Effect of Milk Protein–Polyphenol Conjugate on the Regulation of GLP-1 Hormone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Required Chemicals and Reagents
2.2. Apo-LF-EGCG Conjugate Synthesis
2.3. Characterization of the Apo-LF-EGCG Conjugates
2.4. HPLC-MS Analysis
2.5. Fluorescence Spectroscopy Analysis
2.6. Assessment of Antioxidant Activity
2.7. MTT Assay
2.8. qRT-PCR
2.9. ELISA
2.10. Statistical Analysis
3. Results and Discussion
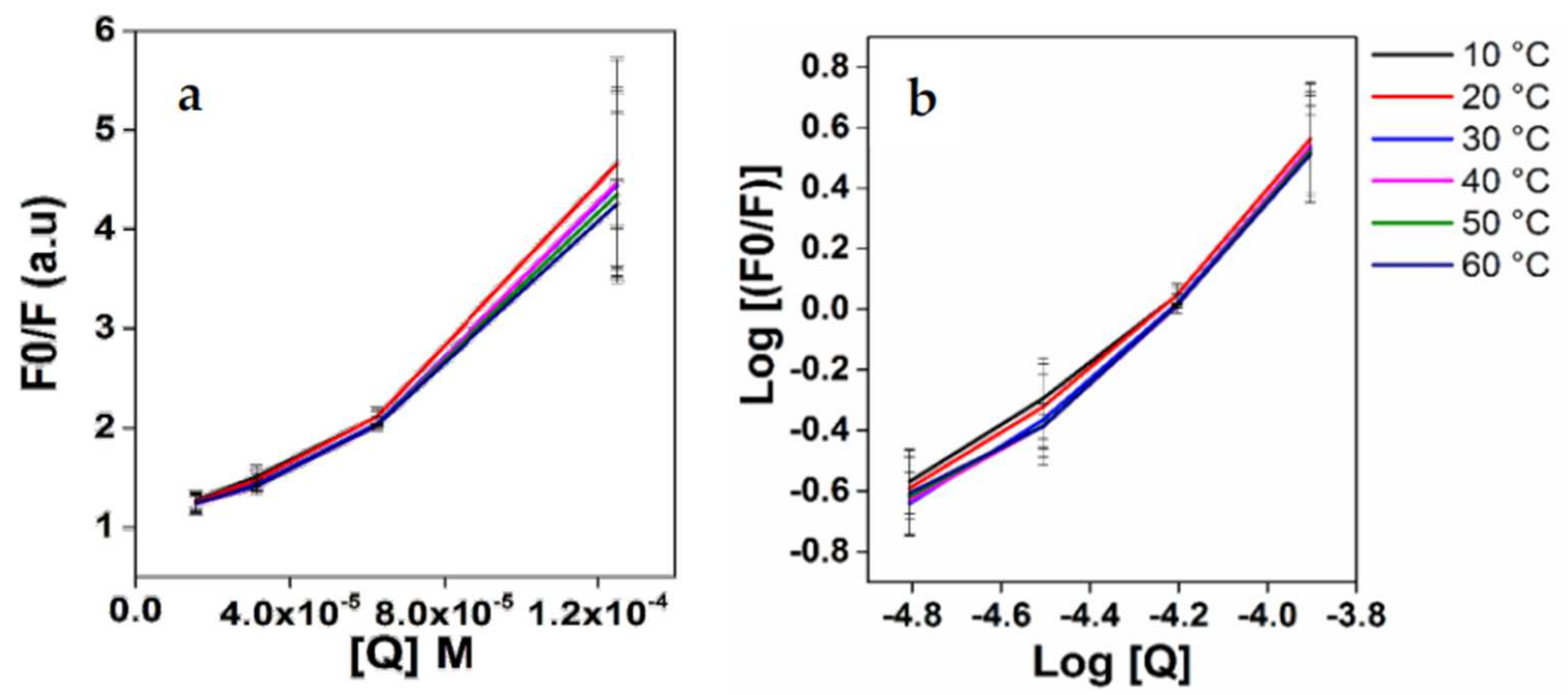
3.1. Interaction between EGCG and Apo-LF
3.2. Changes in the Secondary Structure of Apo-LF
3.3. Antioxidant Capacities of Conjugates
3.4. Mechanism of Interaction in the Conjugates
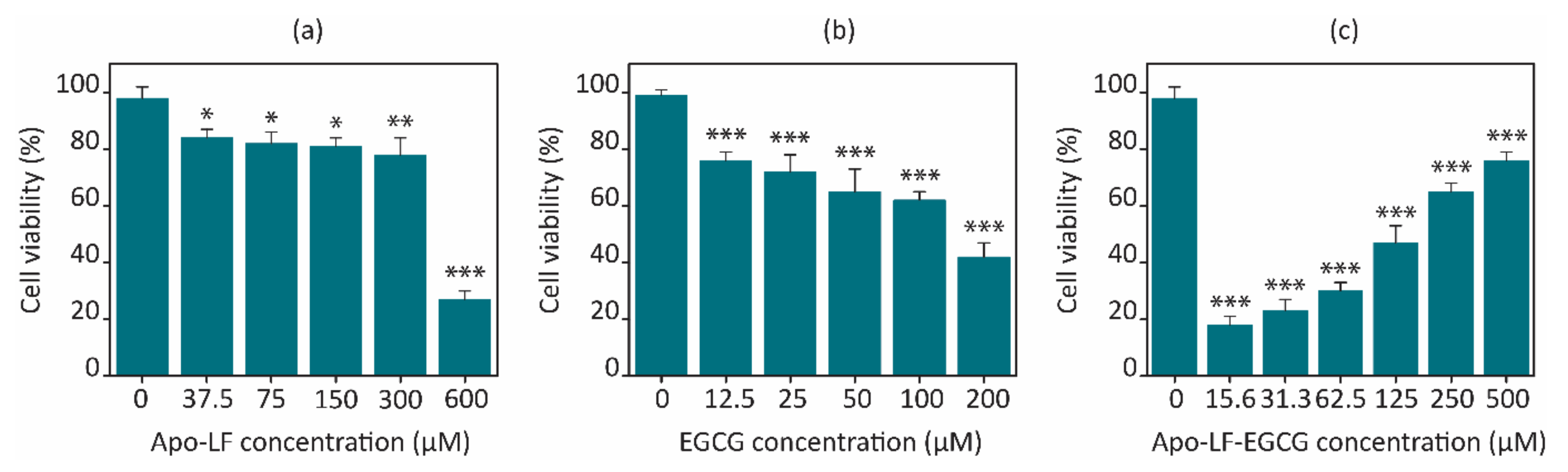
3.5. Effect of Conjugates on Cell Viability
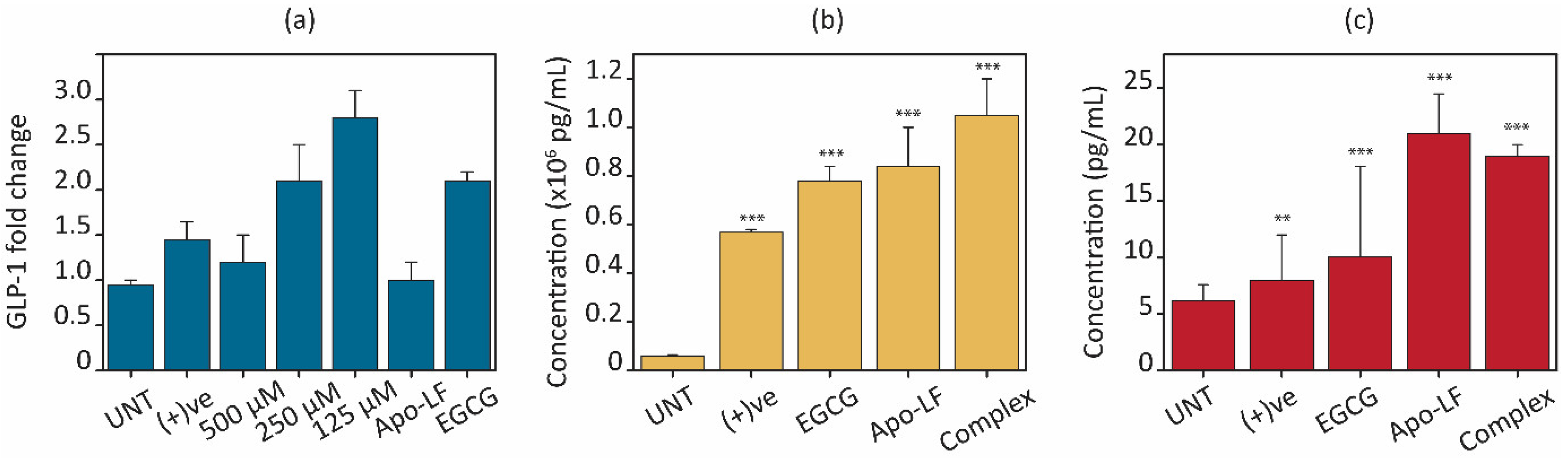
3.6. Effect of the Conjugate on GLP-1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Health Quality Council of Alberta. 2015. Available online: https://hqca.ca/reports/overweight-obesity-in-adult-albertans/ (accessed on 7 August 2023).
- Swinburn, B.A.; Caterson, I.; Seidell, J.C.; James, W.P. Diet, nutrition and the prevention of excess weight gain and obesity. Public Health Nutr. 2004, 7, 123–146. [Google Scholar]
- Zhao, T.C. Glucagon-like peptide-1 (GLP-1) and protective effects in cardiovascular disease: A new therapeutic approach for myocardial protection. Cardiovasc Diabetol. 2013, 12, 90. [Google Scholar] [CrossRef]
- World Obesity Atlas. 2022. Available online: https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2022 (accessed on 7 August 2023).
- Lin, X.; Li, H. Obesity: Epidemiology, pathophysiology, and therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef]
- Tanaka, M. Improving obesity and blood pressure. Hypertens. Res. 2020, 43, 79–89. [Google Scholar] [CrossRef]
- Ernst, N.D.; Obarzanek, E. Child health and nutrition: Obesity and high blood cholesterol. Prev. Med. 1994, 23, 427–436. [Google Scholar] [CrossRef]
- Boutari, C.; Mantzoros, C.S. A 2022 update on the epidemiology of obesity and a call to action: As its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2022, 133, 155217. [Google Scholar] [CrossRef]
- Small, C.J.; Bloom, S.R. Gut hormones and the control of appetite. Trends Endocrinol. Metab. 2004, 15, 259–263. [Google Scholar] [CrossRef]
- Neary, M.T.; Batterham, R.L. Gut hormones: Implications for the treatment of obesity. Pharmacol. Ther. 2009, 124, 44–56. [Google Scholar] [CrossRef]
- Kashyap, S.R.; Gatmaitan, P.; Brethauer, S.; Schauer, P. Bariatric surgery for type 2 diabetes: Weighing the impact for obese patients. Cleve Clin. J. Med. 2010, 77, 468–476. [Google Scholar] [CrossRef]
- Karra, E.; Batterham, R.L. The role of gut hormones in the regulation of body weight and energy homeostasis. Mol. Cell Endocrinol. 2010, 316, 120–128. [Google Scholar] [CrossRef]
- Woods, S.C.; D’Alessio, D.A. Central control of body weight and appetite. J. Clin. Endocrinol. Metab. 2008, 93, S37–S50. [Google Scholar] [CrossRef]
- Popkin, B.M.; Armstrong, L.E.; Bray, G.M.; Caballero, B.; Frei, B.; Willett, W.C. A new proposed guidance system for beverage consumption in the United States. Am. J. Clin. Nutr. 2006, 83, 529–542. [Google Scholar] [CrossRef]
- De Graaf, C.; Hulshof, T.; Weststrate, J.A.; Jas, P. Short-term effects of different amounts of protein, fats, and carbohydrates on satiety. Am. J. Clin. Nutr. 1992, 55, 33–38. [Google Scholar] [CrossRef]
- Hall, W.L.; Millward, D.J.; Long, S.J.; Morgan, L.M. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br. J. Nutr. 2003, 89, 239–248. [Google Scholar] [CrossRef]
- Chen, Q.; Reimer, R.A. Dairy protein and leucine alter GLP-1 release and mRNA of genes involved in intestinal lipid metabolism in vitro. Nutrition 2009, 25, 340–349. [Google Scholar] [CrossRef]
- Veldhorst, M.A.; Nieuwenhuizen, A.G.; Hochstenbach-Waelen, A.; van Vught, A.J.; Westerterp, K.R.; Engelen, M.P.; Brummer, R.J.; Deutz, N.E.; Westerterp-Plantenga, M.S. Dose-dependent satiating effect of whey relative to casein or soy. Physiol. Behav. 2009, 96, 675–682. [Google Scholar] [CrossRef]
- Geraedts, M.C.; Troost, F.J.; Fischer, M.A.; Edens, L.; Saris, W.H. Direct induction of CCK and GLP-1 release from murine endocrine cells by intact dietary proteins. Mol. Nutr. Food Res. 2011, 55, 476–484. [Google Scholar] [CrossRef]
- Luhovyy, B.L.; Akhavan, T.; Anderson, G.H. Whey proteins in the regulation of food intake and satiety. J. Am. Coll. Nutr. 2007, 26, 704s–712s. [Google Scholar] [CrossRef]
- Gillespie, A.L.; Calderwood, D.; Hobson, L.; Green, B.D. Whey proteins have beneficial effects on intestinal enteroendocrine cells stimulating cell growth and increasing the production and secretion of incretin hormones. Food Chem. 2015, 189, 120–128. [Google Scholar] [CrossRef]
- Jang, H.J.; Kokrashvili, Z.; Theodorakis, M.J.; Carlson, O.D.; Kim, B.J.; Zhou, J.; Kim, H.H.; Xu, X.; Chan, S.L.; Juhaszova, M.; et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc. Natl. Acad. Sci. USA 2007, 104, 15069–15074. [Google Scholar] [CrossRef]
- Perez-Montes, D.E.O.A.; Pellitero, S.; Puig-Domingo, M. Obesity and GLP-1. Minerva Endocrinol. 2021, 46, 168–176. [Google Scholar] [CrossRef]
- Westerterp-Plantenga, M.S.; Lejeune, M.P.; Kovacs, E.M. Body weight loss and weight maintenance in relation to habitual caffeine intake and green tea supplementation. Obes. Res. 2005, 13, 1195–1204. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea polyphenols in promotion of human health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef]
- Yin, Z.; Zheng, T.; Ho, C.T.; Huang, Q.; Wu, Q.; Zhang, M. Improving the stability and bioavailability of tea polyphenols by encapsulations: A review. Food Sci. Hum. Wellness 2022, 11, 537–556. [Google Scholar] [CrossRef]
- Liu, J.; Yong, H.; Yao, X.; Hu, H.; Yun, D.; Xiao, L. Recent advances in phenolic–protein conjugates: Synthesis, characterization, biological activities and potential applications. RSC Adv. 2019, 9, 35825–35840. [Google Scholar] [CrossRef]
- Ferrara, L.; Montesano, D.; Senatore, A. The distribution of minerals and flavonoids in the tea plant (Camellia sinensis). Farmaco 2001, 56, 397–401. [Google Scholar] [CrossRef]
- Ki, Y.; Goodner, K.L.; Park, J.-D.; Choi, J.; Talcott, S.T. Changes in antioxidant phytochemicals and volatile composition of Camellia sinensis by oxidation during tea fermentation. Food Chem. 2011, 129, 1331–1342. [Google Scholar]
- Sang, S.; Lambert, J.D.; Ho, C.T.; Yang, C.S. The chemistry and biotransformation of tea constituents. Pharmacol. Res. 2011, 64, 87–99. [Google Scholar] [CrossRef]
- Yang, F.; de Villiers, W.J.; McClain, C.J.; Varilek, G.W. Green tea polyphenols block endotoxin-induced tumor necrosis factor-production and lethality in a murine model. J. Nutr. 1998, 128, 2334–2340. [Google Scholar] [CrossRef]
- Lamothe, S.; Azimy, N.; Bazinet, L.; Couillard, C.; Britten, M. Interaction of green tea polyphenols with dairy matrices in a simulated gastrointestinal environment. Food Funct. 2014, 5, 2621–2631. [Google Scholar] [CrossRef]
- Zeng, L.; Ma, M.; Li, C.; Luo, L. Stability of tea polyphenols solution with different pH at different temperatures. Int. J. Food Prop. 2017, 20, 1–18. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Zhang, T.; McClements, D.J.; Liu, X.; Wu, X.; Liu, F. Enzymatic and nonenzymatic conjugates of lactoferrin and (−)-epigallocatechin gallate: Formation, structure, functionality, and allergenicity. J. Agric. Food Chem. 2021, 69, 6291–6302. [Google Scholar] [CrossRef]
- Hursel, R.; Westerterp-Plantenga, M.S. Green tea catechin plus caffeine supplementation to a high-protein diet has no additional effect on body weight maintenance after weight loss. Am. J. Clin. Nutr. 2009, 89, 822–830. [Google Scholar] [CrossRef]
- Stojadinovic, M.; Radosavljevic, J.; Ognjenovic, J.; Vesic, J.; Prodic, I.; Stanic-Vucinic, D.; Velickovic, T.C. Binding affinity between dietary polyphenols and β-lactoglobulin negatively correlates with the protein susceptibility to digestion and total antioxidant activity of complexes formed. Food Chem. 2013, 136, 1263–1271. [Google Scholar] [CrossRef]
- Ono, T.; Murakoshi, M.; Suzuki, N.; Iida, N.; Ohdera, M.; Iigo, M.; Yoshida, T.; Sugiyama, K.; Nishino, H. Potent anti-obesity effect of enteric-coated lactoferrin: Decrease in visceral fat accumulation in Japanese men and women with abdominal obesity after 8-week administration of enteric-coated lactoferrin tablets. Br. J. Nutr. 2010, 104, 1688–1695. [Google Scholar] [CrossRef]
- Liu, F.; Wang, D.; Sun, C.; Gao, Y. Influence of polysaccharides on the physicochemical properties of lactoferrin–polyphenol conjugates coated β-carotene emulsions. Food Hydrocoll. 2016, 52, 661–669. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, L.Y.; Lü, W.J.; Cao, H.R. Analysis of the spectroscopic characteristics on the binding interaction between tosufloxacin and bovine lactoferrin. J. Lumin. 2011, 131, 768–775. [Google Scholar] [CrossRef]
- Yang, W.; Liu, F.; Xu, C.; Yuan, F.; Gao, Y. Molecular interaction between (−)-epigallocatechin-3-gallate and bovine lactoferrin using multi-spectroscopic method and isothermal titration calorimetry. Food Res. Int. 2014, 64, 141–149. [Google Scholar] [CrossRef]
- Stănciuc, N.; Aprodu, I.; Râpeanu, G.; van der Plancken, I.; Bahrim, G.; Hendrickx, M. Analysis of the thermally induced structural changes of bovine lactoferrin. J. Agric. Food Chem. 2013, 61, 2234–2243. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, Q. Probing the binding between norbixin and dairy proteins by spectroscopy methods. Food Chem. 2013, 139, 611–616. [Google Scholar] [CrossRef]
- Yang, W.; Xu, C.; Liu, F.; Yuan, F.; Gao, Y. Native and thermally modified protein–polyphenol coassemblies: Lactoferrin-based nanoparticles and submicrometer particles as protective vehicles for (−)-epigallocatechin-3-gallate. J. Agric. Food Chem. 2014, 62, 10816–10827. [Google Scholar] [CrossRef]
- Galaon, T.; David, V. Deviation from van’t Hoff dependence in RP-LC induced by tautomeric interconversion observed for four compounds. J. Sep. Sci. 2011, 34, 1423–1428. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Characteristics of bovine lactoferrin powders produced through spray and freeze drying processes. Int. J. Biol. Macromol. 2017, 95, 985–994. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Abdelmoneem, M.A.; Hassanin, I.A.; Elwakil, M.M.A.; Elnaggar, M.A.; Mokhtar, S.; Fang, J.Y.; Elkhodairy, K.A. Lactoferrin, a multi-functional glycoprotein: Active therapeutic, drug nanocarrier & targeting ligand. Biomaterials 2020, 263, 120355. [Google Scholar]
- González-Chávez, S.A.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin: Structure, function and applications. Int. J. Antimicrob. Agents 2009, 33, e301–e308. [Google Scholar] [CrossRef]
- Von Staszewski, M.; Jara, F.L.; Ruiz, A.L.; Jagus, R.J.; Carvalho, J.E.; Pilosof, A.M. Nanocomplex formation between β-lactoglobulin or caseinomacropeptide and green tea polyphenols: Impact on protein gelation and polyphenols antiproliferative activity. J. Funct. Foods 2012, 4, 800–809. [Google Scholar] [CrossRef]
- Liu, F.; Wang, D.; Ma, C.; Gao, Y. Conjugation of polyphenols prevents lactoferrin from thermal aggregation at neutral pH. Food Hydrocoll. 2016, 58, 49–59. [Google Scholar] [CrossRef]
- Pryshchepa, O.; Rafińska, K.; Gołębiowski, A.; Sugajski, M.; Sagandykova, G.; Madajski, P.; Buszewski, B.; Pomastowski, P. Synthesis and physicochemical characterization of bovine lactoferrin supersaturated complex with iron (III) ions. Sci. Rep. 2022, 12, 12695. [Google Scholar] [CrossRef]
- Reddy, V.C.; Sagar, G.V.V.; Sreeramulu, D.; Venu, L.; Raghunath, M. Addition of milk does not alter the antioxidant activity of black tea. Ann. Nutr. Metab. 2005, 49, 189–195. [Google Scholar] [CrossRef]
- Zapata, R.C.; Singh, A.; Pezeshki, A.; Nibber, T.; Chelikani, P.K. Whey protein components-lactalbumin and lactoferrin-improve energy balance and metabolism. Sci. Rep. 2017, 7, 9917. [Google Scholar] [CrossRef]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, function, denaturation and digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Yan, X.; McClements, D.J.; Ma, C.; Liu, X.; Liu, F. Ultrasound-assisted preparation of lactoferrin-EGCG conjugates and their application in forming and stabilizing algae oil emulsions. Ultrason. Sonochem. 2022, 89, 106110. [Google Scholar] [CrossRef]
- Song, W.Y.; Aihara, Y.; Hashimoto, T.; Kanazawa, K.; Mizuno, M. (−)-Epigallocatechin-3-gallate induces secretion of anorexigenic gut hormones. J. Clin. Biochem. Nutr. 2015, 57, 164–169. [Google Scholar] [CrossRef]
- Arts, M.J.; Haenen, G.R.; Wilms, L.C.; Beetstra, S.A.; Heijnen, C.G.; Voss, H.P.; Bast, A. Interactions between flavonoids and proteins: Effect on the total antioxidant capacity. J. Agric. Food Chem. 2002, 50, 1184–1187. [Google Scholar] [CrossRef]
- Kartsova, L.; Alekseeva, A. Effect of milk caseins on the concentration of polyphenolic compounds in tea. J. Anal. Chem. 2008, 63, 1107–1111. [Google Scholar] [CrossRef]
- Serafini, M.; Ghiselli, A.; Ferro-Luzzi, A. In vivo antioxidant effect of green and black tea in man. Eur. J. Clin. Nutr. 1996, 50, 28–32. [Google Scholar]
- Van het Hof, K.H.; Kivits, G.A.; Weststrate, J.A.; Tijburg, L.B. Bioavailability of catechins from tea: The effect of milk. Eur. J. Clin. Nutr. 1998, 52, 356–359. [Google Scholar] [CrossRef]
- Hirun, S.; Roach, P. A study of stability of (−)-epigallocatechin gallate (EGCG) from green tea in a frozen product. Int. Food Res. J. 2011, 18, 1261. [Google Scholar]
- Radhakrishnan, R.; Kulhari, H.; Pooja, D.; Gudem, S.; Bhargava, S.; Shukla, R.; Sistla, R. Encapsulation of biophenolic phytochemical EGCG within lipid nanoparticles enhances its stability and cytotoxicity against cancer. Chem. Phys. Lipids 2016, 198, 51–60. [Google Scholar] [CrossRef]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Mild thermal treatment and in-vitro digestion of three forms of bovine lactoferrin: Effects on functional properties. Int. Dairy J. 2017, 64, 22–30. [Google Scholar] [CrossRef]
- Shpigelman, A.; Israeli, G.; Livney, Y.D. Thermally-induced protein–polyphenol co-assemblies: Beta lactoglobulin-based nanocomplexes as protective nanovehicles for EGCG. Food Hydrocoll. 2010, 24, 735–743. [Google Scholar] [CrossRef]
- Tsai, P.J.; She, C.H. Significance of phenol−protein interactions in modifying the antioxidant capacity of peas. J. Agric. Food Chem. 2006, 54, 8491–8494. [Google Scholar] [CrossRef]
- Kuznetsova, I.M.; Turoverov, K.K.; Uversky, V.N. Use of the phase diagram method to analyze the protein unfolding-refolding reactions: Fishing out the “invisible” intermediates. J. Proteome Res. 2004, 3, 485–494. [Google Scholar] [CrossRef]
- Mariam, J.; Dongre, P.M.; Kothari, D.C. Study of interaction of silver nanoparticles with bovine serum albumin using fluorescence spectroscopy. J. Fluoresc. 2011, 21, 2193–2199. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Q.; Wang, F.; Yuan, L.; Xu, Z.; Jiang, F.; Liu, Y. Comparison of interactions between human serum albumin and silver nanoparticles of different sizes using spectroscopic methods. Luminescence 2015, 30, 397–404. [Google Scholar] [CrossRef]
- De Paula Rezende, J.; Hudson, E.A.; de Paula, H.M.C.; Coelho, Y.L.; da Silva, L.H.M.; dos Santos Pires, A.C. Thermodynamic and kinetic study of epigallocatechin-3-gallate-bovine lactoferrin complex formation determined by surface plasmon resonance (SPR): A comparative study with fluorescence spectroscopy. Food Hydrocoll. 2019, 95, 526–532. [Google Scholar] [CrossRef]
- Shim, J.H.; Su, Z.Y.; Chae, J.I.; Kim, D.J.; Zhu, F.; Ma, W.Y.; Bode, A.M.; Yang, C.S.; Dong, Z. Epigallocatechin gallate suppresses lung cancer cell growth through Ras–GTPase-activating protein SH3 domain-binding protein 1. Cancer Prev. Res. 2010, 3, 670–679. [Google Scholar] [CrossRef]
- Redwanel, R.M.; Matar, S.M.; El-Aziz, G.A.; Serour, E.A. Synthesis of the human insulin gene: Protein expression, scaling up and bioactivity. Prep. Biochem. Biotechnol. 2008, 38, 24–39. [Google Scholar] [CrossRef]
- Reimer, R.A.; Darimont, C.; Gremlich, S.; Nicolas-Métral, V.; Ruëgg, U.T.; Macé, K. A human cellular model for studying the regulation of glucagon-like peptide-1 secretion. Endocrinology 2001, 142, 522–524. [Google Scholar] [CrossRef]
- Sangle, G.V.; Lauffer, L.M.; Grieco, A.; Trivedi, S.; Iakoubov, R.; Brubaker, P.L. Novel biological action of the dipeptidylpeptidase-IV inhibitor, sitagliptin, as a glucagon-like peptide-1 secretagogue. Endocrinology 2012, 153, 564–573. [Google Scholar] [CrossRef]
- Le Nevé, B.; Daniel, H. Selected tetrapeptides lead to a GLP-1 release from the human enteroendocrine cell line NCI-H716. Regul. Pept. 2011, 167, 14–20. [Google Scholar] [CrossRef]




| Sample | Apo-LF (10 µM) | Apo-LF-EGCG Conjugates (µM) | |||||
|---|---|---|---|---|---|---|---|
| 15.63 | 31.25 | 62.50 | 125 | 250 | 500 | ||
| α-helix | 30.7% | 31.33% | 30.45% | 29.29% | 23.28% | 20.53% | 22.63% |
| β-strand | 20.23% | 18.07% | 19.06% | 20.34% | 26.77% | 30.11% | 27.61% |
| Temperature (°C) | Kq (×1012) | Ksv (×104 M−1) | K (×102 M−1) | ΔG° (kJmol−1) | ΔH° (kJmol−1) | ΔS° (Jmol−1K−1 × 1013) |
|---|---|---|---|---|---|---|
| 10 | 3.16 | 3.16 | 22.08 | −20.62 | 4.56 | 3.76 |
| 20 | 3.18 | 3.18 | 29.14 | −21.51 | ||
| 30 | 3.01 | 3.01 | 35.92 | −22.40 | ||
| 40 | 3.03 | 3.03 | 34.64 | −23.29 | ||
| 50 | 2.92 | 2.92 | 25.86 | −24.18 | ||
| 60 | 2.82 | 2.82 | 20.71 | −25.07 | ||
| 70 | 2.89 | 2.89 | 17.32 | −25.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wazzan, H.A.; Abraham, A.N.; Saiara, N.; Anand, S.; Gill, H.; Shukla, R. Effect of Milk Protein–Polyphenol Conjugate on the Regulation of GLP-1 Hormone. Foods 2024, 13, 1935. https://doi.org/10.3390/foods13121935
Wazzan HA, Abraham AN, Saiara N, Anand S, Gill H, Shukla R. Effect of Milk Protein–Polyphenol Conjugate on the Regulation of GLP-1 Hormone. Foods. 2024; 13(12):1935. https://doi.org/10.3390/foods13121935
Chicago/Turabian StyleWazzan, Huda Abdulrahim, Amanda N. Abraham, Noshin Saiara, Sushil Anand, Harsharn Gill, and Ravi Shukla. 2024. "Effect of Milk Protein–Polyphenol Conjugate on the Regulation of GLP-1 Hormone" Foods 13, no. 12: 1935. https://doi.org/10.3390/foods13121935
APA StyleWazzan, H. A., Abraham, A. N., Saiara, N., Anand, S., Gill, H., & Shukla, R. (2024). Effect of Milk Protein–Polyphenol Conjugate on the Regulation of GLP-1 Hormone. Foods, 13(12), 1935. https://doi.org/10.3390/foods13121935







