Ingredient Functionality of Soy, Chickpea, and Pea Protein before and after Dry Heat Pretreatment and Low Moisture Extrusion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methodology
2.3. Ingredient Characterization
2.3.1. Heat Pretreatment
2.3.2. Pasting Properties
2.3.3. Water Absorption Capacity
2.3.4. Particle Size Distribution
2.3.5. Color
2.3.6. DSC
2.4. Extrusion Processing
2.5. Extrudate Characterization
2.5.1. Physical Properties
2.5.2. Textural Properties
2.5.3. Water-Holding Capacity
2.6. Statistical Methodology
2.6.1. Normality Testing
2.6.2. Analysis of Variance (ANOVA)
2.6.3. Kruskal–Wallis Test
2.6.4. Partial Least Squares Regression
2.6.5. Monte Carlo Simulation
3. Results and Discussion
3.1. Ingredient Functionality
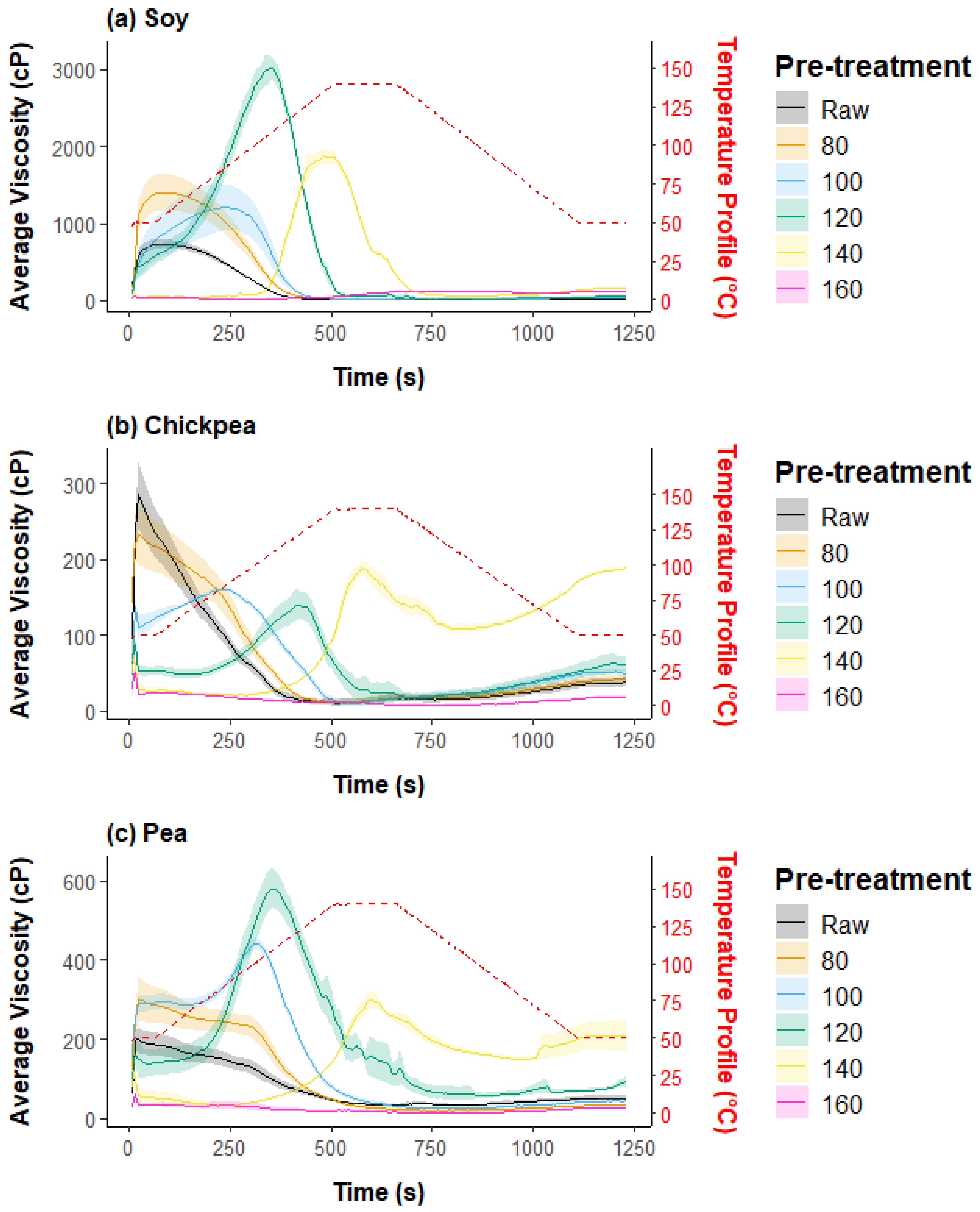
3.1.1. Pasting Properties
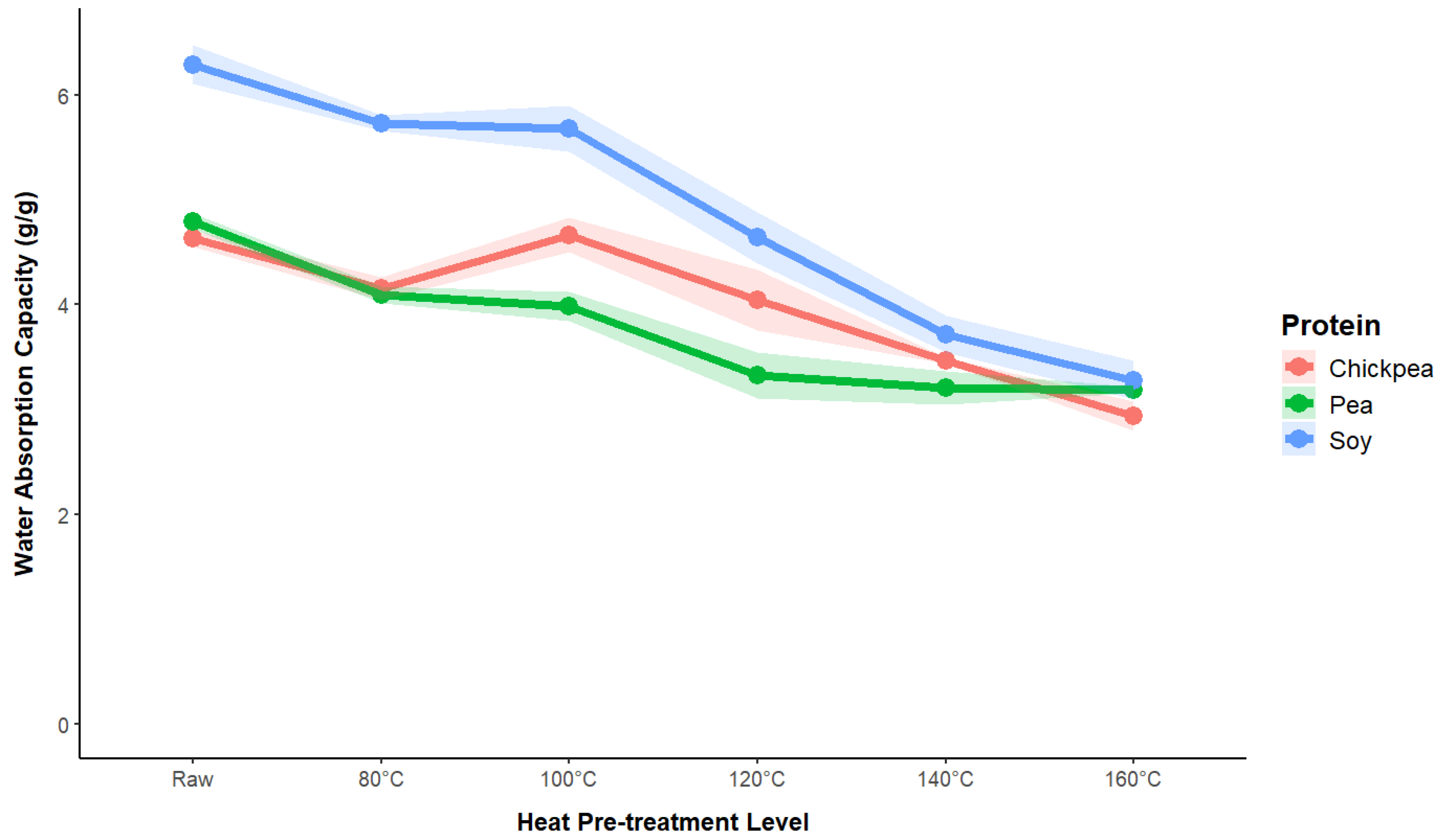
3.1.2. Water Absorption Capacity
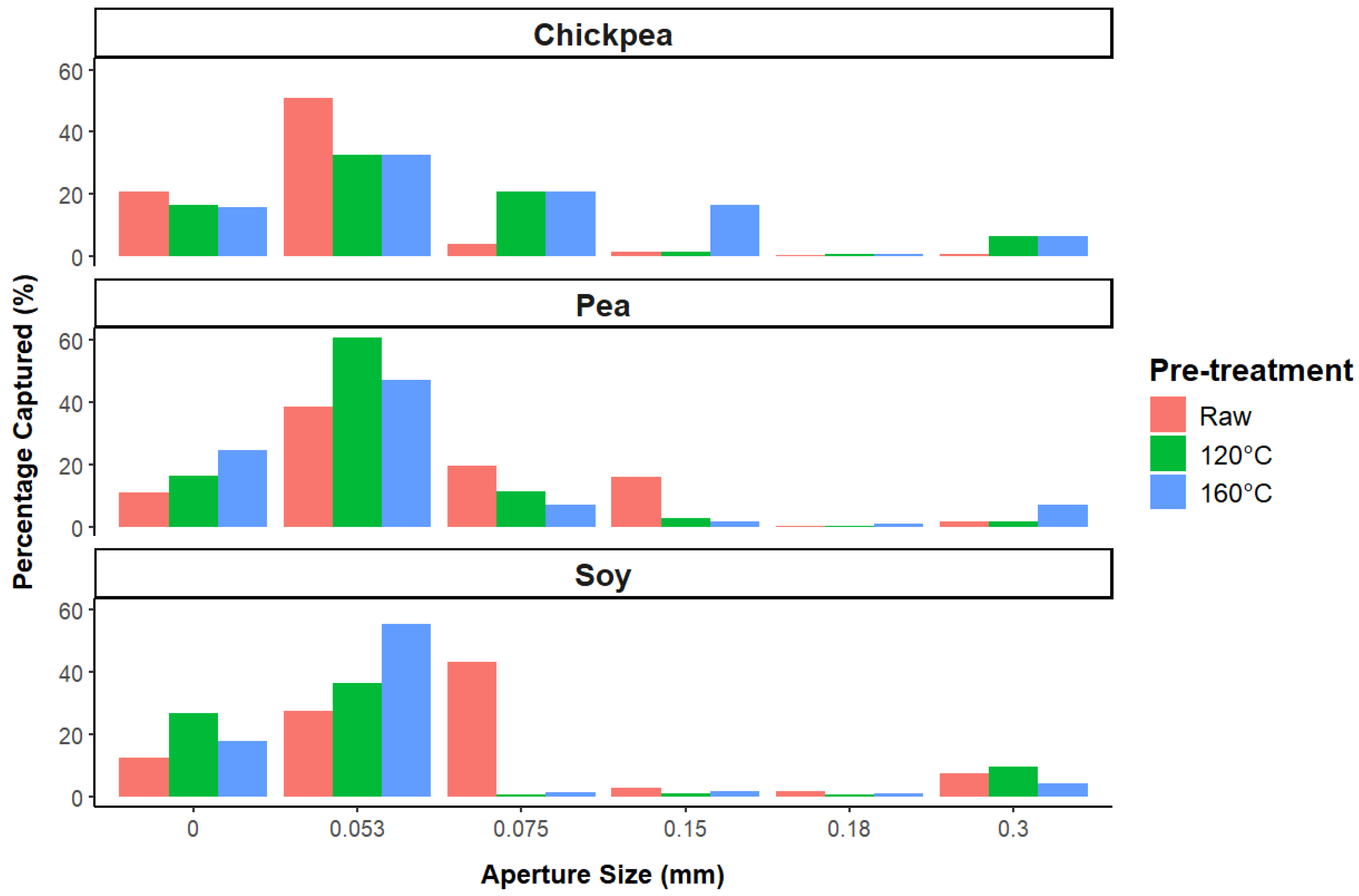
3.1.3. Particle Size Distribution
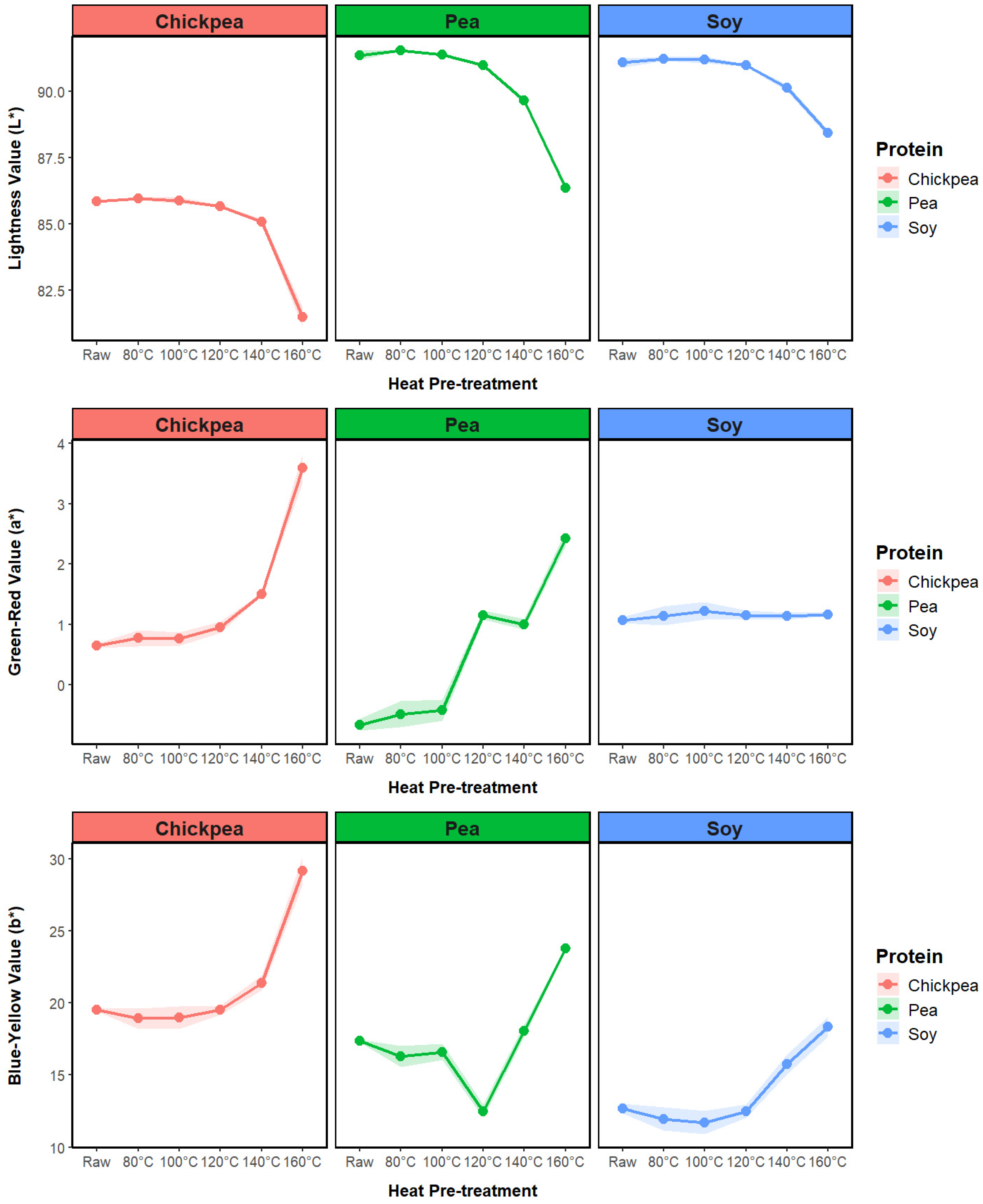
3.1.4. Powder Color
3.1.5. DSC
3.2. Extrusion Outcomes
3.2.1. Visual Appearance
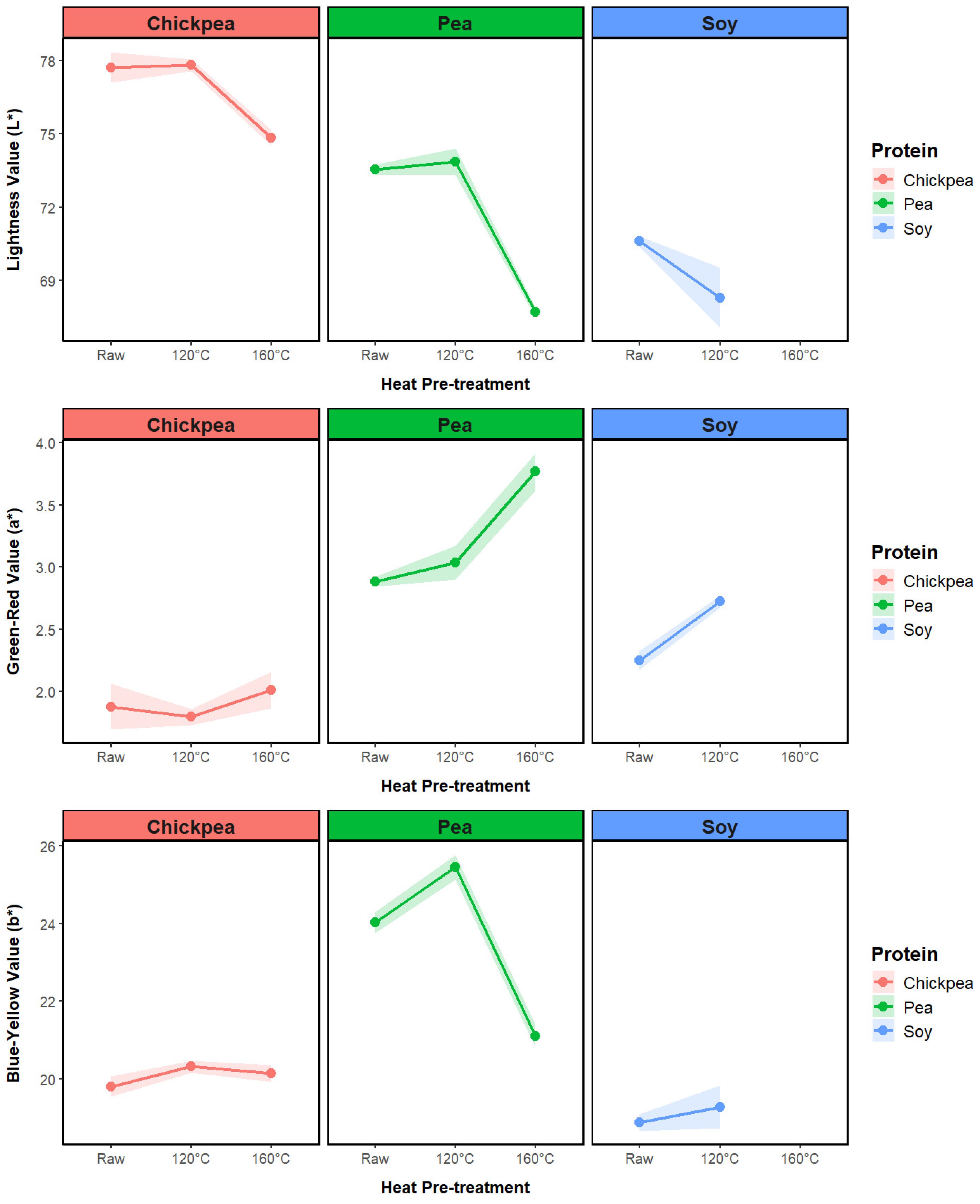
3.2.2. Extrudate Color
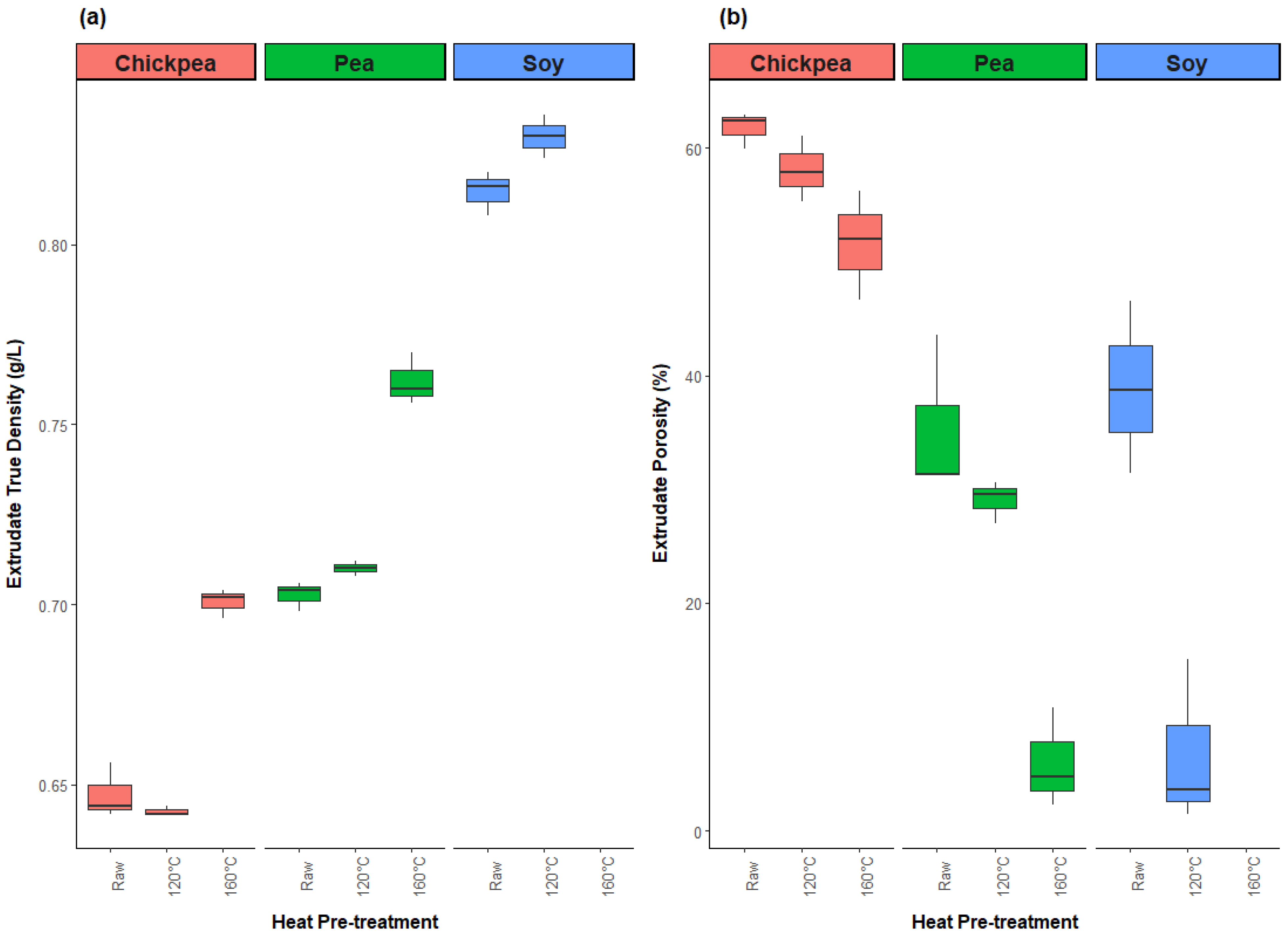
3.2.3. Physical Properties
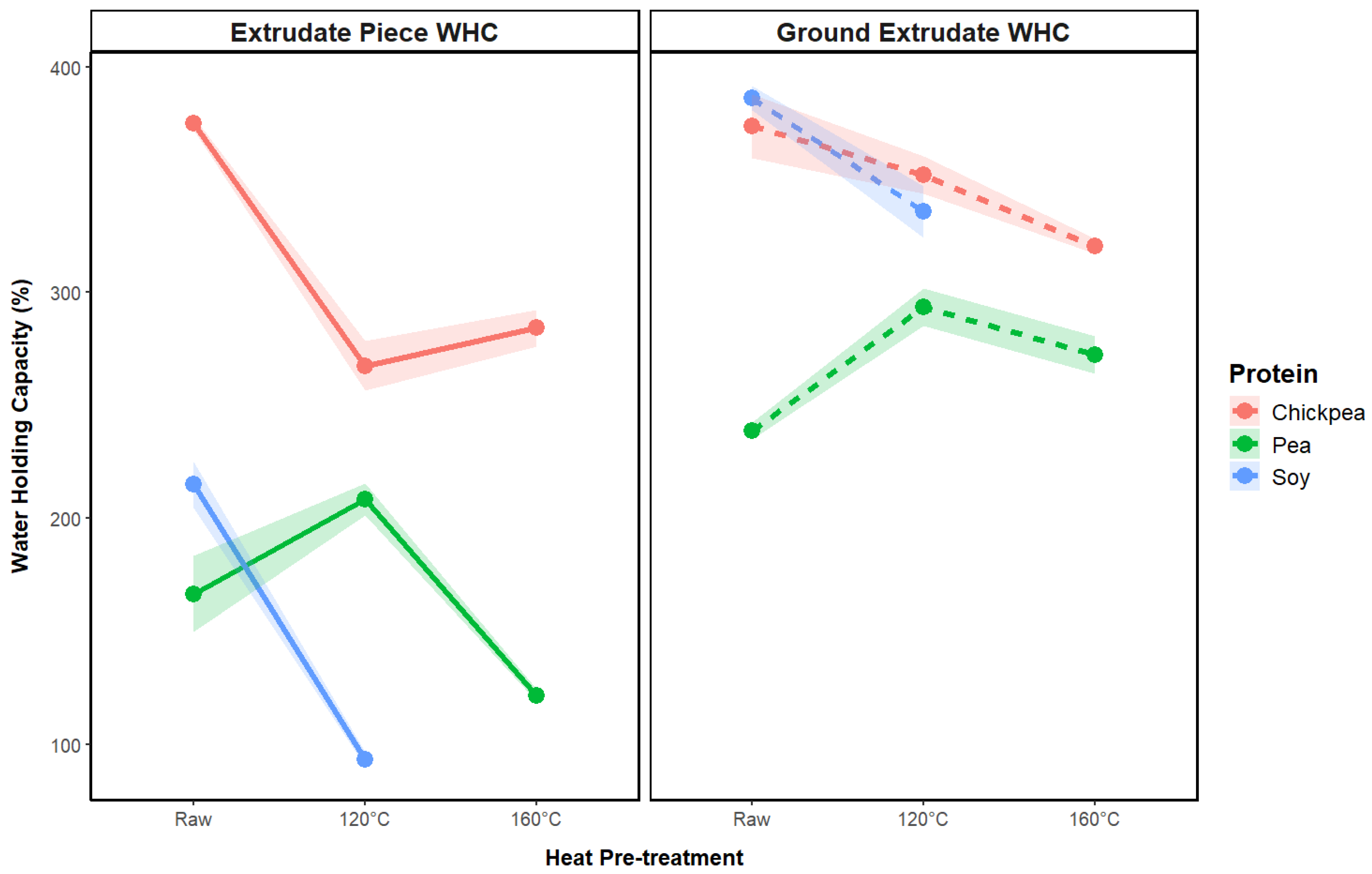
3.2.4. Water-Holding Capacity
3.2.5. Textural Properties
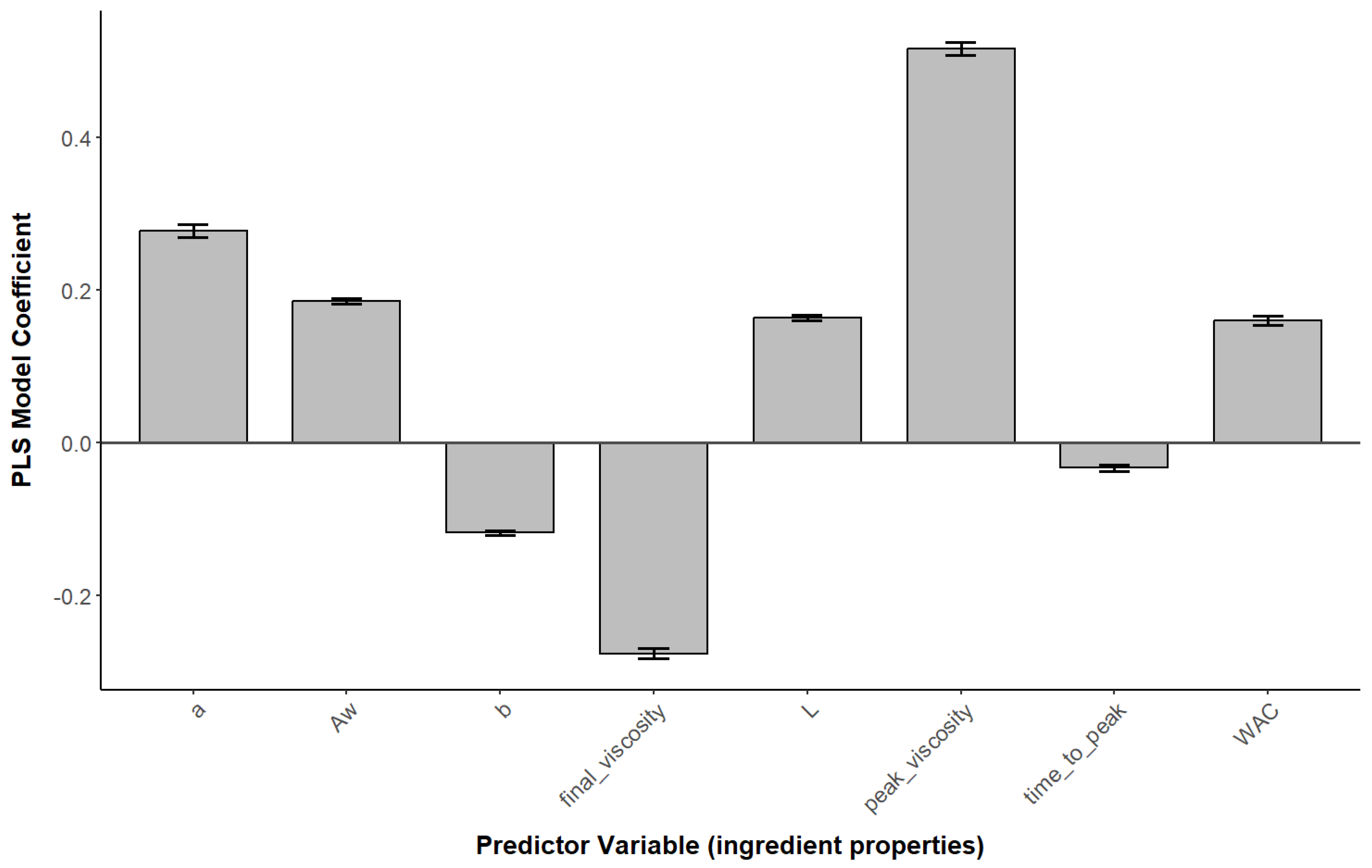
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wittek, P.; Ellwanger, F.; Karbstein, H.P.; Emin, M.A. Morphology development and flow characteristics during high moisture extrusion of a plant-based meat analogue. Foods 2021, 10, 1753. [Google Scholar] [CrossRef]
- Baune, M.-C.; Terjung, N.; Tülbek, M.Ç.; Boukid, F. Textured vegetable proteins (TVP): Future foods standing on their merits as meat alternatives. Future Foods 2022, 6, 100181. [Google Scholar] [CrossRef]
- The Good Food Institute. Plant-Based Meat: Anticipating 2030 Production Requirements; Good Food Institute: Washington, DC, USA, 2022. [Google Scholar]
- Kurek, M.A.; Onopiuk, A.; Pogorzelska-Nowicka, E.; Szpicer, A.; Zalewska, M. Novel protein sources for applications in meat-alternative products—Insight and challenges. Foods 2022, 11, 957. [Google Scholar] [CrossRef]
- Asen, N.D.; Aluko, R.E.; Martynenko, A.; Utioh, A.; Bhowmik, P. Yellow Field Pea Protein (Pisum sativum L.): Extraction Technologies, Functionalities, and Applications. Foods 2023, 12, 3978. [Google Scholar] [CrossRef]
- Sirtori, E.; Isak, I.; Resta, D.; Boschin, G.; Arnoldi, A. Mechanical and thermal processing effects on protein integrity and peptide fingerprint of pea protein isolate. Food Chem. 2012, 134, 113–121. [Google Scholar] [CrossRef]
- Miranda, C.G.; Speranza, P.; Kurozawa, L.E.; Sato, A.C.K. Lentil protein: Impact of different extraction methods on structural and functional properties. Heliyon 2022, 8, 11775. [Google Scholar] [CrossRef]
- Hewage, A.; Olatunde, O.O.; Nimalaratne, C.; Boschin, G.; Arnoldi, A. Novel Extraction technologies for developing plant protein ingredients with improved functionality. Trends Food Sci. Technol. 2022, 129, 492–511. [Google Scholar] [CrossRef]
- Zayas, J.F. Functionality of Proteins in Food; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Loveday, S.M.; Halim, C. Protein isolates made in the laboratory versus the factory: A case study with mung bean and chickpea. Sustain. Food Proteins 2024, 2, 89–98. [Google Scholar] [CrossRef]
- Sari, Y.W.; Mulder, W.J.; Sanders, J.P.M.; Bruins, M.E. Towards plant protein refinery: Review on protein extraction using alkali and potential enzymatic assistance. Biotechnol. J. 2015, 10, 1138–1157. [Google Scholar] [CrossRef] [PubMed]
- Schmid, E.M.; Farahnaky, A.; Adhikari, B.; Torley, P.J. High moisture extrusion cooking of meat analogs: A review of mechanisms of protein texturization. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4573–4609. [Google Scholar] [CrossRef]
- Ubbink, J.; Muhialdin, B.J. Protein physical state in meat analogue processing. Curr. Opin. Food Sci. 2022, 45, 100822. [Google Scholar] [CrossRef]
- Jiang, Z.; Pulkkinen, M.; Wang, Y.; Lampi, A.-M.; Stoddard, F.L.; Salovaara, H.; Piironen, V.; Sontag-Strohm, T. Faba bean flavour and technological property improvement by thermal pre-treatments. LWT-Food Sci. Technol. 2016, 68, 295–305. [Google Scholar] [CrossRef]
- Hossain Brishti, F.; Chay, S.Y.; Muhammad, K.; Ismail-Fitry, M.R.; Zarei, M.; Karthikeyan, S.; Caballero-Briones, F.; Saari, N. Structural and rheological changes of texturized mung bean protein induced by feed moisture during extrusion. Food Chem. 2021, 344, 128643. [Google Scholar] [CrossRef] [PubMed]
- Afizah, M.N.; Rizvi, S.S. Functional properties of whey protein concentrate texturized at acidic pH: Effect of extrusion temperature. LWT-Food Sci. Technol. 2014, 57, 290–298. [Google Scholar] [CrossRef]
- Webb, D.; Dogan, H.; Li, Y.; Alavi, S. Physico-Chemical Properties and Texturization of Pea, Wheat and Soy Proteins Using Extrusion and Their Application in Plant-Based Meat. Foods 2023, 12, 1586. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Oh, H.; Choi, I.; Yoon, C.S.; Han, J. Physico-chemical characteristics of rice protein-based novel textured vegetable proteins as meat analogues produced by low-moisture extrusion cooking technology. LWT 2022, 157, 113056. [Google Scholar] [CrossRef]
- Martin, A.; Schmidt, V.; Osen, R.; Bez, J.; Ortner, E.; Mittermaier, S. Texture, sensory properties and functionality of extruded snacks from pulses and pseudocereal proteins. J. Sci. Food Agric. 2022, 102, 5011–5021. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, D.; Kaleda, A.; Pasqualone, A.; Vaikma, H.; Tamm, M.; Tammik, M.-L.; Squeo, G.; Summo, C. Physicochemical and sensorial evaluation of meat analogues produced from dry-fractionated pea and oat proteins. Foods 2020, 9, 1754. [Google Scholar] [CrossRef] [PubMed]
- Balet, S.; Guelpa, A.; Fox, G.; Manley, M. Rapid Visco Analyser (RVA) as a Tool for Measuring Starch-Related Physiochemical Properties in Cereals: A Review. Food Anal. Methods 2019, 12, 2344–2360. [Google Scholar] [CrossRef]
- Onwulata, C.I.; Tunick, M.H.; Thomas-Gahring, A.E. Rapid Visco Analysis of Food Protein Pastes. J. Food Process Preserv. 2014, 38, 2083–2089. [Google Scholar] [CrossRef]
- Osen, R.; Toelstede, S.; Wild, F.; Eisner, P.; Schweiggert-Weisz, U. High moisture extrusion cooking of pea protein isolates: Raw material characteristics, extruder responses, and texture properties. J. Food Eng. 2014, 127, 67–74. [Google Scholar] [CrossRef]
- Ma, K.K.; Greis, M.; Lu, J.; Nolden, A.A.; McClements, D.J.; Kinchla, A.J. Functional performance of plant proteins. Foods 2022, 11, 594. [Google Scholar] [CrossRef] [PubMed]
- Shevkani, K.; Singh, N.; Chen, Y.; Kaur, A.; Yu, L. Pulse proteins: Secondary structure, functionality and applications. J. Food Sci. Technol. 2019, 56, 2787–2798. [Google Scholar] [CrossRef] [PubMed]
- Chaparro Acuña, S.P.; Gil González, J.H.; Aristizábal Torres, I.D. Physicochemical characteristics and functional properties of vitabosa (mucuna deeringiana) and soybean (glycine max). Food Sci. Technol. 2012, 32, 98–105. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Petersen, I.L.; Joehnke, M.S.; Sørensen, J.C.; Bez, J.; Detzel, A.; Busch, M.; Krueger, M.; O’mahony, J.A.; Arendt, E.K.; et al. Comparison of faba bean protein ingredients produced using dry fractionation and isoelectric precipitation: Techno-functional, nutritional and environmental performance. Foods 2020, 9, 322. [Google Scholar] [CrossRef]
- Bühler, J.M.; Dekkers, B.L.; Bruins, M.E.; Van Der Goot, A.J. Modifying faba bean protein concentrate using dry heat to increase water holding capacity. Foods 2020, 9, 1077. [Google Scholar] [CrossRef] [PubMed]
- Remondetto, G.; Añon, M.C.; González, R.J. Hydration properties of soybean protein isolates. Braz. Arch. Biol. Technol. 2001, 44, 425–431. [Google Scholar] [CrossRef]
- Tan, E.-S.; Ying-Yuan, N.; Gan, C.-Y. A comparative study of physicochemical characteristics and functionalities of pinto bean protein isolate (PBPI) against the soybean protein isolate (SPI) after the extraction optimisation. Food Chem. 2014, 152, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.K.; Grossmann, L.; Nolden, A.A.; McClements, D.J.; Kinchla, A.J. Functional and physical properties of commercial pulse proteins compared to soy derived protein. Future Foods 2022, 6, 100155. [Google Scholar] [CrossRef]
- Lund, M.N.; Ray, C.A. Control of Maillard Reactions in Foods: Strategies and Chemical Mechanisms. J. Agric. Food Chem. 2017, 65, 4537–4552. [Google Scholar] [CrossRef]
- Tang, X.; Shen, Y.; Zhang, Y.; Schilling, M.W.; Li, Y. Parallel comparison of functional and physicochemical properties of common pulse proteins. LWT 2021, 146, 111594. [Google Scholar] [CrossRef]
- Ricci, L.; Umiltà, E.; Righetti, M.C.; Messina, T.; Zurlini, C.; Montanari, A.; Bronco, S.; Bertoldo, M. On the thermal behavior of protein isolated from different legumes investigated by DSC and TGA. J. Sci. Food Agric. 2018, 98, 5368–5377. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; McCurdy, A.; Baik, B. Textural property of 6 legume curds in relation to their protein constituents. J. Food Sci. 2002, 67, 1725–1730. [Google Scholar] [CrossRef]
- Lefèvre, C.; Bohuon, P.; Lullien-Pellerin, V.; Mestres, C. Modeling the Thermal Denaturation of the Protein–Water System in Pulses (Lentils, Beans, and Chickpeas). J. Agric. Food Chem. 2022, 70, 9980–9989. [Google Scholar] [CrossRef] [PubMed]
- Webb, D.; Li, Y.; Alavi, S. Chemical and physicochemical features of common plant proteins and their extrudates for use in plant-based meat. Trends Food Sci. Technol. 2023, 131, 129–138. [Google Scholar] [CrossRef]
- Manzocchi, E.; Ferlay, A.; Mendowski, S.; Chesneau, G.; Chapoutot, P.; Lemosquet, S.; Cantalapiedra-Hijar, G.; Nozière, P. Extrusion of lupines with or without addition of reducing sugars: Effects on the formation of Maillard reaction compounds, partition of nitrogen and Nε-carboxymethyl-lysine, and performance of dairy cows. J. Dairy Sci. 2023, 106, 7675–7697. [Google Scholar] [CrossRef] [PubMed]
- Cornet, S.H.V.; Snel, S.J.E.; Lesschen, J.; van der Goot, A.J.; van der Sman, R.G.M. Enhancing the water holding capacity of model meat analogues through marinade composition. J. Food Eng. 2021, 290, 110283. [Google Scholar] [CrossRef]
- Webb, D.; Plattner, B.J.; Donald, E.; Funk, D.; Plattner, B.S.; Alavi, S. Role of chickpea flour in texturization of extruded pea protein. J. Food Sci. 2020, 85, 4180–4187. [Google Scholar] [CrossRef]
- Mandliya, S.; Pratap-Singh, A.; Vishwakarma, S.; Dalbhagat, C.G.; Mishra, H.N. Incorporation of mycelium (Pleurotus eryngii) in pea protein based low moisture meat analogue: Effect on its physicochemical, rehydration and structural properties. Foods 2022, 11, 2476. [Google Scholar] [CrossRef]
- Webb, D.; Dogan, H.; Li, Y.; Alavi, S. Use of legume flours and fiber for tailoring structure and texture of pea protein-based extruded meat alternatives. J. Food Sci. 2023, 88, 57–71. [Google Scholar] [CrossRef]


| Component | Soy | Chickpea | Yellow Pea |
|---|---|---|---|
| Protein (%) | 93.1 | 81.4 | 86.0 |
| Moisture (%) | 4.5 | 7.0 | 3.0 |
| Fat (%) | 0.3 | 0.4 | N/A |
| Ash (%) | N/A | 2.7 | N/A |
| Crude Fiber (%) | N/A | 0.7 | N/A |
| Protein | Treatment | ΔE |
|---|---|---|
| Chickpea | 80 | 0.63 |
| Chickpea | 100 | 0.55 |
| Chickpea | 120 | 0.35 |
| Chickpea | 140 | 2.19 |
| Chickpea | 160 | 11.00 |
| Pea | 80 | 1.10 |
| Pea | 100 | 0.80 |
| Pea | 120 | 5.21 |
| Pea | 140 | 2.50 |
| Pea | 160 | 8.73 |
| Soy | 80 | 0.72 |
| Soy | 100 | 0.97 |
| Soy | 120 | 0.21 |
| Soy | 140 | 3.23 |
| Soy | 160 | 6.28 |
| Protein | Treatment | Onset Temperature (°C) | Peak Temperature (°C) | ΔH (J/g) | Reduction ΔH (%) |
|---|---|---|---|---|---|
| Chickpea | Raw | 89.5 ± 0.2 | 107.1 ± 0.8 | −0.25 ± 0.25 | 0 |
| Chickpea | 120 °C | 92.4 ± 0.0 | 102.9 ± 0.0 | −0.09 * | 63.0 |
| Chickpea | 160 °C | 97.8 ± 2.1 | 105.1 ± 0.1 | −0.07 ± 0.07 | 73.6 |
| Pea | Raw | 79.9 ± 0.4 | 87.8 ± 1.0 | −0.71 ± 0.07 | 0 |
| Pea | 120 °C | 78.0 ± 0.3 | 85.9 ± 0.3 | −0.60 ± 0.01 | 15.3 |
| Pea | 160 °C | - | - | 0 | 100 |
| Soy | Raw | - | - | 0 | - |
| Soy | 120 °C | - | - | 0 | - |
| Soy | 160 °C | - | - | 0 | - |
| Protein | Treatment | ΔE |
|---|---|---|
| Chickpea | Raw | 8.20 |
| Chickpea | 120 | 8.13 |
| Chickpea | 160 | 11.11 |
| Pea | Raw | 19.35 |
| Pea | 120 | 19.63 |
| Pea | 160 | 24.38 |
| Soy | Raw | 21.44 |
| Soy | 120 | 23.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pennells, J.; Trigona, L.; Patel, H.; Ying, D. Ingredient Functionality of Soy, Chickpea, and Pea Protein before and after Dry Heat Pretreatment and Low Moisture Extrusion. Foods 2024, 13, 2168. https://doi.org/10.3390/foods13142168
Pennells J, Trigona L, Patel H, Ying D. Ingredient Functionality of Soy, Chickpea, and Pea Protein before and after Dry Heat Pretreatment and Low Moisture Extrusion. Foods. 2024; 13(14):2168. https://doi.org/10.3390/foods13142168
Chicago/Turabian StylePennells, Jordan, Louise Trigona, Hetvi Patel, and Danyang Ying. 2024. "Ingredient Functionality of Soy, Chickpea, and Pea Protein before and after Dry Heat Pretreatment and Low Moisture Extrusion" Foods 13, no. 14: 2168. https://doi.org/10.3390/foods13142168
APA StylePennells, J., Trigona, L., Patel, H., & Ying, D. (2024). Ingredient Functionality of Soy, Chickpea, and Pea Protein before and after Dry Heat Pretreatment and Low Moisture Extrusion. Foods, 13(14), 2168. https://doi.org/10.3390/foods13142168





