Fermented Gastrodia elata Bl. Alleviates Cognitive Deficits by Regulating Neurotransmitters and Gut Microbiota in D-Gal/AlCl3-Induced Alzheimer’s Disease-like Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of FGE and Experimental Animals
2.1.1. Preparation of FGE
2.1.2. Animal Experiments
2.2. Behavioral Tests
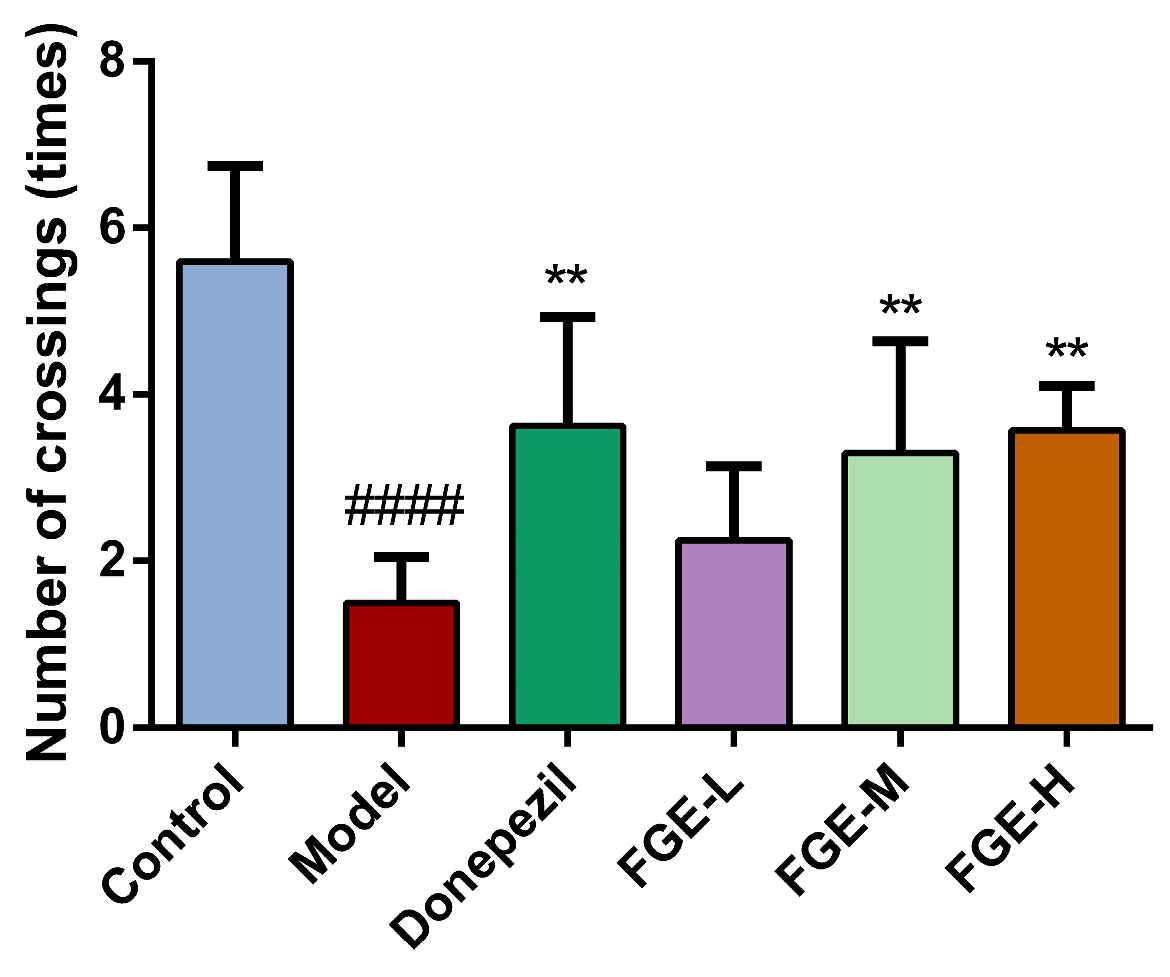
2.2.1. Shuttle Box Test
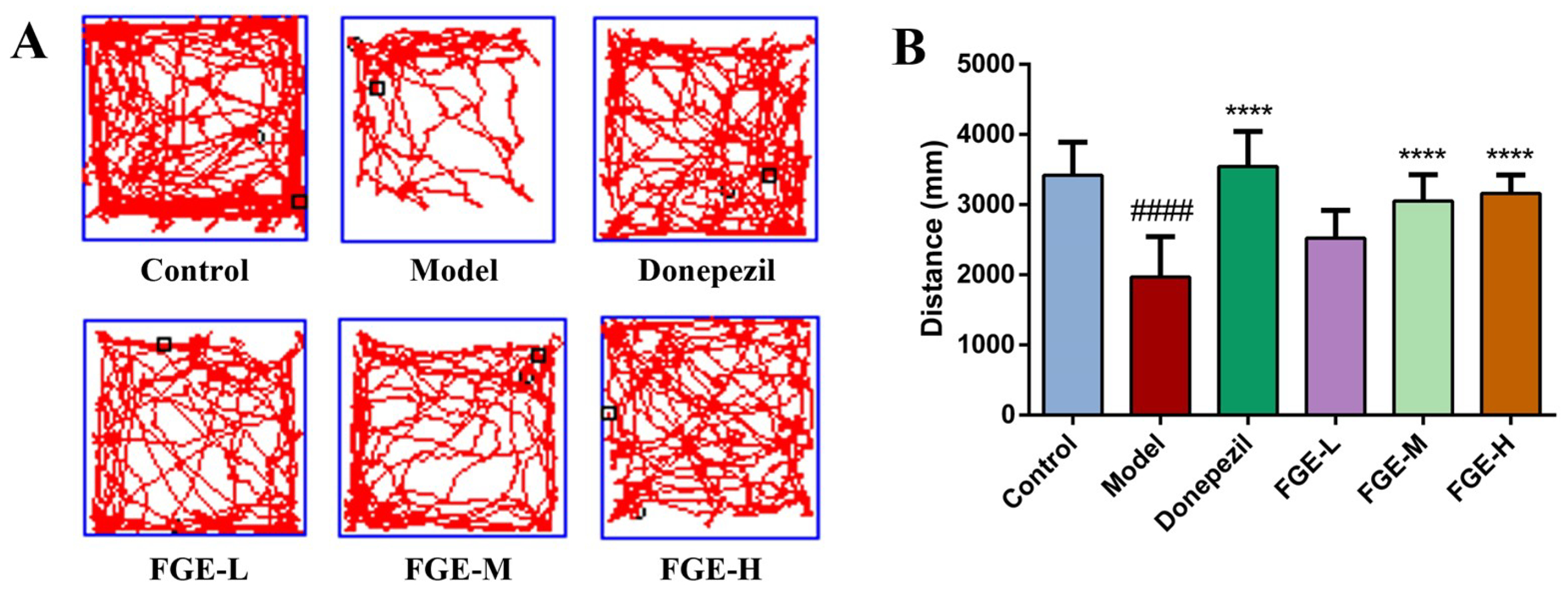
2.2.2. Open Field Test
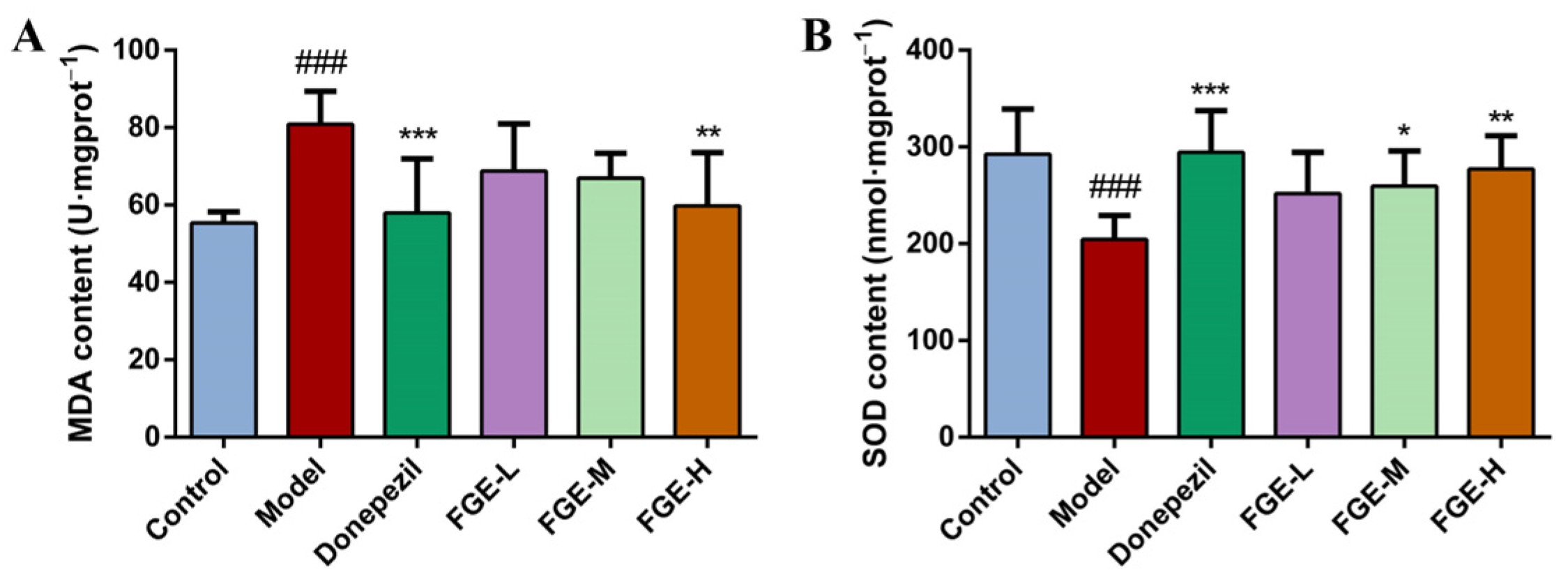
2.3. Determination of Neurotransmitters and Oxidative Stress Indexes
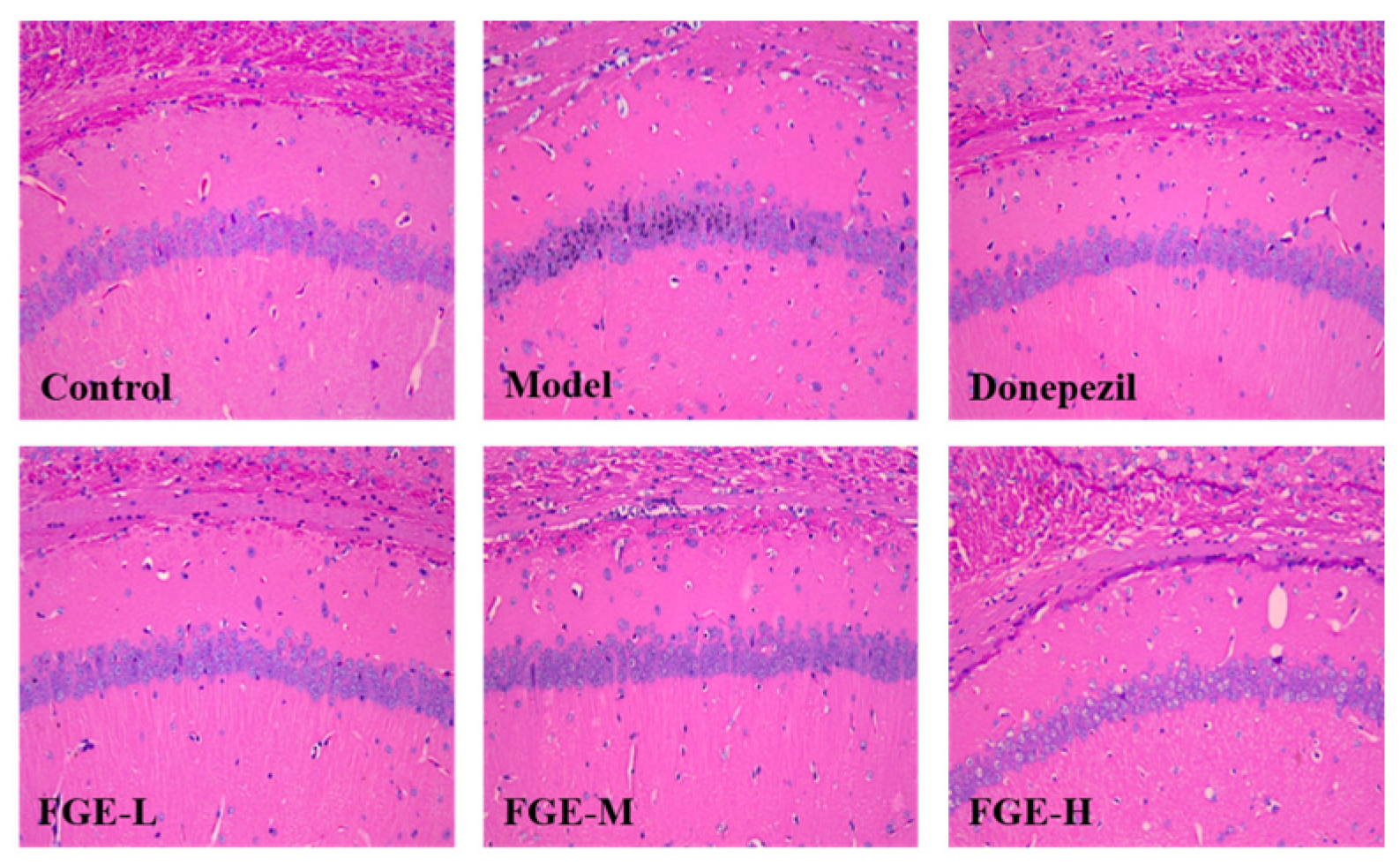
2.4. H&E Staining
2.5. Immunohistochemistry Analysis
2.6. Gut Microbiota DNA Extraction and Analysis of Gut Microbiota
2.7. UPLC-MS Analysis
2.8. Statistical Analysis
3. Results
3.1. Identification of Main Components in FGE
3.2. The Effect of FGE on Cognitive Function in AD-like Mice
3.3. The Effect of FGE on Anxiety-like Behavior in AD-like Mice
3.4. The Effect of FGE on the Levels of Neurotransmitters in Brain Tissue of AD-like Mice
3.5. The Effect of FGE on the Content of Antioxidants in AD-like Mice
3.6. The Effect of FGE on the Damage of Neuronal Cells in the Hippocampus of AD-like Mice
3.7. The Effect of FGE on the Protein Expression of Aβ in the Hippocampus of AD-like Mice
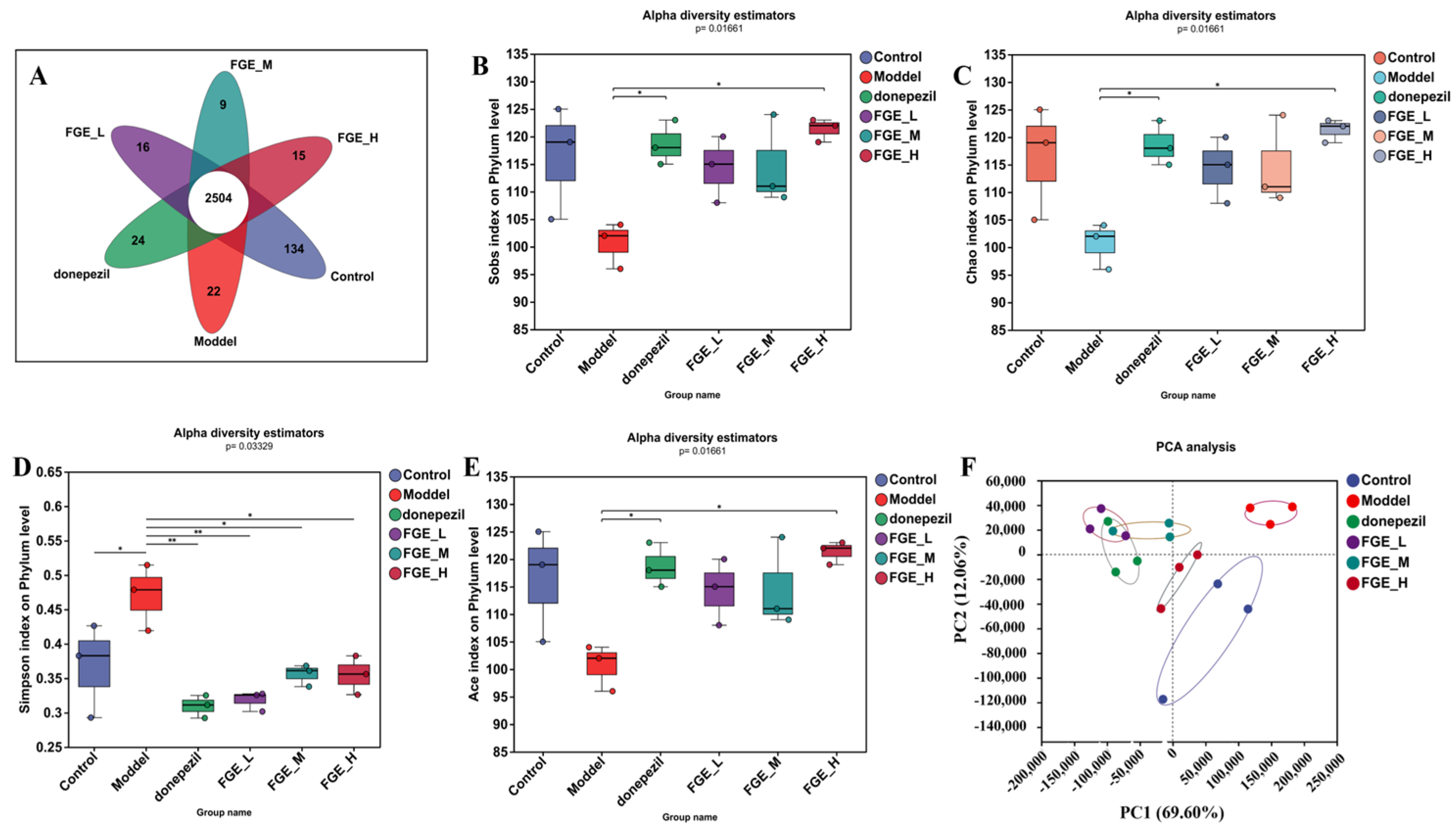
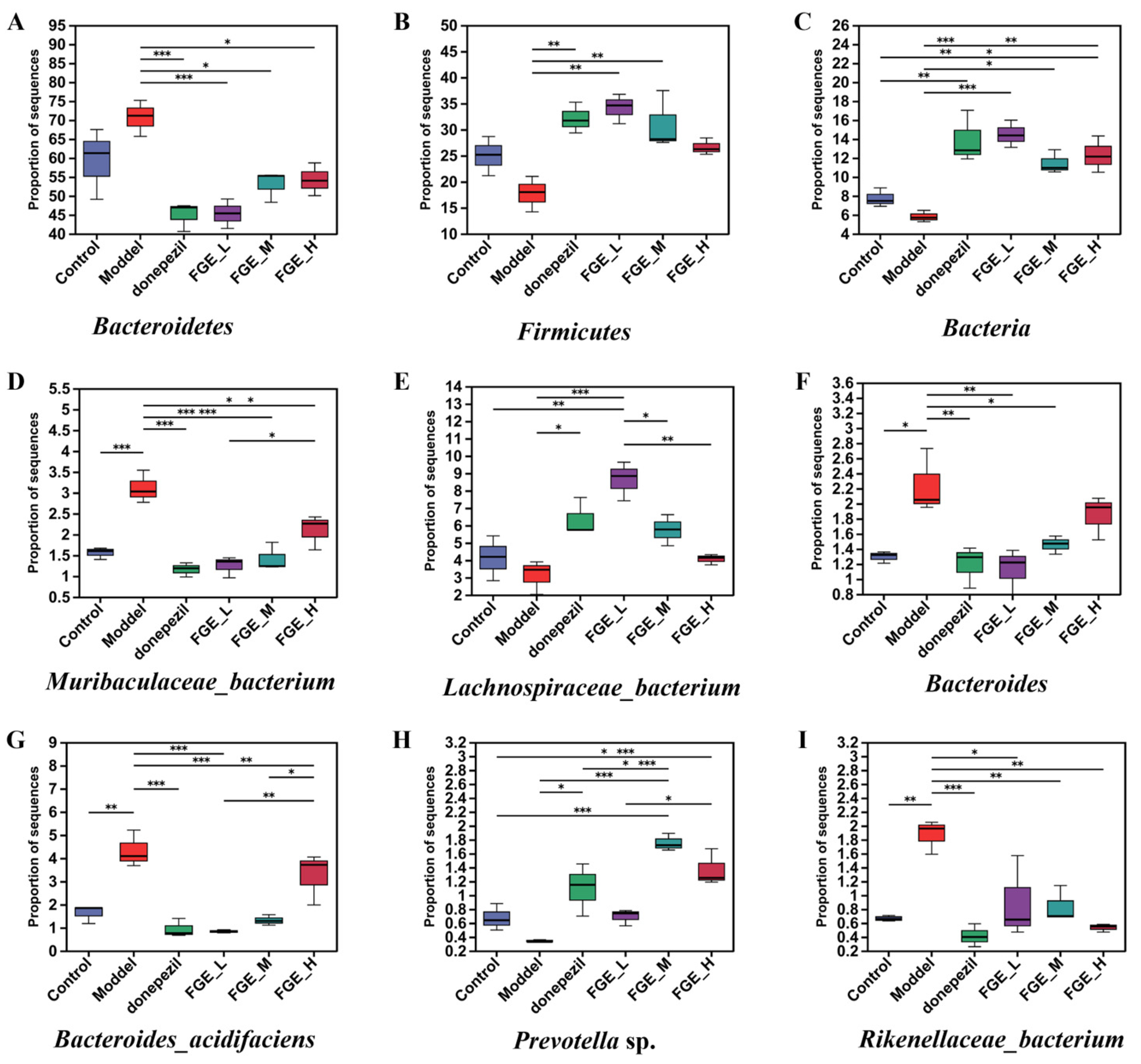
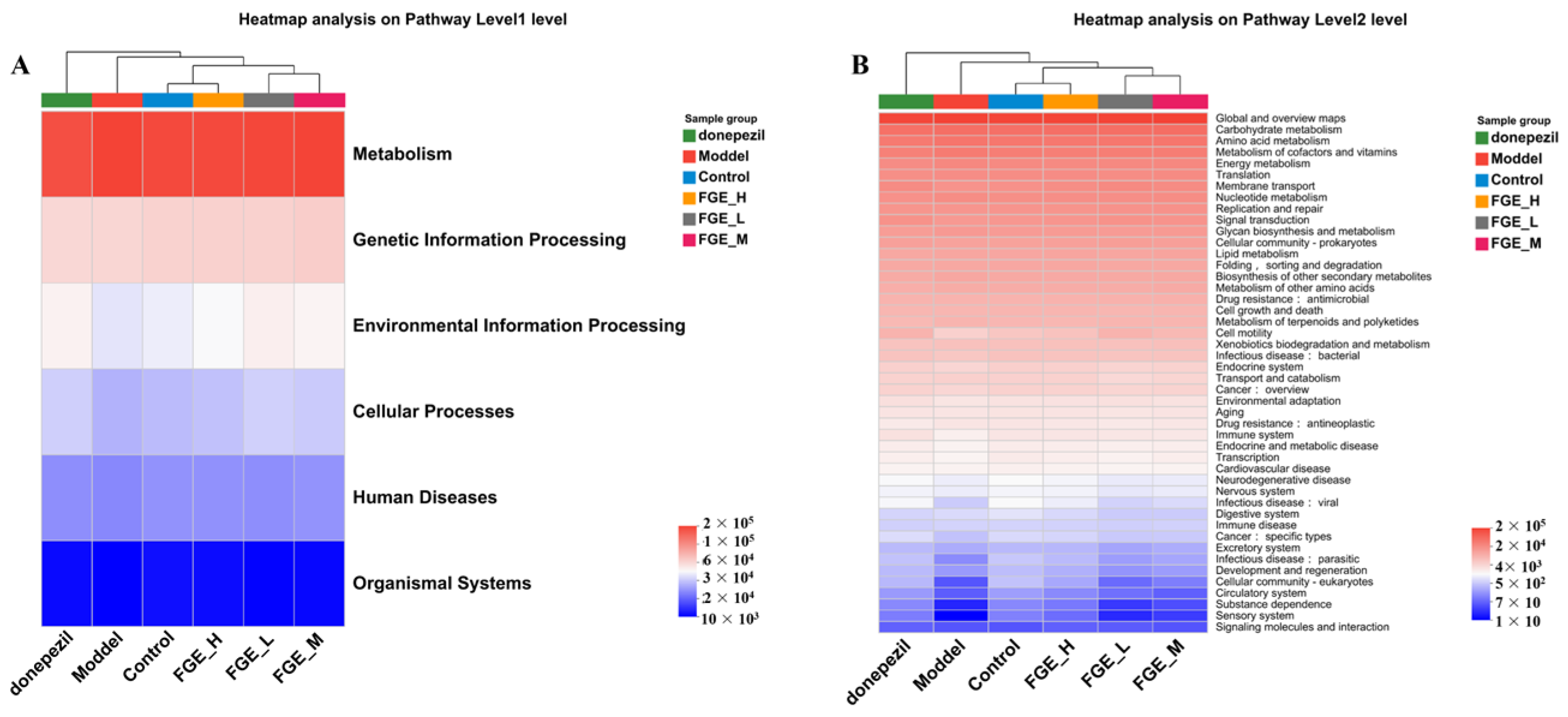
3.8. The Effect of FGE on the Abundance of Intestinal Flora in AD-like Mice
3.9. FGE Regulates the Imbalance of Amino Acid Metabolism
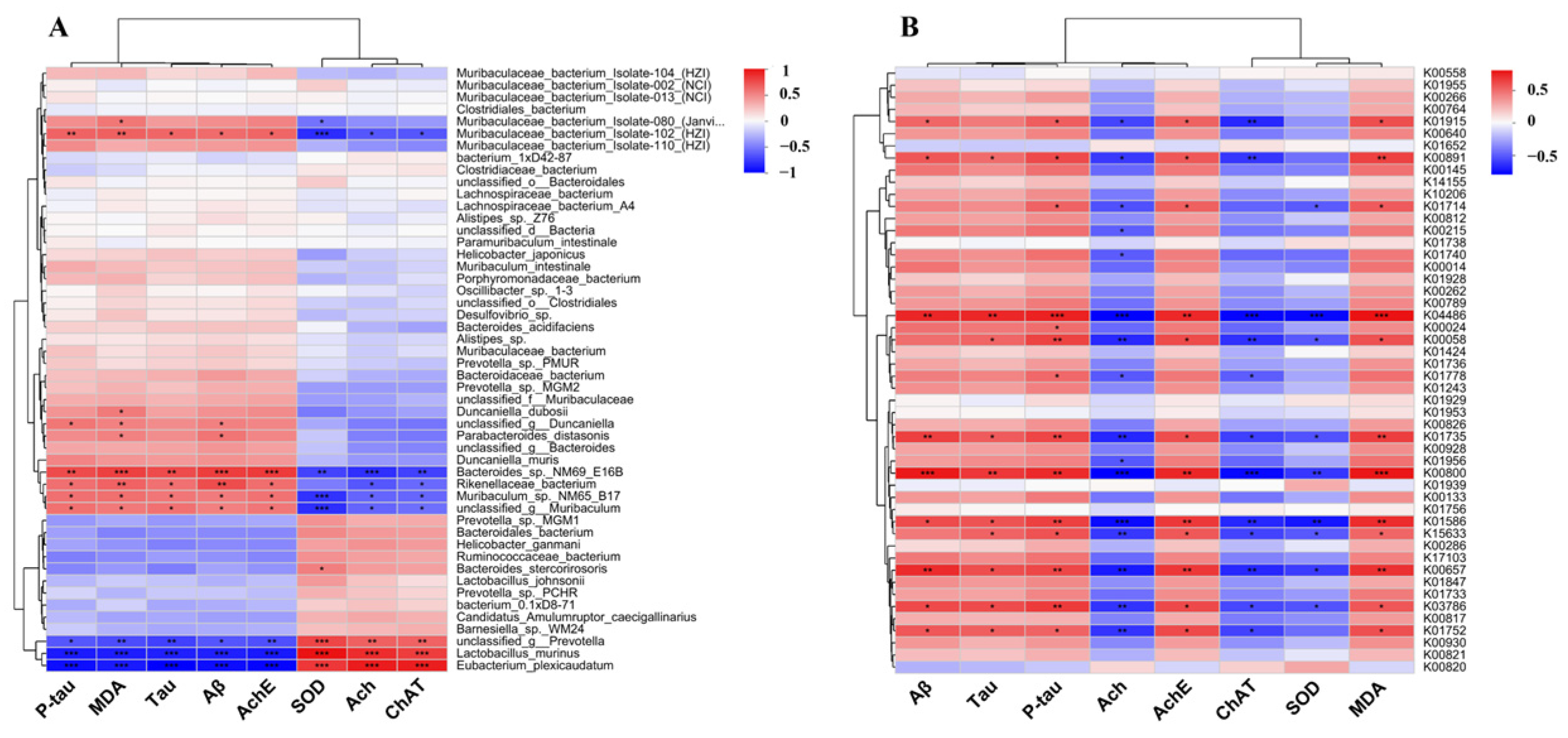
3.10. The Effect of FGE Treatment on Neurotransmitters by Regulating Gut Microbiota
3.11. Correlation Analysis between Amino Acid Metabolism and Oxidative Stress Level in AD-like Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scheltens, P.; Strooper, B.D.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789–5816. [Google Scholar] [CrossRef]
- Ren, R.; Qi, J.; Lin, S.; Liu, X.; Yin, P.; Wang, Z.; Tang, R.; Wang, J.; Huang, Q.; Li, J.; et al. The China Alzheimer Report 2022. Gen. Psychiatry 2022, 35, 100751. [Google Scholar] [CrossRef]
- Sola-Sevilla, N.; Puerta, E. SIRT2 as a potential new therapeutic target for Alzheimer’s disease. Neural Regen. Res. 2024, 19, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Cho, M.; Jang, C.H.; Lee, J.S.; Kim, J.-S.; Oh, J.; Lim, J. Oral Administration of Euonymus alatus Leaf Extract Ameliorates Alzheimer’s Disease Phenotypes in 5xFAD Transgenic Mice. Foods 2024, 13, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.C.B.; Dammer, E.B.; Duong, D.M.; Ping, L.; Zhou, M.; Yin, L.; Higginbotham, L.A.; Guajardo, A.; White, B.; Troncoso, J.C.; et al. Large-scale proteomic analysis of Alzheimer’s disease brain and cerebrospinal fluid reveals early changes in energy metabolism associated with microglia and astrocyte activation. Nat. Med. 2020, 26, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Wang, S.; Gao, X.; Zou, Z.; Hirotsune, S.; Sun, L. Pathological and physiological functional cross-talks of α-synuclein and tau in the central nervous system. Neural Regen. Res. 2024, 19, 855–862. [Google Scholar] [CrossRef]
- Kuragano, M.; Yoshinari, W.; Lin, X.; Shimamori, K.; Uwai, K.; Tokuraku, K. Evaluation of Amyloid β42 Aggregation Inhibitory Activity of Commercial Dressings by A Microliter-Scale High-Throughput Screening System Using Quantum-Dot Nanoprobes. Foods 2020, 9, 825–836. [Google Scholar] [CrossRef]
- Tracy, T.E.; Madero-Pérez, J.; Swaney, D.L.; Chang, T.S.; Moritz, M.; Konrad, C.; Ward, M.E.; Stevenson, E.; Hüttenhain, R.; Kauwe, G.; et al. Tau interactome maps synaptic and mitochondrial processes associated with neurodegeneration. Cell 2022, 185, 712–728. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Chou, P.; Hou, A.; Huang, C.; Shiu, W.; Wang, S. Lactobacillus paracasei PS23 improves cognitive deficits via modulating the hippocampal gene expression and the gut microbiota in d-galactose-induced aging mice. Food Funct. 2022, 13, 5240–5251. [Google Scholar] [CrossRef] [PubMed]
- Verhaar, B.J.H.; Hendriksen, H.M.A.; de Leeuw, F.A.; Doorduijn, A.S.; van Leeuwenstijn, M.; Teunissen, C.E.; Barkhof, F.; Scheltens, P.; Kraaij, R.; van Duijn, C.M.; et al. Gut Microbiota Composition Is Related to AD Pathology. Front. Immunol. 2022, 12, 794519. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, B.; Sun, D.; Chen, S. Gut Microbiota: Implications in Alzheimer’s Disease. J. Clin. Med. 2020, 9, 2042–2059. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Mo, X.; Huang, H.; Chen, X.; Liu, H.; Peng, Z.; Chen, L.; Rong, S.; Yang, W.; Xu, S.; et al. Yeast β-glucan alleviates cognitive deficit by regulating gut microbiota and metabolites in Aβ1–42-induced AD-like mice. Int. J. Biol. Macromol. 2020, 161, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Sheen, L. Gastrodiae Rhizoma (天麻 tiān má): A review of biological activity and antidepressant mechanisms. J. Tradit. Complement. Med. 2011, 1, 31–40. [Google Scholar] [CrossRef]
- Guan, J.; Chen, Z.; Guo, L.; Wang, C.; Xu, T.; Wan, F.; Zhou, T.; Cui, X.; Yang, Y. Evaluation of how sulfur-fumigation reduces the edible quality and flavor of Gastrodia elata Blume Rhizoma. LWT-Food Sci. Technol. 2023, 187, 115296. [Google Scholar] [CrossRef]
- Fasina, O.B.; Wang, J.; Mo, J.; Osada, H.; Ohno, H.; Pan, W.; Xiang, L.; Qi, J. Gastrodin from Gastrodia elata Enhances Cognitive Function and Neuroprotection of AD Mice via the Regulation of Gut Microbiota Composition and Inhibition of Neuron Inflammation. Front. Pharmacol. 2022, 13, 814271. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, J.; Latta, M.; Wang, C.; Liu, Y.; Ma, W.; Zhou, Z.; Hu, S.; Chen, P.; Liu, Y. Rhizoma Gastrodiae Water Extract Modulates the Gut Microbiota and Pathological Changes of P-TauThr231 to Protect against Cognitive Impairment in Mice. Front. Pharmacol. 2022, 13, 903659. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wu, S.; Weng, Z.; Wang, H.; Shi, R.; Tian, M.; Wang, Y.; He, H.; Wang, Y.; Liu, X.; et al. Tianma Formula Alleviates Dementia via ACER2-Mediated Sphingolipid Signaling Pathway Involving Aβ. Evid. -Based Complement. Altern. Med. 2021, 2021, 6029237. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Qiu, J.; Zhang, H.; Gao, N.; Jiang, T.; Gong, Y.; Zhang, W.; Li, Z.; Feng, X.; Hong, Z. PD184352 exerts anti-inflammatory and antioxidant effects by promoting activation of the Nrf2/HO-1 axis. Biochem Pharmacol 2023, 211, 115542. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, W.; Wang, X.; Ge, W. Impact of mitochondrial aldehyde dehydrogenase 2 on cognitive impairment in the AD model mouse. Acta Biochim. Biophys. Sin. 2021, 53, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef]
- Wu, J.; Wang, K.; Wang, X.; Pang, Y.; Jiang, C. The role of the gut microbiome and its metabolites in metabolic diseases. Protein Cell 2020, 12, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lu, S.; Yu, Z.; Zhang, J.; Mei, Z. Mechanisms for the biological activity of Gastrodia elata Blume and its constituents: A comprehensive review on sedative-hypnotic, and antidepressant properties. Phytomedicine 2024, 123, 155251. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Liu, S.; Liu, D.; Wang, X.; Wang, Z. Transformation Mechanisms of Chemical Ingredients in Steaming Process of Gastrodia elata Blume. Molecules 2019, 24, 3159–3172. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Y.; Li, L.; An, Z.; Xie, C.; Lin, L.; Yang, X.; Yang, J. Dynamic study on flavor components and antioxidant activity in the fermentation of Gastrodia elata. Food Ferment. Ind. 2021, 47, 92–98. [Google Scholar]
- Cacciamani, F.; Sambati, L.; Houot, M.; Habert, M.O.; Dubois, B.; Epelbaum, S. Reduced awareness of cognitive decline is associated with brain amyloid load in asymptomatic elderly individuals at risk for AD: A longitudinal study. Alzheimer’s Dement. 2020, 16, 037138. [Google Scholar] [CrossRef]
- Roda, A.R.; Montoliu-Gaya, L.; Serra-Mir, G.; Villegas, S. Both Amyloid-β Peptide and Tau Protein Are Affected by an Anti-Amyloid-β Antibody Fragment in Elderly 3xTg-AD Mice. Int. J. Mol. Sci. 2020, 21, 6630–6652. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Khachaturian, A.S.; Vergallo, A.; Farlow, M.R.; Snyder, P.J.; Giacobini, E.; Khachaturian, Z.S. Revisiting the Cholinergic Hypothesis in Alzheimer’s Disease: Emerging Evidence from Translational and Clinical Research. J. Prev. Alzheimer’s Dis. 2018, 6, 1–14. [Google Scholar] [CrossRef]
- Liskowsky, W.; Schliebs, R. Muscarinic acetylcholine receptor inhibition in transgenic Alzheimer-like Tg2576 mice by scopolamine favours the amyloidogenic route of processing of amyloid precursor protein. Int. J. Dev. Neurosci. 2006, 24, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Snowden, S.G.; Ebshiana, A.A.; Hye, A.; Pletnikova, O.; O’Brien, R.; Yang, A.; Troncoso, J.; Legido-Quigley, C.; Thambisetty, M. Neurotransmitter Imbalance in the Brain and Alzheimer’s Disease Pathology. J. Alzheimer’s Dis. 2019, 72, 35–43. [Google Scholar] [CrossRef]
- Reddy, V.P. Oxidative Stress in Health and Disease. Biomedicines 2023, 11, 2925–2941. [Google Scholar] [CrossRef]
- Ohira, H.; Oikawa, D.; Kurokawa, Y.; Aoki, Y.; Omura, A.; Kiyomoto, K.; Nakagawa, W.; Mamoto, R.; Fujioka, Y.; Nakayama, T. Suppression of colonic oxidative stress caused by chronic ethanol administration and attenuation of ethanol-induced colitis and gut leakiness by oral administration of sesaminol in mice. Food Funct. 2022, 13, 9285–9298. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Amin, M.M.; Rafiei, N.; Poursafa, P.; Ebrahimpour, K.; Mozafarian, N.; Shoshtari-Yeganeh, B.; Hashemi, M.; Kelishadi, R. Association of benzene exposure with insulin resistance, SOD, and MDA as markers of oxidative stress in children and adolescents. Environ. Sci. Pollut. Res. 2018, 25, 34046–34052. [Google Scholar] [CrossRef]
- Mohajeri, M. Brain Aging and Gut–Brain Axis. Nutrients 2019, 11, 424–426. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, D.; Chitrakar, B.; Li, C.; Zhang, N.; Zhang, S.; Wang, X.; Wang, M.; Tian, H.; Luo, Y. Study on Lactiplantibacillus plantarum R6-3 from Sayram Ketteki to prevent chronic unpredictable mild stress-induced depression in mice through the microbiota–gut–brain axis. Food Funct. 2023, 14, 3304–3318. [Google Scholar] [CrossRef]
- Banc, R.; Rusu, M.E.; Filip, L.; Popa, D.-S. The Impact of Ellagitannins and Their Metabolites through Gut Microbiome on the Gut Health and Brain Wellness within the Gut–Brain Axis. Foods 2023, 12, 270–310. [Google Scholar] [CrossRef]
- Feyissa, A.M.; Chandran, A.; Stockmeier, C.A.; Karolewicz, B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Riezzo, G.; Chimienti, G.; Orlando, A.; D’Attoma, B.; Clemente, C.; Russo, F. Effects of long-term administration of Lactobacillus reuteri DSM-17938 on circulating levels of 5-HT and BDNF in adults with functional constipation. Benef. Microbes 2019, 10, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Baranger, K.; Giannoni, P.; Girard, S.D.; Girot, S.; Gaven, F.; Stephan, D.; Migliorati, M.; Khrestchatisky, M.; Bockaert, J.; Marchetti-Gauthier, E.; et al. Chronic treatments with a 5-HT 4 receptor agonist decrease amyloid pathology in the entorhinal cortex and learning and memory deficits in the 5xFAD mouse model of Alzheimer’s disease. Neuropharmacology 2017, 126, 128–141. [Google Scholar] [CrossRef]
- Jalanka, J.; Major, G.; Murray, K.; Singh, G.; Nowak, A.; Kurtz, C.; Silos-Santiago, I.; Johnston, J.; de Vos, W.; Spiller, R. The Effect of Psyllium Husk on Intestinal Microbiota in Constipated Patients and Healthy Controls. Int. J. Mol. Sci. 2019, 20, 433–444. [Google Scholar] [CrossRef]
- Chen, W.; Bi, Z.; Zhu, Q.; Gao, H.; Fan, Y.; Zhang, C.; Liu, X.; Ye, M. An analysis of the characteristics of the intestinal flora in patients with Parkinson’s disease complicated with constipation. Am. J. Transl. Res. 2021, 13, 13710–13722. [Google Scholar]
- Yuan, X.; Chen, B.; Duan, Z.; Xia, Z.; Ding, Y.; Chen, T.; Liu, H.; Wang, B.; Yang, B.; Wang, X.; et al. Depression and anxiety in patients with active ulcerative colitis: Crosstalk of gut microbiota, metabolomics and proteomics. Gut Microbes 2021, 13, 1987779. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2020, 19, 55–71. [Google Scholar] [CrossRef]
- Cui, M.; Jiang, Y.; Zhao, Q.; Zhu, Z.; Liang, X.; Zhang, K.; Wu, W.; Dong, Q.; An, Y.; Tang, H.; et al. Metabolomics and incident dementia in older Chinese adults: The Shanghai Aging Study. Alzheimer’s Dement. 2020, 16, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhao, M.; Xie, C.; Li, L.; Lin, L.; Li, Q.; Li, L.; Chen, F.; Yang, X.; Yang, J.; et al. Fermented Gastrodia elata Bl. Alleviates Cognitive Deficits by Regulating Neurotransmitters and Gut Microbiota in D-Gal/AlCl3-Induced Alzheimer’s Disease-like Mice. Foods 2024, 13, 2154. https://doi.org/10.3390/foods13132154
Wang Y, Zhao M, Xie C, Li L, Lin L, Li Q, Li L, Chen F, Yang X, Yang J, et al. Fermented Gastrodia elata Bl. Alleviates Cognitive Deficits by Regulating Neurotransmitters and Gut Microbiota in D-Gal/AlCl3-Induced Alzheimer’s Disease-like Mice. Foods. 2024; 13(13):2154. https://doi.org/10.3390/foods13132154
Chicago/Turabian StyleWang, Yu, Min Zhao, Chunzhi Xie, Lilang Li, Ling Lin, Qiji Li, Liangqun Li, Faju Chen, Xiaosheng Yang, Juan Yang, and et al. 2024. "Fermented Gastrodia elata Bl. Alleviates Cognitive Deficits by Regulating Neurotransmitters and Gut Microbiota in D-Gal/AlCl3-Induced Alzheimer’s Disease-like Mice" Foods 13, no. 13: 2154. https://doi.org/10.3390/foods13132154
APA StyleWang, Y., Zhao, M., Xie, C., Li, L., Lin, L., Li, Q., Li, L., Chen, F., Yang, X., Yang, J., & Gao, M. (2024). Fermented Gastrodia elata Bl. Alleviates Cognitive Deficits by Regulating Neurotransmitters and Gut Microbiota in D-Gal/AlCl3-Induced Alzheimer’s Disease-like Mice. Foods, 13(13), 2154. https://doi.org/10.3390/foods13132154






