Effects of Transglutaminase-Induced β-Conglycinin Gels on Intestinal Morphology and Intestinal Flora in Mice at Different High-Intensity Ultrasound Pretreatment Time
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mice Care
2.3. Small Intestine Sections Observation
- (1)
- Washing: rinsing with running water for 24 h.
- (2)
- Dehydration: gradient dehydration with anhydrous ethanol.
- (3)
- Transparency: the dehydrated tissue block was immersed in xylene until transparency was achieved.
- (4)
- Wax dipping: dip the tissue blocks into melted paraffin wax: paraffin I (melting point 50–52 °C, 1 h), paraffin II (melting point 52–54 °C, 1 h), paraffin III (melting point 54–56 °C, 1 h).
- (5)
- Embedding: after dipping the wax, placed the paraffin wax into the embedding frame, removed the embedding frame after the paraffin wax cooled and solidified, and trim the wax into a trapezoidal shape for use.
- (6)
- Sectioning: cut thin slices of tissue with a thickness of 4 μm (transverse sections).
- (7)
- Staining, dehydration, and transparency: Hematoxylin-eosin (HE) staining, gradient anhydrous ethanol-xylene dehydration to transparency, sealing with a drop of neutral resin, drying, and then observing the changes in the small intestine tissue sections under a light microscope.
- (8)
- Analysis: The length of the mouse villi was determined using Image-J v1.8.0 image analysis software.
2.4. Fecal Sample Collection
2.5. Intestinal Flora Testing
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effects of Gels on Body Weight, Food Intake, and Water Intake in Mice
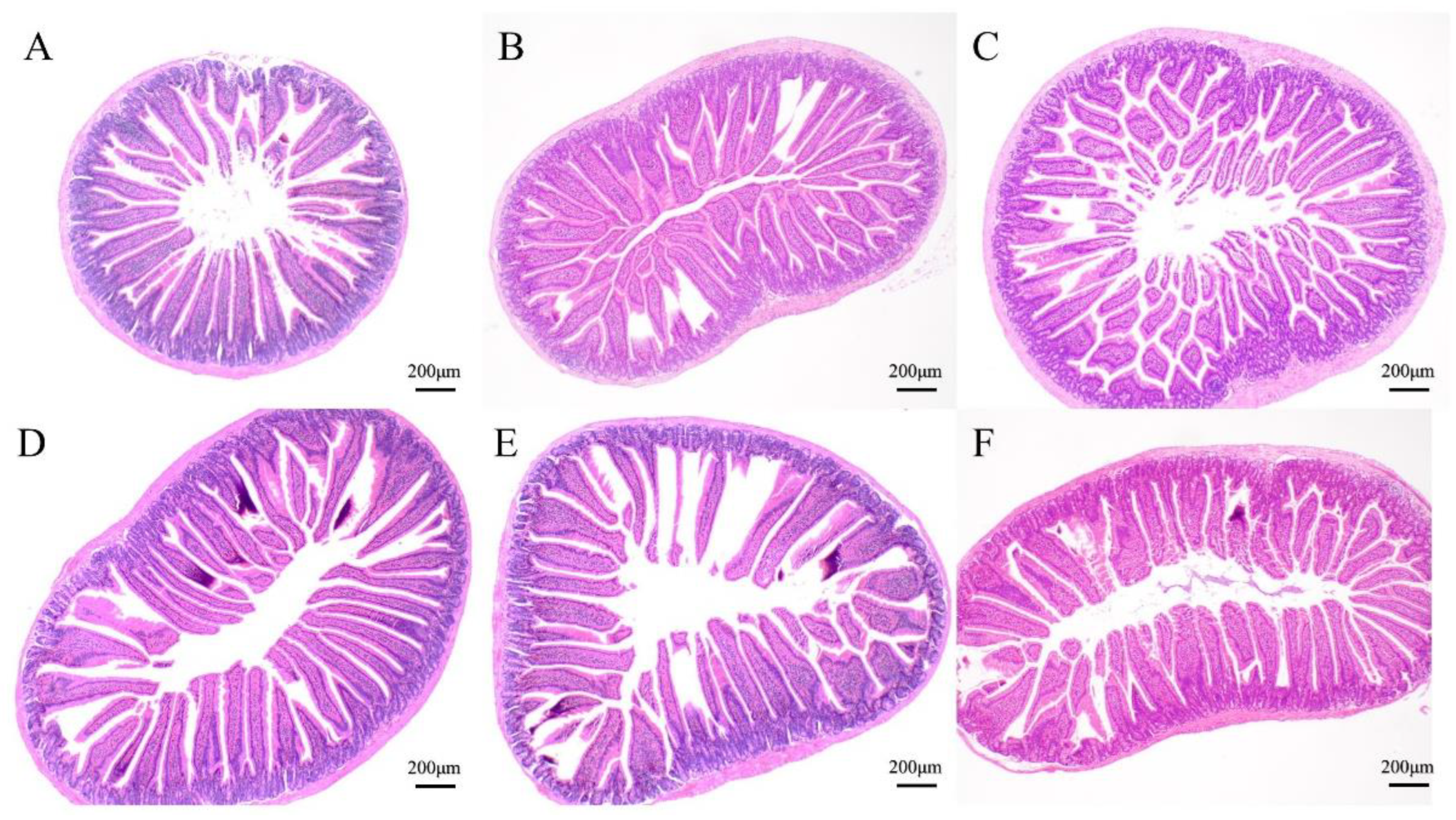
3.2. Effects of Gels on Intestinal Morphology
3.3. Quality Assurance of Intestinal Flora Samples
3.4. Changes in the Diversity of Gut Flora
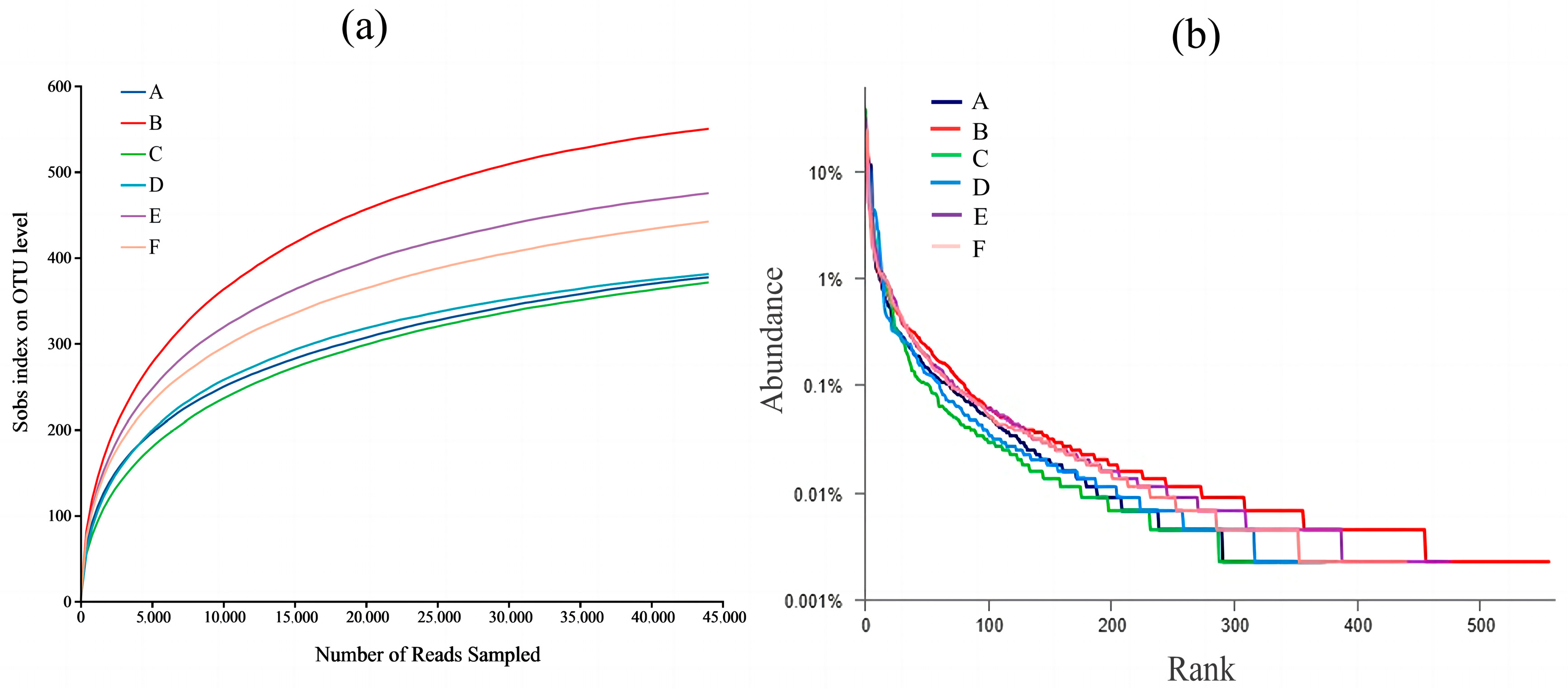
3.4.1. Alpha-Diversity Analysis
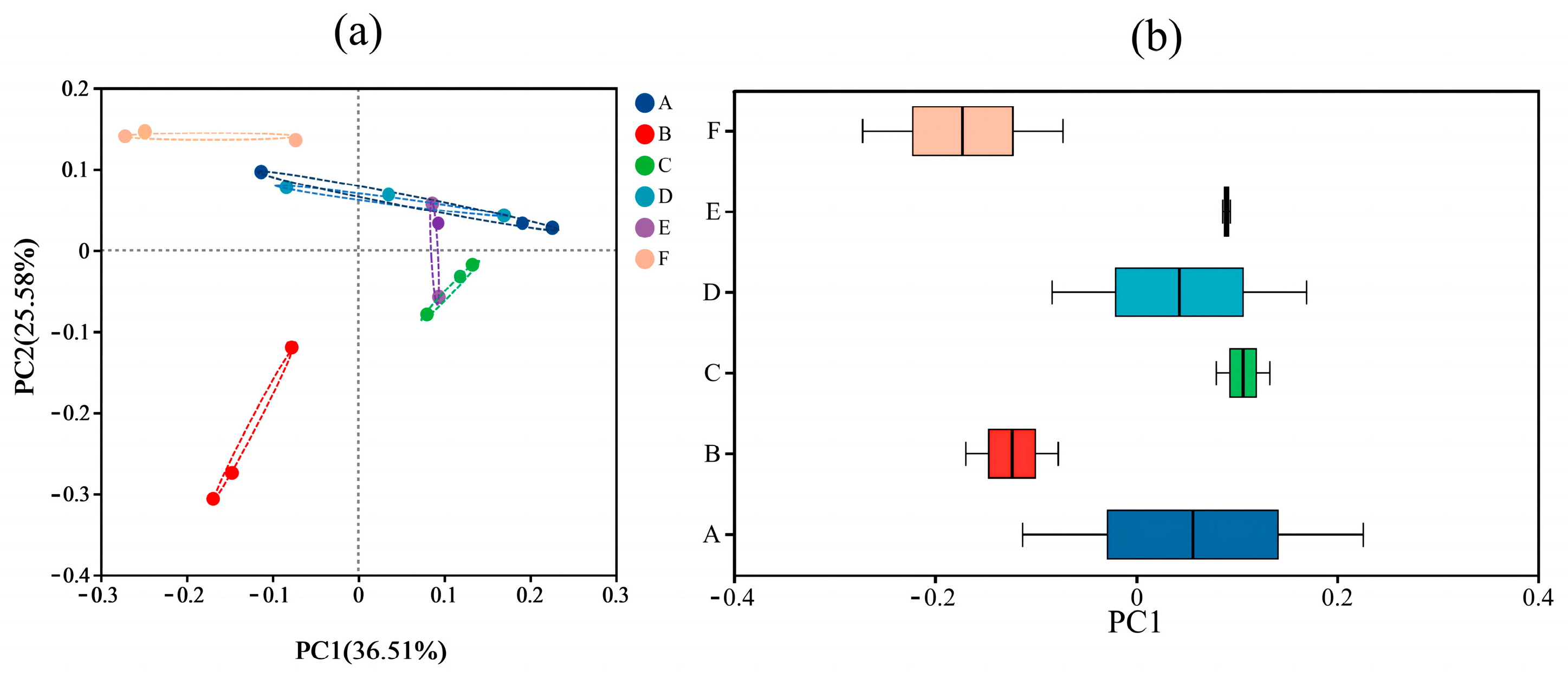
3.4.2. Beta-Diversity Analysis
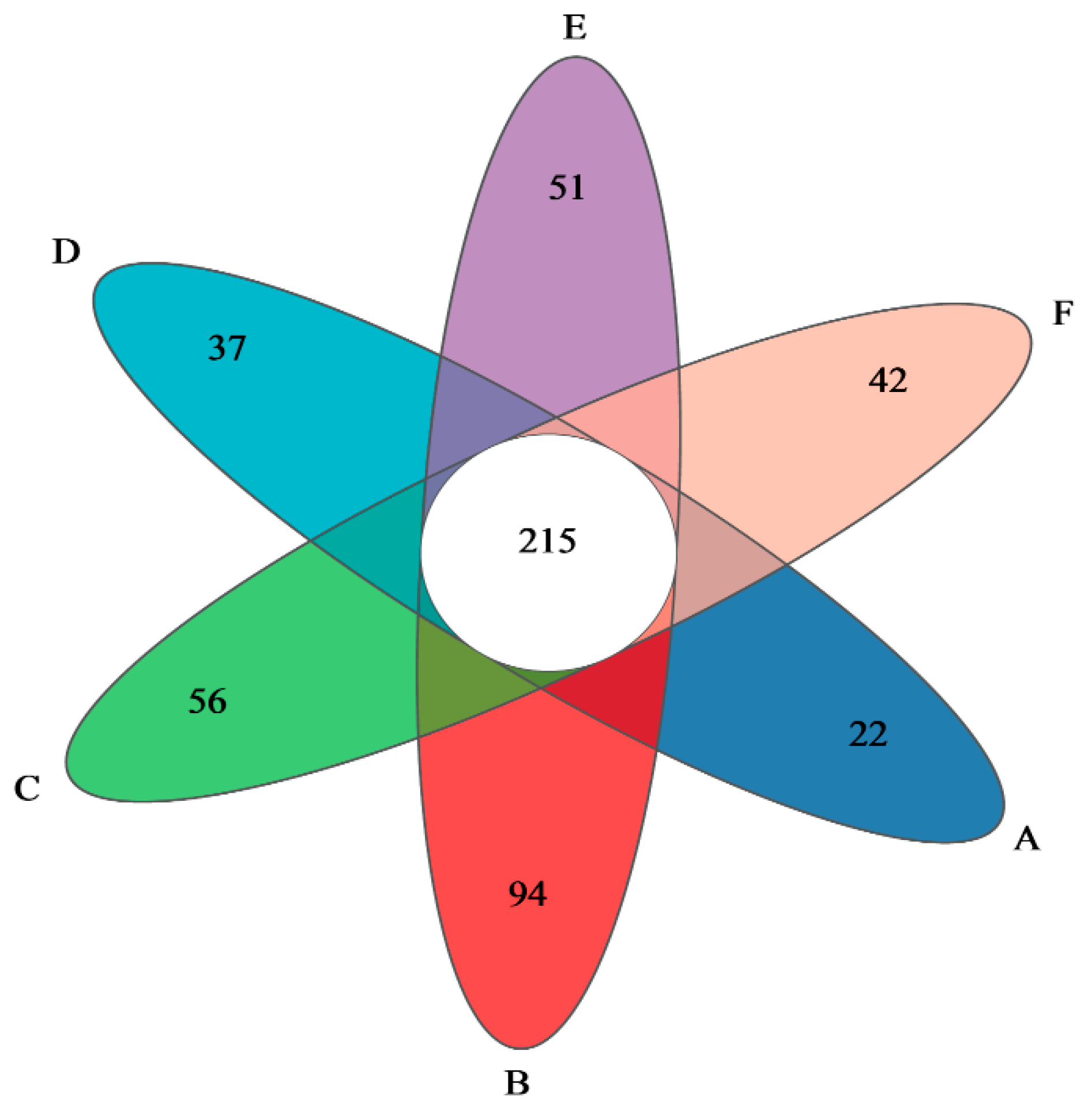
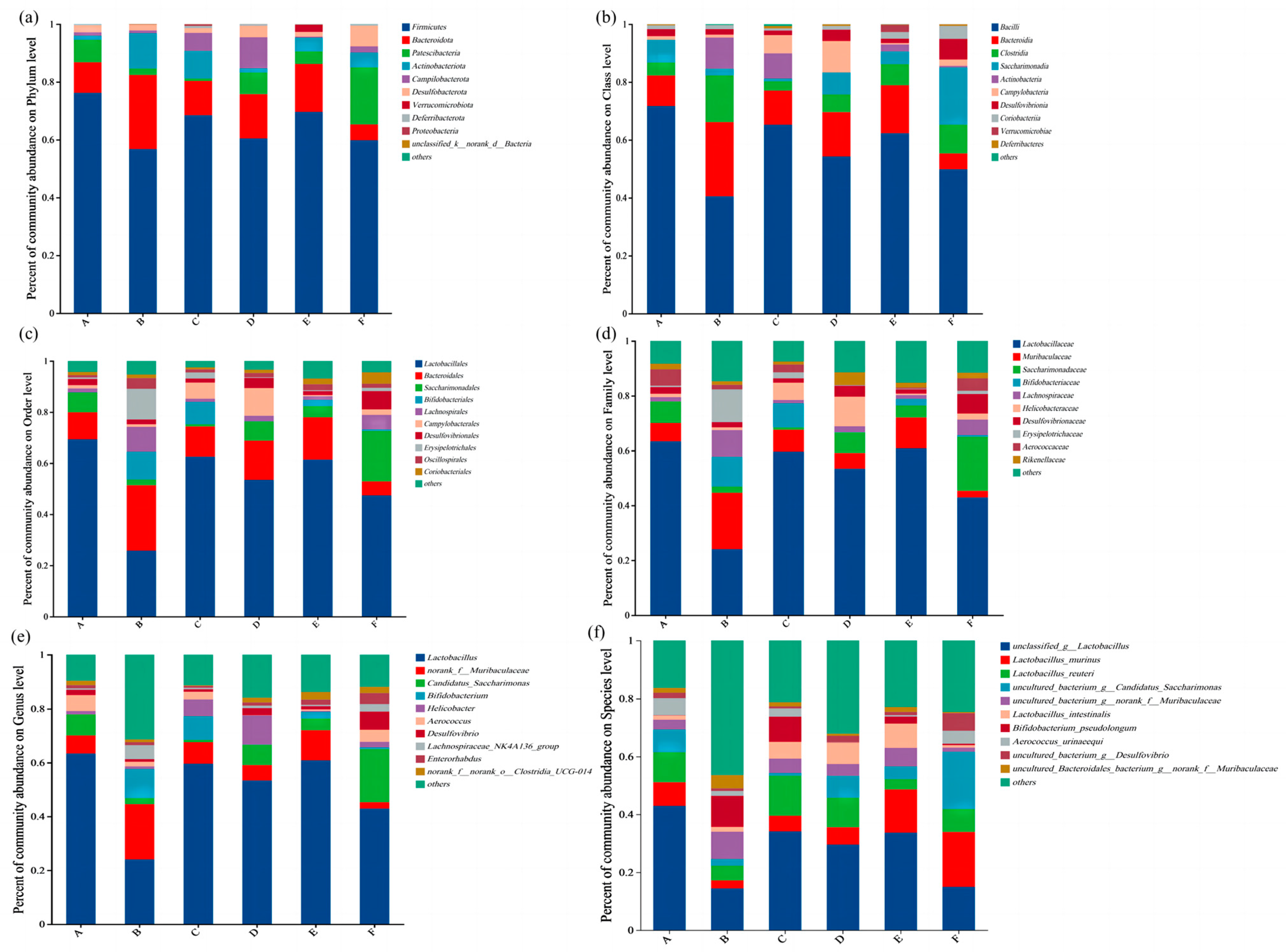
3.5. Analysis of the Composition of Gut Flora
3.6. Prediction of Biological Functions of Intestinal Flora
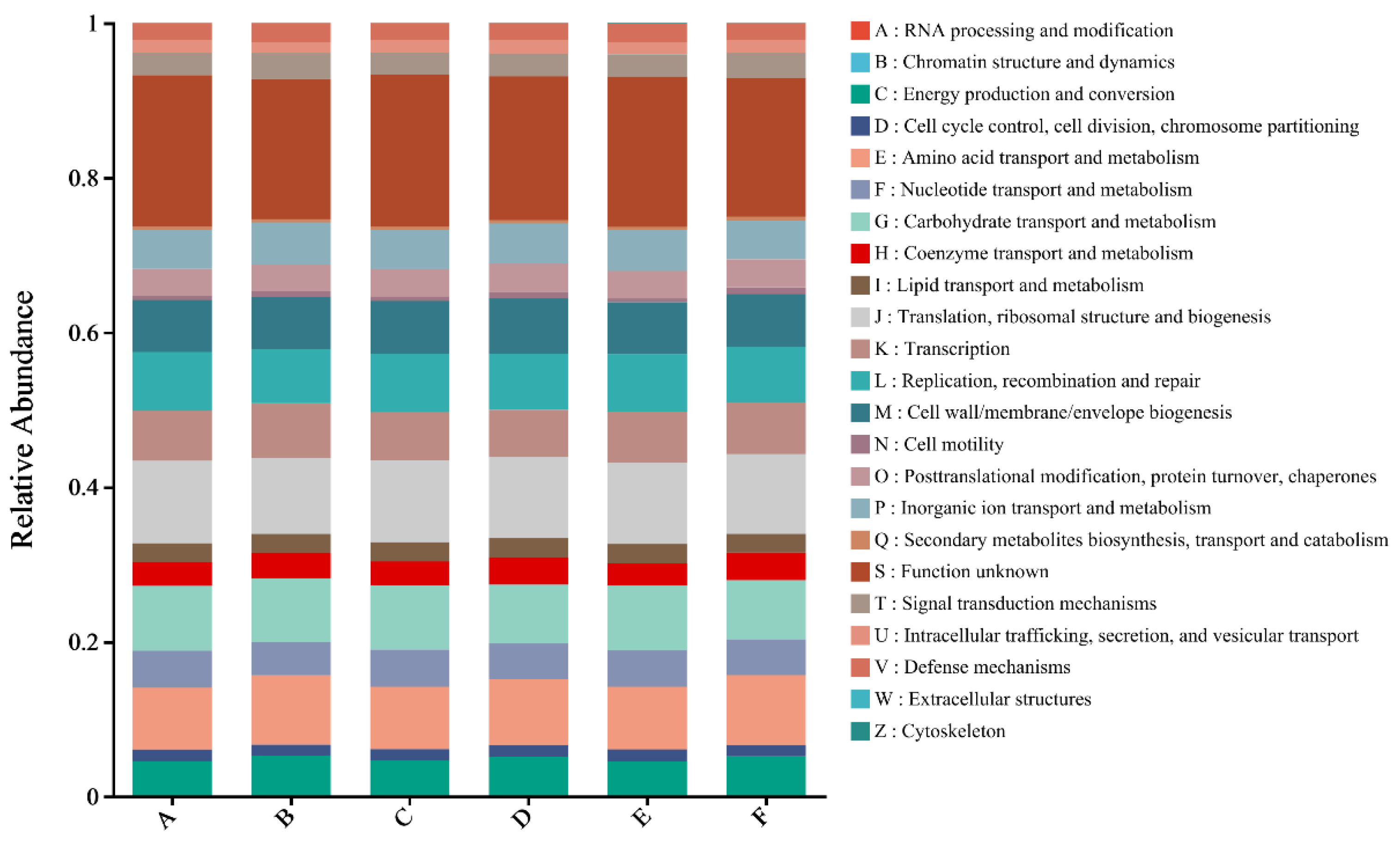
3.6.1. COG Biofunction Prediction
3.6.2. Prediction of KEGG Biological Function
KEGG Tier One Functional Prediction
Analysis of KEGG Second-Level Subfunctional Pathways
Analysis of KEGG Tertiary Metabolic Pathways
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Zhang, J.; Wen, P.; Xu, J.; Xu, H.; Cui, G.; Wang, J. Effect of High-Intensity Ultrasound Pretreatment on the Properties of the Transglutaminase (TGase)-Inducedβ-Conglycinin (7S) Gel. Foods 2023, 12, 2037. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; DeCoffe, D.; Molcan, E.; Gibson, D.L. Diet-induced Dysbiosis of the Intestinal Microbiota and the Effects on Immunity and Disease. Nutrients 2012, 4, 1095–1119. [Google Scholar] [CrossRef] [PubMed]
- Wei, T. Regulation of Intestinal Flora and Metabolic Level in Mice with Type 2 Diabetes by High Quality Protein-Derived Enteral Nutrition Preparation. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2023. [Google Scholar]
- Zhu, Y.; Shi, X.; Lin, X.; Ye, K.; Xu, X.; Li, C.; Zhou, G. Beef, Chicken, and Soy Proteins in Diets Induce Different Gut Microbiota and Metabolites in Rats. Front. Microbiol. 2017, 8, 1395. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yin, R.; Guo, E.; Cheng, R.; Diao, X.; Xue, Y.; Shen, Q. Protein isolates from raw and cooked foxtail millet attenuate development of type 2 diabetes in streptozotocin-induced diabetic mice. Mol. Nutr. Food Res. 2021, 65, 20000365. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.P.; Bhandari, B.; Cichero, J.; Prakash, S. Gastrointestinal digestion of dairy and soy proteins in infant formulas: An in vitro study. Food Res. Int. 2015, 76, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Hultsch, C.; Bergmann, R.; Pawelke, B.; Pietzsch, J.; Wuest, F.; Johannsen, B.; Henle, T. Biodistribution and catabolism of 18F-labelled isopeptide Nɛ-(γ-glutamyl)-L-lysine. Amino Acids 2005, 29, 405–413. [Google Scholar] [CrossRef]
- Fang, M.; Xiong, S.; Yin, T.; Hu, Y.; Liu, R.; Du, H.; Liu, Y.; You, J. In vivo digestion and absorption characteristics of surimi gels with different degrees of cross-linking induced by transglutaminase (TGase). Food Hydrocoll. 2021, 121, 107007. [Google Scholar] [CrossRef]
- Liang, P.; Chen, S.; Fang, X.; Wu, J. Recent advance in modification strategies and applications of soy protein gel properties. Compr. Rev. Food Sci. Food Saf. 2023, 23, e13276. [Google Scholar] [CrossRef]
- Cai, Y.; You, Y.; Liu, J.; Qiu, Y.; Lv, F.; Ding, Y. Progress in the study of soy protein gelation and its modification methods. Food Ferment. Ind. 2021, 47, 298–306. [Google Scholar]
- Fatemi, S.A.; Elliott, K.E.C.; Macklin, K.S.; Bello, A.; Peebles, E.D. Effects of the In Ovo Injection of Vitamin D3 and 25-Hydroxyvitamin D3 in Ross 708 Broilers Subsequently Challenged with Coccidiosis: II Immunological and Inflammatory Responses and Small Intestine Histomorphology. Animals 2022, 12, 1027. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Dong, Y.; Cao, J.; Chen, Y. Blue Light Alters the Composition of the Jejunal Microbiota and Promotes the Development of the Small Intestine by Reducing Oxidative Stress. Antioxidants 2022, 11, 274. [Google Scholar] [CrossRef]
- Canani, R.B.; Filippis, F.D.; Nocerino, R.; Paparo, L.; Scala, C.D.; Cosenza, L.; Gatta, G.D.; Calignano, A.; Caro, C.D.; Laiola, M.; et al. Gut microbiota composition and butyrate production in children affected by non-IgE-mediated cow’s milk allergy. Sci. Rep. 2018, 8, 12500. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, D.; Tian, Z.; Kang, M.; Li, Q.; Zu, F.; Sun, X.; Ding, C. Effects of antibiotic intake in drinking water on intestinal flora in mice. Chin. J. Antibiot. 2023, 48, 833–840. [Google Scholar]
- Yan, J.; Li, J.; Xue, Q.; Xie, S.; Jiang, J.; Li, P.; Du, B. Bacillus sp. DU-106 ameliorates type 2 diabetes by modulating gut microbiota in high-fat-fed and streptozotocin-induced mice. J. Appl. Microbiol. 2022, 133, 3126–3138. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, Y.; Huang, J.; Zhang, H.; Lin, Q.; Han, L.; Liu, J.; Wang, J.; Liu, H. Insoluble dietary fiber from soy hulls regulates the gut microbiota in vitro and increases the abundance of bifidobacteriales and lactobacillales. J. Food Sci. Technol. 2020, 57, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Sprong, R.; Schonewille, A.; Meer, D.V.R. Dietary cheese whey protein protects rats against mild dextran sulfate sodium–induced colitis: Role of mucin and microbiota. J. Dairy Sci. 2010, 93, 1364–1371. [Google Scholar] [CrossRef]
- Kahraman, O.; Gurbuz, E.; Inal, F.; Arık, H.; Alatas, M.S.; Inanc, Z.S.; Ahmed, I. Effects of Bacillus subtilis C-3102 addition on nutrient digestibility, faecal characteristics, blood chemistry and faecal Lactobacilli spp., Enterococci spp., and Escherichia coli in healthy dogs. Ital. J. Anim. Sci. 2023, 22, 568–577. [Google Scholar] [CrossRef]
- Lei, S.; He, S.; Li, X.; Zheng, B.; Zhang, Y.; Zeng, H. Effect of lotus seed resistant starch on small intestinal flora and bile acids in hyperlipidemic rats. Food Chem. 2023, 404, 134599. [Google Scholar] [CrossRef]
- Watanabe, K.; Igarashi, M.; Li, X.; Nakatani, A.; Miyamoto, J.; Inaba, Y.; Sutou, A.; Saito, T.; Sato, T.; Tachibana, N.; et al. Dietary soybean protein ameliorates high-fat diet-induced obesity by modifying the gut microbiota-dependent biotransformation of bile acids. PLoS ONE 2018, 13, e0202083. [Google Scholar] [CrossRef] [PubMed]
- Mouzaki, M.; Comelli, E.M.; Arendt, B.M.; Bonengel, J.; Fung, S.K.; Fischer, S.E.; McGilvray, I.D.; Allard, J.P. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology 2013, 58, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Galvão, F.C.; Xavier, F.V.; Marcin, Ł.G.; Boroni, A.P.M.; Rocha, D.M.U.P.; Alfenas, R.C.G. Impact of dietary fat on gut microbiota and low-grade systemic inflammation: Mechanisms and clinical implications on obesity. Int. J. Food Sci. Nutr. 2018, 69, 125–143. [Google Scholar]
- Li, R.W.; Li, W.; Sun, J.; Yu, P.; Baldwin, R.L.; Urban, J.F. The effect of helminth infection on the microbial composition and structure of the caprine abomasal microbiome. Sci. Rep. 2016, 6, 20606. [Google Scholar] [CrossRef] [PubMed]
- Krautkramer, K.; Fan, J.; Bäckhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol. 2020, 19, 77–94. [Google Scholar] [CrossRef]
- Collins, S.L.; Stine, J.G.; Bisanz, J.E.; Okafor, C.D.; Patterson, A.D. Bile acids and the gut microbiota: Metabolic interactions and impacts on disease. Nat. Rev. Microbiol. 2022, 21, 236–247. [Google Scholar] [CrossRef]



| Index | Group | |||||
|---|---|---|---|---|---|---|
| NC | 0-Gel | 15-Gel | 30-Gel | 45-Gel | 60-Gel | |
| Weight gain (g/mouse) | 3.60 ± 0.82 abc | 2.98 ± 1.04 bc | 1.92 ± 1.19 c | 5.30 ± 0.93 a | 4.02 ± 1.78 ab | 4.51 ± 0.81 ab |
| Food intake (g/day/mouse) | 4.48 ± 0.68 a | 4.63 ± 0.46 a | 4.95 ± 0.71 a | 5.20 ± 1.32 a | 4.77 ± 0.10 a | 5.04 ± 0.62 a |
| Water intake (g/day/mouse) | 7.38 ± 1.66 a | 6.90 ± 2.68 a | 7.90 ± 2.43 a | 8.33 ± 2.80 a | 6.89 ± 1.40 a | 7.00 ± 1.89 a |
| Intestinal villus length (μm) | 428.73 ± 38.03 c | 454.29 ± 72.30 c | 515.06 ± 26.99 b | 599.27 ± 44.28 a | 571.23 ± 48.77 a | 434.67 ± 25.26 c |
| Index | Groups | |||||
|---|---|---|---|---|---|---|
| NC | 0-Gel | 15-Gel | 30-Gel | 45-Gel | 60-Gel | |
| Ace | 421.70 ± 45.49 a | 664.48 ± 79.90 a | 437.29 ± 8.51 a | 431.18 ± 31.12 a | 464.14 ± 97.91 a | 467.66 ± 72.30 a |
| Chao1 | 405.50 ± 43.26 a | 641.11 ± 78.73 a | 424.13 ± 12.47 a | 412.18 ± 29.96 a | 448.54 ± 99.82 a | 456.28 ± 63.74 a |
| Shannon | 2.41 ± 0.94 a | 3.83 ± 0.56 a | 2.79 ± 0.03 a | 2.94 ± 0.34 a | 2.86 ± 0.60 a | 2.94 ± 0.38 a |
| Simpson | 0.28 ± 0.25 a | 0.07 ± 0.04 a | 0.16 ± 0.01 a | 0.14 ± 0.08 a | 0.17 ± 0.07 a | 0.13 ± 0.01 a |
| Metabolic | Groups | |||||
|---|---|---|---|---|---|---|
| Pathway | NC | 0-Gel | 15-Gel | 30-Gel | 45-Gel | 60-Gel |
| ko01100 | 23,439,746 | 19,360,321 | 22,336,993 | 25,354,327 | 22,925,673 | 24,892,139 |
| ko01110 | 10,456,317 | 9,254,657 | 9,910,042 | 11,610,032 | 10,212,205 | 11,975,884 |
| ko01120 | 5,779,879 | 4,734,169 | 5,491,556 | 6,186,953 | 5,589,684 | 6,268,392 |
| ko01230 | 4,132,845 | 4,211,400 | 3,910,724 | 4,821,597 | 4,104,280 | 5,313,082 |
| ko03010 | 4,091,703 | 2,904,285 | 3,773,697 | 4,171,639 | 3,953,634 | 3,987,781 |
| ko01200 | 3,632,795 | 2,968,635 | 3,397,004 | 3,928,264 | 3,529,986 | 3,967,398 |
| ko02010 | 3,386,694 | 2,750,944 | 3,190,401 | 3,285,163 | 3,350,084 | 3,264,017 |
| ko00230 | 2,513,030 | 1,883,457 | 2,389,565 | 2,567,332 | 2,453,262 | 2,669,402 |
| ko02020 | 2,348,392 | 1,940,732 | 2,354,091 | 2,674,206 | 2,322,834 | 2,419,457 |
| ko00010 | 2,398,224 | 1,580,678 | 2,169,217 | 2,247,200 | 2,311,216 | 2,252,075 |
| ko00520 | 2,269,628 | 1,570,781 | 2,084,154 | 2,216,457 | 2,268,899 | 2,013,616 |
| ko00500 | 2,294,951 | 1,551,009 | 2,070,266 | 1,943,892 | 2,247,550 | 1,823,264 |
| ko00240 | 2,017,036 | 1,448,176 | 1,909,123 | 2,033,974 | 1,956,324 | 1,921,928 |
| ko02024 | 1,855,968 | 1,507,906 | 1,688,810 | 1,877,558 | 2,023,999 | 2,036,021 |
| ko00970 | 1,871,519 | 1,341,629 | 1,740,058 | 1,927,406 | 1,820,459 | 1,847,727 |
| ko00620 | 1,581,537 | 1,180,863 | 1,514,130 | 1,672,443 | 1,504,536 | 1,616,424 |
| ko03440 | 1,516,975 | 1,117,059 | 1,402,726 | 1,543,021 | 1,492,658 | 1,504,727 |
| ko02060 | 1,801,536 | 871,704.1 | 1,491,779 | 1,325,477 | 1,644,406 | 1,191,803 |
| ko00550 | 1,408,416 | 1,053,988 | 1,302,987 | 1,402,598 | 1,363,430 | 1,404,262 |
| ko00250 | 1,308,220 | 1,093,091 | 1,258,017 | 1,392,205 | 1,264,763 | 1,338,927 |
| ko00052 | 1,443,285 | 990,006.1 | 1,335,246 | 1,309,394 | 1,398,118 | 1,166,213 |
| ko03430 | 1,344,278 | 962,723.4 | 1,235,516 | 1,370,198 | 1,302,062 | 1,321,198 |
| ko00270 | 1,176,242 | 1,093,446 | 1,176,320 | 1,287,636 | 1,176,006 | 1,368,999 |
| ko00190 | 1,213,440 | 956,018.2 | 1,162,266 | 1,435,505 | 1,178,794 | 1,302,796 |
| ko00680 | 1,274,113 | 930,364.4 | 1,173,188 | 1,324,706 | 1,240,242 | 1,300,340 |
| ko00260 | 1,184,458 | 1,001,140 | 1,100,288 | 1,235,962 | 1,159,757 | 1,346,824 |
| ko00030 | 1,223,938 | 969,190 | 1,129,966 | 1,214,481 | 1,173,019 | 1,237,497 |
| ko00051 | 1,302,862 | 880,650.4 | 1,159,914 | 1,200,922 | 1,286,395 | 1,068,187 |
| ko03030 | 1,139,027 | 810,182.4 | 1,059,922 | 1,165,906 | 1,103,110 | 1,118,433 |
| ko00720 | 1,044,721 | 888,098.8 | 978,078.5 | 1,238,024 | 1,028,684 | 1,202,155 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhang, L.; Xu, H.; Wang, J. Effects of Transglutaminase-Induced β-Conglycinin Gels on Intestinal Morphology and Intestinal Flora in Mice at Different High-Intensity Ultrasound Pretreatment Time. Foods 2024, 13, 2192. https://doi.org/10.3390/foods13142192
Zhang J, Zhang L, Xu H, Wang J. Effects of Transglutaminase-Induced β-Conglycinin Gels on Intestinal Morphology and Intestinal Flora in Mice at Different High-Intensity Ultrasound Pretreatment Time. Foods. 2024; 13(14):2192. https://doi.org/10.3390/foods13142192
Chicago/Turabian StyleZhang, Jixin, Lan Zhang, Huiqing Xu, and Jun Wang. 2024. "Effects of Transglutaminase-Induced β-Conglycinin Gels on Intestinal Morphology and Intestinal Flora in Mice at Different High-Intensity Ultrasound Pretreatment Time" Foods 13, no. 14: 2192. https://doi.org/10.3390/foods13142192
APA StyleZhang, J., Zhang, L., Xu, H., & Wang, J. (2024). Effects of Transglutaminase-Induced β-Conglycinin Gels on Intestinal Morphology and Intestinal Flora in Mice at Different High-Intensity Ultrasound Pretreatment Time. Foods, 13(14), 2192. https://doi.org/10.3390/foods13142192





