Okra (Abelmoschus esculentus L.) Flour Integration in Wheat-Based Sourdough: Effect on Nutritional and Technological Quality of Bread
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Microorganisms
2.3. Microbiological Analysis of Raw Materials
2.4. Production of Sourdough
2.5. Bread-Making Test
2.6. Microbiological Analysis of Sourdough
2.7. Physico-Chemical Analyses of Sourdough and Bread Samples
2.7.1. pH and TTA Measurement
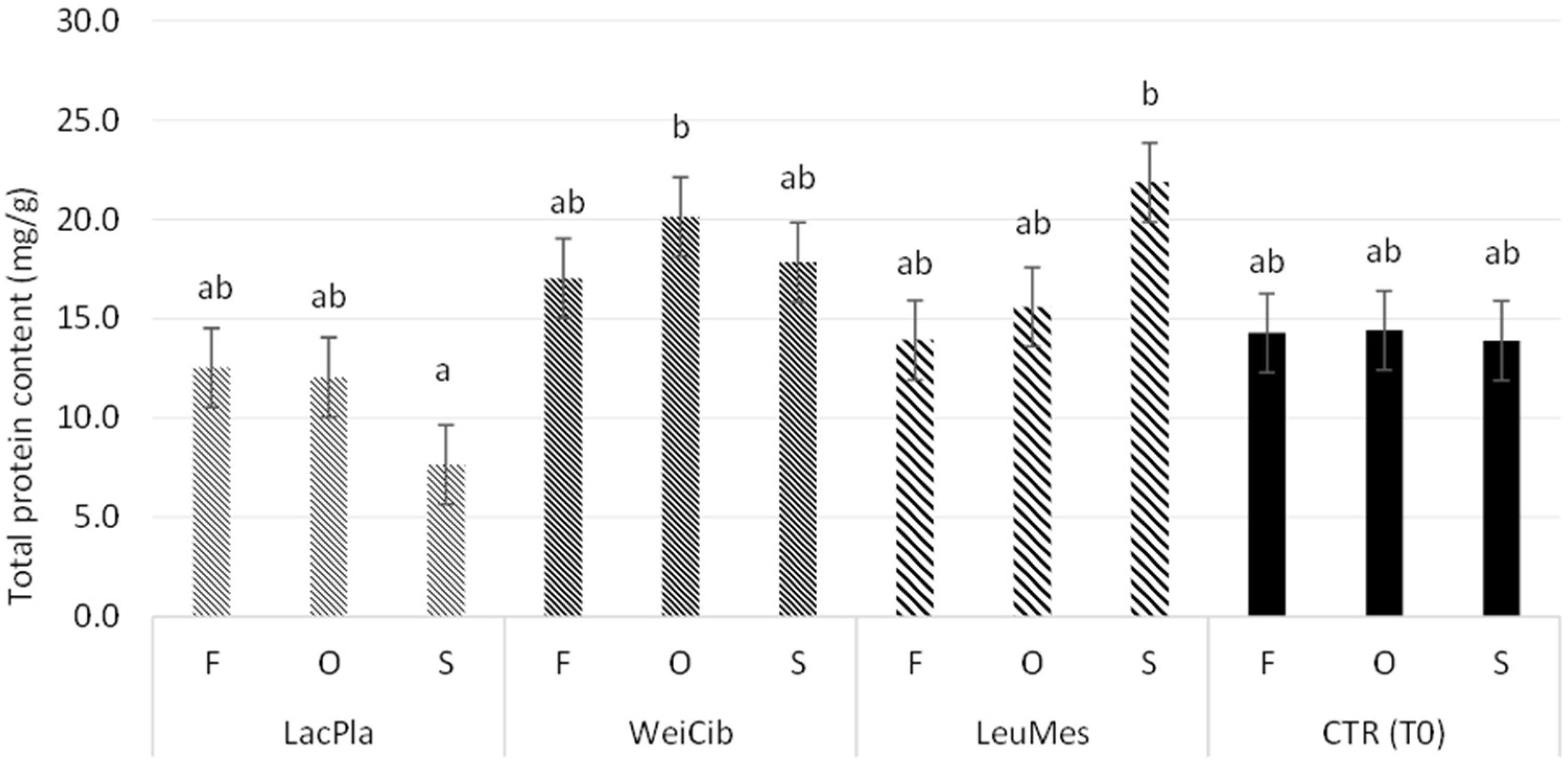
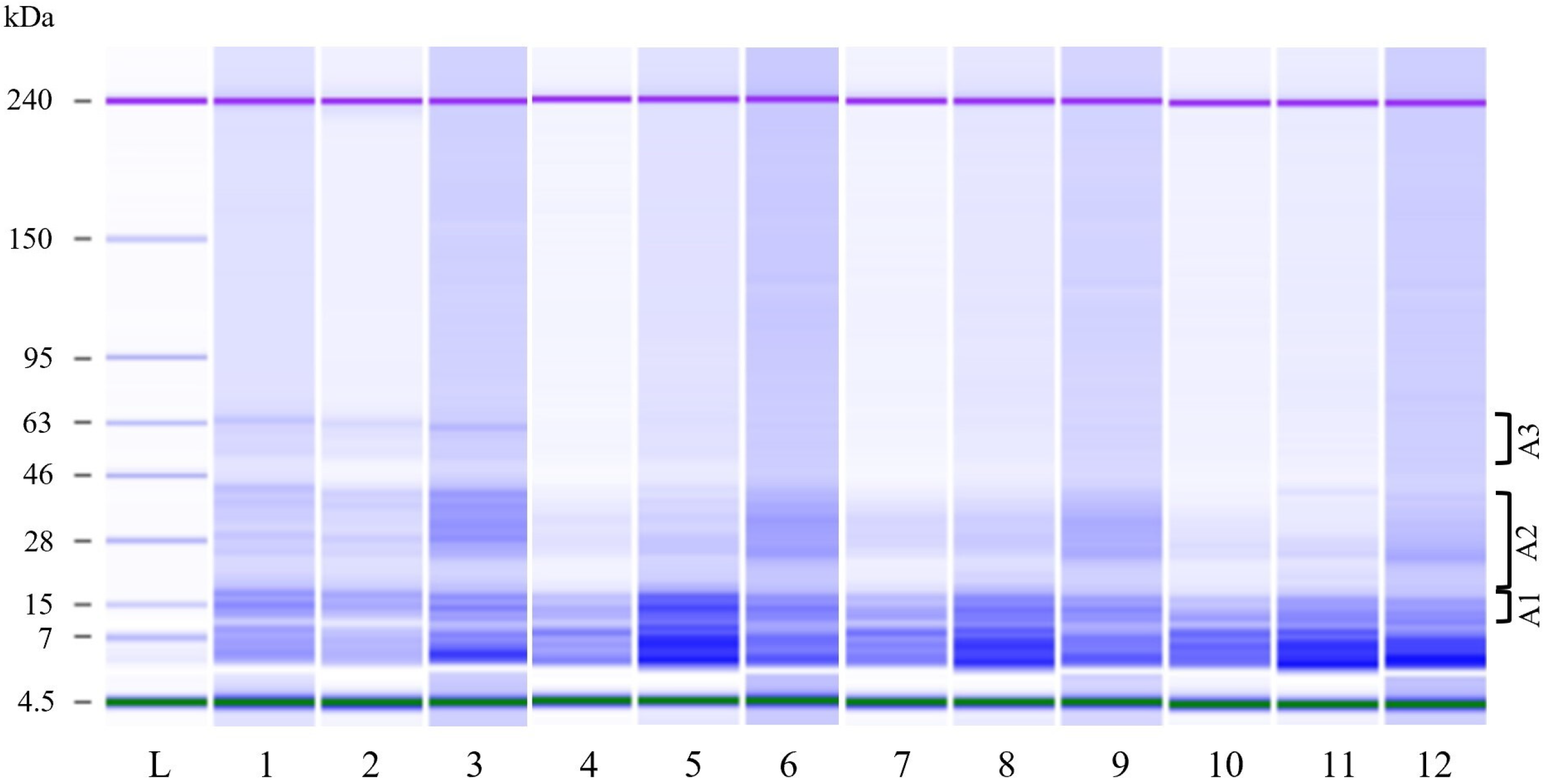
2.7.2. Protein Content and Protein Profile Analysis
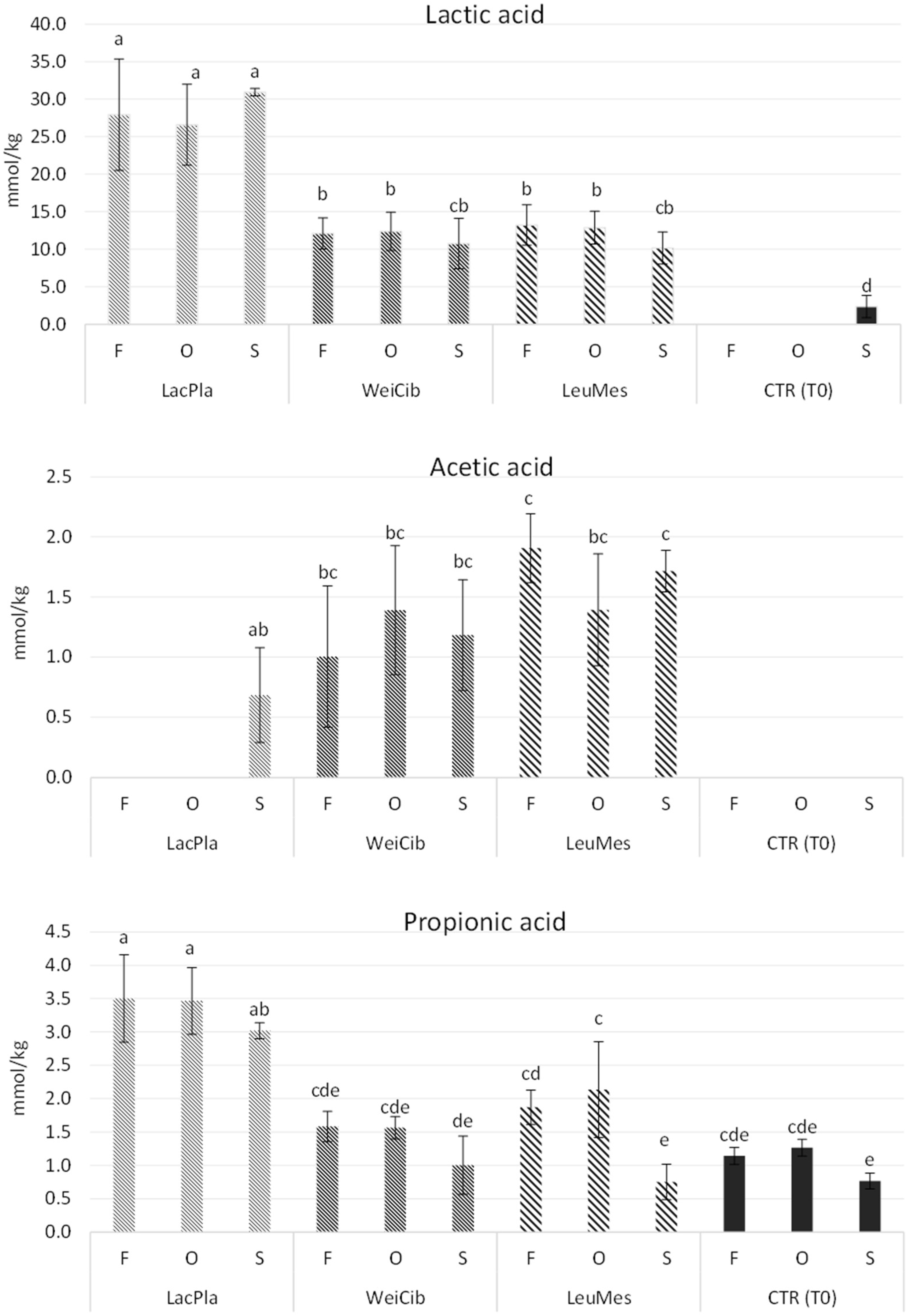
2.7.3. Determination of Organic Acids
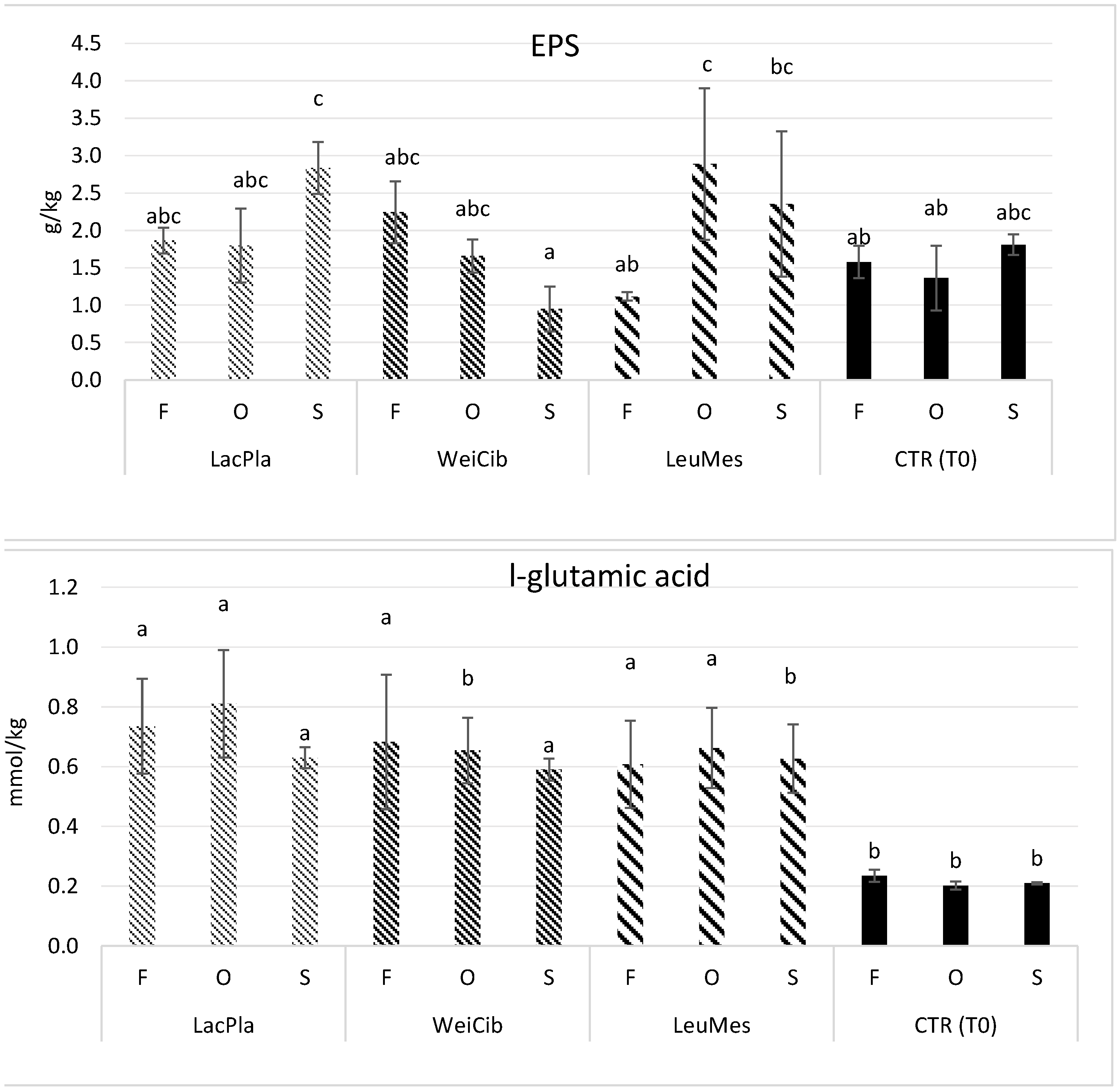
2.7.4. Determination of L-Glutamic Acid
2.7.5. EPS Quantification
2.7.6. Determination of Total Free Amino Acids
2.8. Technological Analyses of Bread Samples
2.8.1. Moisture Content
2.8.2. Water Activity
2.8.3. Bread Crumb Firmness
2.8.4. Crumb Color Evaluation
2.8.5. Bread Volume
2.9. Statistical Analysis
3. Results and Discussion
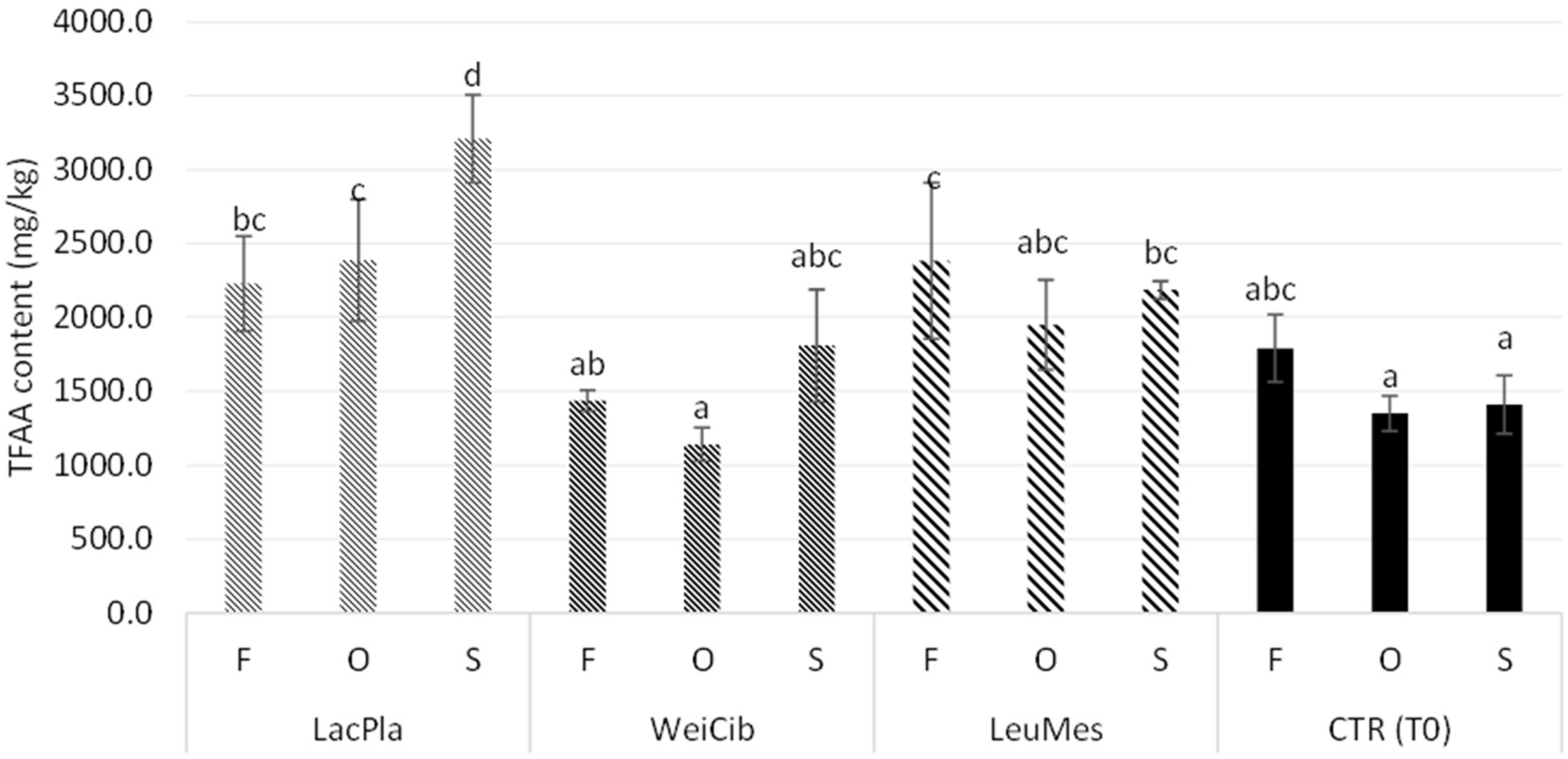
3.1. Fermentation Studies
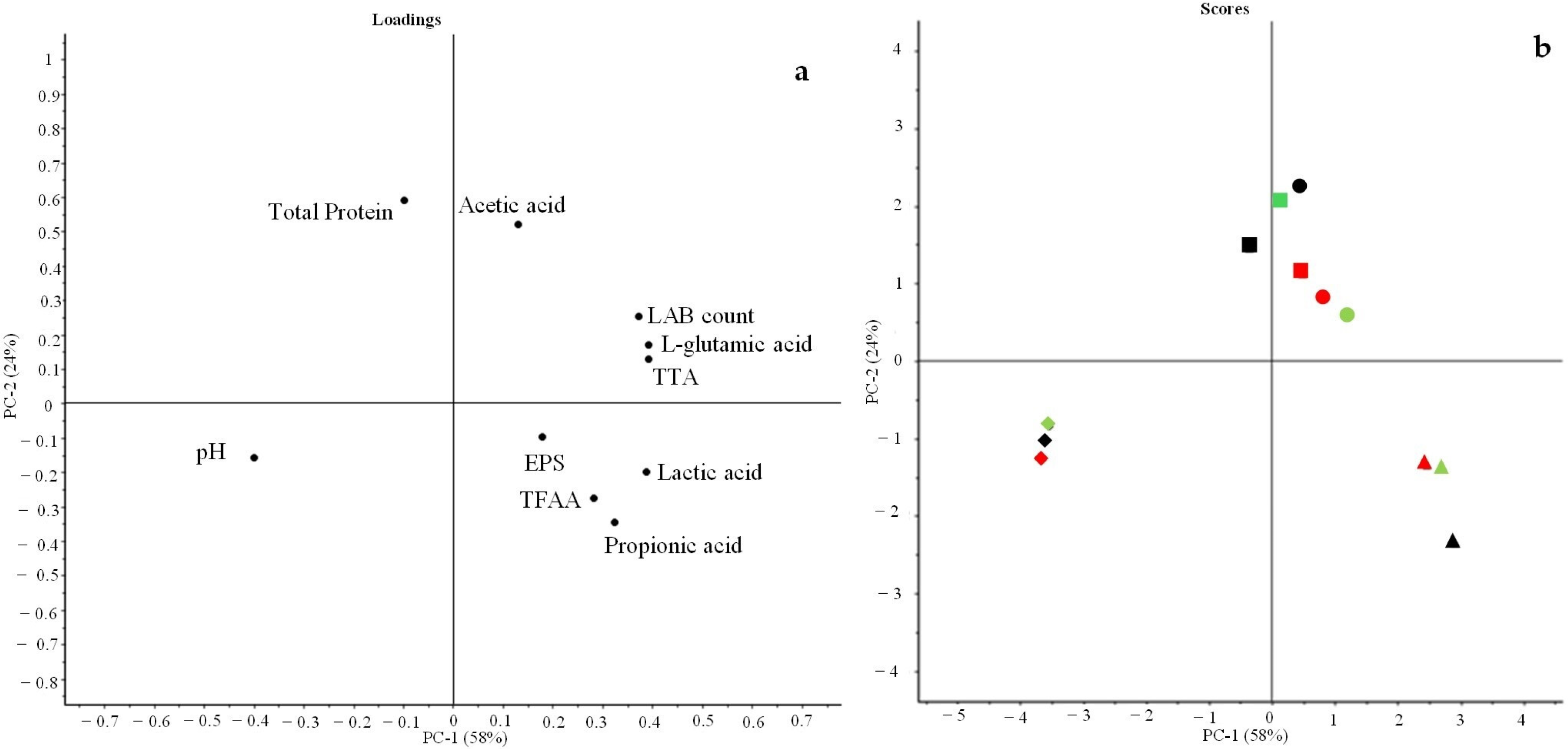
3.2. Principal Component Analysis
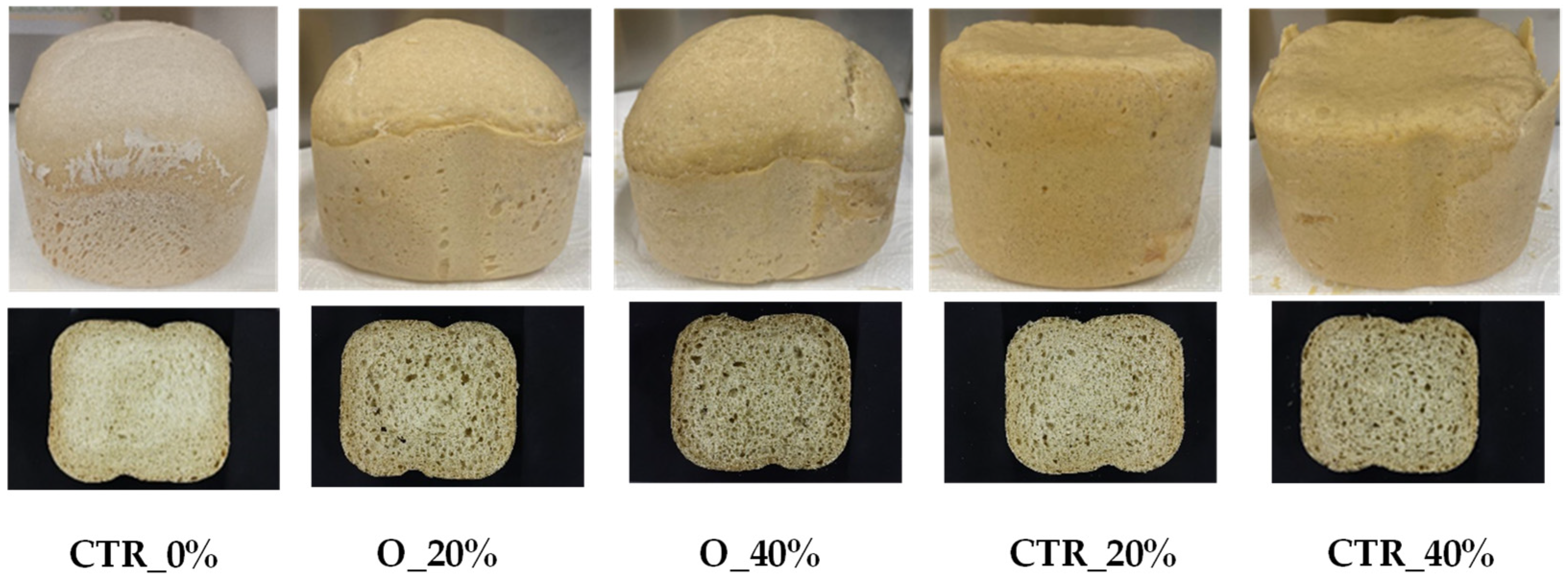
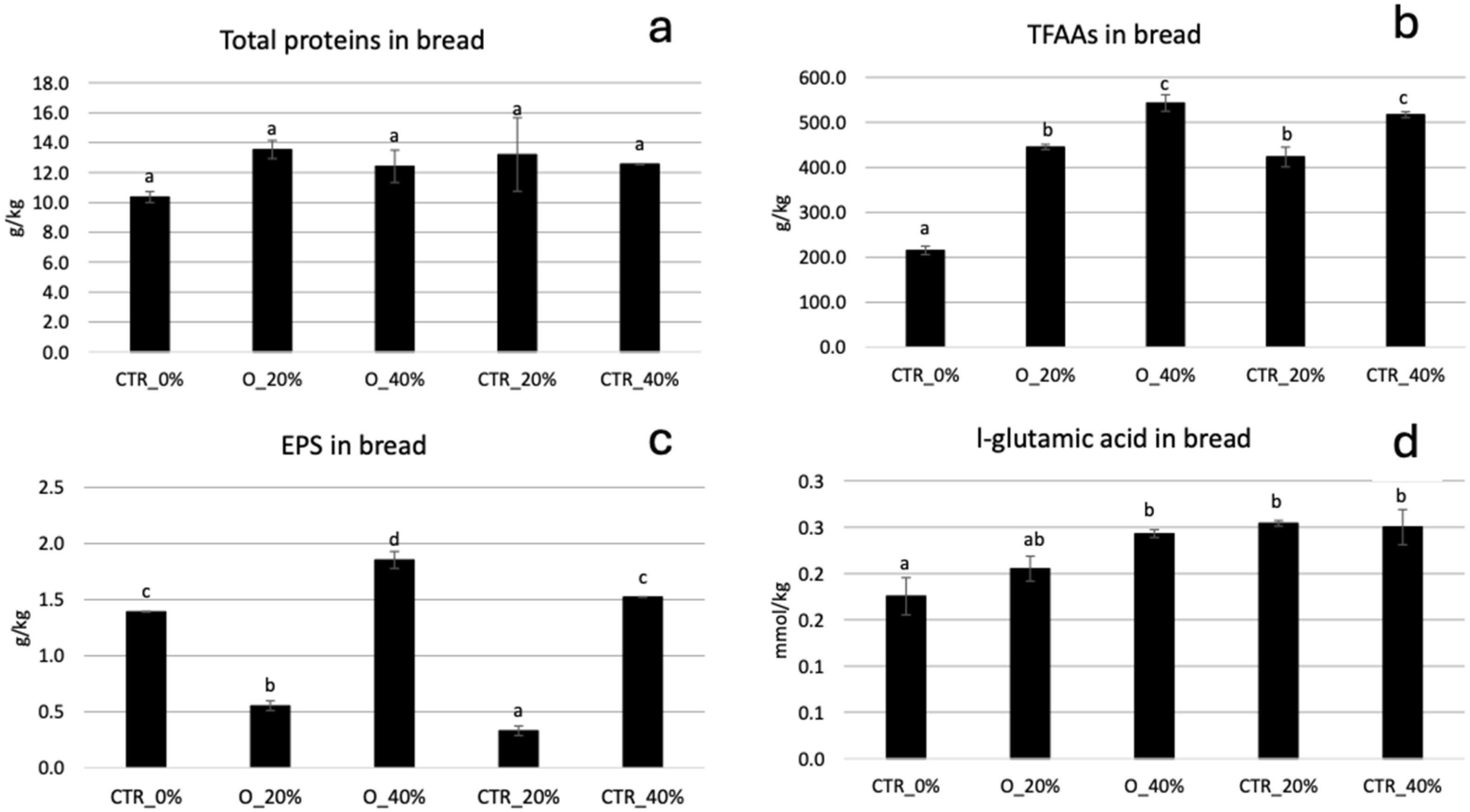
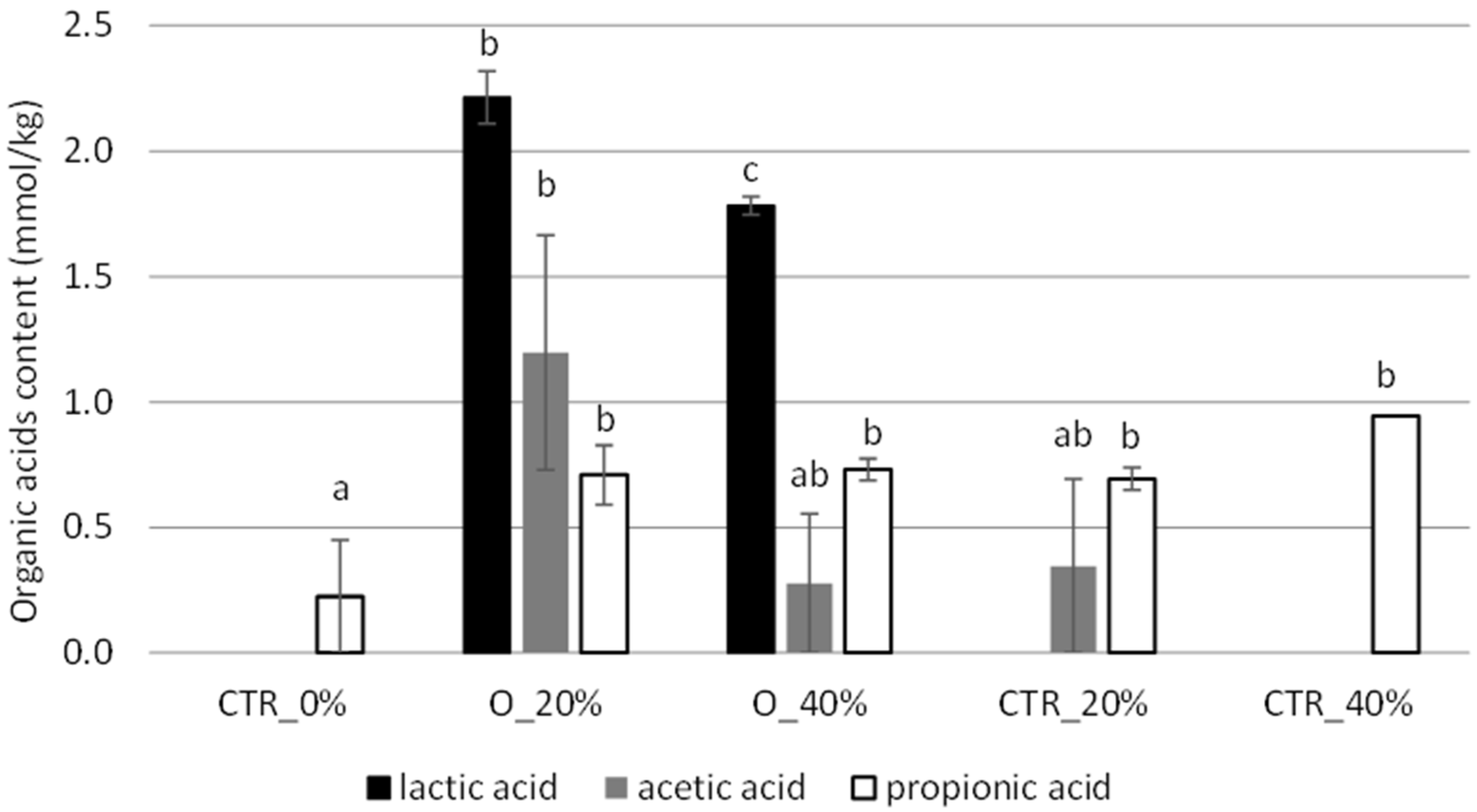
3.3. Bread Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| S.No | Notation | Description |
| 1 | O | oven-dried okra flour |
| 2 | F | freeze-dried okra flour |
| 3 | S | sun-dried okra flour |
| 4 | DY | dough yield |
| 5 | CTR | control |
| 6 | O_CTR_T0 | not inoculated mixtures, containing O flour, before fermentation |
| 7 | O_WeiCib | sourdough inoculated with W. cibaria C43-11 strain, containing O flour, after 14 h of fermentation |
| 8 | O_LeuMes | sourdough inoculated with Leuc. mesenteroides C43-2M strain, containing O flour, after 14 h of fermentation |
| 9 | O_LacPla | sourdough inoculated with L. plantarum ITM21B strain, containing O flour, after 14 h of fermentation |
| 10 | F_CTR_T0 | not inoculated mixtures, containing F flour, before fermentation |
| 11 | F_LeuMes | sourdough inoculated with W. cibaria C43-11 strain, containing F flour, after 14 h of fermentation |
| 12 | F_LeuMes | sourdough inoculated with Leuc. mesenteroides C43-2M strain, containing F flour, after 14 h of fermentation |
| 13 | F_LacPla | sourdough inoculated with L. plantarum ITM21B strain, containing F flour, after 14 h of fermentation |
| 14 | S_CTR_T0 | not inoculated mixtures, containing S flour, before fermentation |
| 15 | S_WeiCib | sourdough inoculated with W. cibaria C43-11 strain, containing S flour, after 14 h of fermentation |
| 16 | S_LeuMes | sourdough inoculated with Leuc. mesenteroides C43-2M strain, containing S flour, after 14 h of fermentation |
| 17 | S_LacPla | sourdough inoculated with L. plantarum ITM21B strain, containing S flour, after 14 h of fermentation |
| 18 | Mw | molecular weight |
| 19 | HMw | high molecular weight |
| 20 | GF | gluten-free |
| 21 | CTR_0% | bread not containing okra flour, produced with standard recipe |
| 22 | O_20% | bread containing 20% of O_LeuMes (w/w flour weight), corresponding to 0.6% (w/w) of O flour replacement |
| 23 | O_40% | bread containing 40% of O_LeuMes (w/w flour weight), corresponding to 1.21% (w/w) of O flour replacement |
| 24 | CTR_20% | bread containing not fermented O flour at 0.6% w/w |
| 25 | CTR_40% | bread containing not fermented O flour at 1.21% w/w |
References
- Islam, M.T. Phytochemical information and pharmacological activities of Okra (Abelmoschus esculentus): A literature-based review. Phytother. Res. 2019, 33, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.T.; Nie, X.R.; Shen, D.D.; Li, H.Y.; Zhao, L.; Zhang, Q.; Lin, D.R.; Qin, W. Phenolic compounds, antioxidant activities, and inhibitory effects on digestive enzymes of different cultivars of okra (Abelmoschus esculentus). Molecules 2020, 25, 1276. [Google Scholar] [CrossRef] [PubMed]
- Romdhane, M.H.; Chahdoura, H.; Barros, L.; Dias, M.I.; Corrêa, R.C.G.; Morales, P.; Ciudad-Mulero, M.; Flamini, G.; Majdoub, H.; Ferreira, I.C. Chemical composition, nutritional value, and biological evaluation of Tunisian okra pods (Abelmoschus esculentus L. Moench). Molecules 2020, 25, 4739. [Google Scholar] [CrossRef] [PubMed]
- Adelakun, O.E.; Oyelade, O.J. Potential use of okra seed (Abelmoschus esculentus Moench) flour for food fortification and effects of processing. In Flour and Breads and Their Fortification in Health and Disease Prevention, 1st ed.; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: London, UK, 2011; pp. 205–212. [Google Scholar]
- Machine, A.P.; Massingue, A.A.; Asaam, S. Physicochemical and sensory properties of bread with sweet potato flour (Ipomea batatas L.) as partial replacer of wheat flour supplemented with okra hydrocolloids. Afr. J. Food Sci. 2020, 14, 385–394. [Google Scholar]
- Gemede, H.F.; Ratta, N.; Haki, G.D.; Woldegiorgis, A.Z.; Beyene, F. Nutritional quality and health benefits of okra (Abelmoschus esculentus): A review. Int. J. Nutr. Food Sci. 2015, 4, 208–215. [Google Scholar] [CrossRef]
- Nie, X.R.; Fu, Y.; Wu, D.T.; Huang, T.T.; Jiang, Q.; Zhao, L.; Zhang, Q.; Lin, D.R.; Chen, H.; Qin, W. Ultrasonic-assisted extraction, structural characterization, chain conformation, and biological activities of a pectic-polysaccharide from okra (Abelmoschus esculentus). Molecules 2020, 25, 1155. [Google Scholar] [CrossRef]
- Nie, X.R.; Li, H.Y.; Du, G.; Lin, S.; Hu, R.; Li, H.Y.; Zhao, L.; Zhang, Q.; Chen, H.; Wu, D.T.; et al. Structural characteristics, rheological properties, and biological activities of polysaccharides from different cultivars of okra (Abelmoschus esculentus) collected in China. Int. J. Biol. Macromol. 2019, 139, 459–467. [Google Scholar] [CrossRef]
- Maalej, H.; Maalej, A.; Bayach, A.; Zykwinska, A.; Colliec-Jouault, S.; Sinquin, C.; Marchand, L.; Ktari, N.; Bardaa, S.; Salah, R.B.; et al. A novel pectic polysaccharide-based hydrogel derived from okra (Abelmoschus esculentus L. Moench) for chronic diabetic wound healing. Eur. Polym. J. 2023, 183, 111763. [Google Scholar] [CrossRef]
- Liu, H.; Gong, F.; Wei, F.; Wu, H. Artificial simulation of salivary and gastrointestinal digestion, and fermentation by human fecal microbiota, of polysaccharides from Dendrobium aphyllum. RSC Adv. 2018, 8, 13954–13963. [Google Scholar] [CrossRef]
- Xia, F.; Zhong, Y.; Li, M.; Chang, Q.; Liao, Y.; Liu, X.; Pan, R. Antioxidant and anti-fatigue constituents of okra. Nutrients 2015, 7, 8846–8858. [Google Scholar] [CrossRef]
- Yuan, Q.; Lin, S.; Fu, Y.; Nie, X.R.; Liu, W.; Su, Y.; Han, Q.H.; Zhao, L.; Zhang, Q.; Lin, D.R.; et al. Effects of extraction methods on the physicochemical characteristics and biological activities of polysaccharides from okra (Abelmoschus esculentus). Int. J. Biol. Macromol. 2019, 127, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.T.; Fu, Y.; Guo, H.; Yuan, Q.; Nie, X.R.; Wang, S.P.; Gan, R.Y. In vitro simulated digestion and fecal fermentation of polysaccharides from loquat leaves: Dynamic changes in physicochemical properties and impacts on human gut microbiota. Int. J. Biol. Macromol. 2021, 168, 733–742. [Google Scholar] [CrossRef]
- Sengkhamparn, N.; Bakx, E.J.; Verhoef, R.; Schols, H.A.; Sajjaanantakul, T.; Voragen, A.G. Okra pectin contains an unusual substitution of its rhamnosyl residues with acetyl and alpha-linked galactosyl groups. Carbohydr. Res. 2009, 344, 1842–1851. [Google Scholar] [CrossRef] [PubMed]
- Manzocco, L.; Basso, F.; Nicoli, M.C. Effect of hyperbaric storage at room temperature on the activity of polyphenoloxidase in model systems and fresh apple juice. Food Bioprocess Technol. 2023, 16, 2247–2256. [Google Scholar] [CrossRef]
- Islam, M.A.; Islam, S. Sourdough bread quality: Facts and Factors. Foods 2024, 13, 2132. [Google Scholar] [CrossRef]
- Padayachee, A.; Netzel, G.; Netzel, M.; Day, L.; Zabaras, D.; Mikkelsen, D.; Gidley, M.J. Binding of polyphenols to plant cell wall analogues–Part 2: Phenolic acids. Food Chem. 2012, 135, 2287–2292. [Google Scholar] [CrossRef]
- Tufaro, D.; Bassoli, A.; Cappa, C. Okra (Abelmoschus esculentus) powder production and application in gluten-free bread: Effect of particle size. Food Bioprocess Technol. 2022, 15, 904–914. [Google Scholar] [CrossRef]
- Machine, A.P.; Massingue, A.A.; Balane, S.G.; Armando, E.J. Enhancing bread quality: Investigating the physicochemical and sensory properties of bread with sweet potato flour and okra hydrocolloids as substitutes for wheat flour. In Current Perspectives in Agriculture and Food Science, 1st ed.; Son, L., Ed.; B P International: Hooghly, India, 2024; Volume 8, pp. 28–48. [Google Scholar]
- Bai-Ngew, S.; Phimolsiripol, Y.; Walter, P. Characterization of hot air drying and microwave vacuum drying of okra powder and application in bread. Food Appl. Biosci. J. 2024, 12, 11–28. [Google Scholar]
- Xu, K.; Guo, M.; Roman, L.; Pico, J.; Martinez, M.M. Okra seed and seedless pod: Comparative study of their phenolics and carbohydrate fractions and their impact on bread-making. Food Chem. 2020, 317, 126387. [Google Scholar] [CrossRef]
- Ou, J.; Wang, M.; Zheng, J.; Ou, S. Positive and negative effects of polyphenol incorporation in baked foods. Food Chem. 2019, 284, 90–99. [Google Scholar] [CrossRef]
- Li, M.; Koecher, K.; Hansen, L.; Ferruzzi, M.G. Phenolics from whole grain oat products as modifiers of starch digestion and intestinal glucose transport. J. Agric. Food Chem. 2017, 65, 6831–6839. [Google Scholar] [CrossRef] [PubMed]
- Pico, J.; Martinez, M.M. Unraveling the inhibition of intestinal glucose transport by dietary phenolics: A review. Curr. Pharm. Des. 2019, 25, 3418–3433. [Google Scholar] [CrossRef] [PubMed]
- Pico, J.; Corbin, S.; Ferruzzi, M.G.; Martinez, M.M. Banana flour phenolics inhibit trans-epithelial glucose transport from wheat cakes in a coupled in vitro digestion/Caco-2 cell intestinal model. Food Funct. 2019, 10, 6300–6311. [Google Scholar] [CrossRef] [PubMed]
- Alamri, M.S. Okra-gum fortified bread: Formulation and quality. J. Food Sci. Technol. 2014, 51, 2370–2381. [Google Scholar] [CrossRef]
- Buczkowski, B.K. Sourdough Bread Enriched with Soluble Fibres: Development, Characterisation and Nutritional Aspects of a Functional Food Product. Doctoral Dissertation, Manchester Metropolitan University, Manchester, UK, 2013. [Google Scholar]
- De Vuyst, L.; Van Kerrebroeck, S.; Harth, H.; Huys, G.; Daniel, H.-M.; Weckx, S. Microbial ecology of sourdough fermentations: Diverse or uniform? Food Microbiol. 2014, 37, 11–29. [Google Scholar] [CrossRef]
- Arendt, E.K.; Ryan, L.A.; Dal Bello, F. Impact of sourdough on the texture of bread. Food Microbiol. 2007, 24, 165–174. [Google Scholar] [CrossRef]
- Ripari, V.; Bai, Y.; Gänzle, M.G. Metabolism of phenolic acids in whole wheat and rye malt sourdoughs. Food Microbiol. 2019, 77, 43–51. [Google Scholar] [CrossRef]
- Gabriele, M.; Sparvoli, F.; Bollini, R.; Lubrano, V.; Longo, V.; Pucci, L. The impact of sourdough fermentation on non-nutritive compounds and antioxidant activities of flours from different Phaseolus vulgaris L. genotypes. J. Food Sci. 2019, 84, 1929–1936. [Google Scholar] [CrossRef]
- Altunkaya, A.; Hedegaard, R.V.; Brimer, L.; Gökmen, V.; Skibsted, L.H. Antioxidant capacity versus chemical safety of wheat bread enriched with pomegranate peel powder. Food Funct. 2013, 4, 722–727. [Google Scholar] [CrossRef]
- Gänzle, M.G. From gene to function: Metabolic traits of starter cultures for improved quality of cereal foods. Int. J. Food Microbiol. 2009, 134, 29–36. [Google Scholar] [CrossRef]
- Korakli, M.; Rossmann, A.; Gänzle, M.G.; Vogel, R.F. Sucrose metabolism and exopolysaccharide production in wheat and rye sourdoughs by Lactobacillus sanfranciscensis. J. Agr. Food Chem. 2001, 49, 5194–5200. [Google Scholar] [CrossRef] [PubMed]
- Adelakun, O.E.; Olanipekun, B.F.; Akingbaso, O.; Adhikar, B.M. Effect of fermentation and variety on quality attributes of okra seed (Abelmoschus esculentus (L.) Moench) flour. Donn. J. Food Sci. Tech. 2017, 3, 001–006. [Google Scholar]
- Adetuyi, F.O.; Ibrahim, T.A. Effect of fermentation time on the phenolic, flavonoid and vitamin C contents and antioxidant activities of okra (Abelmoschus esculentus) seeds. Niger. Food J. 2014, 32, 128–137. [Google Scholar] [CrossRef]
- Wang, X.; Hu, K.; Chen, Y.; Lai, J.; Zhang, M.; Li, J.; Li, Q.; Zhao, N.; Liu, S. Effect of Lactiplantibacillus plantarum fermentation on the physicochemical, antioxidant activity and immunomodulatory ability of polysaccharides from Lvjian okra. Int. J. Biol. Macromol. 2024, 257, 128649. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, A.; Lavermicocca, P.; Morea, M.; Baruzzi, F.; Tosti, N.; Gobbetti, M. Phenotypic and molecular identification and clustering of lactic acid bacteria and yeasts from wheat (species Triticum durum and Triticum aestivum) sourdoughs of southern Italy. Int. J. Food Microbiol. 2001, 64, 95–104. [Google Scholar] [CrossRef]
- Di Biase, M.; Bavaro, A.R.; Lonigro, S.L.; Pontonio, E.; Conte, A.; Padalino, L.; Minisci, A.; Lavermicocca, P.; Valerio, F. Lactobacillus plantarum ITM21B fermentation product and chickpea flour enhance the nutritional profile of salt reduced bakery products. Int. J. Food Sci. Nutr. 2019, 70, 701–713. [Google Scholar] [CrossRef]
- Valerio, F.; Conte, A.; Di Biase, M.; Lattanzio, V.M.; Lonigro, S.L.; Padalino, L.; Pontonio, E.; Lavermicocca, P. Formulation of yeast-leavened bread with reduced salt content by using a Lactobacillus plantarum fermentation product. Food Chem. 2017, 221, 582–589. [Google Scholar] [CrossRef]
- De Bellis, P.; Ferrara, M.; Bavaro, A.R.; Linsalata, V.; Di Biase, M.; Musio, B.; Gallo, V.; Valerio, F. Characterization of dextran produced by the food-related strain Weissella cibaria C43-11 and of the relevant dextransucrase gene. Foods 2022, 11, 2819. [Google Scholar] [CrossRef]
- Valerio, F.; Favilla, M.; De Bellis, P.; Sisto, A.; de Candia, S.; Lavermicocca, P. Antifungal activity of strains of lactic acid bacteria isolated from a semolina ecosystem against Penicillium roqueforti, Aspergillus niger and Endomyces fibuliger contaminating bakery products. Syst. Appl. Microbiol. 2009, 32, 438–448. [Google Scholar] [CrossRef]
- Bavaro, A.R.; Di Biase, M.; Conte, A.; Lonigro, S.L.; Caputo, L.; Cedola, A.; Del Nobile, A.; Logrieco, A.F.; Lavermicocca, P.; Valerio, F. Weissella cibaria short-fermented liquid sourdoughs based on quinoa or amaranth flours as fat replacer in focaccia bread formulation. Int. J. Food Sci. Technol. 2021, 56, 3197–3208. [Google Scholar] [CrossRef]
- Valerio, F.; Bavaro, A.R.; Di Biase, M.; Lonigro, S.L.; Logrieco, A.F.; Lavermicocca, P. Effect of amaranth and quinoa flours on exopolysaccharide production and protein profile of liquid sourdough fermented by Weissella cibaria and Lactobacillus plantarum. Front. Microbiol. 2020, 11, 967. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Álvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Hilbert, F.; Lindqvist, R.; et al. Scientific Opinion on the update of the list of qualified presumption of safety (QPS) recommended microorganisms intentionally added to food or feed as notified to EFSA. EFSA J. 2023, 21, 7747. [Google Scholar]
- De Bellis, P.; Valerio, F.; Sisto, A.; Lonigro, S.L.; Lavermicocca, P. Probiotic table olives: Microbial populations adhering on olive surface in fermentation sets inoculated with the probiotic strain Lactobacillus paracasei IMPC2.1 in an industrial plant. Int. J. Food Microbiol. 2010, 140, 6–13. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Osborne, T.B. The Proteins of the Wheat Kernel (Monograph); Carnegie Inst.: Washington, DC, USA, 1907. [Google Scholar]
- Weiss, W.; Vogelmeier, C.; Gorg, A. Electrophoretic characterization of wheat grain allergens from different cultivars involved in bakers’ asthma. Electrophoresis 1993, 14, 805–816. [Google Scholar] [CrossRef]
- Doi, E.; Shibata, D.; Matoba, T. Modified colorimetric ninhydrin methods for peptidase assay. Anal Biochem. 1981, 118, 173–184. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- American Association of Cereal Chemists (AACC). Approved Methods of the AACC, 10th ed.; St. Paul: Eagan, MN, USA, 2000. [Google Scholar]
- Lavermicocca, P.; Valerio, F.; Evidente, A.; Lazzaroni, S.; Corsetti, A.; Gobbetti, M. Purification and characterization of novel antifungal compounds from the sourdough Lactobacillus plantarum strain 21B. Appl. Environ. Microbiol. 2000, 66, 4084–4090. [Google Scholar] [CrossRef]
- Montemurro, M.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Investigation of the nutritional, functional and technological effects of the sourdough fermentation of sprouted flours. Int. J. Food Microbiol. 2019, 302, 47–58. [Google Scholar] [CrossRef]
- Pétel, C.; Onno, B.; Prost, C. Sourdough volatile compounds and their contribution to bread: A review. Trends Food Sci. Technol. 2017, 59, 105–123. [Google Scholar] [CrossRef]
- Dantas, T.L.; Alonso Buriti, F.C.; Florentino, E.R. Okra (Abelmoschus esculentus L.) as a potential functional food source of mucilage and bioactive compounds with technological applications and health benefits. Plants 2021, 10, 1683. [Google Scholar] [CrossRef]
- Bi, Q.; Hong, T.; Mei, X.; Xu, X.; Xu, D. Effect of high-molecular weight dextran-enriched sourdough fermented using Leuconostoc mesenteroides ATCC 8293 on bread quality and gluten. Food Biosci. 2023, 53, 102777. [Google Scholar] [CrossRef]
- Zhao, C.J.; Kinner, M.; Wismer, W.; Gänzle, M.G. Effect of glutamate accumulation during sourdough fermentation with Lactobacillus reuteri on the taste of bread and sodium-reduced bread. Cereal Chem. 2015, 92, 224–230. [Google Scholar] [CrossRef]
- Vermeulen, N.; Gänzle, M.G.; Vogel, R.F. Glutamine deamidation by cereal-associated lactic acid bacteria. J. Appl. Microbiol. 2007, 103, 1197–1205. [Google Scholar] [CrossRef]
- Peressini, D.; Sensidoni, A. Effect of soluble dietary fibre addition on rheological and breadmaking properties of wheat doughs. J. Cereal Sci. 2009, 49, 190–201. [Google Scholar] [CrossRef]
- Foschia, M.; Peressini, D.; Sensidoni, A.; Brennan, C.S. The effects of dietary fibre addition on the quality of common cereal products. J. Cereal Sci. 2013, 58, 216–227. [Google Scholar] [CrossRef]
- Ameh, M.O.; Gernah, D.I.; Igbabul, B.D. Physico-chemical and sensory evaluation of wheat bread supplemented with stabilized undefatted rice bran. Food Nutr. Sci. 2013, 4, 43–48. [Google Scholar] [CrossRef]
- Chareonthaikij, P.; Uan-On, T.; Prinyawiwatkul, W. Effects of pineapple pomace fibre on physicochemical properties of composite flour and dough, and consumer acceptance of fibre-enriched wheat bread. Int. J. Food Sci. Technol. 2016, 51, 1120–1129. [Google Scholar] [CrossRef]
- Plazzotta, S.; Sillani, S.; Manzocco, L. Exploitation of lettuce waste flour to increase bread functionality: Effect on physical, nutritional, sensory properties and on consumer response. Int. J. Food Sci. Technol. 2018, 53, 2290–2297. [Google Scholar] [CrossRef]
- Nawab, A.; Alam, F.; Haq, M.A.; Hasnain, A. Effect of guar and xanthan gums on functional properties of mango (Mangifera indica) kernel starch. Int. J. Biol. Macromol. 2016, 93, 630–635. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; Limitone, A.; Minervini, F.; Carnevali, P.; Corsetti, A.; Gänzle, M.; Ciati, R.; Gobbetti, M. Glucan and fructan production by sourdough Weissella cibaria and Lactobacillus plantarum. J. Agric. Food Chem. 2006, 54, 9873–9881. [Google Scholar] [CrossRef]
- Dong, Y.; Ronholm, J.; Fliss, I.; Karboune, S. Screening of lactic acid bacteria strains for potential sourdough and bread applications: Enzyme expression and exopolysaccharide production. Probiotics Antimicrob. Proteins 2024, 1–23. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Zhang, Z.; Guan, H.; Zhang, Y.; Xu, D.; Xu, X.; Li, D. Improvement on wheat bread quality by in situ produced dextran—A comprehensive review from the viewpoint of starch and gluten. Compr. Rev. Food Sci. Food Saf. 2024, 23, 13353. [Google Scholar] [CrossRef]
- Nawrocka, A.; Krekora, M.; Niewiadomski, Z.; Miś, A. Characteristics of the chemical processes induced by celluloses in the model and gluten dough studied with application of FTIR spectroscopy. Food Hydrocoll. 2018, 85, 176–184. [Google Scholar] [CrossRef]
- Pepe, O.; Ventorino, V.; Cavella, S.; Fagnano, M.; Brugno, R. Prebiotic content of bread prepared with flour from immature wheat grain and selected dextran-producing lactic acid bacteria. Appl. Environ. Microbiol. 2013, 79, 3779–3785. [Google Scholar] [CrossRef]
- Sami, R.; Lianzhou, J.; Yang, L.; Ma, Y.; Jing, J. Evaluation of fatty acid and amino acid compositions in okra (Abelmoschus esculentus) grown in different geographical locations. Biomed Res. Int. 2013, 1, 574283. [Google Scholar] [CrossRef]
| F | O | S | |
|---|---|---|---|
| Moisture (g/100 g) | 0.22 ± 0.02 | 9.85 ± 0.1 | 0.00 ± 0.0 |
| Protein content (g/100 g db #) | 18.01 ± 0.25 | 19.04 ± 0.18 | 18.39 ± 0.15 |
| Total fats (g/100 g db) | 18.26 ± 0.36 | 2.40 ± 0.276 | 1.18± 0.08 |
| Total carbohydrates (g/100 g db) | 26.71 ± 0.26 | 35.27 ± 0.37 | 24.88 ± 3.34 |
| Bread Variant * | |||||
|---|---|---|---|---|---|
| CTR_0% | O_20% | O_40% | CTR_20% | CTR_40% | |
| Ingredients | Weight (g) | ||||
| Wheat flour | 350 | 326.67 | 303.33 | 347.88 | 345.76 |
| Salt | 6.3 | 6.3 | 6.3 | 6.3 | 6.3 |
| Olive oil | 10.5 | 10.5 | 10.5 | 10.5 | 10.5 |
| Dry yeast | 5.25 | 5.25 | 5.25 | 5.25 | 5.25 |
| Tap water | 210 | 163.33 | 116.67 | 210 | 210 |
| O_LeuMes | - | 70 | 140 | - | - |
| O flour | - | - | - | 2.12 | 4.24 |
| Okra Flour Type | Sourdough | LAB (log CFU/g) ± SE | pH ± SE | TTA (mL NaOH 0.1 N) ± SE |
|---|---|---|---|---|
| Oven-dried | O_CTR_T0 | 5.16 ± 0.26 b | 5.92 ± 0.02 a | 1.00 ± 0.10 a |
| O_WeiCib | 9.33 ± 0.08 cd | 4.03 ± 0.02 c | 4.75 ± 0.25 bc | |
| O_LeuMes | 9.43 ± 0.16 cd | 3.96 ± 0.02 c | 4.45 ± 0.55 b | |
| O_LacPla | 9.26 ± 0.23 cd | 3.57 ± 0.01 d | 6.50 ± 0.50 d | |
| Freeze-dried | F_CTR_T0 | 3.15 ± 0.15 a | 6.00 ± 0.02 a | 0.65 ± 0.15 a |
| F_WeiCib | 9.45 ± 0.26 cd | 4.02 ± 0.01 c | 4.65 ± 0.35 bc | |
| F_LeuMes | 9.57 ± 0.33 cd | 4.00 ± 0.07 c | 4.50 ± 0.50 b | |
| F_LacPla | 9.74 ± 0.03 d | 3.70 ± 0.12 d | 6.00 ± 1.00 cd | |
| Sun-dried | S_CTR_T0 | 3.15 ± 0.15 a | 5.62 ± 0.04 b | 0.95 ± 0.15 a |
| S_WeiCib | 9.17 ± 0.13 c | 3.95 ± 0.09 c | 3.45 ± 0.55 b | |
| S_LeuMes | 9.16 ± 0.09 c | 3.98 ± 0.06 c | 4.75 ± 0.25 bc | |
| S_LacPla | 9.26 ± 0.06 cd | 3.55 ± 0.00 d | 4.40 ± 0.10 b |
| pH | TTA | LAB Count | EPS | Lactic Acid | Acetic Acid | Propionic Acid | L-Glutamic Acid | Total Proteins | TFAA | |
|---|---|---|---|---|---|---|---|---|---|---|
| pH | - | −0.94 | −0.95 | −0.26 | −0.76 | −0.44 | −0.52 | −0.70 | 0.00 | −0.49 |
| TTA | −0.94 | - | 0.91 | 0.17 | 0.75 | 0.33 | 0.57 | 0.72 | 0.04 | 0.40 |
| LAB count | −0.95 | 0.91 | - | 0.17 | 0.65 | 0.51 | 0.44 | 0.70 | 0.09 | 0.37 |
| EPS | −0.26 | 0.17 | 0.17 | - | 0.29 | 0.15 | 0.33 | 0.07 | −0.16 | 0.32 |
| lactic acid | −0.76 | 0.75 | 0.65 | 0.29 | - | 0.11 | 0.76 | 0.32 | −0.41 | 0.68 |
| acetic acid | −0.44 | 0.33 | 0.51 | 0.15 | 0.11 | - | −0.01 | 0.30 | 0.17 | 0.16 |
| propionic acid | −0.52 | 0.57 | 0.44 | 0.33 | 0.76 | −0.01 | - | 0.19 | −0.40 | 0.58 |
| l-glutamic acid | −0.70 | 0.72 | 0.70 | 0.07 | 0.32 | 0.30 | 0.19 | - | 0.22 | 0.11 |
| total proteins | 0.00 | 0.04 | 0.09 | −0.16 | −0.41 | 0.17 | −0.40 | 0.22 | - | −0.37 |
| TFAA | −0.49 | 0.40 | 0.37 | 0.32 | 0.68 | 0.16 | 0.58 | 0.11 | −0.37 | - |
| Bread Samples | Specific Volume (cm3/g) | Moisture | aw | L* | a* | b* | ΔE | Firmness (N) |
|---|---|---|---|---|---|---|---|---|
| CTR_0% | 4.08 ± 0.81 a | 66.87 ± 0.90 a | 0.64 ± 0.01 a | 71.48 ± 3.16 a | −1.44 ± 0.15 d | 15.21 ± 0.81 a | 1.13 ± 0.81 c | |
| O_20% | 2.30 ± 0.81 b | 68.86 ± 0.71 a | 0.63 ± 0.01 ab | 62.29 ± 2.71 b | −1.18 ± 0.20 c | 15.48 ± 0.99 a | 9.92 | 1.87 ± 0.07 b |
| O_40% | 2.44 ± 0.81 b | 65.87 ± 1.46 a | 0.61 ± 0.02 bca | 57.65 ± 2.17 c | −0.69 ± 0.13 a | 15.23 ± 0.92 a | 13.85 | 1.75 ± 0.03 b |
| CTR_20% | 2.38 ± 0.81 b | 65.66 ± 1.13a | 0.60 ± 0.01 bc | 62.58 ± 2.52 b | −1.00 ± 0.11 bc | 15.73 ± 0.74 a | 8.92 | 1.98 ± 0.14 b |
| CTR_40% | 1.75 ± 0.81 c | 68.74 ± 1.78 a | 0.58 ± 0.02 c | 60.77 ± 2.13 bc | −0.85 ± 0.11 ab | 16.10 ± 0.54 a | 10.76 | 3.01 ± 0.11 a |
| Analytical Data | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EPS | Lactic Acid | Acetic Acid | Propionic Acid | L-Glutamic Acid | Total Proteins | TFAA | pH | TTA | ||
| Technological data | L* | −0.07 | −0.18 | −0.11 | −0.93 | −0.79 | −0.58 | −0.88 | 0.60 | −0.30 |
| a* | 0.34 | 0.28 | 0.05 | 0.87 | 0.64 | 0.43 | 0.87 | −0.50 | 0.41 | |
| b* | 0.09 | 0.42 | 0.18 | 0.09 | 0.14 | 0.30 | 0.28 | −0.44 | 0.50 | |
| moisture | 0.07 | −0.06 | −0.27 | −0.09 | 0.40 | 0.00 | 0.13 | −0.54 | 0.17 | |
| aw | −0.47 | −0.74 | −0.24 | −0.54 | −0.36 | −0.37 | −0.79 | 0.71 | −0.89 | |
| firmness | 0.33 | 0.65 | 0.19 | 0.48 | 0.54 | 0.41 | 0.79 | −0.90 | 0.85 | |
| specific volume | −0.01 | −0.52 | −0.30 | −0.80 | −0.79 | −0.72 | −0.94 | 0.95 | −0.66 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valerio, F.; Di Biase, M.; Cifarelli, V.; Lonigro, S.L.; Maalej, A.; Plazzotta, S.; Manzocco, L.; Calligaris, S.; Maalej, H. Okra (Abelmoschus esculentus L.) Flour Integration in Wheat-Based Sourdough: Effect on Nutritional and Technological Quality of Bread. Foods 2024, 13, 3238. https://doi.org/10.3390/foods13203238
Valerio F, Di Biase M, Cifarelli V, Lonigro SL, Maalej A, Plazzotta S, Manzocco L, Calligaris S, Maalej H. Okra (Abelmoschus esculentus L.) Flour Integration in Wheat-Based Sourdough: Effect on Nutritional and Technological Quality of Bread. Foods. 2024; 13(20):3238. https://doi.org/10.3390/foods13203238
Chicago/Turabian StyleValerio, Francesca, Mariaelena Di Biase, Valentina Cifarelli, Stella Lisa Lonigro, Amina Maalej, Stella Plazzotta, Lara Manzocco, Sonia Calligaris, and Hana Maalej. 2024. "Okra (Abelmoschus esculentus L.) Flour Integration in Wheat-Based Sourdough: Effect on Nutritional and Technological Quality of Bread" Foods 13, no. 20: 3238. https://doi.org/10.3390/foods13203238
APA StyleValerio, F., Di Biase, M., Cifarelli, V., Lonigro, S. L., Maalej, A., Plazzotta, S., Manzocco, L., Calligaris, S., & Maalej, H. (2024). Okra (Abelmoschus esculentus L.) Flour Integration in Wheat-Based Sourdough: Effect on Nutritional and Technological Quality of Bread. Foods, 13(20), 3238. https://doi.org/10.3390/foods13203238








