Comparative 1H NMR-Based Metabolomics of Traditional Landrace and Disease-Resistant Chili Peppers (Capsicum annuum L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Source and Sample Preparation
2.2. 1H NMR Spectroscopic Analysis of Chili Pepper Extracts
2.3. NMR Data Processing and Multivariate Statistical Analysis
2.4. Statistical Analysis
3. Results and Discussion
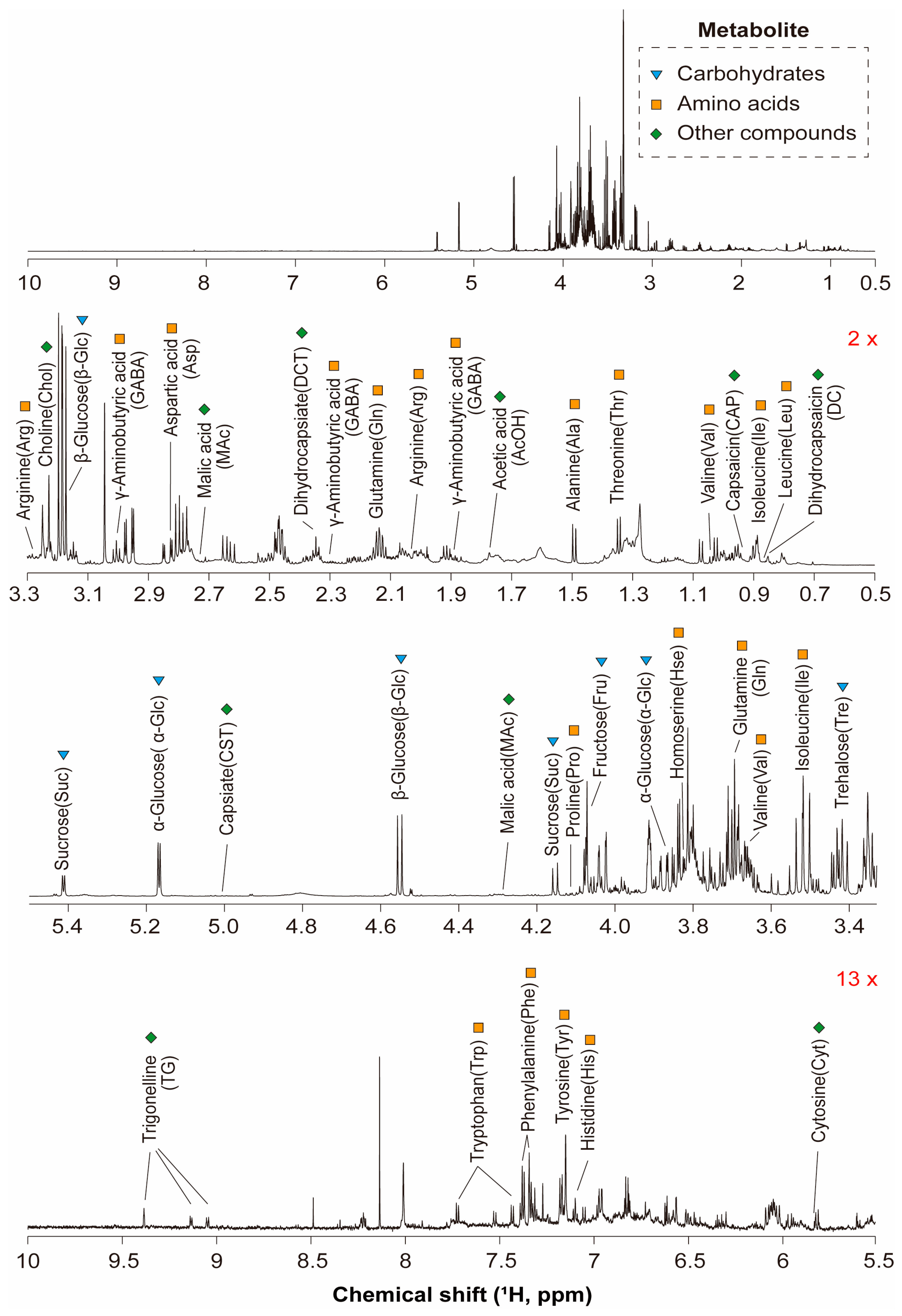
3.1. Identification of Metabolites in the Chili Pepper Extracts by 1D and 2D NMR Spectroscopy
3.2. Multivariate Statistical Analysis of the 1H NMR Spectra
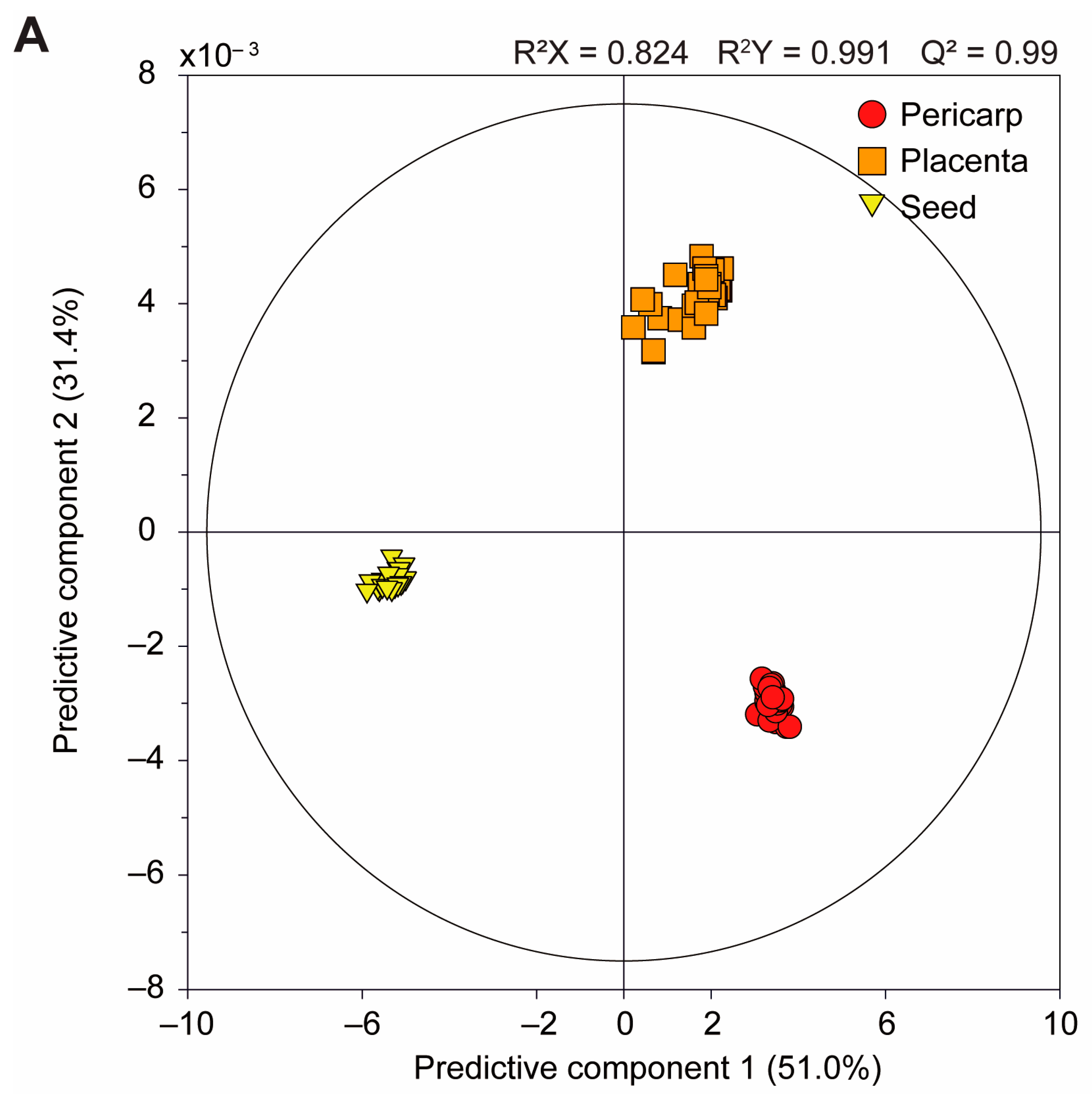
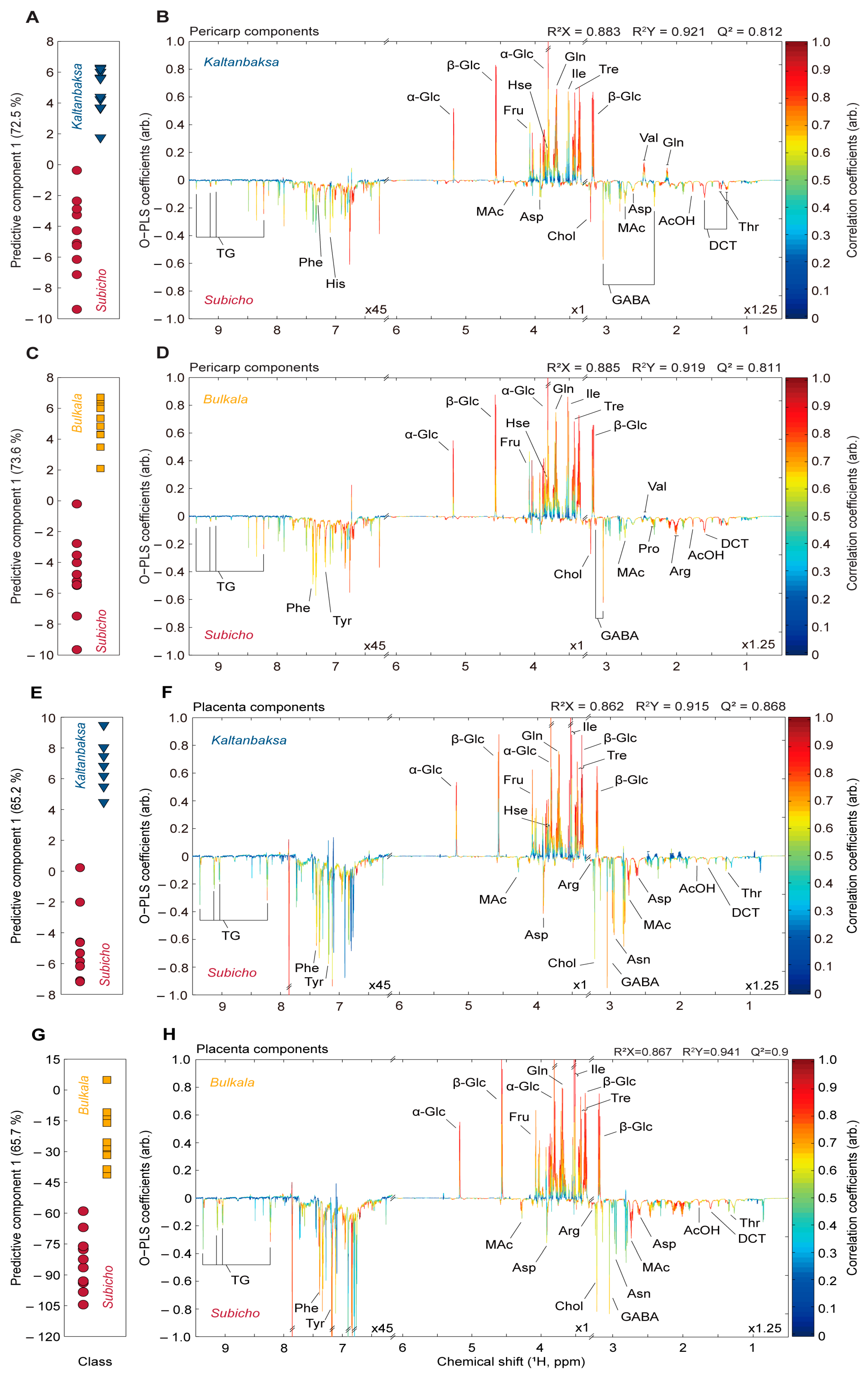
3.2.1. Comparison of the Pericarp, Placenta, and Seed Components
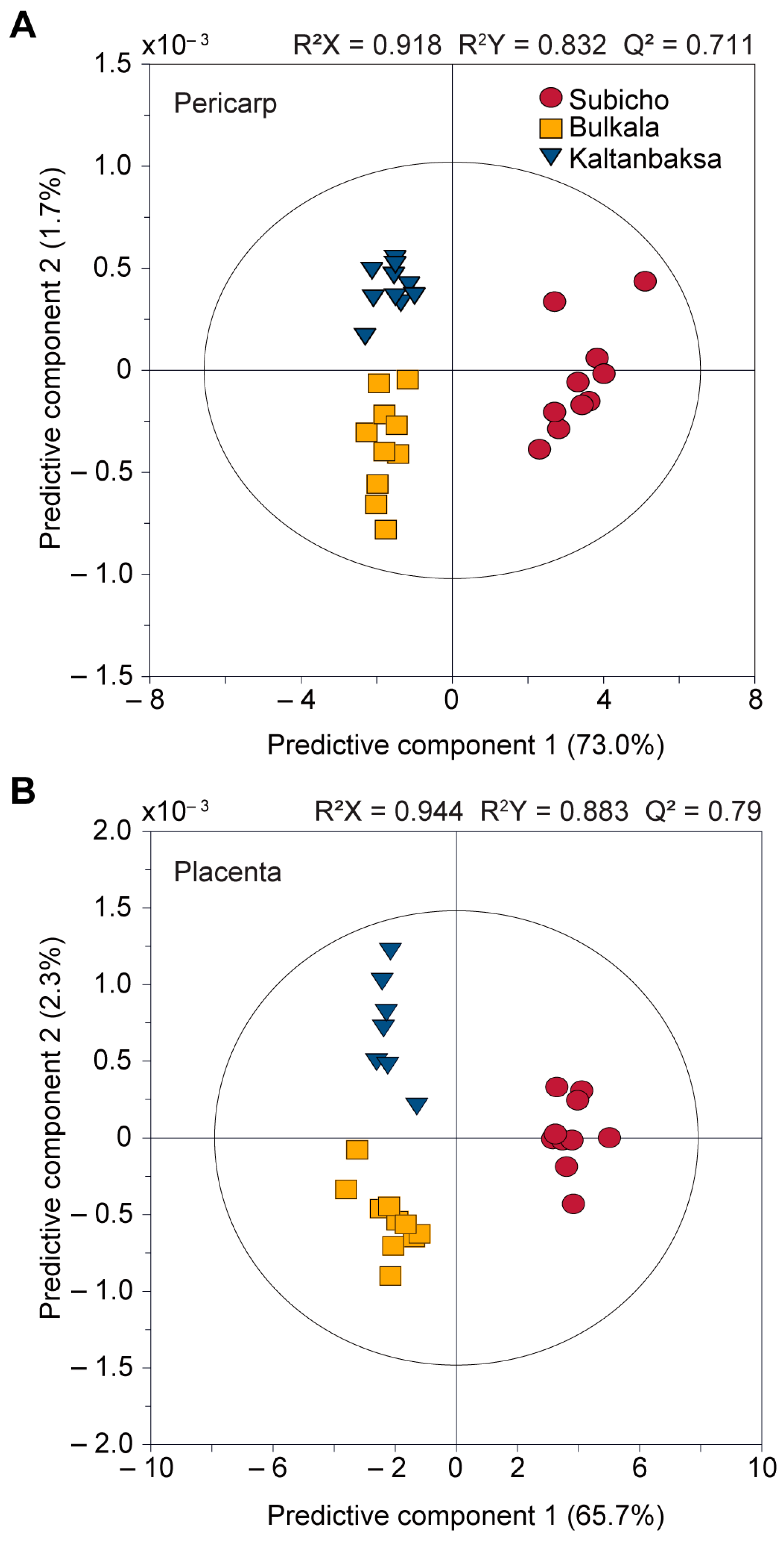
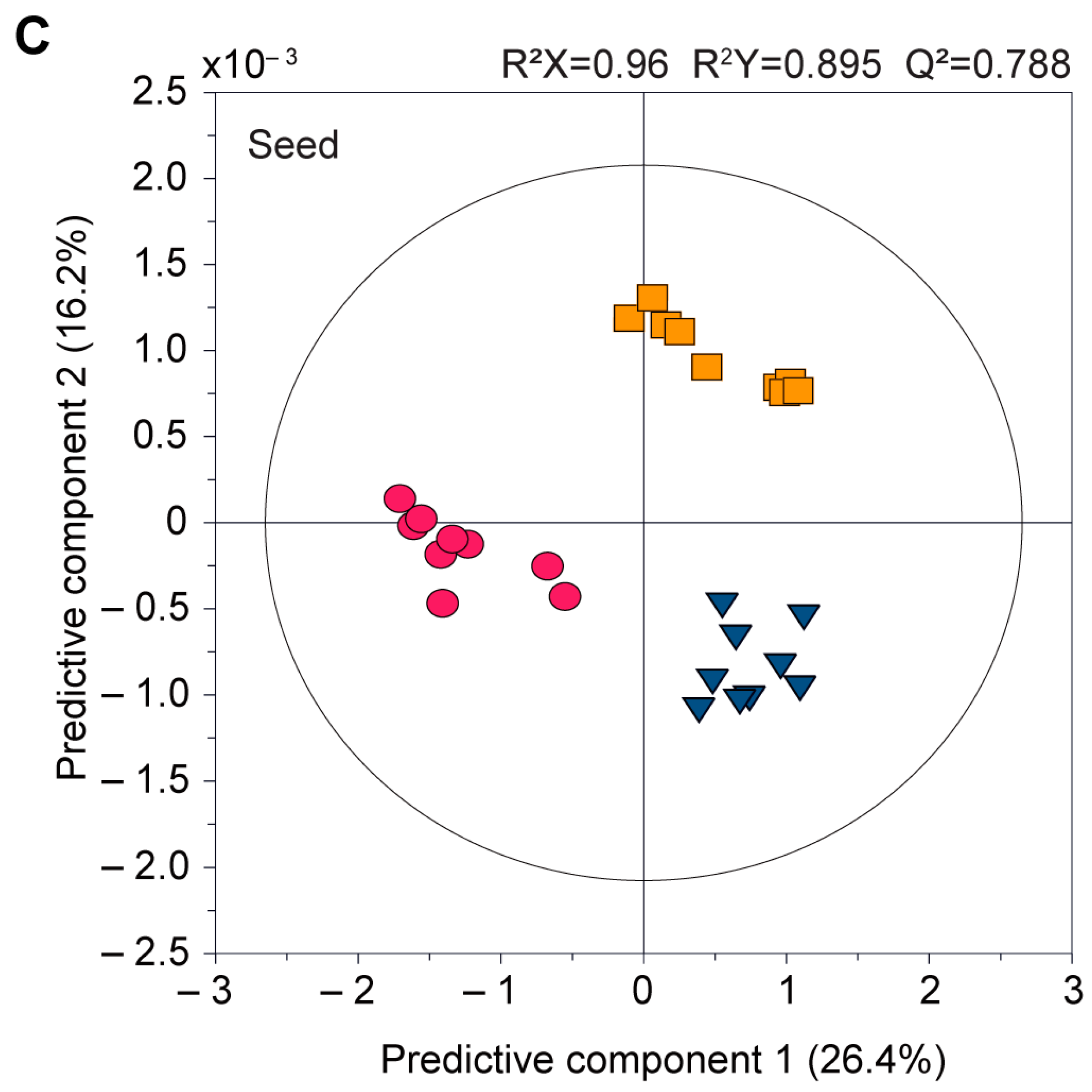
3.2.2. Comparison of the Pericarp, Placenta, and Seed Components of the Cultivars
3.2.3. Comparison of the Landrace and Disease-Resistant Cultivars Based on the Pericarp and Placenta Components
3.3. Relative Amounts of Different Metabolites Based on 1H NMR Spectroscopy
3.3.1. Comparison of the Pericarp, Placenta, and Seed Components
3.3.2. Comparison of the Components in the Pericarp and Placenta of the Cultivars
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moon, S.; Ro, N.; Kim, J.; Ko, H.-C.; Lee, S.; Oh, H.; Kim, B.; Lee, H.-S.; Lee, G.-A. Characterization of diverse pepper (Capsicum spp.) germplasms based on agro-morphological traits and phytochemical contents. Agronomy 2023, 13, 2665. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Jang, D.-J.; Yang, H.J.; Chung, K.R. History of Korean gochu, gochujang, and kimchi. J. Ethn. Foods 2014, 1, 3–7. [Google Scholar] [CrossRef]
- Fattori, V.; Hohmann, M.S.N.; Rossaneis, A.C.; Pinho-Ribeiro, F.A.; Verri, W.A. Capsaicin: Current understanding of its mechanisms and therapy of pain and other pre-clinical and clinical uses. Molecules 2016, 21, 844. [Google Scholar] [CrossRef] [PubMed]
- Azlan, A.; Sultana, S.; Huei, C.S.; Razman, M.R. Antioxidant, anti-obesity, nutritional and other beneficial effects of different chili pepper: A review. Molecules 2022, 27, 898. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-Y.; Cho, J.-S.; Park, K.J.; Choi, J.H.; Lim, J.H. Effect of Moisture Content Difference on the Analysis of Quality Attributes of Red Pepper (Capsicum annuum L.) Powder Using a Hyperspectral System. Foods 2022, 11, 4086. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Bordoh, P.K.; Singh, A.; Siddiqui, Y.; Droby, S. Post-harvest development of anthracnose in pepper (Capsicum spp.): Etiology and management strategies. Crop Prot. 2016, 90, 132–141. [Google Scholar] [CrossRef]
- Kim, D.; Riu, M.; Oh, S.-K.; Ryu, C.-M. Extracellular self-RNA: A danger elicitor in pepper induces immunity against bacterial and viral pathogens in the field. Front. Plant Sci. 2022, 13, 864086. [Google Scholar] [CrossRef] [PubMed]
- Mohan Rao, A.; Anilkumar, C. Conventional and contemporary approaches to enhance efficiency in breeding chilli/hot pepper. In Accelerated Plant Breeding; Springer: Cham, Switzerland, 2020; Volume 2, pp. 223–269. [Google Scholar]
- Kim, H.K.; Choi, Y.H.; Verpoorte, R. NMR-based metabolomic analysis of plants. Nat. Protoc. 2010, 5, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Choi, Y.H.; Verpoorte, R. NMR-based plant metabolomics: Where do we stand, where do we go? Trends Biotechnol. 2011, 29, 267–275. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.-M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Kim, N.; Kim, K.; Choi, B.Y.; Lee, D.; Shin, Y.-S.; Bang, K.-H.; Cha, S.-W.; Lee, J.W.; Choi, H.-K.; Jang, D.S.; et al. Metabolomic approach for age discrimination of Panax ginseng using UPLC-Q-Tof MS. J. Agric. Food Chem. 2011, 59, 10435–10441. [Google Scholar] [CrossRef] [PubMed]
- Raveendar, S.; Jeon, Y.-A.; Lee, J.-R.; Lee, G.-A.; Lee, K.J.; Cho, G.-T.; Ma, K.-H.; Lee, S.-Y.; Chung, J.-W. The complete chloroplast genome sequence of Korean landrace “Subicho” pepper (Capsicum annuum var. annuum). Plant Breed. Biotechnol. 2015, 3, 88–94. [Google Scholar]
- Ro, N.; Haile, M.; Hur, O.; Ko, H.-C.; Yi, J.-Y.; Woo, H.-J.; Choi, Y.-M.; Rhee, J.; Lee, Y.-J.; Kim, D.-A.; et al. Genome-wide association study of resistance to anthracnose in pepper (Capsicum chinense) germplasm. BMC Plant Biol. 2023, 23, 389. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.-Y.; Kang, Y.-G.; Lee, E.J.; Kim, D.; Kim, E.-H.; Hong, Y.-S. Metabolomics study for exploring metabolic perturbations in soybean adventitious roots by fluorescent light irradiation. Appl. Biol. Chem. 2021, 64, 26. [Google Scholar] [CrossRef]
- Engskog, M.K.R.; Ersson, L.; Haglöf, J.; Arvidsson, T.; Pettersson, C.; Brittebo, E. β-N-Methylamino-l-alanine (BMAA) perturbs alanine, aspartate and glutamate metabolism pathways in human neuroblastoma cells as determined by metabolic profiling. Amino Acids 2017, 49, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Savorani, F.; Tomasi, G.; Engelsen, S.B. icoshift: A versatile tool for the rapid alignment of 1D NMR spectra. J. Magn. Reson. 2010, 202, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, G.; van den Berg, F.; Andersson, C. Correlation optimized warping and dynamic time warping as preprocessing methods for chromatographic data. J. Chemom. 2004, 18, 231–241. [Google Scholar] [CrossRef]
- Cervantes-Hernández, F.; Alcalá-González, P.; Martínez, O.; Ordaz-Ortiz, J.J. Placenta, pericarp, and seeds of tabasco chili pepper fruits show a contrasting diversity of bioactive metabolites. Metabolites 2019, 9, 206. [Google Scholar] [CrossRef]
- Kong, X.-M.; Ge, W.-Y.; Wei, B.-D.; Zhou, Q.; Zhou, X.; Zhao, Y.-B.; Ji, S.-J. Melatonin ameliorates chilling injury in green bell peppers during storage by regulating membrane lipid metabolism and antioxidant capacity. Postharvest Biol. Technol. 2020, 170, 111315. [Google Scholar] [CrossRef]
- Zamljen, T.; Jakopič, J.; Hudina, M.; Veberič, R.; Slatnar, A. Influence of intra and inter species variation in chilies (Capsicum spp.) on metabolite composition of three fruit segments. Sci. Rep. 2021, 11, 4932. [Google Scholar] [CrossRef]
- Rodrigues-Salvador, A.; Lana-Costa, J.; Omena-Garcia, R.P.; Batista-Silva, W.; Scossa, F.; Rosado-Souza, L.; Pérez-Díaz, J.L.; Menezes-Silva, P.E.; DaMatta, F.M.; Sulpice, R.; et al. Metabolic shifts during fruit development in pungent and non-pungent peppers. Food Chem. 2022, 375, 131850. [Google Scholar] [CrossRef] [PubMed]
- Snowden, C.J.; Thomas, B.; Baxter, C.J.; Smith, J.A.C.; Sweetlove, L.J. A tonoplast Glu/Asp/GABA exchanger that affects tomato fruit amino acid composition. Plant J. 2015, 81, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Chaban, I.A.; Gulevich, A.A.; Baranova, E.N. Formation of unique placental seed capsules in the maturation process of the tomato fruit. Int. J. Mol. Sci. 2022, 23, 11101. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, L.H. Red pepper (Capsicum spp.) fruit: A model for the study of secondary metabolite product distribution and its management. In AIP Conference Proceedings; AIP Publishing: New York, NY, USA, 2016; p. 020034. [Google Scholar]
- Palma, J.M.; Terán, F.; Contreras-Ruiz, A.; Rodríguez-Ruiz, M.; Corpas, F.J. Antioxidant profile of pepper (Capsicum annuum L.) fruits containing diverse levels of capsaicinoids. Antioxidants 2020, 9, 878. [Google Scholar] [CrossRef] [PubMed]
- Barchenger, D.W.; Bosland, P.W. Exogenous applications of capsaicin inhibits seed germination of Capsicum annuum. Sci. Hortic. 2016, 203, 29–31. [Google Scholar] [CrossRef]
- Silva, L.R.; Azevedo, J.; Pereira, M.J.; Valentão, P.; Andrade, P.B. Chemical assessment and antioxidant capacity of pepper (Capsicum annuum L.) seeds. Food Chem. Toxicol. 2013, 53, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Amir, R.; Galili, G.; Cohen, H. The metabolic roles of free amino acids during seed development. Plant Sci. 2018, 275, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Moreno Curtidor, C.; Annunziata, M.G.; Gupta, S.; Apelt, F.; Richard, S.I.; Kragler, F.; Mueller-Roeber, B.; Olas, J.J. Physiological profiling of embryos and dormant seeds in two arabidopsis accessions reveals a metabolic switch in carbon reserve accumulation. Front. Plant Sci. 2020, 11, 588433. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hao, R.; Chen, W.; Jia, H.; Qin, S.; Wang, Q.; Zhang, D.; Han, Z.; Li, Y. Effect of choline amino acid ionic liquids on maize seed germination and endogenous plant hormone levels. RSC Adv. 2024, 14, 382–389. [Google Scholar] [CrossRef]
- Bolton, M.D. Primary metabolism and plant defense—Fuel for the fire. Mol. Plant-Microbe Interact. 2009, 22, 487–497. [Google Scholar] [CrossRef]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef]
- Gebauer, P.; Korn, M.; Engelsdorf, T.; Sonnewald, U.; Koch, C.; Voll, L.M. Sugar accumulation in leaves of arabidopsis sweet11/sweet12 double mutants enhances priming of the salicylic acid-mediated defense response. Front. Plant Sci. 2017, 8, 1378. [Google Scholar] [CrossRef] [PubMed]
- Bezrutczyk, M.; Yang, J.; Eom, J.-S.; Prior, M.; Sosso, D.; Hartwig, T.; Szurek, B.; Oliva, R.; Vera-Cruz, C.; White, F.F.; et al. Sugar flux and signaling in plant–microbe interactions. Plant J. 2018, 93, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Rojas, C.; Senthil-Kumar, M.; Tzin, V.; Mysore, K. Regulation of primary plant metabolism during plant-pathogen interactions and its contribution to plant defense. Front. Plant Sci. 2014, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Ratajczak, L. The role of sugar signaling in plant defense responses against fungal pathogens. Acta Physiol. Plant. 2014, 36, 1607–1619. [Google Scholar] [CrossRef]
- Krasavina, M.S.; Burmistrova, N.A.; Raldugina, G.N. The role of carbohydrates in plant resistance to abiotic stresses. In Emerging Technologies and Management of Crop Stress Tolerance; Ahmad, P., Rasool, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 229–270. [Google Scholar]
- Liu, G.; Ji, Y.; Bhuiyan, N.H.; Pilot, G.; Selvaraj, G.; Zou, J.; Wei, Y. Amino acid homeostasis modulates salicylic acid–associated redox status and defense responses in Arabidopsis. Plant Cell 2010, 22, 3845–3863. [Google Scholar] [CrossRef]
- Zeier, J. New insights into the regulation of plant immunity by amino acid metabolic pathways. Plant Cell Environ. 2013, 36, 2085–2103. [Google Scholar] [CrossRef] [PubMed]
- Fabro, G.; Kovács, I.; Pavet, V.; Szabados, L.; Alvarez, M.E. Proline accumulation and AtP5CS2 gene activation are induced by plant-pathogen incompatible interactions in Arabidopsis. Mol. Plant-Microbe Interact. 2004, 17, 343–350. [Google Scholar] [CrossRef]
- Alvarez, M.E.; Savouré, A.; Szabados, L. Proline metabolism as regulatory hub. Trends Plant Sci. 2022, 27, 39–55. [Google Scholar] [CrossRef]
- Qamar, A.; Mysore, K.; Senthil-Kumar, M. Role of proline and pyrroline-5-carboxylate metabolism in plant defense against invading pathogens. Front. Plant Sci. 2015, 6, 503. [Google Scholar] [CrossRef] [PubMed]
- Kavi Kishor, P.B.; Sreenivasulu, N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, L.; Zhao, R.; Li, R.; Zhang, S.; Yu, W.; Sheng, J.; Shen, L. Melatonin induces disease resistance to Botrytis cinerea in tomato fruit by activating jasmonic acid signaling pathway. J. Agric. Food Chem. 2019, 67, 6116–6124. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.-S.; Duan, C.-G. Epigenetic regulation of plant immunity: From chromatin codes to plant disease resistance. aBIOTECH 2023, 4, 124–139. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seong, G.-U.; Yun, D.-Y.; Shin, D.-H.; Cho, J.-S.; Lee, G.; Choi, J.H.; Park, K.-J.; Ku, K.-H.; Lim, J.-H. Comparative 1H NMR-Based Metabolomics of Traditional Landrace and Disease-Resistant Chili Peppers (Capsicum annuum L.). Foods 2024, 13, 1966. https://doi.org/10.3390/foods13131966
Seong G-U, Yun D-Y, Shin D-H, Cho J-S, Lee G, Choi JH, Park K-J, Ku K-H, Lim J-H. Comparative 1H NMR-Based Metabolomics of Traditional Landrace and Disease-Resistant Chili Peppers (Capsicum annuum L.). Foods. 2024; 13(13):1966. https://doi.org/10.3390/foods13131966
Chicago/Turabian StyleSeong, Gi-Un, Dae-Yong Yun, Dong-Hyeok Shin, Jeong-Seok Cho, Gyuseok Lee, Jeong Hee Choi, Kee-Jai Park, Kyung-Hyung Ku, and Jeong-Ho Lim. 2024. "Comparative 1H NMR-Based Metabolomics of Traditional Landrace and Disease-Resistant Chili Peppers (Capsicum annuum L.)" Foods 13, no. 13: 1966. https://doi.org/10.3390/foods13131966




