Effectiveness of Flavonoid-Rich Diet in Alleviating Symptoms of Neurodegenerative Diseases
Abstract
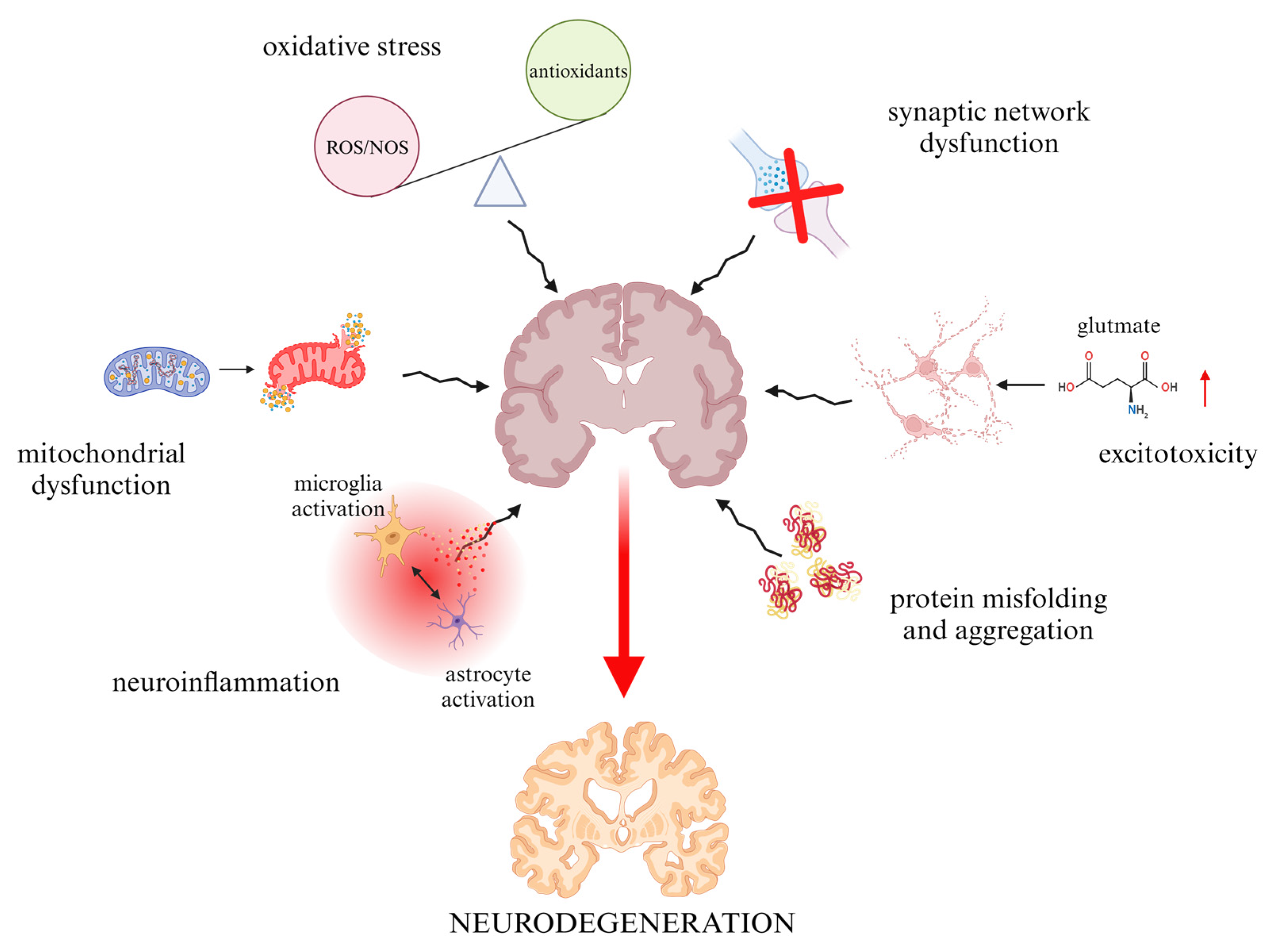
:1. Introduction
2. Overview of the Use of Flavonoids in Neurodegenerative Disorders
2.1. Alzheimer Disease (AD)
2.2. Parkinson Disease (PD)
2.3. Amyotrophic Lateral Sclerosis (ALS)
2.4. Huntington Disease (HD)
| Neurodegenerative Disease | Flavonoid | Study Model | Therapeutic Effects | References |
|---|---|---|---|---|
| Alzheimer’s disease | 7,8-DHF | animal |
| [83,84,85] |
| Catechin | animal |
| [86] | |
| human |
| [87] | ||
| Genistein | animal |
| [45,46] | |
| human |
| [48] | ||
| Luteolin | animal |
| [88,89,90] | |
| cellular |
| [91] | ||
| Nobiletin | animal |
| [92,93,94,95] | |
| Amyotrophic Lateral Sclerosis | 7,8-DHF | animal |
| [96,97,98] |
| Fisetin | animal |
| [66] | |
| Genistein | animal |
| [99,100] | |
| Kaempferide and kaempferol | animal |
| [101,102] | |
| Quercetin | cellular |
| [103] | |
| animal |
| [104] | ||
| Parkinson’s disease | Genistein | animal |
| [58,105,106,107] |
| Baicalein | cellular |
| [58,108,109] | |
| animal |
| [110,111,112,113] | ||
| Hesperetin | animal |
| [114,115,116,117] | |
| cellular |
| [118,119,120] | ||
| Morin | animal |
| [118,119,121] | |
| Nobiletin | animal |
| [92] | |
| Huntington’s disease | 7,8-DHF | animal |
| [122] |
| Anthocyanins | animal |
| [123,124] | |
| Genistein | cellular |
| [76,125] | |
| animal |
| [126] | ||
| Hesperidin | animal |
| [127,128] | |
| Naringin | animal |
| [129,130] |
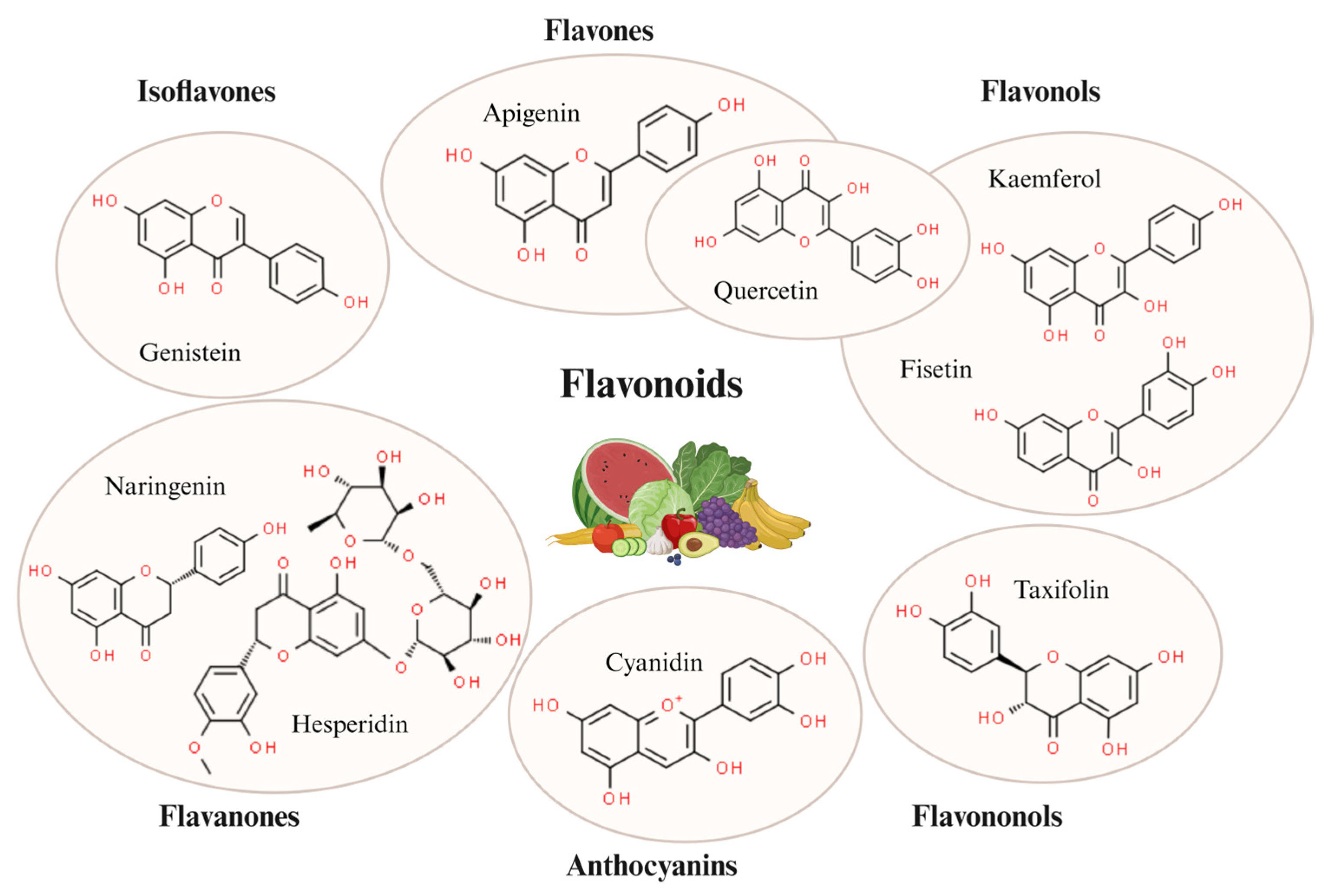
3. General Characteristics and Targets of Flavonoids
3.1. Flavones
3.1.1. Apigenin
3.1.2. Nobiletin
3.2. Flavonols
3.2.1. Quercetine
3.2.2. Fisetin
3.2.3. Kaempferol
3.3. Flavanones
3.3.1. Hesperetin
3.3.2. Naringenin
3.4. Isoflavones
Genistein
3.5. Anthocyanins
Cyanidin
3.6. Flavononols
Taxifolin
4. Effects of a Flavonoid-Rich Diet on Neuroprotection
4.1. Animal Studies
4.1.1. Animal Models of Alzheimer’s Disease (AD)
4.1.2. Animal Models of Parkinson’s Disease (PD)
4.1.3. Animal Models of Amyotrophic Lateral Sclerosis (ALS)
4.1.4. Animal Models of Huntington’s Disease (HD)
4.2. Population Studies
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feigin, V.L.; Abajobir, A.A.; Abate, K.H.; Abd-Allah, F.; Abdulle, A.M.; Abera, S.F.; Abyu, G.Y.; Ahmed, M.B.; Aichour, A.N.; Aichour, I.; et al. Global, Regional, and National Burden of Neurological Disorders during 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef]
- Tahir, M.S.; Almezgagi, M.; Zhang, Y.; Bashir, A.; Abdullah, H.M.; Gamah, M.; Wang, X.; Zhu, Q.; Shen, X.; Ma, Q.; et al. Mechanistic New Insights of Flavonols on Neurodegenerative Diseases. Biomed. Pharmacother. 2021, 137, 111253. [Google Scholar] [CrossRef]
- Wilson, D.M.; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of Neurodegenerative Diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef]
- Maher, P. The Potential of Flavonoids for the Treatment of Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 3056. [Google Scholar] [CrossRef]
- Devi, S.; Kumar, V.; Singh, S.K.; Dubey, A.K.; Kim, J.-J. Flavonoids: Potential Candidates for the Treatment of Neurodegenerative Disorders. Biomedicines 2021, 9, 99. [Google Scholar] [CrossRef]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef]
- Zheng, J.C.; Chen, S. Translational Neurodegeneration in the Era of Fast Growing International Brain Research. Transl. Neurodegener. 2022, 11, 1. [Google Scholar] [CrossRef]
- Nakai, S.; Fujita, M.; Kamei, Y. Health Promotion Effects of Soy Isoflavones. J. Nutr. Sci. Vitaminol. 2020, 66, 502–507. [Google Scholar] [CrossRef]
- Sekikawa, A.; Ihara, M.; Lopez, O.; Kakuta, C.; Lopresti, B.; Higashiyama, A.; Aizenstein, H.; Chang, Y.-F.; Mathis, C.; Miyamoto, Y.; et al. Effect of S-Equol and Soy Isoflavones on Heart and Brain. Curr. Cardiol. Rev. 2019, 15, 114–135. [Google Scholar] [CrossRef]
- Ramdath, D.; Padhi, E.; Sarfaraz, S.; Renwick, S.; Duncan, A. Beyond the Cholesterol-Lowering Effect of Soy Protein: A Review of the Effects of Dietary Soy and Its Constituents on Risk Factors for Cardiovascular Disease. Nutrients 2017, 9, 324. [Google Scholar] [CrossRef]
- Yu, J.; Bi, X.; Yu, B.; Chen, D. Isoflavones: Anti-Inflammatory Benefit and Possible Caveats. Nutrients 2016, 8, 361. [Google Scholar] [CrossRef]
- Kim, I.-S.; Kim, C.-H.; Yang, W.-S. Physiologically Active Molecules and Functional Properties of Soybeans in Human Health—A Current Perspective. Int. J. Mol. Sci. 2021, 22, 4054. [Google Scholar] [CrossRef]
- Ayaz, M.; Mosa, O.F.; Nawaz, A.; Hamdoon, A.A.E.; Elkhalifa, M.E.M.; Sadiq, A.; Ullah, F.; Ahmed, A.; Kabra, A.; Khan, H.; et al. Neuroprotective Potentials of Lead Phytochemicals against Alzheimer’s Disease with Focus on Oxidative Stress-Mediated Signaling Pathways: Pharmacokinetic Challenges, Target Specificity, Clinical Trials and Future Perspectives. Phytomedicine Int. J. Phytother. Phytopharm. 2024, 124, 155272. [Google Scholar] [CrossRef]
- Bellavite, P. Neuroprotective Potentials of Flavonoids: Experimental Studies and Mechanisms of Action. Antioxidants 2023, 12, 280. [Google Scholar] [CrossRef]
- Jazvinšćak Jembrek, M.; Oršolić, N.; Mandić, L.; Sadžak, A.; Šegota, S. Anti-Oxidative, Anti-Inflammatory and Anti-Apoptotic Effects of Flavonols: Targeting Nrf2, NF-κB and P53 Pathways in Neurodegeneration. Antioxidants 2021, 10, 1628. [Google Scholar] [CrossRef]
- Meng-Zhen, S.; Ju, L.; Lan-Chun, Z.; Cai-Feng, D.; Shu-da, Y.; Hao-Fei, Y.; Wei-Yan, H. Potential Therapeutic Use of Plant Flavonoids in AD and PD. Heliyon 2022, 8, e11440. [Google Scholar] [CrossRef]
- Bai, X.; Bian, Z.; Zhang, M. Targeting the Nrf2 Signaling Pathway Using Phytochemical Ingredients: A Novel Therapeutic Road Map to Combat Neurodegenerative Diseases. Phytomed. Int. J. Phytother. Phytopharm. 2023, 109, 154582. [Google Scholar] [CrossRef]
- Evans, J.A.; Mendonca, P.; Soliman, K.F.A. Neuroprotective Effects and Therapeutic Potential of the Citrus Flavonoid Hesperetin in Neurodegenerative Diseases. Nutrients 2022, 14, 2228. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Zhang, Y.-J.; Zhu, G.-Y.; Shi, X.-C.; Chen, X.; Herrera-Balandrano, D.D.; Liu, F.-Q.; Laborda, P. Occurrence of Isoflavones in Soybean Sprouts and Strategies to Enhance Their Content: A Review. J. Food Sci. 2022, 87, 1961–1982. [Google Scholar] [CrossRef]
- Rizzo, G.; Baroni, L. Soy, Soy Foods and Their Role in Vegetarian Diets. Nutrients 2018, 10, 43. [Google Scholar] [CrossRef]
- Jargin, S.V. Soy and Phytoestrogens: Possible Side Effects. GMS Ger. Med. Sci. 2014, 12, Doc18, ISSN: 1612-3174. [Google Scholar] [CrossRef]
- Messina, M.; Redmond, G. Effects of Soy Protein and Soybean Isoflavones on Thyroid Function in Healthy Adults and Hypothyroid Patients: A Review of the Relevant Literature. Thyroid 2006, 16, 249–258. [Google Scholar] [CrossRef]
- Belobrajdic, D.P.; James-Martin, G.; Jones, D.; Tran, C.D. Soy and Gastrointestinal Health: A Review. Nutrients 2023, 15, 1959. [Google Scholar] [CrossRef]
- Pramitha, J.L.; Rana, S.; Aggarwal, P.R.; Ravikesavan, R.; Joel, A.J.; Muthamilarasan, M. Diverse Role of Phytic Acid in Plants and Approaches to Develop Low-Phytate Grains to Enhance Bioavailability of Micronutrients. In Advances in Genetics; Elsevier: Amsterdam, The Netherlands, 2021; Volume 107, pp. 89–120. ISBN 978-0-12-824123-3. [Google Scholar]
- Han, X.; Sun, S.; Sun, Y.; Song, Q.; Zhu, J.; Song, N.; Chen, M.; Sun, T.; Xia, M.; Ding, J.; et al. Small Molecule-Driven NLRP3 Inflammation Inhibition via Interplay between Ubiquitination and Autophagy: Implications for Parkinson Disease. Autophagy 2019, 15, 1860–1881. [Google Scholar] [CrossRef]
- Ko, J.W.; Chung, Y.-S.; Kwak, C.S.; Kwon, Y.H. Doenjang, A Korean Traditional Fermented Soybean Paste, Ameliorates Neuroinflammation and Neurodegeneration in Mice Fed a High-Fat Diet. Nutrients 2019, 11, 1702. [Google Scholar] [CrossRef]
- Jang, C.H.; Oh, J.; Lim, J.S.; Kim, H.J.; Kim, J.-S. Fermented Soy Products: Beneficial Potential in Neurodegenerative Diseases. Foods 2021, 10, 636. [Google Scholar] [CrossRef]
- Reed, K.E.; Camargo, J.; Hamilton-Reeves, J.; Kurzer, M.; Messina, M. Neither Soy nor Isoflavone Intake Affects Male Reproductive Hormones: An Expanded and Updated Meta-Analysis of Clinical Studies. Reprod. Toxicol. 2021, 100, 60–67. [Google Scholar] [CrossRef]
- Genser, D. Food and Drug Interaction: Consequences for the Nutrition/Health Status. Ann. Nutr. Metab. 2008, 52 (Suppl. S1), 29–32. [Google Scholar] [CrossRef]
- Seden, K.; Dickinson, L.; Khoo, S.; Back, D. Grapefruit-Drug Interactions. Drugs 2010, 70, 2373–2407. [Google Scholar] [CrossRef]
- Schilling, L.P.; Balthazar, M.L.F.; Radanovic, M.; Forlenza, O.V.; Silagi, M.L.; Smid, J.; Barbosa, B.J.A.P.; Frota, N.A.F.; Souza, L.C.D.; Vale, F.A.C.; et al. Diagnosis of Alzheimer’s Disease: Recommendations of the Scientific Department of Cognitive Neurology and Aging of the Brazilian Academy of Neurology. Dement. Neuropsychol. 2022, 16, 25–39. [Google Scholar] [CrossRef]
- Sepulveda-Falla, D. Resistant and Resilient Mutations in Protection against Familial Alzheimer’s Disease: Learning from Nature. Mol. Neurodegener. 2023, 18, 36. [Google Scholar] [CrossRef]
- Benek, O.; Korabecny, J.; Soukup, O. A Perspective on Multi-Target Drugs for Alzheimer’s Disease. Trends Pharmacol. Sci. 2020, 41, 434–445. [Google Scholar] [CrossRef]
- Zenaro, E.; Piacentino, G.; Constantin, G. The Blood-Brain Barrier in Alzheimer’s Disease. Neurobiol. Dis. 2017, 107, 41–56. [Google Scholar] [CrossRef]
- Shishtar, E.; Rogers, G.T.; Blumberg, J.B.; Au, R.; Jacques, P.F. Long-Term Dietary Flavonoid Intake and Risk of Alzheimer Disease and Related Dementias in the Framingham Offspring Cohort. Am. J. Clin. Nutr. 2020, 112, 343–353. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective Effects of Quercetin in Alzheimer’s Disease. Biomolecules 2019, 10, 59. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Muñoz-Manco, J.I.; Ramírez-Pineda, J.R.; Lamprea-Rodriguez, M.; Osorio, E.; Cardona-Gómez, G.P. The Flavonoid Quercetin Ameliorates Alzheimer’s Disease Pathology and Protects Cognitive and Emotional Function in Aged Triple Transgenic Alzheimer’s Disease Model Mice. Neuropharmacology 2015, 93, 134–145. [Google Scholar] [CrossRef]
- Abdalla, F.H.; Schmatz, R.; Cardoso, A.M.; Carvalho, F.B.; Baldissarelli, J.; de Oliveira, J.S.; Rosa, M.M.; Gonçalves Nunes, M.A.; Rubin, M.A.; da Cruz, I.B.M.; et al. Quercetin Protects the Impairment of Memory and Anxiogenic-like Behavior in Rats Exposed to Cadmium: Possible Involvement of the Acetylcholinesterase and Na+,K+-ATPase Activities. Physiol. Behav. 2014, 135, 152–167. [Google Scholar] [CrossRef]
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More. Oxid. Med. Cell. Longev. 2016, 2016, 2986796. [Google Scholar] [CrossRef]
- Chen, R.-L.; Rawlinson, C. Pharmacological Properties of Rutin and Its Potential Uses for Alzheimer’s Disease. J. Exp. Stroke Transl. Med. 2021, 13, 1–12. [Google Scholar]
- Bermejo-Bescós, P.; Jiménez-Aliaga, K.L.; Benedí, J.; Martín-Aragón, S. A Diet Containing Rutin Ameliorates Brain Intracellular Redox Homeostasis in a Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 4863. [Google Scholar] [CrossRef]
- Moghbelinejad, S.; Nassiri-Asl, M.; Farivar, T.N.; Abbasi, E.; Sheikhi, M.; Taghiloo, M.; Farsad, F.; Samimi, A.; Hajiali, F. Rutin Activates the MAPK Pathway and BDNF Gene Expression on Beta-Amyloid Induced Neurotoxicity in Rats. Toxicol. Lett. 2014, 224, 108–113. [Google Scholar] [CrossRef]
- Pierzynowska, K.; Rzeszótko, A.; Blendowska, A.; Wieczerzak, E.; Rodziewicz-Motowidło, S.; Piotrowska, E.; Węgrzyn, G. Differential Effects of Various Soy Isoflavone Dietary Supplements (Nutraceuticals) on Bacterial Growth and Human Fibroblast Viability. Acta Biochim. Pol. 2018, 65, 325–332. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Borrás, C.; Viña, J. The Multimodal Action of Genistein in Alzheimer’s and Other Age-Related Diseases. Free Radic. Biol. Med. 2022, 183, 127–137. [Google Scholar] [CrossRef]
- Pierzynowska, K.; Podlacha, M.; Gaffke, L.; Majkutewicz, I.; Mantej, J.; Węgrzyn, A.; Osiadły, M.; Myślińska, D.; Węgrzyn, G. Autophagy-Dependent Mechanism of Genistein-Mediated Elimination of Behavioral and Biochemical Defects in the Rat Model of Sporadic Alzheimer’s Disease. Neuropharmacology 2019, 148, 332–346. [Google Scholar] [CrossRef]
- Duan, X.; Li, Y.; Xu, F.; Ding, H. Study on the Neuroprotective Effects of Genistein on Alzheimer’s Disease. Brain Behav. 2021, 11, e02100. [Google Scholar] [CrossRef]
- Gao, H.; Lei, X.; Ye, S.; Ye, T.; Hua, R.; Wang, G.; Song, H.; Zhou, P.; Wang, Y.; Cai, B. Genistein Attenuates Memory Impairment in Alzheimer’s Disease via ERS-Mediated Apoptotic Pathway In Vivo and In Vitro. J. Nutr. Biochem. 2022, 109, 109118. [Google Scholar] [CrossRef]
- Viña, J.; Escudero, J.; Baquero, M.; Cebrián, M.; Carbonell-Asíns, J.A.; Muñoz, J.E.; Satorres, E.; Meléndez, J.C.; Ferrer-Rebolleda, J.; Cózar-Santiago, M.D.P.; et al. Genistein Effect on Cognition in Prodromal Alzheimer’s Disease Patients. The GENIAL Clinical Trial. Alzheimers Res. Ther. 2022, 14, 164. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s Disease. Lancet Lond. Engl. 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Aryal, S.; Skinner, T.; Bridges, B.; Weber, J.T. The Pathology of Parkinson’s Disease and Potential Benefit of Dietary Polyphenols. Molecules 2020, 25, 4382. [Google Scholar] [CrossRef]
- Dickson, D.W. Parkinson’s Disease and Parkinsonism: Neuropathology. Cold Spring Harb. Perspect. Med. 2012, 2, a009258. [Google Scholar] [CrossRef]
- Magalingam, K.B.; Radhakrishnan, A.K.; Haleagrahara, N. Protective Mechanisms of Flavonoids in Parkinson’s Disease. Oxid. Med. Cell. Longev. 2015, 2015, 314560. [Google Scholar] [CrossRef]
- Ye, H.; Robak, L.A.; Yu, M.; Cykowski, M.; Shulman, J.M. Genetics and Pathogenesis of Parkinson’s Syndrome. Annu. Rev. Pathol. Mech. Dis. 2023, 18, 95–121. [Google Scholar] [CrossRef]
- Foltynie, T.; Bruno, V.; Fox, S.; Kühn, A.A.; Lindop, F.; Lees, A.J. Medical, Surgical, and Physical Treatments for Parkinson’s Disease. Lancet 2024, 403, 305–324. [Google Scholar] [CrossRef]
- Banerjee, C.; Nandy, S.; Chakraborty, J.; Kumar, D. Myricitrin—A Flavonoid Isolated from the Indian Olive Tree (Elaeocarpus floribundus)—Inhibits Monoamine Oxidase in the Brain and Elevates Striatal Dopamine Levels: Therapeutic Implications against Parkinson’s Disease. Food Funct. 2022, 13, 6545–6559. [Google Scholar] [CrossRef]
- Li, J.; Xiang, H.; Huang, C.; Lu, J. Pharmacological Actions of Myricetin in the Nervous System: A Comprehensive Review of Preclinical Studies in Animals and Cell Models. Front. Pharmacol. 2021, 12, 797298. [Google Scholar] [CrossRef]
- Graves, S.M.; Xie, Z.; Stout, K.A.; Zampese, E.; Burbulla, L.F.; Shih, J.C.; Kondapalli, J.; Patriarchi, T.; Tian, L.; Brichta, L.; et al. Dopamine Metabolism by a Monoamine Oxidase Mitochondrial Shuttle Activates the Electron Transport Chain. Nat. Neurosci. 2020, 23, 15–20. [Google Scholar] [CrossRef]
- Jung, U.J.; Kim, S.R. Beneficial Effects of Flavonoids Against Parkinson’s Disease. J. Med. Food 2018, 21, 421–432. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Hölscher, C. Therapeutic Potential of Baicalein in Alzheimer’s Disease and Parkinson’s Disease. CNS Drugs 2017, 31, 639–652. [Google Scholar] [CrossRef]
- Açar, Y.; Ağagündüz, D.; De Cicco, P.; Capasso, R. Flavonoids: Their Putative Neurologic Roles, Epigenetic Changes, and Gut Microbiota Alterations in Parkinson’s Disease. Biomed. Pharmacother. 2023, 168, 115788. [Google Scholar] [CrossRef]
- Song, Q.; Peng, S.; Zhu, X. Baicalein Protects against MPP+/MPTP-Induced Neurotoxicity by Ameliorating Oxidative Stress in SH-SY5Y Cells and Mouse Model of Parkinson’s Disease. NeuroToxicology 2021, 87, 188–194. [Google Scholar] [CrossRef]
- Wei, N.; Wei, Y.; Li, B.; Pang, L. Baicalein Promotes Neuronal and Behavioral Recovery After Intracerebral Hemorrhage Via Suppressing Apoptosis, Oxidative Stress and Neuroinflammation. Neurochem. Res. 2017, 42, 1345–1353. [Google Scholar] [CrossRef]
- Dervishi, I.; Ozdinler, P.H. Incorporating Upper Motor Neuron Health in ALS Drug Discovery. Drug Discov. Today 2018, 23, 696–703. [Google Scholar] [CrossRef]
- Novak, V.; Rogelj, B.; Župunski, V. Therapeutic Potential of Polyphenols in Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Antioxidants 2021, 10, 1328. [Google Scholar] [CrossRef]
- Hassan, S.S.U.; Samanta, S.; Dash, R.; Karpiński, T.M.; Habibi, E.; Sadiq, A.; Ahmadi, A.; Bungau, S. The Neuroprotective Effects of Fisetin, a Natural Flavonoid in Neurodegenerative Diseases: Focus on the Role of Oxidative Stress. Front. Pharmacol. 2022, 13, 1015835. [Google Scholar] [CrossRef]
- Wang, T.H.; Wang, S.Y.; Wang, X.D.; Jiang, H.Q.; Yang, Y.Q.; Wang, Y.; Cheng, J.L.; Zhang, C.T.; Liang, W.W.; Feng, H.L. Fisetin Exerts Antioxidant and Neuroprotective Effects in Multiple Mutant hSOD1 Models of Amyotrophic Lateral Sclerosis by Activating ERK. Neuroscience 2018, 379, 152–166. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, L.; Wang, L. Kaempferol, a Potential Neuroprotective Agent in Neurodegenerative Diseases: From Chemistry to Medicine. Biomed. Pharmacother. 2023, 165, 115215. [Google Scholar] [CrossRef]
- Srinivasan, E.; Rajasekaran, R. Comparative Binding of Kaempferol and Kaempferide on Inhibiting the Aggregate Formation of Mutant (G85R) SOD1 Protein in Familial Amyotrophic Lateral Sclerosis: A Quantum Chemical and Molecular Mechanics Study. BioFactors 2018, 44, 431–442. [Google Scholar] [CrossRef]
- Ueda, T.; Inden, M.; Shirai, K.; Sekine, S.; Masaki, Y.; Kurita, H.; Ichihara, K.; Inuzuka, T.; Hozumi, I. The Effects of Brazilian Green Propolis That Contains Flavonols against Mutant Copper-Zinc Superoxide Dismutase-Mediated Toxicity. Sci. Rep. 2017, 7, 2882. [Google Scholar] [CrossRef]
- Rieux, M.; Alpaugh, M.; Sciacca, G.; Saint-Pierre, M.; Masnata, M.; Denis, H.L.; Lévesque, S.A.; Herrmann, F.; Bazenet, C.; Garneau, A.P.; et al. Shedding a New Light on Huntington’s Disease: How Blood Can Both Propagate and Ameliorate Disease Pathology. Mol. Psychiatry 2021, 26, 5441–5463. [Google Scholar] [CrossRef]
- Irfan, Z.; Khanam, S.; Karmakar, V.; Firdous, S.M.; El Khier, B.S.I.A.; Khan, I.; Rehman, M.U.; Khan, A. Pathogenesis of Huntington’s Disease: An Emphasis on Molecular Pathways and Prevention by Natural Remedies. Brain Sci. 2022, 12, 1389. [Google Scholar] [CrossRef]
- Li, R.; Robinson, M.; Ding, X.; Geetha, T.; Al-Nakkash, L.; Broderick, T.L.; Babu, J.R. Genistein: A Focus on Several Neurodegenerative Diseases. J. Food Biochem. 2022, 46, e14155. [Google Scholar] [CrossRef]
- Fuloria, S.; Yusri, M.A.A.; Sekar, M.; Gan, S.H.; Rani, N.N.I.M.; Lum, P.T.; Ravi, S.; Subramaniyan, V.; Azad, A.K.; Jeyabalan, S.; et al. Genistein: A Potential Natural Lead Molecule for New Drug Design and Development for Treating Memory Impairment. Molecules 2022, 27, 265. [Google Scholar] [CrossRef]
- Singh, N.K.; Verma, N.; Gupta, J.K.; Raghav, J. Anti-Amnesic and Neuroprotective Potential of Genistein Against Alzheimer’s Disease. Rev. Bras. Farmacogn. 2024, 34, 80–92. [Google Scholar] [CrossRef]
- Walczak-Nowicka, Ł.J.; Herbet, M. Acetylcholinesterase Inhibitors in the Treatment of Neurodegenerative Diseases and the Role of Acetylcholinesterase in Their Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9290. [Google Scholar] [CrossRef]
- Pierzynowska, K.; Gaffke, L.; Hać, A.; Mantej, J.; Niedziałek, N.; Brokowska, J.; Węgrzyn, G. Correction of Huntington’s Disease Phenotype by Genistein-Induced Autophagy in the Cellular Model. Neuromol. Med. 2018, 20, 112–123. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Tundis, R.; Belwal, T.; Devkota, H.P.; Daglia, M.; Cetin, Z.; Saygili, E.I.; Campos, M.d.G.; Capanoglu, E.; et al. Dietary Flavonoids in the Management of Huntington’s Disease: Mechanism and Clinical Perspective. eFood 2020, 1, 38–52. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a Flavonoid with Potentials for Cancer Prevention and Therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, M.; Hu, J.; Du, Z.; Su, Q.; Xiang, Z. Luteolin Suppresses Microglia Neuroinflammatory Responses and Relieves Inflammation-Induced Cognitive Impairments. Neurotox. Res. 2021, 39, 1800–1811. [Google Scholar] [CrossRef]
- Kang, K.A.; Piao, M.J.; Ryu, Y.S.; Hyun, Y.J.; Park, J.E.; Shilnikova, K.; Zhen, A.X.; Kang, H.K.; Koh, Y.S.; Jeong, Y.J.; et al. Luteolin Induces Apoptotic Cell Death via Antioxidant Activity in Human Colon Cancer Cells. Int. J. Oncol. 2017, 51, 1169–1178. [Google Scholar] [CrossRef]
- Ramadan, A.; Mohammed, A.; Elnour, A.A.; Sadeq, A.; Al Mazrouei, N.; Alkaabi, M.; Al-Kubaisi, K.A.; Beshir, S.A.; Menon, V.; AlAmoodi, A.; et al. The Flavonoid Luteolin Reduces Mutant Huntingtin Aggregation and Cytotoxicity in Huntingtin-Mutated Neuroblastoma Cells. Saudi Pharm. J. 2023, 31, 101871. [Google Scholar] [CrossRef]
- Siddique, Y.H.; Varshney, H.; Mantasha, I.; Shahid, M. Effect of Luteolin on the Transgenic Drosophila Model of Huntington’s Disease. Comput. Toxicol. 2021, 17, 100148. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Schroeder, J.P.; Chan, C.-B.; Song, M.; Yu, S.P.; Weinshenker, D.; Ye, K. 7,8-Dihydroxyflavone Prevents Synaptic Loss and Memory Deficits in a Mouse Model of Alzheimer’s Disease. Neuropsychopharmacology 2014, 39, 638–650. [Google Scholar] [CrossRef]
- Akhtar, A.; Dhaliwal, J.; Sah, S.P. 7,8-Dihydroxyflavone Improves Cognitive Functions in ICV-STZ Rat Model of Sporadic Alzheimer’s Disease by Reversing Oxidative Stress, Mitochondrial Dysfunction, and Insulin Resistance. Psychopharmacology 2021, 238, 1991–2009. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Zhang, Z.; Liu, X.; Kang, S.S.; Zhang, Y.; Ye, K. The Prodrug of 7,8-Dihydroxyflavone Development and Therapeutic Efficacy for Treating Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2018, 115, 578–583. [Google Scholar] [CrossRef]
- Özduran, G.; Becer, E.; Vatansever, H.S. The Role and Mechanisms of Action of Catechins in Neurodegenerative Diseases. J. Am. Nutr. Assoc. 2023, 42, 67–74. [Google Scholar] [CrossRef]
- Ide, K.; Matsuoka, N.; Yamada, H.; Furushima, D.; Kawakami, K. Effects of Tea Catechins on Alzheimer’s Disease: Recent Updates and Perspectives. Mol. J. Synth. Chem. Nat. Prod. Chem. 2018, 23, 2357. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Cheng, H.; Che, Z. Ameliorating Effect of Luteolin on Memory Impairment in an Alzheimer’s Disease Model. Mol. Med. Rep. 2016, 13, 4215–4220. [Google Scholar] [CrossRef]
- Taheri, Y.; Sharifi-Rad, J.; Antika, G.; Yılmaz, Y.B.; Tumer, T.B.; Abuhamdah, S.; Chandra, S.; Saklani, S.; Kılıç, C.S.; Sestito, S.; et al. Paving Luteolin Therapeutic Potentialities and Agro-Food-Pharma Applications: Emphasis on In Vivo Pharmacological Effects and Bioavailability Traits. Oxid. Med. Cell. Longev. 2021, 2021, e1987588. [Google Scholar] [CrossRef]
- He, Z.; Li, X.; Wang, Z.; Cao, Y.; Han, S.; Li, N.; Cai, J.; Cheng, S.; Liu, Q. Protective Effects of Luteolin against Amyloid Beta-Induced Oxidative Stress and Mitochondrial Impairments through Peroxisome Proliferator-Activated Receptor γ-Dependent Mechanism in Alzheimer’s Disease. Redox Biol. 2023, 66, 102848. [Google Scholar] [CrossRef]
- Vongthip, W.; Nilkhet, S.; Boonruang, K.; Sukprasansap, M.; Tencomnao, T.; Baek, S.J. Neuroprotective Mechanisms of Luteolin in Glutamate-Induced Oxidative Stress and Autophagy-Mediated Neuronal Cell Death. Sci. Rep. 2024, 14, 7707. [Google Scholar] [CrossRef]
- Nakajima, A.; Ohizumi, Y. Potential Benefits of Nobiletin, A Citrus Flavonoid, against Alzheimer’s Disease and Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 3380. [Google Scholar] [CrossRef]
- Nakajima, A.; Aoyama, Y.; Shin, E.-J.; Nam, Y.; Kim, H.-C.; Nagai, T.; Yokosuka, A.; Mimaki, Y.; Yokoi, T.; Ohizumi, Y.; et al. Nobiletin, a Citrus Flavonoid, Improves Cognitive Impairment and Reduces Soluble Aβ Levels in a Triple Transgenic Mouse Model of Alzheimer’s Disease (3XTg-AD). Behav. Brain Res. 2015, 289, 69–77. [Google Scholar] [CrossRef]
- Pang, Y.; Xiong, J.; Wu, Y.; Ding, W. A Review on Recent Advances on Nobiletin in Central and Peripheral Nervous System Diseases. Eur. J. Med. Res. 2023, 28, 485. [Google Scholar] [CrossRef]
- Chai, W.; Zhang, J.; Xiang, Z.; Zhang, H.; Mei, Z.; Nie, H.; Xu, R.; Zhang, P. Potential of Nobiletin against Alzheimer’s Disease through Inhibiting Neuroinflammation. Metab. Brain Dis. 2022, 37, 1145–1154. [Google Scholar] [CrossRef]
- Korkmaz, O.T.; Aytan, N.; Carreras, I.; Choi, J.-K.; Kowall, N.W.; Jenkins, B.G.; Dedeoglu, A. 7,8-Dihydroxyflavone Improves Motor Performance and Enhances Lower Motor Neuronal Survival in a Mouse Model of Amyotrophic Lateral Sclerosis. Neurosci. Lett. 2014, 566, 286–291. [Google Scholar] [CrossRef]
- Rawlings-Mortimer, F.; Lazari, A.; Tisca, C.; Tachrount, M.; Martins-Bach, A.B.; Miller, K.L.; Lerch, J.P.; Johansen-Berg, H. 7,8-Dihydroxyflavone Enhances Long-Term Spatial Memory and Alters Brain Volume in Wildtype Mice. Front. Syst. Neurosci. 2023, 17, 1134594. [Google Scholar] [CrossRef]
- Li, X.; Chen, C.; Zhan, X.; Li, B.; Zhang, Z.; Li, S.; Xie, Y.; Song, X.; Shen, Y.; Liu, J.; et al. R13 Preserves Motor Performance in SOD1G93A Mice by Improving Mitochondrial Function. Theranostics 2021, 11, 7294–7307. [Google Scholar] [CrossRef]
- Zhao, Z.; Fu, J.; Li, S.; Li, Z. Neuroprotective Effects of Genistein in a SOD1-G93A Transgenic Mouse Model of Amyotrophic Lateral Sclerosis. J. Neuroimmune Pharmacol. 2019, 14, 688–696. [Google Scholar] [CrossRef]
- Trieu, V.N.; Uckun, F.M. Genistein Is Neuroprotective in Murine Models of Familial Amyotrophic Lateral Sclerosis and Stroke. Biochem. Biophys. Res. Commun. 1999, 258, 685–688. [Google Scholar] [CrossRef]
- Bangar, S.P.; Chaudhary, V.; Sharma, N.; Bansal, V.; Ozogul, F.; Lorenzo, J.M. Kaempferol: A Flavonoid with Wider Biological Activities and Its Applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 9580–9604. [Google Scholar] [CrossRef]
- López-Sánchez, C.; Lagoa, R.; Poejo, J.; García-López, V.; García-Martínez, V.; Gutierrez-Merino, C. An Update of Kaempferol Protection against Brain Damage Induced by Ischemia-Reperfusion and by 3-Nitropropionic Acid. Molecules 2024, 29, 776. [Google Scholar] [CrossRef]
- Bhatia, N.K.; Modi, P.; Sharma, S.; Deep, S. Quercetin and Baicalein Act as Potent Antiamyloidogenic and Fibril Destabilizing Agents for SOD1 Fibrils. ACS Chem. Neurosci. 2020, 11, 1129–1138. [Google Scholar] [CrossRef]
- Sharma, D.R.; Wani, W.Y.; Sunkaria, A.; Kandimalla, R.J.; Sharma, R.K.; Verma, D.; Bal, A.; Gill, K.D. Quercetin Attenuates Neuronal Death against Aluminum-Induced Neurodegeneration in the Rat Hippocampus. Neuroscience 2016, 324, 163–176. [Google Scholar] [CrossRef]
- Wang, X.; Chen, S.; Ma, G.; Ye, M.; Lu, G. Genistein Protects Dopaminergic Neurons by Inhibiting Microglial Activation. Neuroreport 2005, 16, 267–270. [Google Scholar] [CrossRef]
- Sarkaki, A.; Badavi, M.; Aligholi, H.; Moghaddam, A.Z. Preventive Effects of Soy Meal (+/− Isoflavone) on Spatial Cognitive Deficiency and Body Weight in an Ovariectomized Animal Model of Parkinson’s Disease. Pak. J. Biol. Sci. 2009, 12, 1338–1345. [Google Scholar] [CrossRef]
- Kyuhou, S.-I. Preventive Effects of Genistein on Motor Dysfunction Following 6-Hydroxydopamine Injection in Ovariectomized Rats. Neurosci. Lett. 2008, 448, 10–14. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, S.-J. Mechanism of Anti-α-Synuclein Immunotherapy. J. Mov. Disord. 2016, 9, 14–19. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Yu, H.-T.; Pu, X.-P.; Du, G.-H. Baicalein Prevents 6-Hydroxydopamine-Induced Mitochondrial Dysfunction in SH-SY5Y Cells via Inhibition of Mitochondrial Oxidation and up-Regulation of DJ-1 Protein Expression. Molecules 2013, 18, 14726–14738. [Google Scholar] [CrossRef]
- Hu, Q.; Uversky, V.N.; Huang, M.; Kang, H.; Xu, F.; Liu, X.; Lian, L.; Liang, Q.; Jiang, H.; Liu, A.; et al. Baicalein Inhibits α-Synuclein Oligomer Formation and Prevents Progression of α-Synuclein Accumulation in a Rotenone Mouse Model of Parkinson’s Disease. Biochim. Biophys. Acta 2016, 1862, 1883–1890. [Google Scholar] [CrossRef]
- Braidy, N.; Behzad, S.; Habtemariam, S.; Ahmed, T.; Daglia, M.; Nabavi, S.M.; Sobarzo-Sanchez, E.; Nabavi, S.F. Neuroprotective Effects of Citrus Fruit-Derived Flavonoids, Nobiletin and Tangeretin in Alzheimer’s and Parkinson’s Disease. CNS Neurol. Disord. Drug Targets 2017, 16, 387–397. [Google Scholar] [CrossRef]
- Chen, M.; Peng, L.; Gong, P.; Zheng, X.; Sun, T.; Zhang, X.; Huo, J. Baicalein Induces Mitochondrial Autophagy to Prevent Parkinson’s Disease in Rats via miR-30b and the SIRT1/AMPK/mTOR Pathway. Front. Neurol. 2022, 12, 646817. [Google Scholar] [CrossRef]
- Rui, W.; Li, S.; Xiao, H.; Xiao, M.; Shi, J. Baicalein Attenuates Neuroinflammation by Inhibiting NLRP3/Caspase-1/GSDMD Pathway in MPTP-Induced Mice Model of Parkinson’s Disease. Int. J. Neuropsychopharmacol. 2020, 23, 762–773. [Google Scholar] [CrossRef]
- de Andrade Teles, R.B.; Diniz, T.C.; Costa Pinto, T.C.; de Oliveira Júnior, R.G.; Gama e Silva, M.; de Lavor, É.M.; Fernandes, A.W.C.; de Oliveira, A.P.; de Almeida Ribeiro, F.P.R.; da Silva, A.A.M.; et al. Flavonoids as Therapeutic Agents in Alzheimer’s and Parkinson’s Diseases: A Systematic Review of Preclinical Evidences. Oxid. Med. Cell. Longev. 2018, 2018, e7043213. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Pauze, M.; Fukuyama, K.; Nagai, N.; Funakoshi-Tago, M.; Sugai, T.; Tamura, H. Effect of Hesperetin Derivatives on the Development of Selenite-induced Cataracts in Rats. Mol. Med. Rep. 2018, 18, 1043–1050. [Google Scholar] [CrossRef]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; de Oliveira, A.C.P. Lipopolysaccharide-Induced Neuroinflammation as a Bridge to Understand Neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar] [CrossRef]
- Khan, A.; Ikram, M.; Hahm, J.R.; Kim, M.O. Antioxidant and Anti-Inflammatory Effects of Citrus Flavonoid Hesperetin: Special Focus on Neurological Disorders. Antioxidants 2020, 9, 609. [Google Scholar] [CrossRef]
- Hong, D.; Lee, S.; Kim, J.; Yang, S.; Lee, M.; Ahn, J.; Lee, H.; Chang, S.-C.; Ha, N.-C.; Lee, J. Anti-Inflammatory and Neuroprotective Effects of Morin in an MPTP-Induced Parkinson’s Disease Model. Int. J. Mol. Sci. 2022, 23, 10578. [Google Scholar] [CrossRef]
- Lee, K.M.; Lee, Y.; Chun, H.J.; Kim, A.H.; Kim, J.Y.; Lee, J.Y.; Ishigami, A.; Lee, J. Neuroprotective and Anti-Inflammatory Effects of Morin in a Murine Model of Parkinson’s Disease. J. Neurosci. Res. 2016, 94, 865–878. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, J.; Li, D.; Ran, S.; Huang, J.; Chen, G. Morin Exhibits a Neuroprotective Effect in MPTP-Induced Parkinson’s Disease Model via TFEB/AMPK-Mediated Mitophagy. Phytomedicine 2023, 116, 154866. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, X.; Xiong, N.; Wang, H.; Huang, J.; Sun, S.; Wang, T. Morin Exerts Neuroprotective Actions in Parkinson Disease Models in Vitro and in Vivo. Acta Pharmacol. Sin. 2010, 31, 900–906. [Google Scholar] [CrossRef]
- García-Díaz Barriga, G.; Giralt, A.; Anglada-Huguet, M.; Gaja-Capdevila, N.; Orlandi, J.G.; Soriano, J.; Canals, J.-M.; Alberch, J. 7,8-Dihydroxyflavone Ameliorates Cognitive and Motor Deficits in a Huntington’s Disease Mouse Model through Specific Activation of the PLCγ1 Pathway. Hum. Mol. Genet. 2017, 26, 3144–3160. [Google Scholar] [CrossRef]
- Kreilaus, F.; Spiro, A.S.; Hannan, A.J.; Garner, B.; Jenner, A.M. Therapeutic Effects of Anthocyanins and Environmental Enrichment in R6/1 Huntington’s Disease Mice. J. Huntingt. Dis. 2016, 5, 285–296. [Google Scholar] [CrossRef]
- Møllersen, L.; Moldestad, O.; Rowe, A.D.; Bjølgerud, A.; Holm, I.; Tveterås, L.; Klungland, A.; Retterstøl, L. Effects of Anthocyanins on CAG Repeat Instability and Behaviour in Huntington’s Disease R6/1 Mice. PLoS Curr. 2016, 8. [Google Scholar] [CrossRef]
- Pierzynowska, K.; Gaffke, L.; Cyske, Z.; Węgrzyn, G. Genistein Induces Degradation of Mutant Huntingtin in Fibroblasts from Huntington’s Disease Patients. Metab. Brain Dis. 2019, 34, 715–720. [Google Scholar] [CrossRef]
- Pierzynowska, K.; Podlacha, M.; Gaffke, L.; Rintz, E.; Wiśniewska, K.; Cyske, Z.; Węgrzyn, G. Correction of Symptoms of Huntington Disease by Genistein through FOXO3-Mediated Autophagy Stimulation. Autophagy 2023, 20, 1159–1182. [Google Scholar] [CrossRef]
- Hajialyani, M.; Hosein Farzaei, M.; Echeverría, J.; Nabavi, S.M.; Uriarte, E.; Sobarzo-Sánchez, E. Hesperidin as a Neuroprotective Agent: A Review of Animal and Clinical Evidence. Molecules 2019, 24, 648. [Google Scholar] [CrossRef]
- Kumar, A.; Chaudhary, T.; Mishra, J. Minocycline Modulates Neuroprotective Effect of Hesperidin against Quinolinic Acid Induced Huntington’s Disease like Symptoms in Rats: Behavioral, Biochemical, Cellular and Histological Evidences. Eur. J. Pharmacol. 2013, 720, 16–28. [Google Scholar] [CrossRef]
- Gopinath, K.; Sudhandiran, G. Naringin Modulates Oxidative Stress and Inflammation in 3-Nitropropionic Acid-Induced Neurodegeneration through the Activation of Nuclear Factor-Erythroid 2-Related Factor-2 Signalling Pathway. Neuroscience 2012, 227, 134–143. [Google Scholar] [CrossRef]
- Solanki, I.; Parihar, P.; Mansuri, M.L.; Parihar, M.S. Flavonoid-Based Therapies in the Early Management of Neurodegenerative Diseases. Adv. Nutr. 2015, 6, 64–72. [Google Scholar] [CrossRef]
- Figueira, I.; Garcia, G.; Pimpão, R.C.; Terrasso, A.P.; Costa, I.; Almeida, A.F.; Tavares, L.; Pais, T.F.; Pinto, P.; Ventura, M.R.; et al. Polyphenols Journey through Blood-Brain Barrier towards Neuronal Protection. Sci. Rep. 2017, 7, 11456. [Google Scholar] [CrossRef]
- Manolescu, B.N.; Oprea, E.; Mititelu, M.; Ruta, L.L.; Farcasanu, I.C. Dietary Anthocyanins and Stroke: A Review of Pharmacokinetic and Pharmacodynamic Studies. Nutrients 2019, 11, 1479. [Google Scholar] [CrossRef]
- Hasan, S.; Khatri, N.; Rahman, Z.N.; Menezes, A.A.; Martini, J.; Shehjar, F.; Mujeeb, N.; Shah, Z.A. Neuroprotective Potential of Flavonoids in Brain Disorders. Brain Sci. 2023, 13, 1258. [Google Scholar] [CrossRef]
- Singh, R.B.; Fedacko, J.; Fatima, G.; Magomedova, A.; Watanabe, S.; Elkilany, G. Why and How the Indo-Mediterranean Diet May Be Superior to Other Diets: The Role of Antioxidants in the Diet. Nutrients 2022, 14, 898. [Google Scholar] [CrossRef]
- Coutinho, A.J.; Pinheiro, M.; Neves, A.R.; Pinto, M.M.M. Therapeutic Potential of Genistein: Preclinical Studies, Clinical Evidence, and Nanotechnology Application. Curr. Med. Chem. 2023, 30, 2480–2517. [Google Scholar] [CrossRef]
- Zannou, O.; Oussou, K.F.; Chabi, I.B.; Awad, N.M.H.; Aïssi, M.V.; Goksen, G.; Mortas, M.; Oz, F.; Proestos, C.; Kayodé, A.P.P. Nanoencapsulation of Cyanidin 3-O-Glucoside: Purpose, Technique, Bioavailability, and Stability. Nanomaterials 2023, 13, 617. [Google Scholar] [CrossRef]
- Sajid, M.; Channakesavula, C.N.; Stone, S.R.; Kaur, P. Synthetic Biology towards Improved Flavonoid Pharmacokinetics. Biomolecules 2021, 11, 754. [Google Scholar] [CrossRef]
- Naeem, A.; Ming, Y.; Pengyi, H.; Jie, K.Y.; Yali, L.; Haiyan, Z.; Shuai, X.; Wenjing, L.; Ling, W.; Xia, Z.M.; et al. The Fate of Flavonoids after Oral Administration: A Comprehensive Overview of Its Bioavailability. Crit. Rev. Food Sci. Nutr. 2022, 62, 6169–6186. [Google Scholar] [CrossRef]
- Teng, H.; Zheng, Y.; Cao, H.; Huang, Q.; Xiao, J.; Chen, L. Enhancement of Bioavailability and Bioactivity of Diet-Derived Flavonoids by Application of Nanotechnology: A Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 378–393. [Google Scholar] [CrossRef]
- Rodríguez-Arce, E.; Saldías, M. Antioxidant Properties of Flavonoid Metal Complexes and Their Potential Inclusion in the Development of Novel Strategies for the Treatment against Neurodegenerative Diseases. Biomed. Pharmacother. 2021, 143, 112236. [Google Scholar] [CrossRef]
- Rendeiro, C.; Rhodes, J.S.; Spencer, J.P.E. The Mechanisms of Action of Flavonoids in the Brain: Direct versus Indirect Effects. Neurochem. Int. 2015, 89, 126–139. [Google Scholar] [CrossRef]
- Solnier, J.; Chang, C.; Pizzorno, J. Consideration for Flavonoid-Containing Dietary Supplements to Tackle Deficiency and Optimize Health. Int. J. Mol. Sci. 2023, 24, 8663. [Google Scholar] [CrossRef]
- Kang, Y.; Lee, J.-H.; Seo, Y.H.; Jang, J.-H.; Jeong, C.-H.; Lee, S.; Jeong, G.-S.; Park, B. Epicatechin Prevents Methamphetamine-Induced Neuronal Cell Death via Inhibition of ER Stress. Biomol. Ther. 2019, 27, 145–151. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Olaokun, O.O. The Beneficial Role of Apigenin against Cognitive and Neurobehavioural Dysfunction: A Systematic Review of Preclinical Investigations. Biomedicines 2024, 12, 178. [Google Scholar] [CrossRef]
- Jameie, S.B.; Pirasteh, A.; Naseri, A.; Jameie, M.S.; Farhadi, M.; Babaee, J.F.; Elyasi, L. β-Amyloid Formation, Memory, and Learning Decline Following Long-Term Ovariectomy and Its Inhibition by Systemic Administration of Apigenin and β-Estradiol. Basic Clin. Neurosci. 2021, 12, 383–394. [Google Scholar] [CrossRef]
- Gaur, K.; Siddique, Y.H. Effect of Apigenin on Neurodegenerative Diseases. CNS Neurol. Disord. Drug Targets 2024, 23, 468–475. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Khan, H.; D’onofrio, G.; Šamec, D.; Shirooie, S.; Dehpour, A.R.; Argüelles, S.; Habtemariam, S.; Sobarzo-Sanchez, E. Apigenin as Neuroprotective Agent: Of Mice and Men. Pharmacol. Res. 2018, 128, 359–365. [Google Scholar] [CrossRef]
- Chesworth, R.; Gamage, R.; Ullah, F.; Sonego, S.; Millington, C.; Fernandez, A.; Liang, H.; Karl, T.; Münch, G.; Niedermayer, G.; et al. Spatial Memory and Microglia Activation in a Mouse Model of Chronic Neuroinflammation and the Anti-Inflammatory Effects of Apigenin. Front. Neurosci. 2021, 15, 699329. [Google Scholar] [CrossRef]
- Ahmedy, O.A.; Abdelghany, T.M.; El-Shamarka, M.E.A.; Khattab, M.A.; El-Tanbouly, D.M. Apigenin Attenuates LPS-Induced Neurotoxicity and Cognitive Impairment in Mice via Promoting Mitochondrial Fusion/Mitophagy: Role of SIRT3/PINK1/Parkin Pathway. Psychopharmacology 2022, 239, 3903–3917. [Google Scholar] [CrossRef]
- Anusha, C.; Sumathi, T.; Joseph, L.D. Protective Role of Apigenin on Rotenone Induced Rat Model of Parkinson’s Disease: Suppression of Neuroinflammation and Oxidative Stress Mediated Apoptosis. Chem. Biol. Interact. 2017, 269, 67–79. [Google Scholar] [CrossRef]
- Yarim, G.F.; Kazak, F.; Yarim, M.; Sozmen, M.; Genc, B.; Ertekin, A.; Gokceoglu, A. Apigenin Alleviates Neuroinflammation in a Mouse Model of Parkinson’s Disease. Int. J. Neurosci. 2022. ahead of print. [Google Scholar] [CrossRef]
- Hashemi, P.; Fahanik Babaei, J.; Vazifekhah, S.; Nikbakht, F. Evaluation of the Neuroprotective, Anticonvulsant, and Cognition-Improvement Effects of Apigenin in Temporal Lobe Epilepsy: Involvement of the Mitochondrial Apoptotic Pathway. Iran. J. Basic Med. Sci. 2019, 22, 752–758. [Google Scholar] [CrossRef]
- Chen, L.; Xie, W.; Xie, W.; Zhuang, W.; Jiang, C.; Liu, N. Apigenin Attenuates Isoflurane-Induced Cognitive Dysfunction via Epigenetic Regulation and Neuroinflammation in Aged Rats. Arch. Gerontol. Geriatr. 2017, 73, 29–36. [Google Scholar] [CrossRef]
- Lotfi, M.-S.; Rassouli, F.B. Natural Flavonoid Apigenin, an Effective Agent Against Nervous System Cancers. Mol. Neurobiol. 2024. ahead of print. [Google Scholar] [CrossRef]
- Calis, Z.; Mogulkoc, R.; Baltaci, A.K. The Roles of Flavonols/Flavonoids in Neurodegeneration and Neuroinflammation. Mini Rev. Med. Chem. 2020, 20, 1475–1488. [Google Scholar] [CrossRef]
- Deepika; Maurya, P.K. Health Benefits of Quercetin in Age-Related Diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef]
- Ulusoy, H.G.; Sanlier, N. A Minireview of Quercetin: From Its Metabolism to Possible Mechanisms of Its Biological Activities. Crit. Rev. Food Sci. Nutr. 2020, 60, 3290–3303. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef]
- Wróbel-Biedrawa, D.; Grabowska, K.; Galanty, A.; Sobolewska, D.; Podolak, I. A Flavonoid on the Brain: Quercetin as a Potential Therapeutic Agent in Central Nervous System Disorders. Life 2022, 12, 591. [Google Scholar] [CrossRef]
- Ebrahimpour, S.; Zakeri, M.; Esmaeili, A. Crosstalk between Obesity, Diabetes, and Alzheimer’s Disease: Introducing Quercetin as an Effective Triple Herbal Medicine. Ageing Res. Rev. 2020, 62, 101095. [Google Scholar] [CrossRef]
- Sayed, W.M. Quercetin Alleviates Red Bull Energy Drink-Induced Cerebral Cortex Neurotoxicity via Modulation of Nrf2 and HO-1. Oxid. Med. Cell. Longev. 2021, 2021, 9482529. [Google Scholar] [CrossRef]
- Bardestani, A.; Ebrahimpour, S.; Esmaeili, A.; Esmaeili, A. Quercetin Attenuates Neurotoxicity Induced by Iron Oxide Nanoparticles. J. Nanobiotechnol. 2021, 19, 327. [Google Scholar] [CrossRef]
- Dora, M.F.; Taha, N.M.; Lebda, M.A.; Hashem, A.E.; Elfeky, M.S.; El-Sayed, Y.S.; Jaouni, S.A.; El-Far, A.H. Quercetin Attenuates Brain Oxidative Alterations Induced by Iron Oxide Nanoparticles in Rats. Int. J. Mol. Sci. 2021, 22, 3829. [Google Scholar] [CrossRef]
- Cui, Z.; Zhao, X.; Amevor, F.K.; Du, X.; Wang, Y.; Li, D.; Shu, G.; Tian, Y.; Zhao, X. Therapeutic Application of Quercetin in Aging-Related Diseases: SIRT1 as a Potential Mechanism. Front. Immunol. 2022, 13, 943321. [Google Scholar] [CrossRef]
- Benameur, T.; Soleti, R.; Porro, C. The Potential Neuroprotective Role of Free and Encapsulated Quercetin Mediated by miRNA against Neurological Diseases. Nutrients 2021, 13, 1318. [Google Scholar] [CrossRef]
- Han, X.; Xu, T.; Fang, Q.; Zhang, H.; Yue, L.; Hu, G.; Sun, L. Quercetin Hinders Microglial Activation to Alleviate Neurotoxicity via the Interplay between NLRP3 Inflammasome and Mitophagy. Redox Biol. 2021, 44, 102010. [Google Scholar] [CrossRef]
- He, W.-B.; Abe, K.; Akaishi, T. Oral Administration of Fisetin Promotes the Induction of Hippocampal Long-Term Potentiation in Vivo. J. Pharmacol. Sci. 2018, 136, 42–45. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, A.; Ali, W.; Jo, M.H.; Park, J.; Ikram, M.; Kim, M.O. Fisetin Rescues the Mice Brains Against D-Galactose-Induced Oxidative Stress, Neuroinflammation and Memory Impairment. Front. Pharmacol. 2021, 12, 612078. [Google Scholar] [CrossRef]
- Goujon, M.; Liang, Z.; Soriano-Castell, D.; Currais, A.; Maher, P. The Neuroprotective Flavonoids Sterubin and Fisetin Maintain Mitochondrial Health under Oxytotic/Ferroptotic Stress and Improve Bioenergetic Efficiency in HT22 Neuronal Cells. Antioxidants 2024, 13, 460. [Google Scholar] [CrossRef]
- Ahmad, A.; Ali, T.; Rehman, S.U.; Kim, M.O. Phytomedicine-Based Potent Antioxidant, Fisetin Protects CNS-Insult LPS-Induced Oxidative Stress-Mediated Neurodegeneration and Memory Impairment. J. Clin. Med. 2019, 8, 850. [Google Scholar] [CrossRef]
- Yang, W.; Tian, Z.-K.; Yang, H.-X.; Feng, Z.-J.; Sun, J.-M.; Jiang, H.; Cheng, C.; Ming, Q.-L.; Liu, C.-M. Fisetin Improves Lead-Induced Neuroinflammation, Apoptosis and Synaptic Dysfunction in Mice Associated with the AMPK/SIRT1 and Autophagy Pathway. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2019, 134, 110824. [Google Scholar] [CrossRef]
- Sharma, N.; Biswas, S.; Al-Dayan, N.; Alhegaili, A.S.; Sarwat, M. Antioxidant Role of Kaempferol in Prevention of Hepatocellular Carcinoma. Antioxidants 2021, 10, 1419. [Google Scholar] [CrossRef]
- Uysal, M.; Celikten, M.; Beker, M.; Polat, N.; Huseyinbas, O.; Terzioglu-Usak, S.; Elibol, B. Kaempferol Treatment Ameliorates Memory Impairments in STZ-induced Neurodegeneration by Acting on Reelin Signaling. Acta Neurobiol. Exp. 2023, 83, 236–245. [Google Scholar] [CrossRef]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and Anti-Inflammatory Properties of the Citrus Flavonoids Hesperidin and Hesperetin: An Updated Review of Their Molecular Mechanisms and Experimental Models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef]
- Nielsen, I.L.F.; Chee, W.S.S.; Poulsen, L.; Offord-Cavin, E.; Rasmussen, S.E.; Frederiksen, H.; Enslen, M.; Barron, D.; Horcajada, M.-N.; Williamson, G. Bioavailability Is Improved by Enzymatic Modification of the Citrus Flavonoid Hesperidin in Humans: A Randomized, Double-Blind, Crossover Trial. J. Nutr. 2006, 136, 404–408. [Google Scholar] [CrossRef]
- Muhammad, T.; Ikram, M.; Ullah, R.; Rehman, S.U.; Kim, M.O. Hesperetin, a Citrus Flavonoid, Attenuates LPS-Induced Neuroinflammation, Apoptosis and Memory Impairments by Modulating TLR4/NF-κB Signaling. Nutrients 2019, 11, 648. [Google Scholar] [CrossRef]
- Jo, S.H.; Kim, M.E.; Cho, J.H.; Lee, Y.; Lee, J.; Park, Y.-D.; Lee, J.S. Hesperetin Inhibits Neuroinflammation on Microglia by Suppressing Inflammatory Cytokines and MAPK Pathways. Arch. Pharm. Res. 2019, 42, 695–703. [Google Scholar] [CrossRef]
- Hollman, P.C.H. Absorption, Bioavailability, and Metabolism of Flavonoids. Pharm. Biol. 2004, 42, 74–83. [Google Scholar] [CrossRef]
- Muhammad, T.; Ali, T.; Ikram, M.; Khan, A.; Alam, S.I.; Kim, M.O. Melatonin Rescue Oxidative Stress-Mediated Neuroinflammation/ Neurodegeneration and Memory Impairment in Scopolamine-Induced Amnesia Mice Model. J. Neuroimmune Pharmacol. Off. J. Soc. Neuroimmune Pharmacol. 2019, 14, 278–294. [Google Scholar] [CrossRef]
- Sadeghi Nejad, Z.; Kazemian, S.; Galedari, A.; Maneshian, M.; Esmaeilpour, K.; Kalantaripour, T.P.; Asadi-Shekaari, M. Naringenin Mitigates Reserpine-Induced Anxiety-like Behavior, Neurodegeneration, and Oxidative Stress in Male Rats. Neurosci. Behav. Physiol. 2023. ahead of print. [Google Scholar] [CrossRef]
- Zou, L.; Ning, M.; Wang, W.; Zheng, Y.; Ma, L.; Lv, J. Naringenin Prevents Propofol Induced Neurodegeneration in Neonatal Mice Brain and Long-Term Neurocognitive Impacts on Adults. Drug Des. Devel. Ther. 2020, 14, 5469–5482. [Google Scholar] [CrossRef]
- Ahmad, M.H.; Fatima, M.; Ali, M.; Rizvi, M.A.; Mondal, A.C. Naringenin Alleviates Paraquat-Induced Dopaminergic Neuronal Loss in SH-SY5Y Cells and a Rat Model of Parkinson’s Disease. Neuropharmacology 2021, 201, 108831. [Google Scholar] [CrossRef]
- Emran, T.B.; Islam, F.; Nath, N.; Sutradhar, H.; Das, R.; Mitra, S.; Alshahrani, M.M.; Alhasaniah, A.H.; Sharma, R. Naringin and Naringenin Polyphenols in Neurological Diseases: Understandings from a Therapeutic Viewpoint. Life 2022, 13, 99. [Google Scholar] [CrossRef]
- Kesh, S.; Kannan, R.R.; Balakrishnan, A. Naringenin Alleviates 6-Hydroxydopamine Induced Parkinsonism in SHSY5Y Cells and Zebrafish Model. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2021, 239, 108893. [Google Scholar] [CrossRef]
- Petry, F.D.S.; Hoppe, J.B.; Klein, C.P.; Dos Santos, B.G.; Hözer, R.M.; Bifi, F.; Matté, C.; Salbego, C.G.; Trindade, V.M.T. Genistein Attenuates Amyloid-Beta-Induced Cognitive Impairment in Rats by Modulation of Hippocampal Synaptotoxicity and Hyperphosphorylation of Tau. J. Nutr. Biochem. 2021, 87, 108525. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.-J.; Chen, R.-J.; Chen, L.; Chen, S.; Yang, X.-F.; Min, J.-W. Genistein Mitigates Oxidative Stress and Inflammation by Regulating Nrf2/HO-1 and NF-κB Signaling Pathways in Hypoxic-Ischemic Brain Damage in Neonatal Mice. Ann. Transl. Med. 2022, 10, 32. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, X. Genistein Attenuates Cognitive Deficits and Neuroapoptosis in Hippocampus Induced by Ketamine Exposure in Neonatal Rats. Synapse 2021, 75, e22181. [Google Scholar] [CrossRef]
- Jantaratnotai, N.; Utaisincharoen, P.; Sanvarinda, P.; Thampithak, A.; Sanvarinda, Y. Phytoestrogens Mediated Anti-Inflammatory Effect through Suppression of IRF-1 and pSTAT1 Expressions in Lipopolysaccharide-Activated Microglia. Int. Immunopharmacol. 2013, 17, 483–488. [Google Scholar] [CrossRef]
- Paramanik, V.; Kurrey, K.; Singh, P.; Tiwari, S. Nisha Roles of Genistein in Learning and Memory during Aging and Neurological Disorders. Biogerontology 2023, 24, 329–346. [Google Scholar] [CrossRef]
- Du, Z.-R.; Feng, X.-Q.; Li, N.; Qu, J.-X.; Feng, L.; Chen, L.; Chen, W.-F. G Protein-Coupled Estrogen Receptor Is Involved in the Anti-Inflammatory Effects of Genistein in Microglia. Phytomed. Int. J. Phytother. Phytopharm. 2018, 43, 11–20. [Google Scholar] [CrossRef]
- Ariyani, W.; Miyazaki, W.; Amano, I.; Hanamura, K.; Shirao, T.; Koibuchi, N. Soy Isoflavones Accelerate Glial Cell Migration via GPER-Mediated Signal Transduction Pathway. Front. Endocrinol. 2020, 11, 554941. [Google Scholar] [CrossRef]
- Du, Z.-R.; Gu, Y.; Xie, X.-M.; Zhang, M.; Jiang, G.-Y.; Chen, W.-F. GPER and IGF-1R Mediate the Anti-Inflammatory Effect of Genistein against Lipopolysaccharide (LPS)-Induced Nigrostriatal Injury in Rats. J. Steroid Biochem. Mol. Biol. 2021, 214, 105989. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Wang, J.; Ma, L.; Zhao, J.; Wang, J.; Fang, Z.; Hou, W.; Guo, H. Neuronal GPER Participates in Genistein-Mediated Neuroprotection in Ischemic Stroke by Inhibiting NLRP3 Inflammasome Activation in Ovariectomized Female Mice. Mol. Neurobiol. 2022, 59, 5024–5040. [Google Scholar] [CrossRef]
- Xiao, Y.Q.; Shao, D.; Tong, H.B.; Shi, S.R. Genistein Increases Progesterone Secretion by Elevating Related Enzymes in Chicken Granulosa Cells. Poult. Sci. 2019, 98, 1911–1917. [Google Scholar] [CrossRef]
- Żabińska, M.; Wiśniewska, K.; Węgrzyn, G.; Pierzynowska, K. Exploring the Physiological Role of the G Protein-Coupled Estrogen Receptor (GPER) and Its Associations with Human Diseases. Psychoneuroendocrinology 2024, 166, 107070. [Google Scholar] [CrossRef]
- Ariyani, W.; Amano, I.; Koibuchi, N. Isoflavones Mediate Dendritogenesis Mainly through Estrogen Receptor α. Int. J. Mol. Sci. 2023, 24, 9011. [Google Scholar] [CrossRef]
- Wnuk, A.; Przepiórska, K.; Pietrzak, B.A.; Kajta, M. Emerging Evidence on Membrane Estrogen Receptors as Novel Therapeutic Targets for Central Nervous System Pathologies. Int. J. Mol. Sci. 2023, 24, 4043. [Google Scholar] [CrossRef]
- Sanjay; Shin, J.-H.; Park, M.; Lee, H.-J. Cyanidin-3-O-Glucoside Regulates the M1/M2 Polarization of Microglia via PPARγ and Aβ42 Phagocytosis Through TREM2 in an Alzheimer’s Disease Model. Mol. Neurobiol. 2022, 59, 5135–5148. [Google Scholar] [CrossRef]
- Baek, H.; Sanjay, null; Park, M.; Lee, H.-J. Cyanidin-3-O-Glucoside Protects the Brain and Improves Cognitive Function in APPswe/PS1ΔE9 Transgenic Mice Model. J. Neuroinflamm. 2023, 20, 268. [Google Scholar] [CrossRef]
- Fan, Z.; Wen, H.; Zhang, X.; Li, J.; Zang, J. Cyanidin 3-O-β-Galactoside Alleviated Cognitive Impairment in Mice by Regulating Brain Energy Metabolism During Aging. J. Agric. Food Chem. 2022, 70, 1111–1121. [Google Scholar] [CrossRef]
- Sukprasansap, M.; Chanvorachote, P.; Tencomnao, T. Cyanidin-3-Glucoside Activates Nrf2-Antioxidant Response Element and Protects against Glutamate-Induced Oxidative and Endoplasmic Reticulum Stress in HT22 Hippocampal Neuronal Cells. BMC Complement. Med. Ther. 2020, 20, 46. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, X.-T.; Li, D.-W.; Wang, K.; Wang, X.-Z.; Li, Y.; Sun, B.-L.; Yang, X.-Y.; Zheng, Z.-C.; Cho, N.C. Cyanidin Suppresses Amyloid Beta-Induced Neurotoxicity by Inhibiting Reactive Oxygen Species-Mediated DNA Damage and Apoptosis in PC12 Cells. Neural Regen. Res. 2016, 11, 795–800. [Google Scholar] [CrossRef]
- Qu, D.; Ye, Z.; Zhang, W.; Dai, B.; Chen, G.; Wang, L.; Shao, X.; Xiang, A.; Lu, Z.; Shi, J. Cyanidin Chloride Improves LPS-Induced Depression-Like Behavior in Mice by Ameliorating Hippocampal Inflammation and Excitotoxicity. ACS Chem. Neurosci. 2022, 13, 3023–3033. [Google Scholar] [CrossRef]
- Suresh, S.; Vellapandian, C. Restoring Impaired Neurogenesis and Alleviating Oxidative Stress by Cyanidin against Bisphenol A-Induced Neurotoxicity: In Vivo and In Vitro Evidence. Curr. Drug Discov. Technol. 2024, 21, e250124226256. [Google Scholar] [CrossRef]
- Yang, R.; Yang, X.; Zhang, F. New Perspectives of Taxifolin in Neurodegenerative Diseases. Curr. Neuropharmacol. 2023, 21, 2097–2109. [Google Scholar] [CrossRef]
- Inoue, T.; Saito, S.; Tanaka, M.; Yamakage, H.; Kusakabe, T.; Shimatsu, A.; Ihara, M.; Satoh-Asahara, N. Pleiotropic Neuroprotective Effects of Taxifolin in Cerebral Amyloid Angiopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 10031–10038. [Google Scholar] [CrossRef]
- Lovekamp-Swan, T.; Glendenning, M.; Schreihofer, D.A. A High Soy Diet Reduces Programmed Cell Death and Enhances Bcl-xL Expression in Experimental Stroke. Neuroscience 2007, 148, 644–652. [Google Scholar] [CrossRef]
- Soltani, Z.; Khaksari, M. Can Soy Diet Be Protective in Severe and Diffuse Traumatic Brain Injury? J. Neurol. Neurophysiol. 2014, 5, 2. [Google Scholar] [CrossRef]
- Pan, M.; Li, Z.; Yeung, V.; Xu, R.-J. Dietary Supplementation of Soy Germ Phytoestrogens or Estradiol Improves Spatial Memory Performance and Increases Gene Expression of BDNF, TrkB Receptor and Synaptic Factors in Ovariectomized Rats. Nutr. Metab. 2010, 7, 75. [Google Scholar] [CrossRef]
- Rendeiro, C.; Vauzour, D.; Kean, R.J.; Butler, L.T.; Rattray, M.; Spencer, J.P.E.; Williams, C.M. Blueberry Supplementation Induces Spatial Memory Improvements and Region-Specific Regulation of Hippocampal BDNF mRNA Expression in Young Rats. Psychopharmacology 2012, 223, 319–330. [Google Scholar] [CrossRef]
- Resnick, S.M.; Metter, E.J.; Zonderman, A.B. Estrogen Replacement Therapy and Longitudinal Decline in Visual Memory: A Possible Protective Effect? Neurology 1997, 49, 1491–1497. [Google Scholar] [CrossRef]
- Park, Y.-J.; Ko, J.; Jeon, S.; Kwon, Y. Protective Effect of Genistein against Neuronal Degeneration in ApoE−/− Mice Fed a High-Fat Diet. Nutrients 2016, 8, 692. [Google Scholar] [CrossRef]
- Menze, E.T.; Tadros, M.G.; Abdel-Tawab, A.M.; Khalifa, A.E. Potential Neuroprotective Effects of Hesperidin on 3-Nitropropionic Acid-Induced Neurotoxicity in Rats. Neurotoxicology 2012, 33, 1265–1275. [Google Scholar] [CrossRef]
- Currais, A.; Goldberg, J.; Farrokhi, C.; Chang, M.; Prior, M.; Dargusch, R.; Daugherty, D.; Armando, A.; Quehenberger, O.; Maher, P.; et al. A Comprehensive Multiomics Approach toward Understanding the Relationship between Aging and Dementia. Aging 2015, 7, 937–955. [Google Scholar] [CrossRef]
- Currais, A.; Farrokhi, C.; Dargusch, R.; Armando, A.; Quehenberger, O.; Schubert, D.; Maher, P. Fisetin Reduces the Impact of Aging on Behavior and Physiology in the Rapidly Aging SAMP8 Mouse. J. Gerontol. Ser. A 2018, 73, 299–307. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.-L.; Liu, R.; Li, X.-X.; Li, J.-F.; Zhang, L. Neuroprotective, Anti-Amyloidogenic and Neurotrophic Effects of Apigenin in an Alzheimer’s Disease Mouse Model. Molecules 2013, 18, 9949–9965. [Google Scholar] [CrossRef]
- Blesa, J.; Przedborski, S. Parkinson’s Disease: Animal Models and Dopaminergic Cell Vulnerability. Front. Neuroanat. 2014, 8, 123289. [Google Scholar] [CrossRef]
- Ay, M.; Luo, J.; Langley, M.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Molecular Mechanisms Underlying Protective Effects of Quercetin against Mitochondrial Dysfunction and Progressive Dopaminergic Neurodegeneration in Cell Culture and MitoPark Transgenic Mouse Models of Parkinson’s Disease. J. Neurochem. 2017, 141, 766–782. [Google Scholar] [CrossRef]
- Cheng, Y.; He, G.; Mu, X.; Zhang, T.; Li, X.; Hu, J.; Xu, B.; Du, G. Neuroprotective Effect of Baicalein against MPTP Neurotoxicity: Behavioral, Biochemical and Immunohistochemical Profile. Neurosci. Lett. 2008, 441, 16–20. [Google Scholar] [CrossRef]
- Luo, D.; Shi, Y.; Wang, J.; Lin, Q.; Sun, Y.; Ye, K.; Yan, Q.; Zhang, H. 7,8-Dihydroxyflavone Protects 6-OHDA and MPTP Induced Dopaminergic Neurons Degeneration through Activation of TrkB in Rodents. Neurosci. Lett. 2016, 620, 43–49. [Google Scholar] [CrossRef]
- He, J.; Xiang, Z.; Zhu, X.; Ai, Z.; Shen, J.; Huang, T.; Liu, L.; Ji, W.; Li, T. Neuroprotective Effects of 7, 8-Dihydroxyflavone on Midbrain Dopaminergic Neurons in MPP+-Treated Monkeys. Sci. Rep. 2016, 6, 34339. [Google Scholar] [CrossRef]
- Goes, A.T.R.; Jesse, C.R.; Antunes, M.S.; Lobo Ladd, F.V.; Lobo Ladd, A.A.B.; Luchese, C.; Paroul, N.; Boeira, S.P. Protective Role of Chrysin on 6-Hydroxydopamine-Induced Neurodegeneration a Mouse Model of Parkinson’s Disease: Involvement of Neuroinflammation and Neurotrophins. Chem. Biol. Interact. 2018, 279, 111–120. [Google Scholar] [CrossRef]
- Jeong, K.H.; Jeon, M.-T.; Kim, H.D.; Jung, U.J.; Jang, M.C.; Chu, J.W.; Yang, S.J.; Choi, I.Y.; Choi, M.-S.; Kim, S.R. Nobiletin Protects Dopaminergic Neurons in the 1-Methyl-4-Phenylpyridinium-Treated Rat Model of Parkinson’s Disease. J. Med. Food 2015, 18, 409–414. [Google Scholar] [CrossRef]
- Lutz, C. Mouse Models of ALS: Past, Present and Future. Brain Res. 2018, 1693, 1–10. [Google Scholar] [CrossRef]
- Rangel-Barajas, C.; Rebec, G.V. Overview of Huntington’s Disease Models: Neuropathological, Molecular, and Behavioral Differences. Curr. Protoc. Neurosci. 2018, 83, e47. [Google Scholar] [CrossRef]
- Thangarajan, S.; Ramachandran, S.; Krishnamurthy, P. Chrysin Exerts Neuroprotective Effects against 3-Nitropropionic Acid Induced Behavioral Despair—Mitochondrial Dysfunction and Striatal Apoptosis via Upregulating Bcl-2 Gene and Downregulating Bax—Bad Genes in Male Wistar Rats. Biomed. Pharmacother. 2016, 84, 514–525. [Google Scholar] [CrossRef]
- Sandhir, R.; Mehrotra, A. Quercetin Supplementation Is Effective in Improving Mitochondrial Dysfunctions Induced by 3-Nitropropionic Acid: Implications in Huntington’s Disease. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2013, 1832, 421–430. [Google Scholar] [CrossRef]
- Maher, P.; Dargusch, R.; Bodai, L.; Gerard, P.E.; Purcell, J.M.; Marsh, J.L. ERK Activation by the Polyphenols Fisetin and Resveratrol Provides Neuroprotection in Multiple Models of Huntington’s Disease. Hum. Mol. Genet. 2011, 20, 261–270. [Google Scholar] [CrossRef]
- Chang, L.M.; Song, Y.; Li, X.-M.; Sampson, H.A.; Masilamani, M. Dietary Elimination of Soybean Components Enhances Allergic Immune Response to Peanuts in BALB/c Mice. Int. Arch. Allergy Immunol. 2015, 166, 304–310. [Google Scholar] [CrossRef]
- Fontenla, M.; Prchal, A.; Cena, A.M.; Albarracín, A.L.; Pintos, S.; Benvenuto, S.; Sosa, M.L.; Fontenla de Petrino, S. Effects of Soy Milk as a Dietary Complement during the Natural Aging Process. Nutr. Hosp. 2008, 23, 607–613. [Google Scholar]
- Messina, M.; Nagata, C.; Wu, A.H. Estimated Asian Adult Soy Protein and Isoflavone Intakes. Nutr. Cancer 2006, 55, 1–12. [Google Scholar] [CrossRef]
- Murai, U.; Sawada, N.; Charvat, H.; Inoue, M.; Yasuda, N.; Yamagishi, K.; Tsugane, S.; JPHC Study Group. Soy Product Intake and Risk of Incident Disabling Dementia: The JPHC Disabling Dementia Study. Eur. J. Nutr. 2022, 61, 4045–4057. [Google Scholar] [CrossRef]
- Talaei, M.; Feng, L.; Yuan, J.-M.; Pan, A.; Koh, W.-P. Dairy, Soy, and Calcium Consumption and Risk of Cognitive Impairment: The Singapore Chinese Health Study. Eur. J. Nutr. 2020, 59, 1541–1552. [Google Scholar] [CrossRef]
- Lin, H.-C.; Peng, C.-H.; Huang, C.-N.; Chiou, J.-Y. Soy-Based Foods Are Negatively Associated with Cognitive Decline in Taiwan’s Elderly. J. Nutr. Sci. Vitaminol. 2018, 64, 335–339. [Google Scholar] [CrossRef]
- File, S.; Jarrett, N.; Fluck, E.; Duffy, R.; Casey, K.; Wiseman, H. Eating Soya Improves Human Memory. Psychopharmacology 2001, 157, 430–436. [Google Scholar] [CrossRef]
- Gao, Q.; Dong, J.-Y.; Cui, R.; Muraki, I.; Yamagishi, K.; Sawada, N.; Iso, H.; Tsugane, S.; Japan Public Health Center-based Prospective Study Group. Consumption of Flavonoid-Rich Fruits, Flavonoids from Fruits and Stroke Risk: A Prospective Cohort Study. Br. J. Nutr. 2021, 126, 1717–1724. [Google Scholar] [CrossRef]
- Cui, C.; Birru, R.L.; Snitz, B.E.; Ihara, M.; Kakuta, C.; Lopresti, B.J.; Aizenstein, H.J.; Lopez, O.L.; Mathis, C.A.; Miyamoto, Y.; et al. Effects of Soy Isoflavones on Cognitive Function: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Rev. 2020, 78, 134–144. [Google Scholar] [CrossRef]
- Gleason, C.E.; Fischer, B.L.; Dowling, N.M.; Setchell, K.D.R.; Atwood, C.S.; Carlsson, C.M.; Asthana, S. Cognitive Effects of Soy Isoflavones in Patients with Alzheimer’s Disease. J. Alzheimers Dis. 2015, 47, 1009–1019. [Google Scholar] [CrossRef]
- Sekikawa, A.; Wharton, W.; Butts, B.; Veliky, C.V.; Garfein, J.; Li, J.; Goon, S.; Fort, A.; Li, M.; Hughes, T.M. Potential Protective Mechanisms of S-Equol, a Metabolite of Soy Isoflavone by the Gut Microbiome, on Cognitive Decline and Dementia. Int. J. Mol. Sci. 2022, 23, 11921. [Google Scholar] [CrossRef]
- Igase, M.; Igase, K.; Tabara, Y.; Ohyagi, Y.; Kohara, K. Cross-sectional Study of Equol Producer Status and Cognitive Impairment in Older Adults. Geriatr. Gerontol. Int. 2017, 17, 2103–2108. [Google Scholar] [CrossRef]
- Wu, L.; Chu, L.; Pang, Y.; Huo, J.; Cao, H.; Tian, Q.; Gao, Q. Effects of Dietary Supplements, Foods, and Dietary Patterns in Parkinson’s Disease: Meta-Analysis and Systematic Review of Randomized and Crossover Studies. Eur. J. Clin. Nutr. 2024, 78, 365–375. [Google Scholar] [CrossRef]
- Paknahad, Z.; Sheklabadi, E.; Moravejolahkami, A.R.; Chitsaz, A.; Hassanzadeh, A. The Effects of Mediterranean Diet on Severity of Disease and Serum Total Antioxidant Capacity (TAC) in Patients with Parkinson’s Disease: A Single Center, Randomized Controlled Trial. Nutr. Neurosci. 2022, 25, 313–320. [Google Scholar] [CrossRef]
- Gao, X.; Cassidy, A.; Schwarzschild, M.A.; Rimm, E.B.; Ascherio, A. Habitual Intake of Dietary Flavonoids and Risk of Parkinson Disease. Neurology 2012, 78, 1138–1145. [Google Scholar] [CrossRef]
- Vagadia, B.H.; Vanga, S.K.; Raghavan, V. Inactivation Methods of Soybean Trypsin Inhibitor—A Review. Trends Food Sci. Technol. 2017, 64, 115–125. [Google Scholar] [CrossRef]
- Hemetsberger, F.; Hauser, T.; Domig, K.J.; Kneifel, W.; Schedle, K. Interaction of Soybean Varieties and Heat Treatments and Its Effect on Growth Performance and Nutrient Digestibility in Broiler Chickens. Animals 2021, 11, 2668. [Google Scholar] [CrossRef]
- Otun, J.; Sahebkar, A.; Östlundh, L.; Atkin, S.L.; Sathyapalan, T. Systematic Review and Meta-Analysis on the Effect of Soy on Thyroid Function. Sci. Rep. 2019, 9, 3964. [Google Scholar] [CrossRef]
- Duffy, C.; Cyr, M. Phytoestrogens: Potential Benefits and Implications for Breast Cancer Survivors. J. Womens Health 2003, 12, 617–631. [Google Scholar] [CrossRef]
- Suen, A.A.; Kenan, A.C.; Williams, C.J. Developmental Exposure to Phytoestrogens Found in Soy: New Findings and Clinical Implications. Biochem. Pharmacol. 2022, 195, 114848. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, B.; Xiang, J. The Association between Soy Intake and Risk of Gestational Diabetes Mellitus: A Prospective Cohort Study. BMC Pregnancy Childbirth 2021, 21, 695. [Google Scholar] [CrossRef]
- Martínez-Poveda, B.; Torres-Vargas, J.A.; Ocaña, M.D.C.; García-Caballero, M.; Medina, M.Á.; Quesada, A.R. The Mediterranean Diet, a Rich Source of Angiopreventive Compounds in Cancer. Nutrients 2019, 11, 2036. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Farooqui, T. Importance of Fruit and Vegetable-Derived Flavonoids in the Mediterranean Diet. In Role of the Mediterranean Diet in the Brain and Neurodegenerative Diseases; Elsevier: Amsterdam, The Netherlands, 2018; pp. 417–427. ISBN 978-0-12-811959-4. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szulc, A.; Wiśniewska, K.; Żabińska, M.; Gaffke, L.; Szota, M.; Olendzka, Z.; Węgrzyn, G.; Pierzynowska, K. Effectiveness of Flavonoid-Rich Diet in Alleviating Symptoms of Neurodegenerative Diseases. Foods 2024, 13, 1931. https://doi.org/10.3390/foods13121931
Szulc A, Wiśniewska K, Żabińska M, Gaffke L, Szota M, Olendzka Z, Węgrzyn G, Pierzynowska K. Effectiveness of Flavonoid-Rich Diet in Alleviating Symptoms of Neurodegenerative Diseases. Foods. 2024; 13(12):1931. https://doi.org/10.3390/foods13121931
Chicago/Turabian StyleSzulc, Aneta, Karolina Wiśniewska, Magdalena Żabińska, Lidia Gaffke, Maria Szota, Zuzanna Olendzka, Grzegorz Węgrzyn, and Karolina Pierzynowska. 2024. "Effectiveness of Flavonoid-Rich Diet in Alleviating Symptoms of Neurodegenerative Diseases" Foods 13, no. 12: 1931. https://doi.org/10.3390/foods13121931







