Abstract
As consumer demand for meat analogs continues to grow, various plant proteins are being explored for their production. This study uses isolated mung bean protein (IMBP) to replace isolated soy protein (ISP), investigating the effects of IMBP content (0%, 10%, 20%, 30%, 40%, and 50%) on the physicochemical and textural properties of high-moisture meat analogs (HMMAs) and exploring the potential of IMBP in the development and production of meat analogs. The results show that IMBP can bind water and cause protein denaturation, thus requiring more time and higher temperatures to be formed compared to HMMAs without IMBP. Additionally, increasing the IMBP content improves the gelling ability, thereby increasing the input of specific mechanical energy. As the IMBP content increases, the fibrous structure of the HMMA also increases. When the IMBP content reaches 40–50%, the most meat-like fibrous structure is observed. The water-holding capacity, water absorption capacity, springiness, and cohesiveness are negatively correlated with the IMBP content, while the oil absorption capacity is positively correlated with it. The integrity index and nitrogen solubility index show opposite trends with the increase in the IMBP content. When the IMBP content is 50%, the springiness and chewiness are the lowest, and the cutting strength is also the lowest, but the sample has a rich fibrous content, indicating that the HMMA with 50% IMBP content is soft and juicy. In conclusion, IMBP has the potential to be a substitute for ISP in the production of HMMAs.
1. Introduction
According to the United Nations’ [1] projections, the global population is expected to reach 10 billion by 2050, with the global demand for meat reaching approximately 455 million tons. This represents a 76% increase compared to 2005 [2]. To meet the continuously growing demand for meat, it is necessary to increase livestock production by 50–73% [3]. However, livestock production emits three major greenhouse gases: carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O), leading to environmental issues [4,5]. Therefore, considering the disparity between future meat demand and current meat supply capabilities, there is an increasing need to consider meat analogs as a new source of protein [6].
Meat analogs are plant-based foods that mimic the appearance, flavor, and texture of meat [7]. Extrusion cooking is one of the most used manufacturing processes for producing meat analogs, which can be classified into low-moisture extrusion cooking (LMEC) and high-moisture extrusion cooking (HMEC) based on different moisture contents. Moisture is considered a crucial factor in the extrusion process. Generally, the moisture content is in the range of 10–30% for LMEC and in the range of 50–70% for HMEC. It has been reported that LMEC using short dies may result in expansion [8], while HMEC using long cooling dies can generate meat-like fibrous structures without expansion [9].
Soybeans [10], peas [11], wheat gluten [12], and peanut protein are major plant-based proteins utilized in the production of contemporary meat analogs [13]. Isolated soy protein (ISP) has garnered attention due to its high protein content exceeding 90%, achieved through the removal of oligosaccharides and water-soluble polysaccharides [14]. Moreover, its inherent attributes, such as isoflavones and saponins, contribute to its antioxidative and immunomodulatory functionalities [15]. Nevertheless, concerns regarding the distinctive flavor and bitterness of soybeans [16], as well as issues related to allergies and potential genetically modified organisms, have spurred interest in alternative plant proteins, including mung beans [17].
Mung beans, being a staple crop widely cultivated and consumed in most Asian countries, hold undeniable significance [18]. Studies indicate that mung beans possess various health benefits, including lowering blood sugar levels, inhibiting melanin production, immunomodulation, and hepatoprotection [19]. Isolated mung bean protein (IMBP) is recognized as a high-quality protein, abundant in essential amino acids such as proline, glutamic acid, arginine, leucine, and phenylalanine [20]. Furthermore, it has been reported that the amino acid composition of mung bean protein is like that of soybean protein [21]. The primary component of mung bean protein, constituting over 80%, is 8S globulin. The sequence similarity of the 8S globulin to β-conglycinin, a major protein in soybeans, is 68%, with a structural similarity of 68% as well. The potential health benefits of mung bean protein are estimated to be four times that of β-conglycinin, which accounts for 20% of total soybean protein [22]. The research conducted by Brishti et al. [23] successfully demonstrated the feasibility of optimizing the production of texturized mung bean protein (TMBP) from mung bean protein. TMBP can serve as a meat thickener and is considered a healthier alternative to animal protein. Additionally, Brishti et al. [23] reported that extrusion at 49.3% moisture content can result in the production of TMBP with desirable characteristics, including partial denaturation, the formation of small aggregates, enhanced solubility, and digestibility. TMBP also exhibits strong gel-forming behavior.
However, there is currently a lack of research on high-moisture meat analogs based on isolated mung bean protein content. Therefore, this study aims to investigate the physicochemical properties of extruded high-moisture meat analogs based on varying levels of isolated mung bean content.
2. Materials and Methods
2.1. Materials
The raw material ratio of isolated mung bean protein (IMBP)/ isolated soybean protein (ISP): wheat gluten (WG): corn starch (CS) was 50: 40: 10. ISP (Pingdingshan Tianjing Plant Albumen Co., Ltd., Pingdingshan, China) was replaced with IMBP (Harbin Hada Starch Co., Ltd., Harbin, China) at inclusion levels of 0%, 10%, 20%, 30%, 40%, and 50%, while WG (Roquette Freres, Lestrem, France) and CS (Samyang Ltd., Ulsan, Republic of Korea) were fixed and mixed in a ratio of 40:10. The dry basis crude protein content, ash content, and crude fat content of IMBP are 80%, 7%, and 2%, respectively, while those of ISP are 90%, 5%, and 1%, respectively.
2.2. High-Moisture Extrusion Process
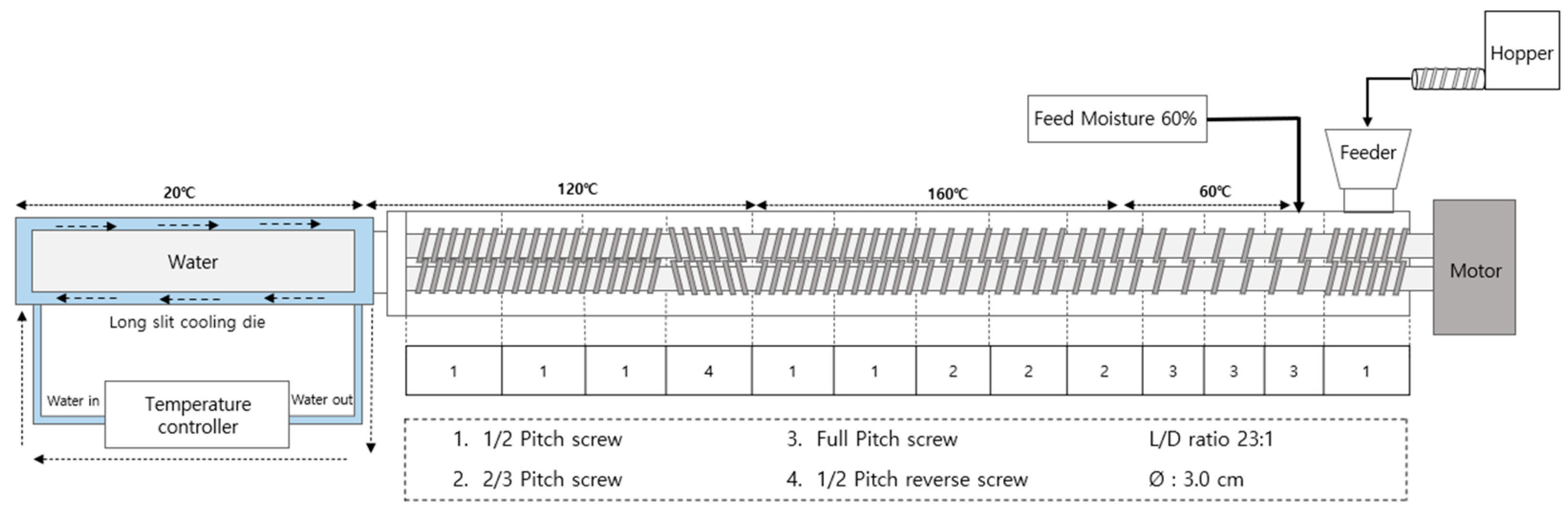
The experiment was conducted using a co-rotating twin-screw extruder (THK31T-No.5, Incheon Machinery Co., Incheon, Republic of Korea), with a screw length-to-diameter ratio of 23:1 and a screw diameter of 3 cm. The cooling die and screw configuration of extruder are illustrated in Figure 1. The extrusion parameters included: a feed rate of 100 g/min, feed moisture content of 60%, and screw speed of 150 rpm. The cooling die temperature was maintained at 20 °C, utilizing a water circulator (Duksan Cotran Co., Daegu, Republic of Korea). Following extrusion, the HMMA samples were cut into 1 × 1 × 1 cm blocks, with a portion stored under sealed conditions at 4 °C for integrity index and texture profile analysis (TPA) measurements, while another portion was freeze-dried for water holding capacity (WHC) assessment. Subsequently, a portion of the freeze-dried HMMAs was ground using a grinder (FM-909T, Hanil Electric Co., Wonju, Republic of Korea), sieved, and samples with a particle size of 50–70 mesh were utilized for measuring the water absorption capacity (WAC), oil absorption capacity (OAC), and nitrogen solubility index (NSI).
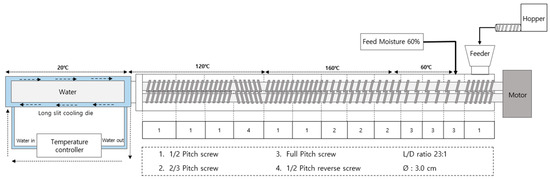
Figure 1.
Schematic diagram of the high-moisture extrusion system with details of the cooling die and screw configuration.
2.3. Viscosity
The viscosities of ISP, IMBP, and their mixtures (IMBP 0%, 30%, and 50%) were measured using a Rapid Visco Analyzer (TecMaster, Perten Instruments, Perkin Elmer, NSW, Australia). The measurement protocol was adapted from the method of Liu et al. [24], with slight modifications. A total of 3 g of samples were uniformly mixed with 25 mL of distilled water and stirred at a speed of 160 rpm for 13 min. Heating and cooling conditions involved maintaining the initial temperature at 50 °C for 1 min, followed by gradual increase to 95 °C over 4 min and then holding for 3 min. Subsequently, the temperature was reduced to the initial temperature of 50 °C over 4 min and maintained for an additional 2 min. The analysis of the RVA curve data was conducted to measure the peak viscosity, final viscosity, and setback.
2.4. Specific Mechanical Energy
The specific mechanical energy (SME), defined as the electrical energy consumed per unit mass during the extrusion process, was measured following the methodology proposed by Ryu and Mulvaney [25]. The SME is represented by Equation (1).
where E is the electric power when input to the material (kJ/s), E0 is the electric power when idle (kJ/s), and PR is the production rate (kg/s).
SME input (kJ/kg) = (E − E0)/PR,
2.5. Water Holding Capacity
To assess the WHC of the HMMAs, modifications were made to the method proposed by [9]. A total of 3 g of freeze-dried block-shaped HMMAs (on a dry basis) were mixed with 100 mL of distilled water. The mixture was hydrated at 50 °C using a water bath (SHWB-45, Lab House Co., Ltd., Pocheon, Republic of Korea) for 16 h. Subsequently, the sample was filtered through a 20-mesh sieve for 15 min to remove excess moisture. The WHC is expressed by Equation (2). Three replicate measurements were conducted, and the average value was calculated.
WHC (g/g) = (Wet sample wt. − Dry sample wt.)/Dry sample wt.
2.6. Texture Profile Analysis and Cutting Strength
The HMMA texture was assessed using a rheometer (Compac-100Ⅱ, Sun Sci Corporation, Tokyo, Japan). A probe with a diameter of 2.5 cm was utilized to measure the springiness, cohesiveness, and chewiness of the samples. The measurements were conducted at a maximum stress of 10 kg using the probe. Additionally, the cutting strength in both the vertical and parallel directions were measured using a probe with the dimensions of 7.5 mm × 38.3 mm at a maximum stress of 2 kg. Subsequently, the springiness, cohesiveness, chewiness, and cutting strength were calculated using Equation (3), Equation (4), Equation (5), and Equation (6), respectively. Ten measurements were conducted for each sample, and after excluding the maximum and minimum values, the average was derived from the remaining six data points.
where D1 is the distance of the first-occurred maximum stress and D2 is the distance of the second-occurred maximum stress.
where A1 is the area of the first-occurred maximum stress and A2 is the area of the second-occurred maximum stress.
Springiness (%) = D2/D1 × 100,
Cohesiveness (%) = A2/A1 × 100,
Chewiness (g) = Springiness × Cohesiveness × Maximum stress/10000,
Cutting strength (g/cm2) = Maximum stress/Cross-sectional area.
2.7. Water Absorption Capacity and Oil Absorption Capacity
The water absorption capacity (WAC) and oil absorption capacity (OAC) were measured according to the modified method by Samard and Ryu [26]. A total of 0.5 g of ground samples were mixed with 5 mL of distilled water using a vortex mixer (SI-0246A, Vortex-Genie-2, Scientific Industries Inc., Bohemia, NY, USA) for 1 min. Subsequently, the mixture was vibrated on a shaker (SI-300R, Jelotech, Gangneung, Republic of Korea) at 30 °C for 30 min, followed by centrifugation at 3000 rpm for 30 min and discarding of the supernatant. Simultaneously, the same procedure was repeated using soybean oil instead of distilled water. The WAC and OAC of the sediment were calculated using Equations (7) and Equation (8), respectively. The density of water used in the experiment was 1 g/cm3, and the density of soybean oil was 0.90 g/cm3. The experiment was repeated three times, and the average value was calculated.
WAC (g/g) = Weight of sediment/Weight of dry solid,
OAC (g/g) = Weight of sediment/Weight of dry solid.
2.8. Integrity Index
The integrity index was measured following the method by Gu and Ryu [27]. It evaluates the resistance of the HMMA’s fibrous structure to high temperature and pressure, as well as its homogeneity. A total of 4 g of freeze-dried block-shaped sample (on a dry basis) were placed in 100 mL of distilled water and heated under high pressure at 121 °C for 15 min using an autoclave. After cooling the samples in running water for 30 s, they were homogenized in a beaker containing 100 mL of distilled water using a homogenizer (IKA-T10B, IKA Co., Staufen, Germany) and filtered through a 20-mesh sieve. The residue was rinsed once with running water for 30 s and then dried at 105 °C for 10 h. The experiment was repeated three times, and the average value was calculated.
Integrity index (%) = Dry residue wt./Sample wt. × 100.
2.9. Nitrogen Solubility Index
The nitrogen solubility index (NSI) was determined using the modified method by Căpriță, and Crețescu [28]. To measure the content of soluble nitrogen, 0.1 g of powdered samples were mixed with 5 mL of 0.5% KOH solution and stirred at 120 rpm for 20 min using a shaker (SI-300R, Jelotech, Gangneung, Republic of Korea) at 30 °C. The mixture was then centrifuged at 3000 rpm for 30 min. Subsequently, 0.05 mL of supernatant were collected, and the content of soluble nitrogen was determined using the anthrone method according to Starcher [29].
The method for determining the total nitrogen content involved completely hydrolyzing 0.1 g of powdered sample in 6 N hydrochloric acid (250 rpm, 100 °C, 24 h). After adding 5 mL of distilled water, the mixture was centrifuged at 3000 rpm for 30 min, and 0.05 mL of supernatant were collected. The total nitrogen content was then determined using the anthrone method. The calculation formula for NSI is as follows in Equation (10).
NSI (%) = Soluble nitrogen content/Total nitrogen content × 100.
2.10. Statistical Analysis
The statistical analysis of the results was conducted using the SPSS software (version 27.0, IBM-SPSS, Somers, New York, NY, USA), which is a statistical package commonly used in the social sciences. A one-way analysis of variance (ANOVA) was performed, followed by Duncan’s multiple range test to assess significant differences among groups, with a significance level set at p < 0.05.
3. Results
3.1. Viscosity
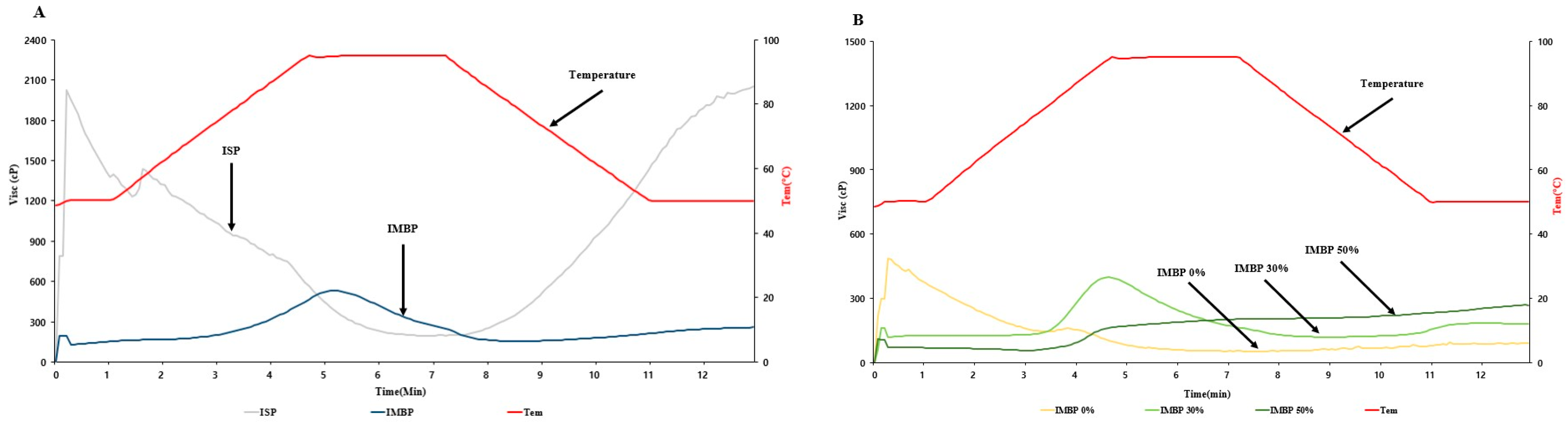
The Rapid Visco Analyzer (RVA) is utilized for predicting viscosity changes in protein before extrusion [30]. Additionally, viscosity is an important parameter to consider during extrusion processes as it affects the flow behavior and mechanical energy input [31]. The viscosity of the raw materials of isolated soy protein (ISP) and isolated mung bean protein (IMBP), as well as mixtures with 0% (no added mung bean protein), 30%, and 50% added IMBP, is illustrated in Figure 2. Panel A of Figure 2 shows the viscosity of the raw materials. ISP initially exhibits a high initial viscosity of approximately 2000 cP, which then decreases as the powder absorbs water during hydration and subsequently decreases with increased mechanical and thermal energy. In contrast, the initial viscosity of IMBP is lower, at less than 200 cP, and rapidly increases upon heating to approximately 95 °C. Therefore, the lower initial viscosity of IMBP compared to ISP may be attributed to its higher solubility [32]. According to Benjakul, and Kishimura [33], IMBP has the highest hydrophobic amino acid content compared to isolated black bean protein and isolated peanut protein, reaching 53.1%. Additionally, hydrophobic amino acids play a crucial role in the thermal stability of globulins [34]. Thus, the sudden increase in the viscosity of IMBP at high temperatures can be attributed to its higher thermal stability. Panel B of Figure 2 displays the viscosity of mixtures containing IMBP. Compared to IMBP 30% and IMBP 50%, IMBP 0% reaches the shortest peak viscosity peak time at 1.07 ± 0.00 min. However, IMBP 50% has a peak time of 6.98 ± 0.04 min, requiring a longer time. This indicates that the addition of IMBP binds moisture and results in protein denaturation, thus requiring more time and higher temperatures compared to samples without added IMBP [35].
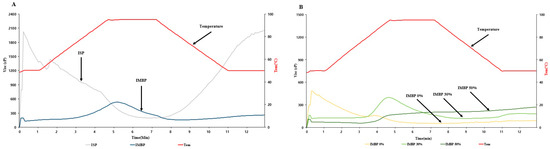
Figure 2.
RVA viscosity curves of ISP and IMBP (A). RVA viscosity curves of IMBP 0%, IMBP 30%, and IMBP 50% (B). Isolated soy protein (ISP); isolated mung bean protein (IMBP).
3.2. Specific Mechanical Energy
During the extrusion process, the specific mechanical energy (SME) input varies with the processing variables, such as moisture content, barrel temperature, screw speed, and feed rate. Therefore, the SME input is a key process parameter affecting the final extrudate’s appearance and physicochemical properties [36]. Table 1 illustrates the SME input as it varies with the addition of IMBP. Table 2 shows the F-values and p-values of the physicochemical and textural properties of high-moisture meat analogs with different isolated mung bean protein contents. When ISP content is replaced by 50% IMBP, the SME input reaches its highest value at 99.20 ± 0.19 kJ/kg. Brishti et al. [37] reported that, under the same experimental conditions, the minimum gelation concentrations for IMBP and ISP are 12% and 14%, respectively. A higher minimum gelation concentration indicates the lower gelation properties of the raw materials. Based on these results, it can be inferred that IMBP exhibits a relatively higher gelation ability compared to ISP, and as the content of IMBP increases, the viscosity of the mixture inside the barrel also increases. Additionally, the gelation ability of proteins indicates the degree of interaction between protein molecules, which correlates positively with viscosity [38]. The stronger the gelation ability of proteins, the higher the viscosity of the mixture after mixing with water in the barrel and the longer the residence time in the barrel, leading to a higher SME input required during the extrusion process. Therefore, it can be concluded that increasing the addition of IMBP leads to higher specific mechanical energy input due to enhanced gelation ability [39].

Table 1.
Specific mechanical energy input and water holding capacity of the high-moisture extrusion meat analog according to the content of isolated mung bean protein.

Table 2.
The F-values and p-values of the physicochemical and textural properties of the high-moisture meat analogs with different isolated mung bean protein contents.
3.3. Appearance
Figure 3 illustrates the appearance of the HMMAs produced based on varying levels of IMBP content. It can be observed that the content of IMBP is positively correlated with the fiber content of the HMMAs, with the highest fiber content achieved when the IMBP content reaches 40%. During the extrusion process, plant-based proteins exist as globular proteins before mixing with water. Upon hydration in the mixing zone, the size of globular protein particles increases, and a dispersed morphology is formed due to the encapsulation of free water by protein networks expanded by heating and shear forces [40]. It has been reported that the formation of fibrous structures in high-moisture meat analogs depends on the relative dynamics of protein aggregation and phase separation [41]. In the extrusion process with cooling dies, when the rate of protein aggregation is lower than the rate of phase separation, a stratified structure is observed [42]. Conversely, when the rate of protein aggregation exceeds the rate of phase separation, a non-oriented gel structure is formed [43]. According to Brishti et al. [37], compared to isolated soy protein, the isolated mung bean protein exhibits superior gelation ability. Increasing the content of isolated mung bean protein increases the viscosity of the protein melt inside the barrel, reducing the flowability of protein chains along the screw direction [44]. Therefore, the rate of protein aggregation through cooling dies is nearly equal to the rate of phase separation, thereby enhancing the formation of fibrous structures [13].

Figure 3.
Photographs of the fibrous structure of the high-moisture extrusion meat analogs at different isolated mung bean different contents. Isolated mung bean protein (IMBP).
3.4. Water Holding Capacity
Water holding capacity (WHC) refers to the amount of water that can be bound to the structure of meat analogs during hydration processes [9]. Wi et al. [45] reported that a higher water holding capacity of meat analogs results in juicier products. Table 1 presents the water holding capacity of high-moisture meat analogs calculated based on the isolated mung bean protein content. The highest water holding capacity was observed when the IMBP content was 0%, reaching 4.39 ± 0.12 g/g, while the lowest was observed when the IMBP replaced 50% of ISP, at 2.12 ± 0.07 g/g. It is inferred that the increase in the viscosity of the mixture inside the barrel after adding the isolated mung bean protein reduces the space for water permeation through capillaries, thus decreasing the water holding capacity. Additionally, the water holding capacity of meat analogs is positively correlated with the resilience and viscosity, consistent with the findings of Samard and Ryu [26].
3.5. Water Absorption Capacity and Oil Absorption Capacity
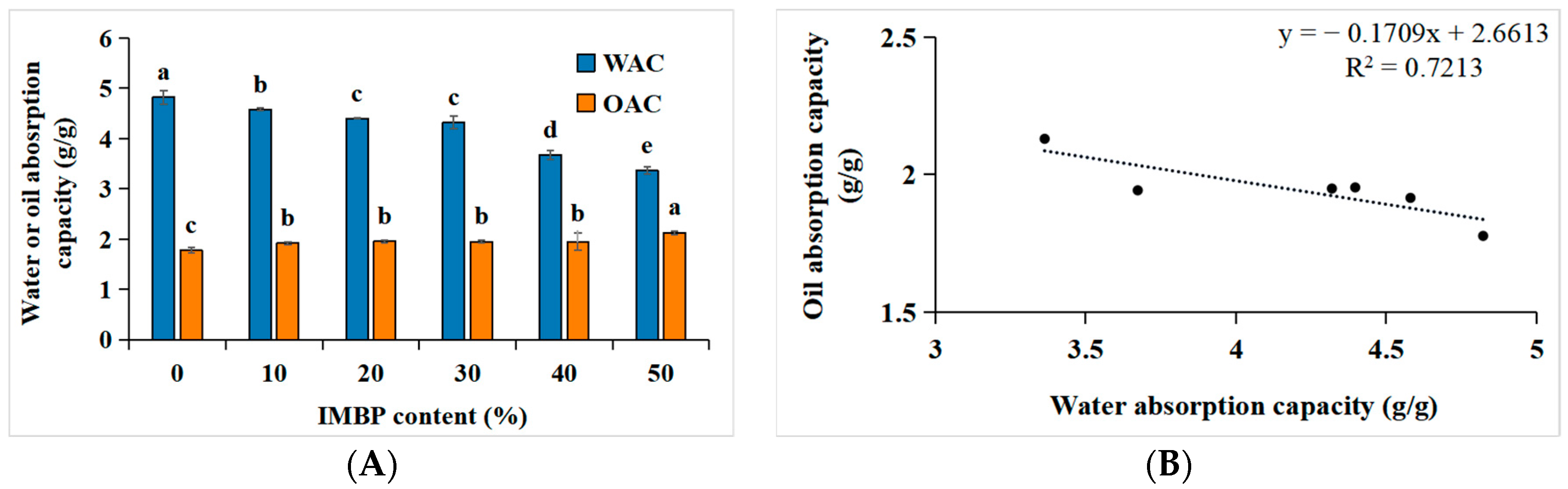
The water absorption capacity (WAC) and oil absorption capacity (OAC) of proteins are crucial for the product quality, including juiciness, texture, mouthfeel, reduction in moisture loss, preservation, and flavor retention [46]. Figure 4A,B depict the correlation between the WAC and OAC of high-moisture meat analogs and the content of IMBP. When IMBP is at 0%, the WAC is highest, at 4.82 ± 0.13 g/g, gradually decreasing with the increase in the content of isolated mung bean protein. Conversely, when IMBP reaches 50%, the OAC is highest, at 2.13 ± 0.04 g/g, gradually increasing with the content of IMBP. The decrease in WAC may be attributed to shear forces and pressures during the extrusion process leading to protein structural denaturation [9], exposing hydrophobic amino acids originally located internally [47]. Consequently, hydrogen bonding between hydrophilic amino acids and water weakens [48]. However, with the increase in the IMBP content, the OAC also increases. This is because the exposed hydrophobic amino acids in denatured proteins increase, allowing them to bind with oil [49]. Furthermore, the correlation between the WAC and OAC with the content of IMBP is illustrated in Figure 4B. As the WAC increases, the OAC decreases, showing a negative correlation (R2 = 0.7213). In summary, as the OAC increases with the increase in IMBP content, meat analogs containing IMBP are more suitable as intermediates compared to those containing ISP, particularly for flavor enhancement and texture improvement [50].
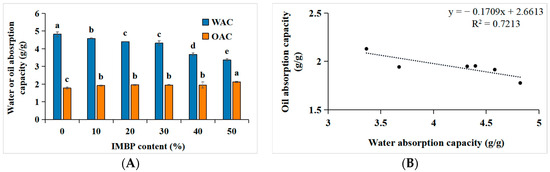
Figure 4.
Water and oil absorption capacity of high-moisture extruded meat analogs according to the isolated mung bean protein (IMBP) content (A). Relationship between the oil absorption capacity (OAC) and water absorption capacity (WAC) of high-moisture extruded meat analogs according to the isolated mung bean protein content (B). Values are means of triplicates ± standard deviation. Values with different letters (a–d) in the same bar indicate significant differences (p < 0.05) by Duncan’s multiple range test.
3.6. Integrity Index and Nitrogen Solubility Index
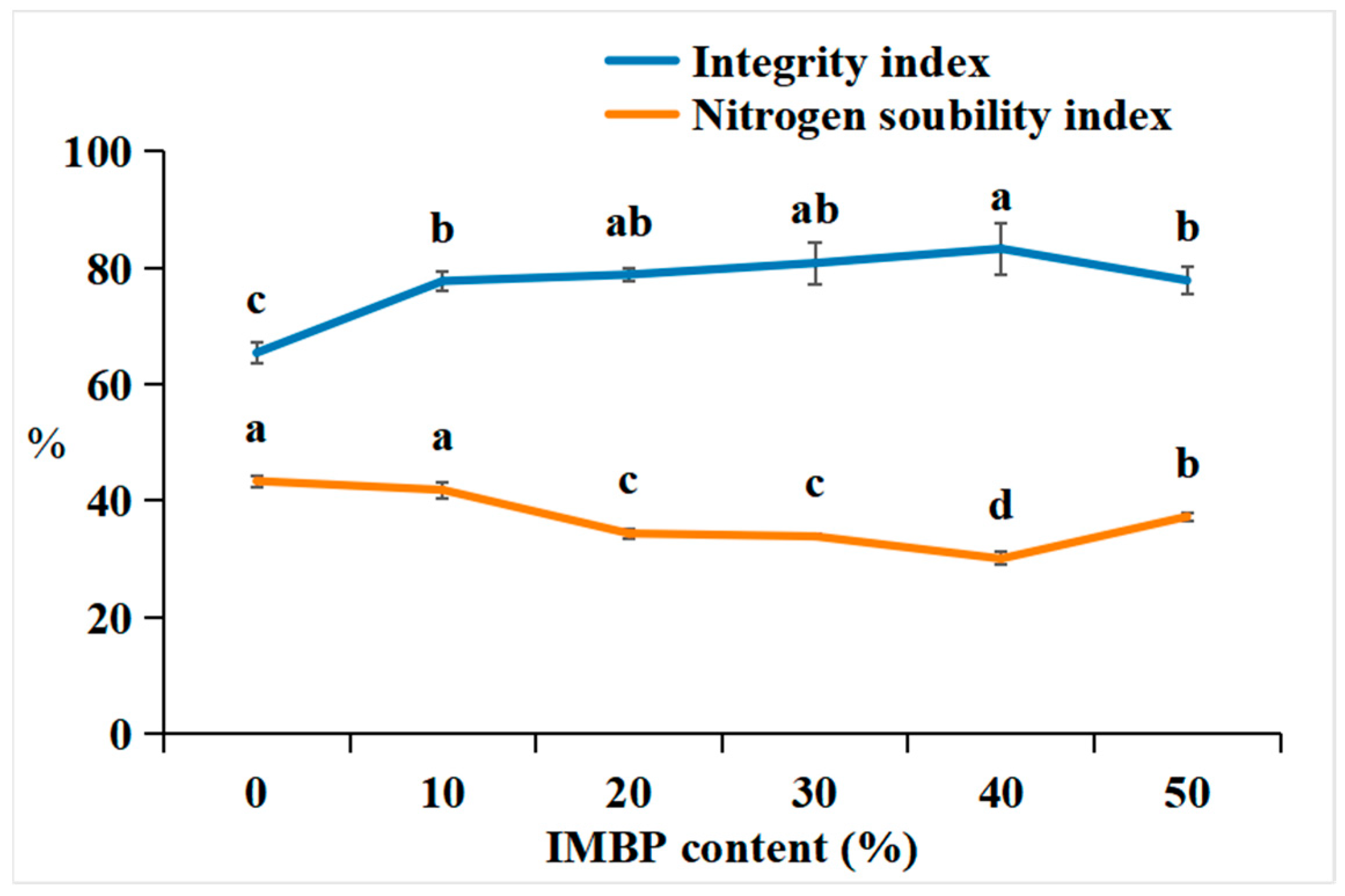
The integrity index is determined by measuring the residual residue of meat analogs after the hydration, pressing, homogenization, and drying processes, which ultimately affects the quality and yield of the meat analogs [51]. Figure 5 shows the integrity index and nitrogen solubility index (NSI) of the HMMAs with different contents of IMBP. The integrity index of the HMMAs increases when the content of IMBP reaches 40% and decreases when it reaches 50%. In texture analysis, the addition of IMBP increased the texture factor (chewiness) by 40%, attributed to the formation of a strong gel network between protein molecules, which can maintain and preserve the simulated meat structure even under high-temperature and high-pressure conditions. A lower integrity index was observed in samples with 50% IMBP content compared to those with 40%, possibly due to a reduction in disulfide bonds.
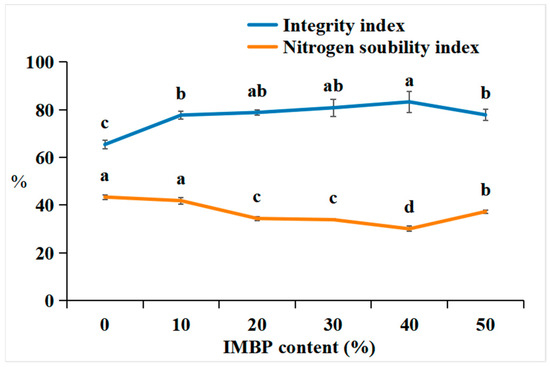
Figure 5.
Correlation between the integrity index and nitrogen solubility index of high-moisture extruded meat analogs according to the isolated mung bean protein (IMBP) content. Values are means of triplicates ± standard deviation. Values with different letters (a–d) in the same lineindicate significant differences (p < 0.05) by Duncan’s multiple range test.
The NSI is an important indicator for assessing the degree of protein denaturation during tissue processing [52]. The NSI of the HMMAs decreases until the content of IMBP reaches 40% and then increases again at 50%. The decrease in NSI at 40% is attributed to protein denaturation during high-moisture extrusion. This denaturation is believed to increase protein–protein interactions [51], and IMBP forms relatively stronger gels in the subsequent 50%. This results in a decrease in shear forces acting on the protein gel network dispersed in the barrel due to reduced intra- and intermolecular interactions. Therefore, the binding strength of the new protein network decreases [53].
3.7. Texture Profile Analysis and Cutting Strength
The texture of meat analogs is a critical factor in mimicking the sensory taste of real meat [45]. Resilience measures the extent to which a sample recovers after deformation caused by external forces, while cohesion represents the strength of internal binding [54]. Chewiness is considered the most crucial factor in expressing texture sensory impressions [41]. The texture and cutting strength of high-moisture meat analogs are shown in Table 3. When the content of IMBP is 0%, the resilience is the highest, at 91.26 ± 0.82%. However, the resilience decreases with the increase in the IMBP content. As the IMBP content increases, the protein content of meat analogs decreases, resulting in decreased resilience. This is consistent with the findings of Jeon, Gu, and Ryu [55], which showed that the resilience of high-moisture meat analogs decreases with the increase in the yeast content. Moreover, when the IMBP content is 0%, cohesion is the highest, at 76.89 ± 2.45%. However, cohesion decreases with the increase in the IMBP content to 50%, reaching 55.81 ± 1.77%. This is because protein–protein interactions between ISP molecules are stronger than those between IMBP molecules, leading to a decrease in the internal binding strength with the increase in the IMBP content [39]. There were no significant differences in chewiness between 10% and 40% (including ISP and IMBP). These results may be attributed to the enhanced interactions between ISP and IMBP, forming a strong gel network [33]. On the other hand, the chewiness of 50% IMBP is the lowest, at 3649.69 ± 281.40 g. This is due to a reduction in disulfide bonds, which are key components in forming the three-dimensional internal structure of meat analogs [56]. A study comparing the sulfur amino acid content of ISP and IMBP also supports these results, indicating a relatively lower sulfur amino acid content in IMBP compared to ISP [57].

Table 3.
Texture properties of high-moisture extrusion meat analogs according to the IMBP content.
The cutting strength of meat analogs determined by the IMBP content indicates that all samples have a higher cutting strength in the vertical direction than in the flow direction. These results suggest that all samples exhibit a fibrous structure, consistent with the report by Webb, Li, and Alavi [58]. Compared to samples containing both ISP and IMBP, samples with 50% IMBP replacing ISP have lower values in resilience, cohesion, chewiness, and cutting strength. These results are aligned with the findings of Samard and Ryu [59], who reported that overall texture characteristics of meat analogs based on IMBP are lower compared to those based on ISP. In conclusion, the complete replacement of ISP with IMBP may lead to a decrease in the overall texture characteristics. However, it holds promise as an intermediate material helpful in adjusting texture characteristics [58].
4. Conclusions
The addition of isolated mung bean protein leads to changes in the fibrous structure of high-moisture meat analogs. When the content of isolated mung bean protein is 40–50%, the fibrous structure closely resembles that of real meat. In the texture profile analysis (TPA), the chewiness of the HMMAs is relatively high when the content of isolated mung bean protein is in the range of 10–40%. This suggests its potential direct use in products. However, when isolated mung bean protein replaces 50% of isolated soy protein, the resulting HMMA texture is softer with a lower chewiness. Therefore, it is recommended as an intermediate material to be mixed with various ingredients. In plant proteins, oil plays a crucial role in achieving texture like that of animal proteins. The highest oil absorption capacity is observed when the content of isolated mung bean protein is 50%, indicating that isolated mung bean protein is more suitable than isolated soy protein for making plant-based meat analogs and dairy products. Further research is needed to optimize the variables by changing independent variables, such as the moisture content, screw speed, and barrel temperature, to find suitable meat analogs for processing. This study demonstrates the potential and feasibility of using isolated mung bean protein instead of isolated soy protein, which is currently the main ingredient used in meat analog production.
Author Contributions
Conceptualization, N.-K.H., B.-J.G., and G.-H.R.; methodology, N.-K.H. and Y.Z.; software, N.-K.H.; validation, N.-K.H. and Y.Z.; formal analysis, N.-K.H.; investigation, N.-K.H. and Y.Z.; resources, B.-J.G. and G.-H.R.; data curation, N.-K.H.; writing—original draft preparation, N.-K.H., B.-J.G., and Y.Z.; writing—review and editing, B.-J.G. and Y.Z.; visualization, N.-K.H.; supervision, B.-J.G. and G.-H.R.; project administration, B.-J.G. and G.-H.R.; funding acquisition, B.-J.G. and G.-H.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through the High Value-added Food Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA), grant number 321021033HD020. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF-2022R1C1C1011094).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- UN P.D. World Population Prospects: 2019: Highlights; UN: New York, NY, USA, 2019; ISBN 978-92-1-148316-1. [Google Scholar]
- Rubio, N.R.; Xiang, N.; Kaplan, D.L. Plant-Based and Cell-Based Approaches to Meat Production. Nat. Commun. 2020, 11, 6276. [Google Scholar] [CrossRef] [PubMed]
- Bonny, S.P.F.; Gardner, G.E.; Pethick, D.W.; Hocquette, J.-F. Artificial Meat and the Future of the Meat Industry. Anim. Prod. Sci. 2017, 57, 2216–2223. [Google Scholar] [CrossRef]
- Jeon, Y.-H.; Gu, B.-J.; Ryu, G.-H. Investigating the Potential of Full-Fat Soy as an Alternative Ingredient in the Manufacture of Low- and High-Moisture Meat Analogs. Foods 2023, 12, 1011. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Seo, M.S.; Jung, S.Y.; Park, B.; Park, S.Y. Effects of Soy Defatting on Texturization of Texturized Vegetable Proteins. Food Sci. Preserv. 2023, 30, 875–884. [Google Scholar] [CrossRef]
- Asgar, M.A.; Fazilah, A.; Huda, N.; Bhat, R.; Karim, A.A. Nonmeat Protein Alternatives as Meat Extenders and Meat Analogs. Compr. Rev. Food Sci. Food Saf. 2010, 9, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, V.L.; Emin, M.A.; Schuchmann, H.P. Process Conditions Influencing Wheat Gluten Polymerization during High Moisture Extrusion of Meat Analog Products. J. Food Eng. 2017, 198, 28–35. [Google Scholar] [CrossRef]
- Gu, B.-J.; Kerr, C.J.; Morris, C.F.; Ganjyal, G.M. Soft Durum Wheat as a Potential Ingredient for Direct Expanded Extruded Products. J. Cereal Sci. 2021, 98, 103184. [Google Scholar] [CrossRef]
- Lin, S.; Huff, H.E.; Hsieh, F. Extrusion Process Parameters, Sensory Characteristics, and Structural Properties of a High Moisture Soy Protein Meat Analog. J. Food Sci. 2002, 67, 1066–1072. [Google Scholar] [CrossRef]
- Chiang, J.H.; Loveday, S.M.; Hardacre, A.K.; Parker, M.E. Effects of Soy Protein to Wheat Gluten Ratio on the Physicochemical Properties of Extruded Meat Analogues. Food Struct. 2019, 19, 100102. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Zhang, Y.; Meng, S.; Wang, Q. Rheological Properties of Pea Protein Isolate-Amylose/Amylopectin Mixtures and the Application in the High-Moisture Extruded Meat Substitutes. Food Hydrocoll. 2021, 117, 106732. [Google Scholar] [CrossRef]
- Cornet, S.H.V.; Bühler, J.M.; Gonçalves, R.; Bruins, M.E.; van der Sman, R.G.M.; van der Goot, A.J. Apparent Universality of Leguminous Proteins in Swelling and Fibre Formation When Mixed with Gluten. Food Hydrocoll. 2021, 120, 106788. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Q.; Liu, L.; Zhang, Y.; He, N.; Wang, Q. High-Moisture Extrusion Process of Transglutaminase-Modified Peanut Protein: Effect of Transglutaminase on the Mechanics of the Process Forming a Fibrous Structure. Food Hydrocoll. 2021, 112, 106346. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Jeon, K.-H.; Park, J.-D.; Sung, J.-M.; Seo, D.-H.; Ku, S.-K.; Oh, N.-S.; Kim, Y.-B. Comparison of Pork Patty Quality Characteristics with Various Binding Agents. Korean J. Food Cook. Sci. 2015, 31, 588–595. [Google Scholar] [CrossRef]
- Kerwin, S.M. Soy Saponins and the Anticancer Effects of Soybeans and Soy-Based Foods. Curr. Med. Chem.—Anti-Cancer Agents 2004, 4, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Jiang, W.; Zhang, D.; Liu, H.; Sun, B. Textural, Sensory and Volatile Compounds Analyses in Formulations of Sausages Analogue Elaborated with Edible Mushrooms and Soy Protein Isolate as Meat Substitute. Foods 2022, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Villaluenga, C.; Gulewicz, P.; Frias, J.; Gulewicz, K.; Vidal-Valverde, C. Assessment of Protein Fractions of Three Cultivars of Pisum sativum L.: Effect of Germination. Eur. Food Res. Technol. 2008, 226, 1465–1478. [Google Scholar] [CrossRef]
- Hou, D.; Zhao, Q.; Yousaf, L.; Khan, J.; Xue, Y.; Shen, Q. Consumption of Mung Bean (Vigna radiata L.) Attenuates Obesity, Ameliorates Lipid Metabolic Disorders and Modifies the Gut Microbiota Composition in Mice Fed a High-Fat Diet. J. Funct. Foods 2020, 64, 103687. [Google Scholar] [CrossRef]
- Chai, W.-M.; Wei, Q.-M.; Deng, W.-L.; Zheng, Y.-L.; Chen, X.-Y.; Huang, Q.; Ou-Yang, C.; Peng, Y.-Y. Anti-Melanogenesis Properties of Condensed Tannins from Vigna angularis Seeds with Potent Antioxidant and DNA Damage Protection Activities. Food Funct. 2019, 10, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Xie, J.; Gong, B.; Xu, X.; Tang, W.; Li, X.; Li, C.; Xie, M. Extraction, Physicochemical Characteristics and Functional Properties of Mung Bean Protein. Food Hydrocoll. 2018, 76, 131–140. [Google Scholar] [CrossRef]
- Mubarak, A.E. Nutritional Composition and Antinutritional Factors of Mung Bean Seeds (Phaseolus aureus) as Affected by Some Home Traditional Processes. Food Chem. 2005, 89, 489–495. [Google Scholar] [CrossRef]
- Kohno, M.; Sugano, H.; Shigihara, Y.; Shiraishi, Y.; Motoyama, T. Improvement of Glucose and Lipid Metabolism via Mung Bean Protein Consumption: Clinical Trials of GLUCODIATM Isolated Mung Bean Protein in the USA and Canada. J. Nutr. Sci. 2018, 7, e2. [Google Scholar] [CrossRef] [PubMed]
- Brishti, F.H.; Chay, S.Y.; Muhammad, K.; Ismail-Fitry, M.R.; Zarei, M.; Saari, N. Texturized Mung Bean Protein as a Sustainable Food Source: Effects of Extrusion on Its Physical, Textural and Protein Quality. Innov. Food Sci. Emerg. Technol. 2021, 67, 102591. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, T.Z.; Wang, X.; Reimer, M.; Isaak, C.; Ai, Y. Behaviors of Starches Evaluated at High Heating Temperatures Using a New Model of Rapid Visco Analyzer—RVA 4800. Food Hydrocoll. 2019, 94, 217–228. [Google Scholar] [CrossRef]
- Ryu, G.-H.; Mulvaney, S.J. Analysis of Physical Properties and Mechanical Energy Input of Cornmeal Extrudates Fortified with Dairy Products by Carbon Dioxide Injection. Korean J. Food Sci. Technol. 1997, 29, 947–954. [Google Scholar]
- Samard, S.; Ryu, G.-H. Physicochemical and Functional Characteristics of Plant Protein-Based Meat Analogs. J. Food Process. Preserv. 2019, 43, e14123. [Google Scholar] [CrossRef]
- Gu, B.Y.; Ryu, G.H. Effects of Moisture Content and Screw Speed on Physical Properties of Extruded Soy Protein Isolate. J. Korean Soc. Food Sci. Nutr. 2017, 46, 751–758. [Google Scholar] [CrossRef]
- Căpriță, R.; Căpriță, A.; Crețescu, I. Protein Solubility as Quality Index for Processed Soybean. Sci. Pap. Anim. Sci. Biotechnol. 2010, 43, 375. [Google Scholar]
- Starcher, B. A Ninhydrin-Based Assay to Quantitate the Total Protein Content of Tissue Samples. Anal. Biochem. 2001, 292, 125–129. [Google Scholar] [CrossRef]
- Almeida-Dominguez, H.D.; Suhendro, E.L.; Rooney, L.W. Factors Affecting Rapid Visco Analyser Curves for the Determination of Maize Kernel Hardness. J. Cereal Sci. 1997, 25, 93–102. [Google Scholar] [CrossRef]
- Webb, D.; Plattner, B.J.; Donald, E.; Funk, D.; Plattner, B.S.; Alavi, S. Role of Chickpea Flour in Texturization of Extruded Pea Protein. J. Food Sci. 2020, 85, 4180–4187. [Google Scholar] [CrossRef]
- Osen, R.; Toelstede, S.; Wild, F.; Eisner, P.; Schweiggert-Weisz, U. High Moisture Extrusion Cooking of Pea Protein Isolates: Raw Material Characteristics, Extruder Responses, and Texture Properties. J. Food Eng. 2014, 127, 67–74. [Google Scholar] [CrossRef]
- Kudre, T.G.; Benjakul, S.; Kishimura, H. Comparative Study on Chemical Compositions and Properties of Protein Isolates from Mung Bean, Black Bean and Bambara Groundnut. J. Sci. Food Agric. 2013, 93, 2429–2436. [Google Scholar] [CrossRef]
- Gorinstein, S.; Zemser, M.; Paredes-López, O. Structural Stability of Globulins. J. Agric. Food Chem. 1996, 44, 100–105. [Google Scholar] [CrossRef]
- Zahari, I.; Ferawati, F.; Helstad, A.; Ahlström, C.; Östbring, K.; Rayner, M.; Purhagen, J.K. Development of High-Moisture Meat Analogues with Hemp and Soy Protein Using Extrusion Cooking. Foods 2020, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.H. Texturization of Plant Protein by Using Extrusion Process. Food Eng. Prog. 2003, 7, 73–79. [Google Scholar]
- Brishti, F.H.; Zarei, M.; Muhammad, S.K.S.; Ismail-Fitry, M.R.; Shukri, R.; Saari, N. Evaluation of the Functional Properties of Mung Bean Protein Isolate for Development of Textured Vegetable Protein. Int. Food Res. J. 2017, 24, 1595–1605. [Google Scholar]
- Chen, X.; Xu, X.; Liu, D.; Zhou, G.; Han, M.; Wang, P. Rheological Behavior, Conformational Changes and Interactions of Water-Soluble Myofibrillar Protein during Heating. Food Hydrocoll. 2018, 77, 524–533. [Google Scholar] [CrossRef]
- Lee, J.-S.; Choi, I.; Han, J. Construction of Rice Protein-Based Meat Analogues by Extruding Process: Effect of Substitution of Soy Protein with Rice Protein on Dynamic Energy, Appearance, Physicochemical, and Textural Properties of Meat Analogues. Food Res. Int. 2022, 161, 111840. [Google Scholar] [CrossRef] [PubMed]
- Mession, J.-L.; Assifaoui, A.; Lafarge, C.; Saurel, R.; Cayot, P. Protein Aggregation Induced by Phase Separation in a Pea Proteins–Sodium Alginate–Water Ternary System. Food Hydrocoll. 2012, 28, 333–343. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Q.; Kaplan, D.L.; Wang, Q. High-Moisture Extruded Protein Fiber Formation toward Plant-Based Meat Substitutes Applications: Science, Technology, and Prospect. Trends Food Sci. Technol. 2022, 128, 202–216. [Google Scholar] [CrossRef]
- Sandoval Murillo, J.L.; Osen, R.; Hiermaier, S.; Ganzenmüller, G. Towards Understanding the Mechanism of Fibrous Texture Formation during High-Moisture Extrusion of Meat Substitutes. J. Food Eng. 2019, 242, 8–20. [Google Scholar] [CrossRef]
- Dekkers, B.L.; Boom, R.M.; van der Goot, A.J. Structuring Processes for Meat Analogues. Trends Food Sci. Technol. 2018, 81, 25–36. [Google Scholar] [CrossRef]
- Verbeek, C.J.R.; van den Berg, L.E. Extrusion Processing and Properties of Protein-Based Thermoplastics. Macromol. Mater. Eng. 2010, 295, 10–21. [Google Scholar] [CrossRef]
- Wi, G.; Bae, J.; Kim, H.; Cho, Y.; Choi, M.-J. Evaluation of the Physicochemical and Structural Properties and the Sensory Characteristics of Meat Analogues Prepared with Various Non-Animal Based Liquid Additives. Foods 2020, 9, 461. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Gao, Z.; Fang, B.; Rao, J.; Chen, B. Ferreting out the Secrets of Industrial Hemp Protein as Emerging Functional Food Ingredients. Trends Food Sci. Technol. 2021, 112, 1–15. [Google Scholar] [CrossRef]
- Schmid, E.-M.; Farahnaky, A.; Adhikari, B.; Torley, P.J. High Moisture Extrusion Cooking of Meat Analogs: A Review of Mechanisms of Protein Texturization. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4573–4609. [Google Scholar] [CrossRef]
- Mandliya, S.; Pratap-Singh, A.; Vishwakarma, S.; Dalbhagat, C.G.; Mishra, H.N. Incorporation of Mycelium (Pleurotus eryngii) in Pea Protein Based Low Moisture Meat Analogue: Effect on Its Physicochemical, Rehydration and Structural Properties. Foods 2022, 11, 2476. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, J.E.; Melachouris, N. Functional Properties of Proteins in Foods: A Survey. CRC Crit. Rev. Food Sci. Nutr. 1976, 7, 219–280. [Google Scholar] [CrossRef]
- Brishti, F.H.; Chay, S.Y.; Muhammad, K.; Ismail-Fitry, M.R.; Zarei, M.; Karthikeyan, S.; Saari, N. Effects of Drying Techniques on the Physicochemical, Functional, Thermal, Structural and Rheological Properties of Mung Bean (Vigna radiata) Protein Isolate Powder. Food Res. Int. 2020, 138, 109783. [Google Scholar] [CrossRef] [PubMed]
- Samard, S.; Gu, B.-Y.; Ryu, G.-H. Effects of Extrusion Types, Screw Speed and Addition of Wheat Gluten on Physicochemical Characteristics and Cooking Stability of Meat Analogues. J. Sci. Food Agric. 2019, 99, 4922–4931. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, J.; Wang, S.; Qi, M.; Yue, M.; Zhang, S.; Song, J.; Wang, C.; Zhang, D.; Wang, X.; et al. High Moisture Extrusion of Pea Protein: Effect of l-Cysteine on Product Properties and the Process Forming a Fibrous Structure. Food Hydrocoll. 2022, 129, 107633. [Google Scholar] [CrossRef]
- Meng, A.; Li, F.; Chen, F.; Luan, B.; Sun, T.; Zhang, B. Relationship between the Physicochemical Properties of Soybean Protein Isolate and Its Extrudate Based on High-Moisture Extrusion Torque. J. Texture Stud. 2023, 54, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Chandra, M.V.; Shamasundar, B.A. Texture Profile Analysis and Functional Properties of Gelatin from the Skin of Three Species of Fresh Water Fish. Int. J. Food Prop. 2015, 18, 572–584. [Google Scholar] [CrossRef]
- Jeon, Y.-H.; Gu, B.-J.; Ryu, G.-H. Effects of Brewer’s Spent Yeast Content on the Physicochemical Properties of Extruded High-Moisture Meat Analog. J. Korean Soc. Food Sci. Nutr. 2022, 51, 1084–1090. [Google Scholar] [CrossRef]
- Kaleda, A.; Talvistu, K.; Vaikma, H.; Tammik, M.-L.; Rosenvald, S.; Vilu, R. Physicochemical, Textural, and Sensorial Properties of Fibrous Meat Analogs from Oat-Pea Protein Blends Extruded at Different Moistures, Temperatures, and Screw Speeds. Future Foods 2021, 4, 100092. [Google Scholar] [CrossRef]
- Ge, J.; Sun, C.-X.; Mata, A.; Corke, H.; Gan, R.-Y.; Fang, Y. Physicochemical and pH-Dependent Functional Properties of Proteins Isolated from Eight Traditional Chinese Beans. Food Hydrocoll. 2021, 112, 106288. [Google Scholar] [CrossRef]
- Webb, D.; Li, Y.; Alavi, S. Chemical and Physicochemical Features of Common Plant Proteins and Their Extrudates for Use in Plant-Based Meat. Trends Food Sci. Technol. 2023, 131, 129–138. [Google Scholar] [CrossRef]
- Samard, S.; Ryu, G.-H. A Comparison of Physicochemical Characteristics, Texture, and Structure of Meat Analogue and Meats. J. Sci. Food Agric. 2019, 99, 2708–2715. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).