Impact of Rare Sugar D-Allulose on Hardening of Starch Gels during Refrigerated Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Moisture Content of the Starch Powders
2.2.2. Total Starch Content of the Starch Powders
2.2.3. Total Amylose Content of the Starch Powders
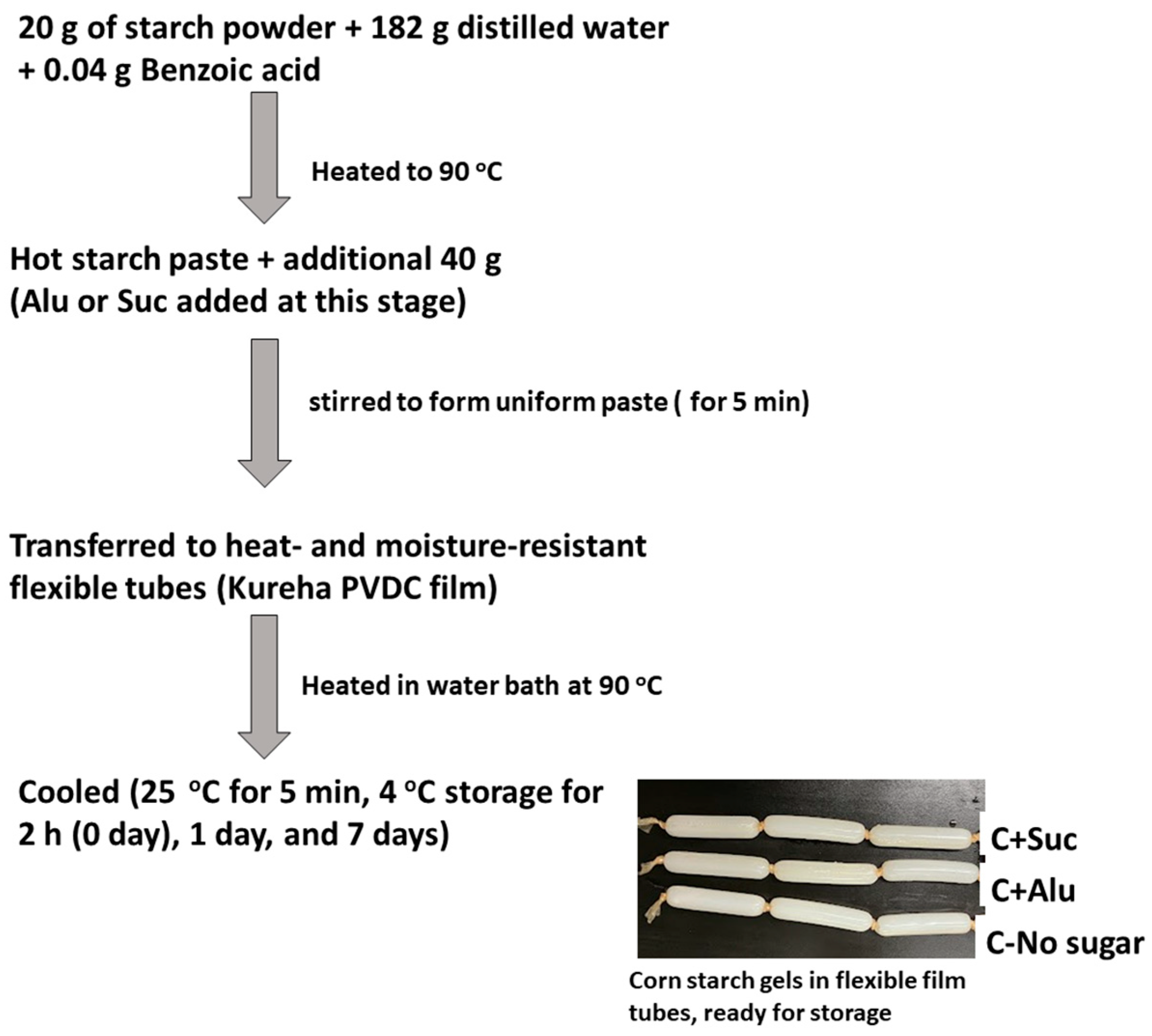
2.2.4. Starch Gel Preparation
Texture Analysis
Scanning Electron Microscopy (SEM)
Fourier Transform Infrared (FTIR) Spectroscopy
2.3. Statistical Analysis
3. Results and Discussion
3.1. Composition of the Starch Sources
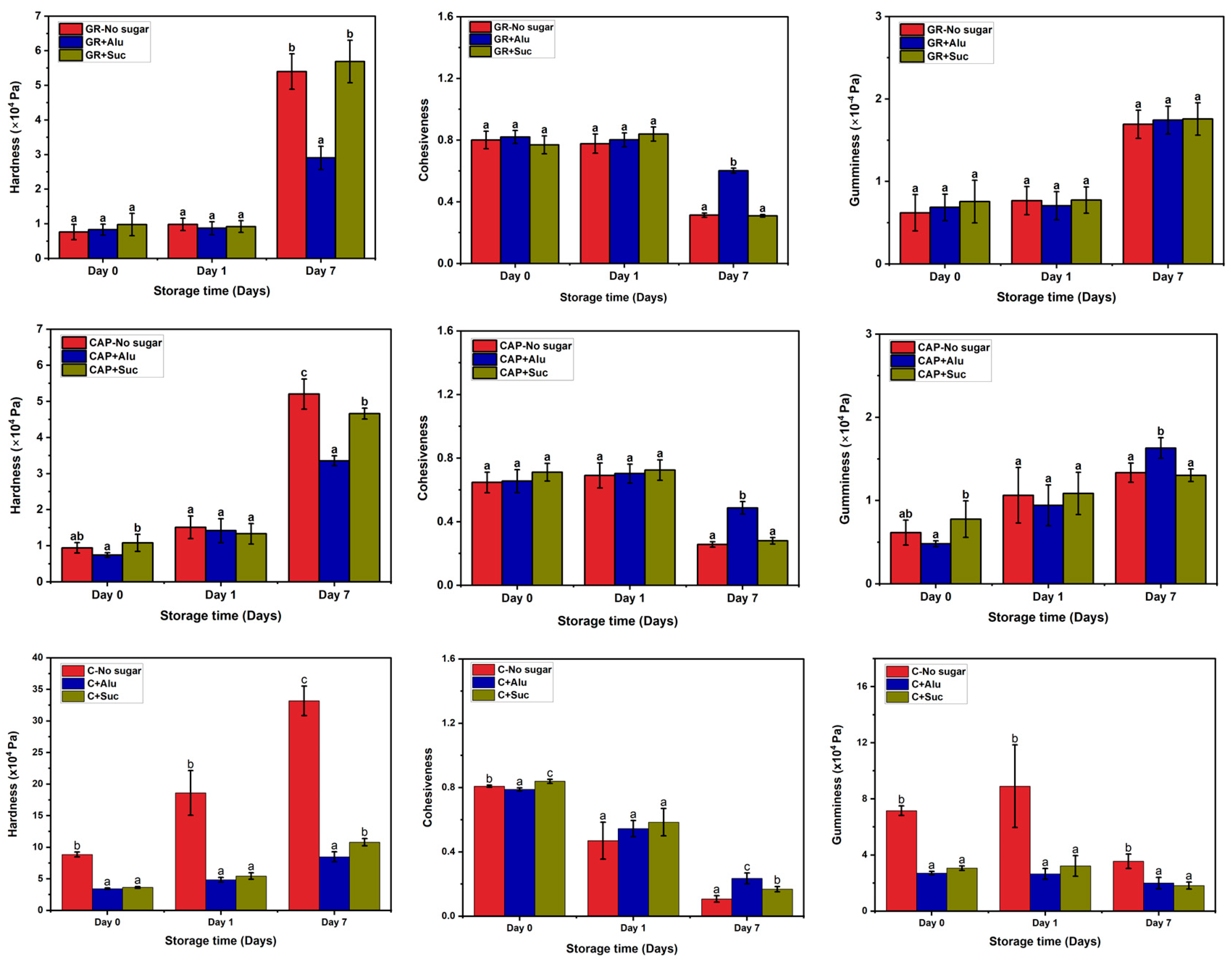
3.2. Effects of Alu on the Textural Properties of the Starch Gels
3.3. Microstructure of the Starch Gels during Storage
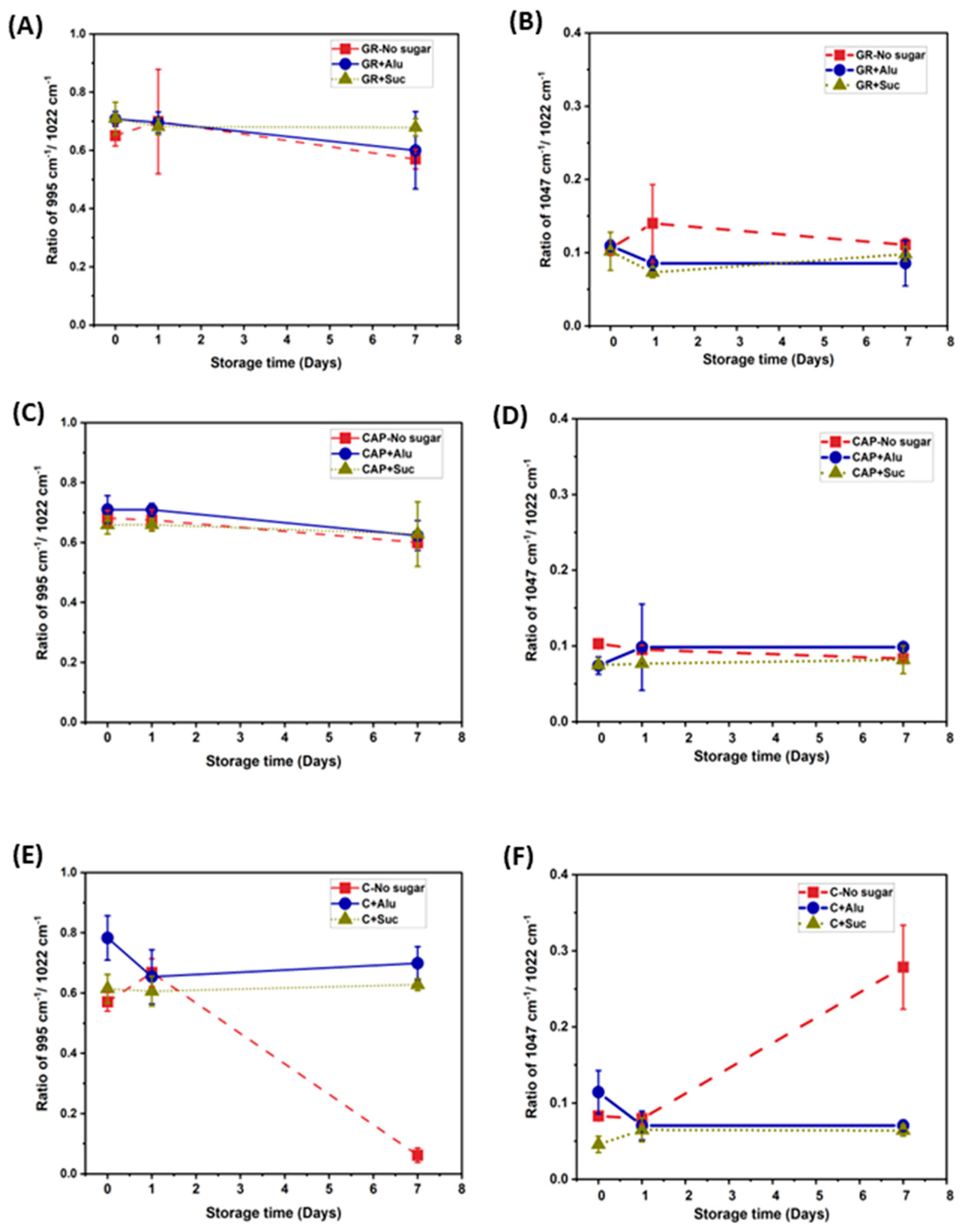
3.4. FTIR of Freeze-Dried Starch Gels (Starch Region)
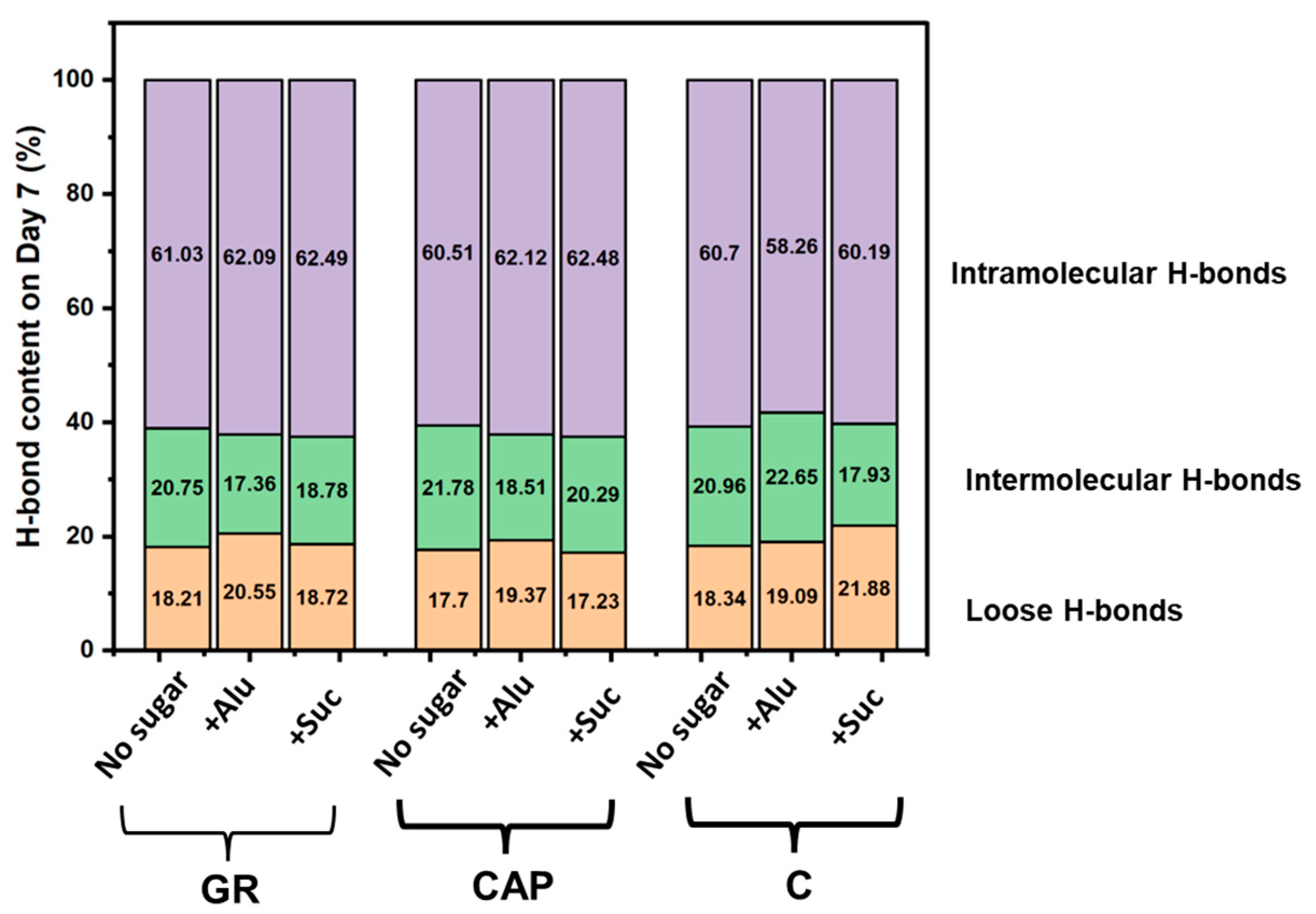
3.5. FTIR of Freeze-Dried Starch Gels (O–H Stretching Region)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horstmann, S.W.; Lynch, K.M.; Arendt, E.K. Starch Characteristics Linked to Gluten-Free Products. Foods 2017, 6, 29. [Google Scholar] [CrossRef]
- Berski, W.; Ziobro, R.; Witczak, M.; Gambuś, H. The Retrogradation Kinetics of Starches of Different Botanical Origin in the Presence of Glucose Syrup. Int. J. Biol. Macromol. 2018, 114, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Kohyama, K.; Nishinari, K. Effect of Soluble Sugars on Gelatinization and Retrogradation of Sweet Potato Starch. J. Agric. Food Chem. 1991, 39, 1406–1410. [Google Scholar] [CrossRef]
- Zhang, X.; Li, R.; Kang, H.; Luo, D.; Fan, J.; Zhu, W.; Liu, X.; Tong, Q. Effects of Low Molecular Sugars on the Retrogradation of Tapioca Starch Gels during Storage. PLoS ONE 2017, 12, e0190180. [Google Scholar] [CrossRef]
- Woodbury, T.J.; Mauer, L.J. Oligosaccharide, Sucrose, and Allulose Effects on the Pasting and Retrogradation Behaviors of Wheat Starch. Food Res. Int. 2023, 171, 113002. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, S.; Shahidi, F.; Koocheki, A.; Farahnaky, A.; Majzoobi, M. Comparing the Effects of Sucrose and Glucose on Functional Properties of Pregelatinized Maize Starch. Int. J. Biol. Macromol. 2016, 88, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Goldfein, K.R.; Slavin, J.L. Why Sugar Is Added to Food: Food Science 101. Compr. Rev. Food Sci. Food Saf. 2015, 14, 644–656. [Google Scholar] [CrossRef]
- Yuma, T.; Tokuda, M.; Nishimoto, N.; Yokoi, H.; Izumori, K. Allulose for the Attenuation of Postprandial Blood Glucose Levels in Healthy Humans: A Systematic Review and Meta-Analysis. PLoS ONE 2023, 18, e0281150. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, K.; Yoshimura, T.; Imachi, H.; Kobayashi, T.; Saheki, T.; Sato, S.; Saheki, N.; Jiang, W.; Murao, K. A Pilot Study on the Efficacy of a Diabetic Diet Containing the Rare Sugar D-Allulose in Patients with Type 2 Diabetes Mellitus: A Prospective, Randomized, Single-Blind, Crossover Study. Nutrients 2023, 15, 2802. [Google Scholar] [CrossRef]
- Hossain, A.; Yamaguchi, F.; Matsuo, T.; Tsukamoto, I.; Toyoda, Y.; Ogawa, M.; Nagata, Y.; Tokuda, M. Rare Sugar D-Allulose: Potential Role and Therapeutic Monitoring in Maintaining Obesity and Type 2 Diabetes Mellitus. Pharmacol. Ther. 2015, 155, 49–59. [Google Scholar] [CrossRef]
- O’Charoen, S.; Hayakawa, S.; Matsumoto, Y.; Ogawa, M. Effect of D-Psicose Used as Sucrose Replacer on the Characteristics of Meringue. J. Food Sci. 2014, 79, E2463–E2469. [Google Scholar] [CrossRef]
- Sun, Y.; Hayakawa, S.; Ogawa, M.; Izumori, K. Antioxidant Properties of Custard Pudding Dessert Containing Rare Hexose, d-Psicose. Food Control 2007, 18, 220–227. [Google Scholar] [CrossRef]
- Ilhan, E.; Pocan, P.; Ogawa, M.; Oztop, M.H. Role of ‘D-Allulose’ in a Starch Based Composite Gel Matrix. Carbohydr. Polym. 2020, 228, 115373. [Google Scholar] [CrossRef]
- Bolger, A.M.; Rastall, R.A.; Oruna-Concha, M.J.; Rodriguez-Garcia, J. Effect of D-Allulose, in Comparison to Sucrose and D-Fructose, on the Physical Properties of Cupcakes. LWT 2021, 150, 111989. [Google Scholar] [CrossRef]
- Allan, M.C.; Mauer, L.J. Variable Effects of Twenty Sugars and Sugar Alcohols on the Retrogradation of Wheat Starch Gels. Foods 2022, 11, 3008. [Google Scholar] [CrossRef] [PubMed]
- Sawettanun, S.; Ogawa, M. Physicochemical Parameters, Volatile Compounds and Organoleptic Properties of Bread Prepared with Substituted Sucrose with Rare Sugar D-Allulose. Int. J. Food Sci. Technol. 2022, 57, 5931–5942. [Google Scholar] [CrossRef]
- Ikeda, S.; Furuta, C.; Fujita, Y.; Gohtani, S. Effects of D-Psicose on Gelatinization and Retrogradation of Rice Flour. Starch-Stärke 2014, 66, 773–779. [Google Scholar] [CrossRef]
- Kwakye, A.O.; Fukada, K.; Ishii, T.; Ogawa, M. The Effects of D-Allulose on the Gelatinization and Recrystallization Properties of Starches from Different Botanical Sources. Starch-Staerke 2024. early view. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Hoover, R.; Ratnayake, W.S. Determination of Total Amylose Content of Starch. Handb. Food Anal. Chem. 2005, 1–2, 689–693. [Google Scholar] [CrossRef]
- Ma, Z.; Yin, X.; Chang, D.; Hu, X.; Boye, J.I. Long- and Short-Range Structural Characteristics of Pea Starch Modified by Autoclaving, α-Amylolysis, and Pullulanase Debranching. Int. J. Biol. Macromol. 2018, 120, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Montealvo, G.; Velazquez, G.; Fonseca-Florido, H.A.; Morales-Sanchez, E.; Soler, A. Insights on the Acid Hydrolysis of Achira (Canna edulis) Starch: Crystalline and Double-Helical Structure Changes Impacting Functionality. LWT 2021, 153, 112509. [Google Scholar] [CrossRef]
- Lu, H.; Ma, R.; Chang, R.; Tian, Y. Evaluation of Starch Retrogradation by Infrared Spectroscopy. Food Hydrocoll. 2021, 120, 106975. [Google Scholar] [CrossRef]
- Zhao, D.; Deng, Y.; Han, D.; Tan, L.; Ding, Y.; Zhou, Z.; Xu, H.; Guo, Y. Exploring Structural Variations of Hydrogen-Bonding Patterns in Cellulose during Mechanical Pulp Refining of Tobacco Stems. Carbohydr. Polym. 2019, 204, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Juliano, B.O. Rice Starch Properties and Grain Quality. Denpun Kagaku 1992, 39, 11–21. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, R.; Wang, D.; Bean, S.R.; Seib, P.A.; Tuinstra, M.R.; Campbell, M.; O’Brien, A. Effects of Amylose, Corn Protein, and Corn Fiber Contents on Production of Ethanol from Starch-Rich Media. Cereal Chem. 2006, 83, 569–575. [Google Scholar] [CrossRef]
- Wang, S.; Guo, P. Botanical Sources of Starch BT. In Starch Structure, Functionality and Application in Foods; Wang, S., Ed.; Springer: Singapore, 2020; pp. 9–27. ISBN 978-981-15-0622-2. [Google Scholar]
- Tian, J.; Qin, L.; Zeng, X.; Ge, P.; Fan, J.; Zhu, Y. The Role of Amylose in Gel Forming of Rice Flour. Foods 2023, 12, 1210. [Google Scholar] [CrossRef] [PubMed]
- Arik Kibar, E.A.; Gönenç, I.; Us, F. Modeling of Retrogradation of Waxy and Normal Corn Starches. Int. J. Food Prop. 2011, 14, 954–967. [Google Scholar] [CrossRef]
- Ogawa, M.; Iritani, S.; Hayakawa, S.; Gohtani, S.; Akazawa, T.; Yamamoto, I.; Ogawa, M. Effects of Rare Sugar D-Allulose on Texture Profile of Glutinous Rice Flour Gel. Nippon Shokuhin Hozou Kagaku Kaishi 2020, 45, 261–270. [Google Scholar] [CrossRef]
- Ogawa, M.; Inoue, M.; Hayakawa, S.; O’Charoen, S.; Ogawa, M. Effects of Rare Sugar D-Allulose on Heat-Induced Gelation of Surimi Prepared from Marine Fish. J. Sci. Food Agric. 2017, 97, 5014–5020. [Google Scholar] [CrossRef]
- Cho, S.A.; Yoo, B. Comparison of the Effect of Sugars on the Viscoelastic Properties of Sweet Potato Starch Pastes. Int. J. Food Sci. Technol. 2010, 45, 410–414. [Google Scholar] [CrossRef]
- Woodbury, T.J.; Grush, E.; Allan, M.C.; Mauer, L.J. The Effects of Sugars and Sugar Alcohols on the Pasting and Granular Swelling of Wheat Starch. Food Hydrocoll. 2022, 126, 107433. [Google Scholar] [CrossRef]
- Apostolidis, E.; Kioupis, D.; Kakali, G.; Stoforos, N.G.; Mandala, I. Effect of Starch Concentration and Resistant Starch Filler Addition on the Physical Properties of Starch Hydrogels. J. Food Sci. 2021, 86, 5340–5352. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, A.J. Texture Profile Analysis—How Important Are the Parameters? J. Texture Stud. 2010, 41, 672–684. [Google Scholar] [CrossRef]
- Jiang, J.; Zeng, J.; Gao, H.; Zhang, L.; Wang, F.; Su, T.; Xiang, F.; Li, G. Effect of Low Temperature on the Aging Characteristics of a Potato Starch Gel. Int. J. Biol. Macromol. 2020, 150, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Arunyanart, T.; Charoenrein, S. Effect of Sucrose on the Freeze-Thaw Stability of Rice Starch Gels: Correlation with Microstructure and Freezable Water. Carbohydr. Polym. 2008, 74, 514–518. [Google Scholar] [CrossRef]
- Mutungi, C.; Onyango, C.; Doert, T.; Paasch, S.; Thiele, S.; Machill, S.; Jaros, D.; Rohm, H. Long- and Short-Range Structural Changes of Recrystallised Cassava Starch Subjected to in Vitro Digestion. Food Hydrocoll. 2011, 25, 477–485. [Google Scholar] [CrossRef]
- Yang, S.; Dhital, S.; Shan, C.S.; Zhang, M.N.; Chen, Z.G. Ordered Structural Changes of Retrograded Starch Gel over Long-Term Storage in Wet Starch Noodles. Carbohydr. Polym. 2021, 270, 118367. [Google Scholar] [CrossRef] [PubMed]
- Warren, F.J.; Perston, B.B.; Royall, P.G.; Butterworth, P.J.; Ellis, P.R. Infrared Spectroscopy with Heated Attenuated Total Internal Reflectance Enabling Precise Measurement of Thermally Induced Transitions in Complex Biological Polymers. Anal. Chem. 2013, 85, 3999–4006. [Google Scholar] [CrossRef]
- Chi, C.; Li, X.; Zhang, Y.; Miao, S.; Chen, L.; Li, L.; Liang, Y. Understanding the Effect of Freeze-Drying on Microstructures of Starch Hydrogels. Food Hydrocoll. 2020, 101, 105509. [Google Scholar] [CrossRef]
- Luo, Y.; Xiao, Y.; Shen, M.; Wen, H.; Ren, Y.; Yang, J.; Han, X.; Xie, J. Effect of Mesona Chinensis Polysaccharide on the Retrogradation Properties of Maize and Waxy Maize Starches during Storage. Food Hydrocoll. 2020, 101, 105538. [Google Scholar] [CrossRef]
- Ottenhof, M.A.; Farhat, I.A. Starch Retrogradation. Biotechnol. Genet. Eng. Rev. 2004, 21, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, H.; Jin, X.; Wang, H.; Lin, T.; Zhu, Z. Effects of Hydrogen Bonding on Starch Granule Dissolution, Spinnability of Starch Solution, and Properties of Electrospun Starch Fibers. Polymer 2018, 153, 643–652. [Google Scholar] [CrossRef]
- Maréchal, Y.; Chanzy, H. The Hydrogen Bond Network in I b Cellulose as Observed by Infrared Spectrometry. J. Mol. Struct. 2000, 523, 183–196. [Google Scholar] [CrossRef]
- Brubach, J.B.; Mermet, A.; Filabozzi, A.; Gerschel, A.; Roy, P. Signatures of the Hydrogen Bonding in the Infrared Bands of Water. J. Chem. Phys. 2005, 122, 184509. [Google Scholar] [CrossRef] [PubMed]
- Queneau, Y.; Jarosz, S.; Lewandowski, B.; Fitremann, J. Sucrose Chemistry and Applications of Sucrochemicals. Adv. Carbohydr. Chem. Biochem. 2007, 61, 217–292. [Google Scholar] [CrossRef]




| Starch Source | Moisture (%) | Total Starch Content (%) | Total Amylose Content (%) | Amylose/Amylopectin Ratio |
|---|---|---|---|---|
| Glutinous Rice (GR) | 11.07 ± 0.04 b | 82.27 ± 3.21 a | 2.13 ± 0.21 a | 1/80 |
| Corn Amylopectin (CAP) | 9.86 ± 0.10 a | 82.27 ± 3.21 a | 0.28 ± 0.01 a | 1/290 |
| Corn (C) | 11.05 ± 0.10 b | 81.65 ± 4.92 a | 27.04 ± 1.53 b | 1/2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwakye, A.O.; Fukada, K.; Ishii, T.; Ogawa, M. Impact of Rare Sugar D-Allulose on Hardening of Starch Gels during Refrigerated Storage. Foods 2024, 13, 2183. https://doi.org/10.3390/foods13142183
Kwakye AO, Fukada K, Ishii T, Ogawa M. Impact of Rare Sugar D-Allulose on Hardening of Starch Gels during Refrigerated Storage. Foods. 2024; 13(14):2183. https://doi.org/10.3390/foods13142183
Chicago/Turabian StyleKwakye, Alexandra Obenewaa, Kazuhiro Fukada, Toya Ishii, and Masahiro Ogawa. 2024. "Impact of Rare Sugar D-Allulose on Hardening of Starch Gels during Refrigerated Storage" Foods 13, no. 14: 2183. https://doi.org/10.3390/foods13142183
APA StyleKwakye, A. O., Fukada, K., Ishii, T., & Ogawa, M. (2024). Impact of Rare Sugar D-Allulose on Hardening of Starch Gels during Refrigerated Storage. Foods, 13(14), 2183. https://doi.org/10.3390/foods13142183





