components and Activities of the Methanol Extracts of Commercially Grown Hemerocallis Fulva L. (Daylily) in Taiwan. J. Food Biochem. 2010, 34, 90–104. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the Peoples Republic of China; Chinese Pharmacopoeia Commission: Beijing, China, 2020; Volume 66.
- Hong, S.S.; Lee, J.H.; Choi, Y.H.; Jeong, W.; Ahn, E.K.; Lym, S.H.; Oh, J.S. Amotsaokonal A–C, benzaldehyde and cycloterpenal from Amomum tsao-ko. Tetrahedron Lett. 2015, 56, 6681–6684. [Google Scholar] [CrossRef]
- Wen, H.; Yang, T.; Yang, W.; Yang, M.; Wang, Y.; Zhang, J. Comparison of Metabolites and Species Classification of Thirteen Zingiberaceae Spices Based on GC-MS and Multi-Spectral Fusion Technology. Foods 2023, 12, 3714. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Pichersky, E. Focus Issue on Metabolism: Metabolites, Metabolites Everywhere; American Society of Plant Biologists: Rockville, MD, USA, 2015; Volume 169, pp. 1421–1423. [Google Scholar]
- Theis, N.; Adler, L.S. Advertising to the enemy: Enhanced floral fragrance increases beetle attraction and reduces plant reproduction. Ecology 2012, 93, 430–435. [Google Scholar] [CrossRef]
- Issa, M.Y.; Mohsen, E.; Younis, I.Y.; Nofal, E.S.; Farag, M.A. Volatiles distribution in jasmine flowers taxa grown in Egypt and its commercial products as analyzed via solid-phase microextraction (SPME) coupled to chemometrics. Ind. Crops Prod. 2020, 144, 112002. [Google Scholar] [CrossRef]
- Guan, W.; Li, S.; Yan, R.; Tang, S.; Quan, C. Comparison of essential oils of clove buds extracted with supercritical carbon dioxide and other three traditional extraction methods. Food Chem. 2007, 101, 1558–1564. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.; Kong, J.; Yue, Y.; Dong, Y.; Zhang, J.; Liu, L. Application of UHPLC-Q-TOF MS based untargeted metabolomics reveals variation and correlation amongst different tissues of Eucommia ulmoides Oliver. Microchem. J. 2022, 172, 106919. [Google Scholar] [CrossRef]
- Yisimayili, Z.; Chao, Z. A review on phytochemicals, metabolic profiles and pharmacokinetics studies of the different parts (juice, seeds, peel, flowers, leaves and bark) of pomegranate (Punica granatum L.). Food Chem. 2022, 395, 133600. [Google Scholar] [CrossRef] [PubMed]
- Ch, R.; Chevallier, O.; McCarron, P.; McGrath, T.F.; Wu, D.; Nguyen Doan Duy, L.; Kapil, A.P.; McBride, M.; Elliott, C.T. Metabolomic fingerprinting of volatile organic compounds for the geographical discrimination of rice samples from China, Vietnam and India. Food Chem. 2021, 334, 127553. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ma, X.; Cheng, J.; Luo, Y. Quantitative studies of floral color and floral scent. Biodivers. Sci. 2013, 20, 308–316. [Google Scholar]
- Zhou, X.; Zhu, S.; Wei, J.; Zhou, Y. Volatile metabolomics and chemometric study provide insight into the formation of the characteristic cultivar aroma of Hemerocallis. Food Chem. 2023, 404, 134495. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zheng, P.; Gong, Z.; Feng, L.; Gao, S.; Wang, X.; Teng, J.; Zheng, L.; Liu, Z. Comparing characteristic aroma components of bead-shaped green teas from different regions using headspace solid-phase microextraction and gas chromatography-mass spectrometry/olfactometry combined with chemometrics. Eur. Food Res. Technol. 2020, 246, 1703–1714. [Google Scholar] [CrossRef]
- Zidi, K.; Kati, D.E.; Bachir-bey, M.; Genva, M.; Fauconnier, M.L. Comparative Study of Fig Volatile Compounds Using Headspace Solid-Phase Microextraction-Gas Chromatography/Mass Spectrometry: Effects of Cultivars and Ripening Stages. Front. Plant Sci. 2021, 12, 667809. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, S.; Liao, P.; Chen, L.; Sun, J.; Sun, B.; Zhao, D.; Wang, B.; Li, H. HS-SPME Combined with GC-MS/O to Analyze the Flavor of Strong Aroma Baijiu Daqu. Foods 2022, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O.; Kind, T. Metabolite Profiling in Blood Plasma. In Metabolomics: Methods and Protocols; Weckwerth, W., Ed.; Humana Press: Totowa, NJ, USA, 2007; pp. 3–17. [Google Scholar]
- Fiehn, O.; Wohlgemuth, G.; Scholz, M.; Kind, T.; Lee, D.Y.; Lu, Y.; Moon, S.; Nikolau, B. Quality control for plant metabolomics: Reporting MSI-compliant studies. Plant J. 2008, 53, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Saccenti, E.; Smilde, A.K.; Westerhuis, J.A. Double-check: Validation of diagnostic statistics for PLS-DA models in metabolomics studies. Metabolomics 2011, 8, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Wang, Y.; Yang, W.; Yang, S.; Zhang, J. Comparison of metabolites and variety authentication of Amomum tsao-ko and Amomum paratsao-ko using GC-MS and NIR spectroscopy. Sci. Rep. 2021, 11, 15200. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.M.; Peng, J.Q.; Chen, Y.; Tao, L.; Zhang, Y.Y.; Fu, L.Y.; Long, Q.D.; Shen, X.C. 1, 8-Cineole: A review of source, biological activities, and application. J. Asian Nat. Prod. Res. 2021, 23, 938–954. [Google Scholar] [CrossRef] [PubMed]
- Caboni, P.; Ntalli, N.G.; Aissani, N.; Cavoski, I.; Angioni, A. Nematicidal activity of (E, E)-2, 4-decadienal and (E)-2-decenal from Ailanthus altissima against Meloidogyne javanica. J. Agric. Food Chem. 2012, 60, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Ren, J.N.; Li, X.; Fan, G.; Qu, S.S.; Song, Y.; Li, Y.; Pan, S.Y. Recent updates on bioactive properties of linalool. Food Funct. 2021, 12, 10370–10389. [Google Scholar] [CrossRef] [PubMed]
- Erasto, P.; Viljoen, A.M. Limonene–A review: Biosynthetic, ecological and pharmacological relevance. Nat. Prod. Commun. 2008, 3, 1934578X0800300728. [Google Scholar] [CrossRef]
- Ma, D.; Lin, T.; Zhao, H.; Li, Y.; Wang, X.; Di, S.; Liu, Z.; Liu, M.; Qi, P.; Zhang, S.; et al. Development and comprehensive SBSE-GC/Q-TOF-MS analysis optimization, comparison, and evaluation of different mulberry varieties volatile flavor. Food Chem. 2024, 443, 138578. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lu, Q.; Wang, J.; Hu, Q.; Liu, P.; Yang, Y.; Li, Y.; Tang, H.; Xie, H. Correlation Analysis of Compounds in Essential Oil of Amomum tsaoko Seed and Fruit Morphological Characteristics, Geographical Conditions, Locality of Growth. Agronomy 2021, 11, 744. [Google Scholar] [CrossRef]
- Hou, Z.W.; Wang, Y.J.; Xu, S.S.; Wei, Y.M.; Bao, G.H.; Dai, Q.Y.; Deng, W.W.; Ning, J.M. Effects of dynamic and static withering technology on volatile and nonvolatile components of Keemun black tea using GC-MS and HPLC combined with chemometrics. LWT 2020, 130, 109547. [Google Scholar] [CrossRef]
- Zhang, S.S.; Guo, S.; Zheng, Z.J.; Liu, S.J.; Hou, Y.F.; Ho, C.T.; Bai, N.S. Characterization of volatiles in Allium tenuissimum L. flower by headspace-gas chromatography-olfactometry-mass spectrometry, odor activity values, and the omission and recombination experiments. LWT 2021, 151, 112144. [Google Scholar] [CrossRef]
- Pu, Z.H.; Wang, B.S.; Zhang, S.Y.; Sun, F.H.; Dai, M. A review on quality control, toxicity and clinical application of Amomum tsao-ko Crevost & Lemarié. Pharmacol. Res.-Mod. Chin. Med. 2022, 5, 100165. [Google Scholar]
- Yang, Z.; Xu, S.; Zhang, W.; An, T.; Liu, L. Analysis on the content and main chemical components of volatile oils from Amomum tsao-ko Stems and Leaves in Yunnan province. J. Chin. Med. Mater. 2019, 42, 339–343. [Google Scholar]
- Gilles, M.; Zhao, J.; An, M.; Agboola, S. Chemical composition and antimicrobial properties of essential oils of three Australian Eucalyptus species. Food Chem. 2010, 119, 731–737. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, D. Supercritical CO2 extraction of Eucalyptus leaves oil and comparison with Soxhlet extraction and hydro-distillation methods. Sep. Purif. Technol. 2014, 133, 443–451. [Google Scholar] [CrossRef]
- Park, Y.J.; Baskar, T.B.; Yeo, S.K.; Arasu, M.V.; Al-Dhabi, N.A.; Lim, S.S.; Park, S.U. Composition of volatile compounds and in vitro antimicrobial activity of nine Mentha spp. SpringerPlus 2016, 5, 1628. [Google Scholar] [CrossRef]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Blázquez, M.A.; Granell, A. Volatile Compounds in Citrus Essential Oils: A Comprehensive Review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef]
- Liang, M.; Zhang, Z.; Wu, Y.; Wang, R.; Liu, Y. Comparison of Amomum tsaoko crevost et Lemaire from four regions via headspace solid-phase microextraction: Variable optimization and volatile characterization. Ind. Crops Prod. 2023, 191, 115924. [Google Scholar] [CrossRef]
- Vyry Wouatsa, N.A.; Misra, L.; Venkatesh Kumar, R. Antibacterial Activity of Essential Oils of Edible Spices, Ocimum canum and Xylopia aethiopica. J. Food Sci. 2014, 79, M972–M977. [Google Scholar] [CrossRef] [PubMed]
- Zhogoleva, A.; Alas, M.; Rosenvald, S. Characterization of odor-active compounds of various pea preparations by GC-MS, GC-O, and their correlation with sensory attributes. Future Foods 2023, 8, 100243. [Google Scholar] [CrossRef]
- Wen, S.; Sun, L.; Zhang, S.; Chen, Z.; Chen, R.; Li, Z.; Lai, X.; Zhang, Z.; Cao, J.; Li, Q.; et al. The formation mechanism of aroma quality of green and yellow teas based on GC-MS/MS metabolomics. Food Res. Int. 2023, 172, 113137. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, C.; Bottoni, M.; Santagostini, L.; Spada, A.; Papini, A.; Milani, F.; Fico, G. Teucrium fruticans L., a Multi-Scale Study: From Trichomes to Essential Oil. Chem. Biodivers. 2023, 20, e202200913. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Shen, S.; Huang, L.; Deng, G.; Wei, Y.; Ning, J.; Wang, Y. Revelation of volatile contributions in green teas with different aroma types by GC-MS and GC-IMS. Food Res. Int. 2023, 169, 112845. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Yang, M.; Qi, M.; Yang, T.; Wang, L.; Yang, W.; Zhang, J. Analysis of the Volatile Components in Different Parts of Three Species of the Genus Amomum via Combined HS–SPME–GC–TOF–MS and Multivariate Statistical Analysis. Foods 2024, 13, 1925. https://doi.org/10.3390/foods13121925
Gu J, Yang M, Qi M, Yang T, Wang L, Yang W, Zhang J. Analysis of the Volatile Components in Different Parts of Three Species of the Genus Amomum via Combined HS–SPME–GC–TOF–MS and Multivariate Statistical Analysis. Foods. 2024; 13(12):1925. https://doi.org/10.3390/foods13121925
Chicago/Turabian StyleGu, Jingjing, Meiquan Yang, Mingju Qi, Tianmei Yang, Li Wang, Weize Yang, and Jinyu Zhang. 2024. "Analysis of the Volatile Components in Different Parts of Three Species of the Genus Amomum via Combined HS–SPME–GC–TOF–MS and Multivariate Statistical Analysis" Foods 13, no. 12: 1925. https://doi.org/10.3390/foods13121925




