Microencapsulation to Harness the Antimicrobial Potential of Essential Oils and Their Applicability in Dairy Products: A Comprehensive Review of the Literature
Abstract
:1. Introduction
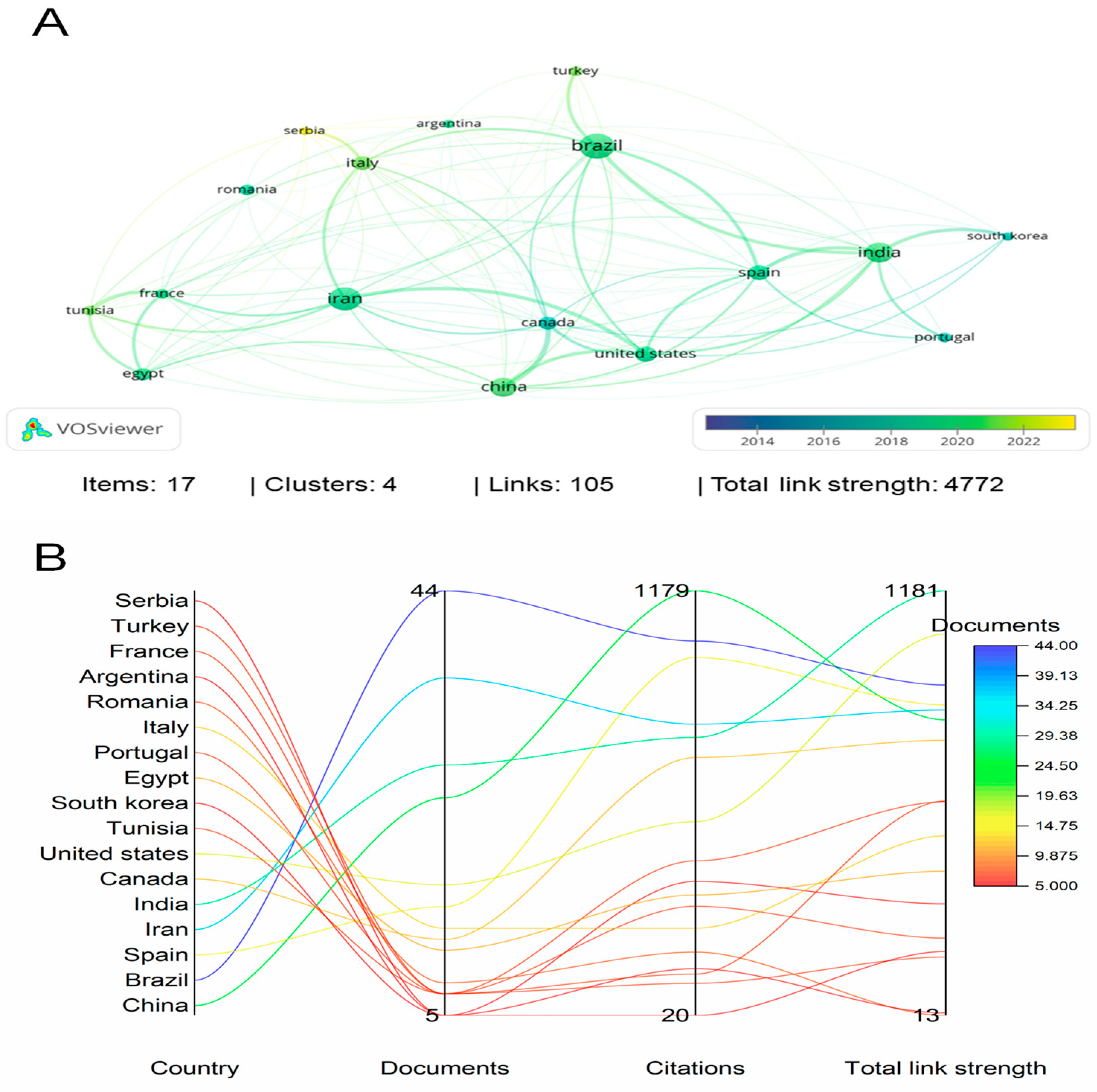
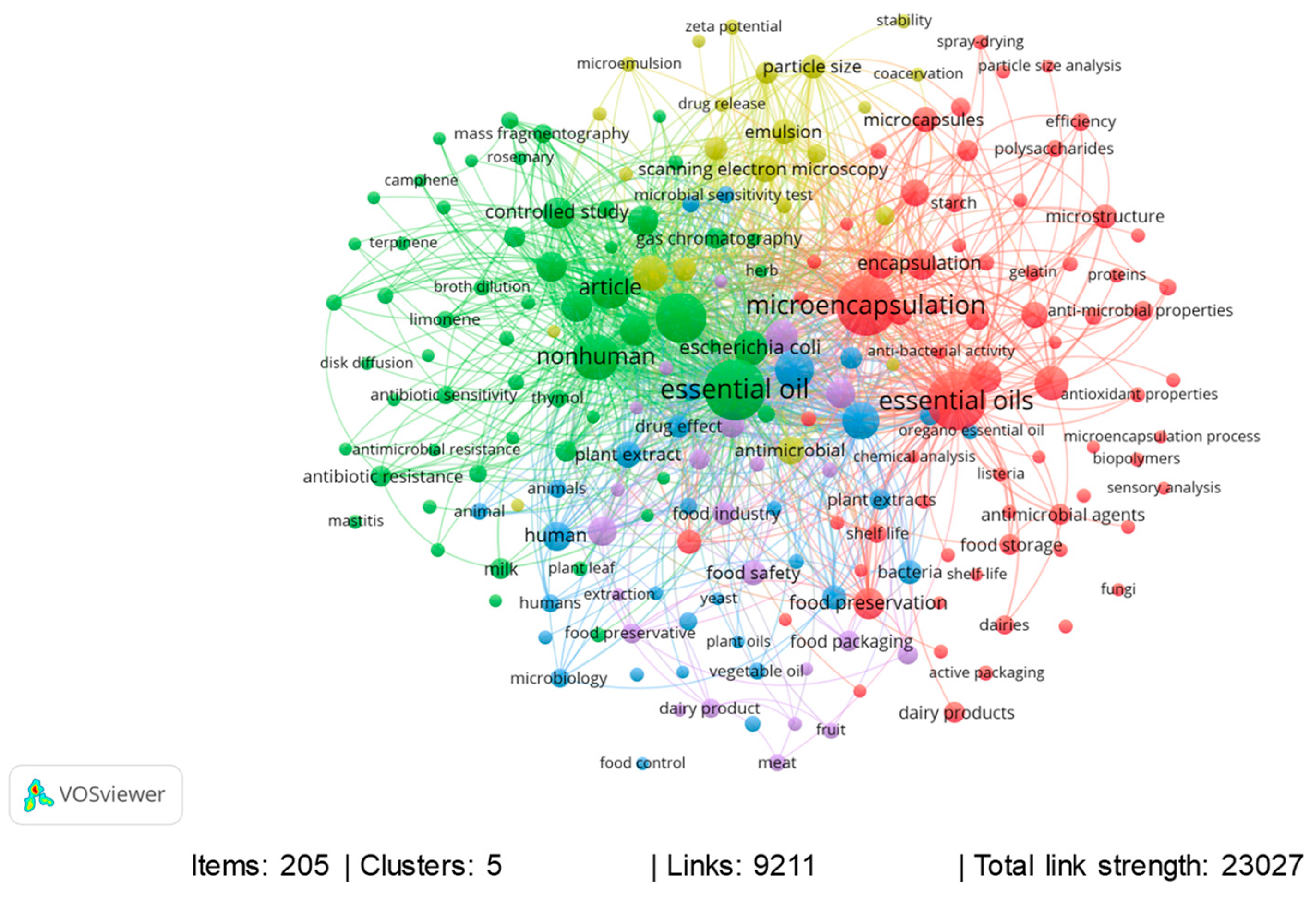
2. Bibliographic Research Methodology
3. Bibliometric Analysis of the Last 11 Years: Period between 2013 and 2023
4. Essential Oils (EOs) and Their Antimicrobial Action
5. Microencapsulation of Essential Oils (EOs) with Antimicrobial Potential
5.1. Microencapsulation Techniques
5.1.1. Interfacial Polymerization
5.1.2. Complex Coacervation
5.1.3. Ionic Gelation
5.1.4. Spray Drying
5.2. Nanoencapsulation Techniques
6. Application in the Dairy Industry
6.1. Milk
6.2. Cheese
6.3. Yogurt
6.4. Other Products
7. Limitations of This Review Study
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haseli, A.; Pourahmad, R.; Eshaghi, M.R.; Rajaei, P.; Akbari-Adergani, B. Application of nanoencapsulated Mofarrah (Nepeta crispa) essential oil as a natural preservative in yogurt drink (doogh). LWT Food Sci. Technol. 2023, 186, 115256. [Google Scholar] [CrossRef]
- Mishra, A.P.; Devkota, H.P.; Nigam, M.; Adetunji, C.O.; Srivastava, N.; Saklani, S.; Khaneghah, A.M. Combination of essential oils in dairy products: A review of their functions and potential benefits. LWT Food Sci. Technol. 2020, 133, 110116. [Google Scholar] [CrossRef]
- Pérez-Soto, E.; Cenobio-Galindo, A.J.; Espino-Manzano, S.O.; Franco-Fernández, M.J.; Ludeña-Urquizo, F.E.; Jiménez-Alvarado, R.; Zepeda-Velázquez, A.P.; Campos-Montiel, F.G. The Addition of Microencapsulated or Nanoemulsified Bioactive Compounds Influences the Antioxidant and Antimicrobial Activities of a Fresh Cheese. Molecules 2021, 26, 2170. [Google Scholar] [CrossRef] [PubMed]
- Dervisoglu, M.; Gul, O.; Aydemir, O.; Yazici, F.; Kahyaoglu, T. Natamycin content and quality evaluation of yoghurt from small- and large-scale brands in Turkey. Food Addit. Contam. Part B 2014, 7, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Varga, G.; Block, E.; Williams, P.; Cassidy, T.; Losa, R. Effect of Crina ruminants, a mixture of essential oil components, on continuous culture fermentation and milk production of lactating cows. J. Dairy Sci. 2004, 87, 334. [Google Scholar]
- Tornambé, G.; Cornu, A.; Verdier-Metz, I.; Pradel, P.; Kondjoyan, N.; Figueredo, G.; Hulin, S.; Martin, B. Addition of pasture plant essential oil in milk: Influence on chemical and sensory properties of milk and cheese. J. Dairy Sci. 2008, 91, 58–69. [Google Scholar] [CrossRef]
- Ben Jemaa, M.; Falleh, H.; Saada, M.; Oueslati, M.; Snoussi, M.; Ksouri, R. Thymus capitatus essential oil ameliorates pasteurization efficiency. J. Food Sci. Technol. 2018, 55, 3446–3452. [Google Scholar] [CrossRef] [PubMed]
- Gouvea, F.D.S.; Rosenthal, A.; Ferreira, E.H.D.R. Plant extract and essential oils added as antimicrobials to cheeses: A review. Ciência Rural 2017, 47, e20160908. [Google Scholar] [CrossRef]
- EL-Kholy, W.; Aamer, R.; Mailam, A. Effect of some essential oils on the quality of UF-soft cheese during storage. Alexandria J. Food Sci. Technol. 2017, 14, 13–28. [Google Scholar] [CrossRef]
- Singh, G.; Kapoor, I.P.S.; Singh, P. Effect of volatile oil and oleoresin of anise on the shelf life of yogurt. J. Food Process. Preserv. 2011, 35, 778–783. [Google Scholar] [CrossRef]
- Azizkhani, M.; Parsaeimehr, M. Probiotics survival, antioxidant activity and sensory properties of yogurt flavored with herbal essential oils. Int. Food Res. J. 2018, 25, 921–927. [Google Scholar]
- Tomar, O.; Akarca, G. Effects of ice cream produced with Lemon, mandarin, and orange peel essential oils on some physicochemical, microbiological and sensorial properties. Kocatepe Vet. J. 2019, 12, 62–70. [Google Scholar] [CrossRef]
- Yilmaztekin, M.; Lević, S.; Kalušević, A.; Cam, M.; Bugarski, B.; Rakić, V.; Nedović, V. Characterisation of peppermint (Mentha piperita L.) essential oil encapsulates. J. Microencapsul. 2019, 36, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Ehsani, A.; Hashemi, M.; Jazani, N.H.; Aliakbarlu, J.; Shokri, S.; Naghibi, S.S. Effect of Echinophora platyloba DC. essential oil and lycopene on the stability of pasteurized cream obtained from cow milk. Vet. Res. Forum 2016, 7, 139. [Google Scholar] [PubMed]
- Ozkan, G.; Simsek, B.; Kuleasan, H. Antioxidant activities of Satureja cilicica essential oil in butter and in vitro. J. Food Eng. 2007, 79, 1391–1396. [Google Scholar] [CrossRef]
- Kouamé, K.J.E.P.; Bora, A.F.M.; Li, X.; Sun, Y.; Liu, L. Novel trends and opportunities for microencapsulation of flaxseed oil in foods: A review. J. Funct. Foods 2021, 87, 104812. [Google Scholar] [CrossRef]
- Jeyakumari, A.; Janarthanan, G.; Chouksey, M.K.; Venkateshwarlu, G. Effect of fish oil encapsulates incorporation on the physico-chemical and sensory properties of cookies. J. Food Sci. Technol. 2016, 53, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Sharma, V.; Sihag, M.K.; Singh, A.K.; Arora, S.; Sabikhi, L. Oxidative stability of alpha-linolenic acid (ω-3) in flaxseed oil microcapsules fortified market milk. Int. J. Dairy Technol. 2017, 70, 188–196. [Google Scholar] [CrossRef]
- Gowda, A.; Sharma, V.; Goyal, A.; Singh, A.K.; Arora, S.; Sabikhi, L. Process optimization and oxidative stability of omega-3 ice cream fortified with flaxseed oil microcapsules. J. Food Sci. Technol. 2018, 55, 1705–1715. [Google Scholar] [CrossRef]
- Shah, B.; Davidson, P.M.; Zhong, Q. Nanodispersed eugenol has improved antimicrobial activity against Escherichia coli O157:H7 and Listeria monocytogenes in bovine milk. Int. J. Food Microbiol. 2013, 161, 53–59. [Google Scholar] [CrossRef]
- Pinilla, C.M.B.; Brandelli, A. Antimicrobial activity of nanoliposomes co-encapsulating nisin and garlic extract against Gram-positive and Gram-negative bacteria in milk. Innov. Food Sci. Emerg. Technol. 2016, 36, 287–293. [Google Scholar] [CrossRef]
- Souza, H.F.; Monteiro, G.F.; Bogáz, L.T.; Freire, E.N.S.; Pereira, K.N.; Carvalho, M.V.; da Cruz, A.G.; Brandi, I.V.; Kamimura, E.S. Bibliometric analysis of water kefir and milk kefir in probiotic foods from 2013 to 2022: A critical review of recent applications and prospects. Food Res. Int. 2023, 175, 113716. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Teixeira, G.L.; Ferreira, B.L. The impact of Brazilian food science over the past two decades. A critical review and meta-analysis. Food Sci. Today 2023, 1, 1–29. [Google Scholar] [CrossRef]
- Chandrakasan, G.; Rodríguez-Hernández, A.I.; del Rocío López-Cuellar, M.; Palma-Rodríguez, H.M.; Chavarría-Hernández, N. Bacteriocin encapsulation for food and pharmaceutical applications: Advances in the past 20 years. Biotechnol. Lett. 2019, 41, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Rocha, R.S.; Ramos, G.L.P.A.; Xavier-Santos, D.; Pimentel, T.C.; Lorenzo, J.M.; Campelo, P.H.; Silva, M.C.; Esmerino, E.A.; Freitas, M.Q.; et al. What are the challenges for ohmic heating in the food industry? Insights of a bibliometric analysis. Food Res. Int. 2022, 157, 111272. [Google Scholar] [CrossRef] [PubMed]
- Xavier-Santos, D.; Padilha, M.; Fabiano, G.A.; Vinderola, G.; Cruz, A.G.; Sivieri, K.; Antunes, A.E.C. Evidences and perspectives of the use of probiotics, prebiotics, synbiotics, and postbiotics as adjuvants for prevention and treatment of COVID-19: A bibliometric analysis and systematic review. Trends Food Sci. Technol. 2022, 120, 174–192. [Google Scholar] [CrossRef]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Yang, C.; Chowdhury, M.A.K.; Huo, Y.; Gong, J. Phytogenic Compounds as Alternatives to In-Feed Antibiotics: Potentials and Challenges in Application. Pathogens 2015, 4, 137–156. [Google Scholar] [CrossRef]
- Omonijo, F.A.; Ni, L.; Gong, J.; Wang, Q.; Lahaye, L.; Yang, C. Essential oils as alternatives to antibiotics in swine production. Anim. Nutr. 2018, 4, 126–136. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, S.; Youssef, A.M. Potential application of herbs and spices and their effects in functional dairy products. Heliyon 2019, 5, e01989. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Hill, L.E.; Peng, Y.; Gomes, C.L. Synthesis and characterization of β-cyclodextrin inclusion complexes of thymol and thyme oil for antimicrobial delivery applications. LWT Food Sci. Technol. 2014, 59, 247–255. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Koteswararao, R.; Sinha, M.; Baral, E.; Cho, M.H. Citrus essential oils: Extraction, authentication and application in food preservation. Crit. Rev. Food Sci. Nutr. 2019, 59, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Cotârlet, M.; Alexe, P.; Dima, S. Microencapsulation of essential oil of pimento [Pimenta dioica (L) Merr.] by chitosan/k-carrageenan complex coacervation method. Innov. Food Sci. Emerg. Technol. 2014, 22, 203–211. [Google Scholar] [CrossRef]
- Bora, H.; Kamle, M.; Mahato, D.K.; Tiwari, P.; Kumar, P. Citrus Essential Oils (CEOs) and Their Applications in Food: An Overview. Plants 2020, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, M.; Carniel, T.K.; Dalcanton, F.; Anjos, R.S.; Riella, H.G.; Araújo, P.H.H.; Oliveira, D.; Fiori, M.A. Use of encapsulated natural compounds as antimicrobial additives in food packaging: A brief review. Trends Food Sci. Technol. 2018, 81, 51–60. [Google Scholar] [CrossRef]
- Silva, B.D.; Bernardes, P.C.; Pinheiro, P.F.; Fantuzzi, E.; Roberto, C.D. Chemical composition, extraction sources and action mechanisms of essential oils: Natural preservative and limitations of use in meat products. Meat Sci. 2021, 176, 108463. [Google Scholar] [CrossRef] [PubMed]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential oils and their major components: An updated review on antimicrobial activities, mechanism of action and their potential application in the food industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Baptista-Silva, S.; Borges, S.; Ramos, O.L.; Pintado, M.; Sarmento, B. The progress of essential oils as potential therapeutic agents: A review. J. Essent. Oil Res. 2020, 32, 279–295. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Rahimi-Nasrabadi, M.; Pourmortazavi, S.M.; Wysokowski, M.; Jesionowski, T.; Ehrlich, H.; Mirsadeghi, S. Supercritical fluid extraction of essential oils. TrAC Trends Anal. Chem. 2019, 118, 182–193. [Google Scholar] [CrossRef]
- Li, Y.X.; Zhang, C.; Pan, S.; Chen, L.; Liu, M.; Yang, K.; Tian, J. Analysis of chemical components and biological activities of essential oils from black and white pepper (Piper nigrum L.) in five provinces of southern China. LWT Food Sci. Technol. 2020, 117, 108644. [Google Scholar] [CrossRef]
- Reis, D.R.; Ambrosi, A.; Di Luccio, M. Encapsulated essential oils: A perspective in food preservation. Future Foods 2022, 5, 100126. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef] [PubMed]
- Mutlu-Ingok, A.; Devecioglu, D.; Dikmetas, D.N.; Karbancioglu-Guler, F.; Capanoglu, E. Antibacterial, antifungal, antimycotoxigenic, and antioxidant activities of essential oils: An updated review. Molecules 2020, 25, 4711. [Google Scholar] [CrossRef]
- Maurya, A.; Prasad, J.; Das, S.; Dwivedy, A.K. Essential oils and their application in food safety. Front. Sustain. Food Syst. 2021, 5, 653420. [Google Scholar] [CrossRef]
- Hou, T.; Sana, S.S.; Li, H.; Xing, Y.; Nanda, A.; Netala, V.R.; Zhang, Z. Essential oils and its antibacterial, antifungal and anti-oxidant activity applications: A review. Food Biosci. 2022, 47, 101716. [Google Scholar] [CrossRef]
- Borges, R.S.; Ortiz, B.L.S.; Pereira, A.C.M.; Keita, H.; Carvalho, J.C.T. Rosmarinus officinalis essential oil: A review of its phytochemistry, anti-inflammatory activity, and mechanisms of action involved. J. Ethnopharmacol. 2019, 229, 29–45. [Google Scholar] [CrossRef]
- Sadeh, D.; Nitzan, N.; Chaimovitsh, D.; Shachter, A.; Ghanim, M.; Dudai, N. Interactive effects of genotype, seasonality and extraction method on chemical compositions and yield of essential oil from rosemary (Rosmarinus officinalis L.). Ind. Crops Prod. 2019, 138, 111419. [Google Scholar] [CrossRef]
- Benkeblia, N. Antimicrobial activity of essential oil extracts of various onions (Allium cepa) and garlic (Allium sativum). LWT Food Sci. Technol. 2004, 37, 263–268. [Google Scholar] [CrossRef]
- Satyal, P.; Craft, J.D.; Dosoky, N.S.; Setzer, W.N. The chemical compositions of the volatile oils of garlic (Allium sativum) and wild garlic (Allium vineale). Foods 2017, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Miao, X.; Lan, X.; Luo, J.; Luo, T.; Zhong, Z.; Tang, Y. Angelica essential oil loaded electrospun gelatin nanofibers for active food packaging application. Polymers 2020, 12, 299. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, M.; Rat, M.; Pezo, L.; Lončar, B.; Pezo, M.; Miljković, A.; Lazarević, J. Biological and chemical diversity of Angelica archangelica L.—Case study of essential oil and its biological activity. Agronomy 2022, 12, 1570. [Google Scholar] [CrossRef]
- Najaf Najafi, M.; Arianmehr, A.; Mohammadi Sani, A. Encapsulation of Barije (Ferula gummosa) essential oil in nanoliposomal system and evaluation of its physical and antimicrobial properties. Innov. Food Technol. 2019, 7, 71–83. [Google Scholar]
- Najafi, M.N.; Arianmehr, A.; Sani, A.M. Preparation of barije (Ferula gummosa) essential oil–loaded liposomes and evaluation of physical and antibacterial effect on Escherichia coli O157:H7. J. Food Prot. 2020, 83, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Raeisi, M.; Tajik, H.; Yarahmadi, A.; Sanginabadi, S. Antimicrobial effect of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Health Scope 2015, 4, e21808. [Google Scholar] [CrossRef]
- Saki, M.; Seyed-Mohammadi, S.; Montazeri, E.A.; Siahpoosh, A.; Moosavian, M.; Latifi, S.M. In vitro antibacterial properties of Cinnamomum zeylanicum essential oil against clinical extensively drug-resistant bacteria. Eur. J. Integr. Med. 2020, 37, 101146. [Google Scholar] [CrossRef]
- Ekpenyong, C.E.; Akpan, E.E. Use of Cymbopogon citratus essential oil in food preservation: Recent advances and future perspectives. Crit. Rev. Food Sci. Nutr. 2017, 57, 2541–2559. [Google Scholar] [CrossRef]
- Majewska, E.; Kozlowska, M.; Gruszczynska-Sekowska, E.; Kowalska, D.; Tarnowska, K. Lemongrass (Cymbopogon citratus) essential oil: Extraction, composition, bioactivity and uses for food preservation–A review. Pol. J. Food Nutr. Sci. 2019, 69, 4. [Google Scholar] [CrossRef]
- Verma, R.S.; Verma, S.K.; Tandon, S.; Padalia, R.C.; Darokar, M.P. Chemical composition and antimicrobial activity of Java citronella (Cymbopogon winterianus Jowitt ex Bor) essential oil extracted by different methods. J. Essent. Oil Res. 2020, 32, 449–455. [Google Scholar] [CrossRef]
- Staudt, A.; Duarte, P.F.; Amaral, B.P.D.; Peixoto Andrade, B.C.D.O.; Simas, N.K.; Correa Ramos Leal, I.; Paroul, N. Biological properties of functional flavoring produced by enzymatic esterification of citronellol and geraniol present in Cymbopogon winterianus essential oil. Nat. Prod. Res. 2021, 35, 5981–5987. [Google Scholar] [CrossRef] [PubMed]
- Kačániová, M.; Galovičová, L.; Ivanišová, E.; Vukovic, N.L.; Štefániková, J.; Valková, V.; Tvrdá, E. Antioxidant, antimicrobial and antibiofilm activity of coriander (Coriandrum sativum L.) essential oil for its application in foods. Foods 2020, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Banadka, A.; Nandhini, M.; Nagella, P.; Al-Mssallem, M.Q.; Alessa, F.M. Essential oil from Coriandrum sativum: A review on its phytochemistry and biological activity. Molecules 2023, 28, 696. [Google Scholar] [CrossRef] [PubMed]
- Dalli, M.; Azizi, S.E.; Benouda, H.; Azghar, A.; Tahri, M.; Bouammali, B.; Gseyra, N. Molecular composition and antibacterial effect of five essential oils extracted from Nigella sativa L. seeds against multidrug-resistant bacteria: A comparative study. Evid. Based Complement. Alternat. Med. 2021, 2021, 6643765. [Google Scholar] [CrossRef] [PubMed]
- Albakry, Z.; Karrar, E.; Ahmed, I.A.M.; Oz, E.; Proestos, C.; El Sheikha, A.F.; Wang, X. Nutritional composition and volatile compounds of black cumin (Nigella sativa L.) seed, fatty acid composition and tocopherols, polyphenols, and antioxidant activity of its essential oil. Horticulturae 2022, 8, 575. [Google Scholar] [CrossRef]
- Abdoul-Latif, F.M.; Elmi, A.; Merito, A.; Nour, M.; Risler, A.; Ainane, A.; Ainane, T. Essential oils of Tagetes minuta and Lavandula coronopifolia from Djibouti: Chemical composition, antibacterial activity and cytotoxic activity against various human cancer cell lines. Int. J. Plant Biol. 2022, 13, 315–329. [Google Scholar] [CrossRef]
- Walasek-Janusz, M.; Grzegorczyk, A.; Zalewski, D.; Malm, A.; Gajcy, S.; Gruszecki, R. Variation in the Antimicrobial Activity of Essential Oils from Cultivars of Lavandula angustifolia and L.× intermedia. Agronomy 2022, 12, 2955. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.T.; Khan, M.; Mousa, A.A.; Mahmood, A.; Alkhathlan, H.Z. Chemical diversity in leaf and stem essential oils of Origanum vulgare L. and their effects on microbicidal activities. AMB Express 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Burton, D.; Parra, F.; López, J.; Muñoz, P.; Escobar, H.; Parra, C. Antioxidant and antibacterial capacities of Origanum vulgare L. essential oil from the arid Andean Region of Chile and its chemical characterization by GC-MS. Metabolites 2020, 10, 414. [Google Scholar] [CrossRef]
- Mérida-Reyes, M.S.; Muñoz-Wug, M.A.; Oliva-Hernández, B.E.; Gaitán-Fernández, I.C.; Simas, D.L.R.; Ribeiro da Silva, A.J.; Pérez-Sabino, J.F. Composition and antibacterial activity of the essential oil from Pimenta dioica (L.) Merr. from Guatemala. Medicines 2020, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- ALrashidi, A.A.; Noumi, E.; Snoussi, M.; Feo, V.D. Chemical composition, antibacterial and anti-quorum sensing activities of Pimenta dioica L. essential oil and its major compound (eugenol) against foodborne pathogenic bacteria. Plants 2022, 11, 540. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.L.B.; Pinto, L.C.; da Costa, J.S.; da Silva, A.R.C.; Mourão, R.H.V.; Montenegro, R.C.; Maia, J.G.S. Composition, antioxidant capacity and cytotoxic activity of Eugenia uniflora L. chemotype-oils from the Amazon. J. Ethnopharmacol. 2019, 232, 30–38. [Google Scholar] [CrossRef]
- Fidelis, E.M.; Savall, A.S.P.; de Oliveira Pereira, F.; Quines, C.B.; Ávila, D.S.; Pinton, S. Pitanga (Eugenia uniflora L.) as a source of bioactive compounds for health benefits: A review. Arab. J. Chem. 2022, 15, 103691. [Google Scholar] [CrossRef]
- Pejčić, M.; Stojanović-Radić, Z.; Genčić, M.; Dimitrijević, M.; Radulović, N. Anti-virulence potential of basil and sage essential oils: Inhibition of biofilm formation, motility and pyocyanin production of Pseudomonas aeruginosa isolates. Food Chem. Toxicol. 2020, 141, 111431. [Google Scholar] [CrossRef] [PubMed]
- Assaggaf, H.M.; Naceiri Mrabti, H.; Rajab, B.S.; Attar, A.A.; Alyamani, R.A.; Hamed, M.; Bouyahya, A. Chemical analysis and investigation of biological effects of Salvia officinalis essential oils at three phenological stages. Molecules 2022, 27, 5157. [Google Scholar] [CrossRef]
- Galovičová, L.; Borotová, P.; Valková, V.; Vukovic, N.L.; Vukic, M.; Štefániková, J.; Kačániová, M. Thymus vulgaris essential oil and its biological activity. Plants 2021, 10, 1959. [Google Scholar] [CrossRef]
- Liu, T.; Kang, J.; Liu, L. Thymol as a critical component of Thymus vulgaris L. essential oil combats Pseudomonas aeruginosa by intercalating DNA and inactivating biofilm. LWT Food Sci. Technol. 2021, 136, 110354. [Google Scholar] [CrossRef]
- Yemiş, G.P.; Candoğan, K. Antibacterial activity of soy edible coatings incorporated with thyme and oregano essential oils on beef against pathogenic bacteria. Food Sci. Biotechnol. 2017, 26, 1113–1121. [Google Scholar] [CrossRef]
- Gaba, A.B.M.; Hassan, M.A.; Abd EL-Tawab, A.A.; Abdelmonem, M.A.; Morsy, M.K. Protective impact of chitosan film loaded oregano and thyme essential oil on the microbial profile and quality attributes of beef meat. Antibiotics 2022, 11, 583. [Google Scholar] [CrossRef]
- Zheng, K.; Li, B.; Liu, Y.; Wu, D.; Bai, Y.; Xiang, Q. Effect of chitosan coating incorporated with oregano essential oil on microbial inactivation and quality properties of refrigerated chicken breasts. LWT 2023, 176, 114547. [Google Scholar] [CrossRef]
- Kazemi, S.M.; Rezaei, M. Antimicrobial effectiveness of gelatin-alginate film containing oregano essential oil for fish preservation. J. Food Saf. 2015, 35, 482–490. [Google Scholar] [CrossRef]
- Wu, J.; Ge, S.; Liu, H.; Wang, S.; Chen, S.; Wang, J.; Li, J.; Zhang, Q. Properties and antimicrobial activity of silver carp (Hypophthalmichthys molitrix) skin gelatin-chitosan films incorporated with oregano essential oil for fish preservation. Food Packag. Shelf 2014, 2, 7–16. [Google Scholar] [CrossRef]
- Teixeira, R.F.; Balbinot Filho, C.A.; Borges, C.D. Essential oils as natural antimicrobials for application in edible coatings for minimally processed apple and melon: A review on antimicrobial activity and characteristics of food models. Food Packag. Shelf 2022, 31, 100781. [Google Scholar] [CrossRef]
- Kour, J.; Gupta, P.; Zahra, S.; Bansal, V.; Sharma, R.; Ajaz, M.; Khajuria, A.K. Introduction to Spices and Their Bioactive Components. In Spice Bioactive Compounds; CRC Press: Boca Raton, FL, USA, 2022; pp. 1–22. [Google Scholar]
- Sundar, S.K.; Parikh, J.K. Advances and trends in encapsulation of essential oils. Int. J. Pharm. 2023, 635, 122668. [Google Scholar] [CrossRef] [PubMed]
- Solanki, K.P.; Desai, M.A.; Parikh, J.K. Improved hydrodistillation process using amphiphilic compounds for extraction of essential oil from java citronella grass. Chem. Pap. 2020, 74, 145–156. [Google Scholar] [CrossRef]
- Kaya, D.A.; Ghica, M.V.; Dănilă, E.; Öztürk, Ş.; Türkmen, M.; Albu Kaya, M.G.; Dinu-Pîrvu, C.E. Selection of optimal operating conditions for extraction of Myrtus communis L. essential oil by the steam distillation method. Molecules 2020, 25, 2399. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Sun, F.; Wang, S.; Wang, W.; Dong, J.; Gao, F. Enhanced extraction of essential oil from Cinnamomum cassia bark by ultrasound-assisted hydrodistillation. Chin. J. Chem. Eng. 2021, 36, 38–46. [Google Scholar] [CrossRef]
- Kaderides, K.; Papaoikonomou, L.; Serafim, M.; Goula, A.M. Microwave-assisted extraction of phenolics from pomegranate peels: Optimization, kinetics, and comparison with ultrasounds extraction. Chem. Eng. Process. Process Intensif. 2019, 137, 1–11. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.J.; Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.G.; Wei, Z.J. Recent updates on the chemistry, bioactivities, mode of action, and industrial applications of plant essential oils. Trends Food Sci. Technol. 2021, 110, 78–89. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antibacterial mechanism of oregano essential oil. Ind. Crops Prod. 2019, 139, 111498. [Google Scholar] [CrossRef]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential oils as antimicrobial agents—Myth or real alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Baek, K.H. Biological efficacy and application of essential oils in foods—A review. J. Essent. Oil Bear. Plants 2016, 19, 1–19. [Google Scholar] [CrossRef]
- Behbahani, B.A.; Noshad, M.; Falah, F. Cumin essential oil: Phytochemical analysis, antimicrobial activity and investigation of its mechanism of action through scanning electron microscopy. Microb. Pathog. 2019, 136, 103716. [Google Scholar] [CrossRef] [PubMed]
- Mukurumbira, A.R.; Shellie, R.A.; Keast, R.; Palombo, E.A.; Jadhav, S.R. Encapsulation of essential oils and their application in antimicrobial active packaging. Food Control 2022, 136, 108883. [Google Scholar] [CrossRef]
- Rao, J.; Chen, B.; McClements, D.J. Improving the efficacy of essential oils as antimicrobials in foods: Mechanisms of action. Annu. Rev. Food Sci. Technol. 2019, 10, 365–387. [Google Scholar] [CrossRef] [PubMed]
- Cutro, A.C.; Castelli, M.V.; López, S.N.; Rosales, M.A.; Hollmann, A.; Rodriguez, S.A. Chemical composition of Schinus areira essential oil and antimicrobial action against Staphylococcus aureus. Nat. Prod. Res. 2021, 35, 2931–2936. [Google Scholar] [CrossRef]
- Jugreet, B.S.; Suroowan, S.; Rengasamy, R.K.; Mahomoodally, M.F. Chemistry, bioactivities, mode of action and industrial applications of essential oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Lopez-Romero, J.C.; González-Ríos, H.; Borges, A.; Simões, M. Antibacterial effects and mode of action of selected essential oils components against Escherichia coli and Staphylococcus aureus. Evid. Based Complement. Alternat. Med. 2015, 2015, 795435. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Mani-López, E.; Cortés-Zavaleta, O.; López-Malo, A. A review of the methods used to determine the target site or the mechanism of action of essential oils and their components against fungi. SN Appl. Sci. 2021, 3, 44. [Google Scholar] [CrossRef]
- Alderees, F.; Mereddy, R.; Were, S.; Netzel, M.E.; Sultanbawa, Y. Anti-yeast synergistic effects and mode of action of Australian Native Plant Essential Oils. Appl. Sci. 2021, 11, 10670. [Google Scholar] [CrossRef]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.A.; Casabianca, H.; Elaissari, A. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef] [PubMed]
- Donsì, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef]
- Biddeci, G.; Cavallaro, G.; Di Blasi, F.; Lazzara, G.; Massaro, M.; Milioto, S.; Spinelli, G. Halloysite nanotubes loaded with peppermint essential oil as filler for functional biopolymer film. Carbohydr. Polym. 2016, 152, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, L.V.V. Microencapsulated Bioactive Components as a Source of Health. In Encapsulations; Academic Press: Cambridge, MA, USA, 2016; pp. 455–501. [Google Scholar]
- Vinceković, M.; Viskić, M.; Jurić, S.; Giacometti, J.; Kovačević, D.B.; Putnik, P.; Jambrak, A.R. Innovative technologies for encapsulation of Mediterranean plants extracts. Trends Food Sci. Technol. 2017, 69, 1–12. [Google Scholar] [CrossRef]
- Vijeth, S.; Heggannavar, G.B.; Kariduraganavar, M.Y. Encapsulating wall materials for micro-/nanocapsules. Microencapsul. Processes Technol. Ind. Appl. 2019, 2019, 1–19. [Google Scholar]
- Bayryamov, S.G. Microencapsulation of natural oils by a coacervation technique using gelatin as shell material. J. Chem. Technol. Metall. 2020, 55, 1985–1989. [Google Scholar]
- Lee, J.H.; Yeo, Y. Controlled drug release from pharmaceutical nanocarriers. Chem. Eng. Sci. 2020, 125, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.B.; Zhang, H.; Yue, C.Y.; Yang, J. Fabrication and release behavior of microcapsules with double-layered shell containing clove oil for antibacterial applications. ACS Appl. Mater. Interfaces. 2018, 10, 15532–15541. [Google Scholar] [CrossRef] [PubMed]
- Marcela, F.; Lucía, C.; Eva, B.; David, G.; Ángeles, B.M.; Luis, B. Microencapsulation of essential oils by interfacial polymerization using polyurea as a wall material. J. Encapsulation Adsorpt. Sci. 2015, 5, 165–177. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, M.; Adhikari, B.; Wang, M. Microencapsulation of Sichuan pepper essential oil in soybean protein isolate-Sichuan pepper seed soluble dietary fiber complex coacervates. Food Hydrocoll. 2022, 125, 107421. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Adhikari, B.; Chang, L. Microencapsulation of rose essential oil in mung bean protein isolate-apricot peel pectin complex coacervates and characterization of microcapsules. Food Hydrocoll. 2022, 124, 107366. [Google Scholar] [CrossRef]
- De Matos, E.F.; Scopel, B.S.; Dettmer, A. Citronella essential oil microencapsulation by complex coacervation with leather waste gelatin and sodium alginate. J. Environ. Chem. Eng. 2018, 6, 1989–1994. [Google Scholar] [CrossRef]
- Mazza, K.E.L.; Costa, A.M.M.; da Silva, J.P.L.; Alviano, D.S.; Bizzo, H.R.; Tonon, R.V. Microencapsulation of marjoram essential oil as a food additive using sodium alginate and whey protein isolate. Int. J. Biol. Macromol. 2023, 233, 123478. [Google Scholar] [CrossRef] [PubMed]
- Benavides, S.; Cortés, P.; Parada, J.; Franco, W. Development of alginate microspheres containing thyme essential oil using ionic gelation. Food Chem. 2016, 204, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Menin, A.; Zanoni, F.; Vakarelova, M.; Chignola, R.; Donà, G.; Rizzi, C.; Zoccatelli, G. Effects of microencapsulation by ionic gelation on the oxidative stability of flaxseed oil. Food Chem. 2018, 269, 293–299. [Google Scholar] [CrossRef]
- Hu, Q.; Li, X.; Chen, F.; Wan, R.; Yu, C.W.; Li, J.; Deng, Z. Microencapsulation of an essential oil (cinnamon oil) by spray drying: Effects of wall materials and storage conditions on microcapsule properties. J. Food Process. Preserv. 2020, 44, e14805. [Google Scholar] [CrossRef]
- Bajac, J.; Nikolovski, B.; Lončarević, I.; Petrović, J.; Bajac, B.; Đurović, S.; Petrović, L. Microencapsulation of juniper berry essential oil (Juniperus communis L.) by spray drying: Microcapsule characterization and release kinetics of the oil. Food Hydrocoll. 2022, 125, 107430. [Google Scholar] [CrossRef]
- Mehran, M.; Masoum, S.; Memarzadeh, M. Microencapsulation of Mentha spicata essential oil by spray drying: Optimization, characterization, release kinetics of essential oil from microcapsules in food models. Ind. Crops Prod. 2020, 154, 112694. [Google Scholar] [CrossRef]
- Bahrampour, Z.; Peighambardoust, S.H.; Amini, A.M.; Soltanzadeh, M. Application of low-, and medium-molecular weight chitosan for preparation of spray-dried microparticles loaded with Ferulago angulata essential oil: Physicochemical, antioxidant, antibacterial and in-vitro release properties. Int. J. Biol. Macromol. 2023, 253, 126554. [Google Scholar] [CrossRef] [PubMed]
- Allaw, M.; Manconi, M.; Caboni, P.; Bacchetta, G.; Escribano-Ferrer, E.; Peris, J.E.; Manca, M.L. Formulation of liposomes loading lentisk oil to ameliorate topical delivery, attenuate oxidative stress damage and improve cell migration in scratch assay. Biomed. Pharmacother. 2021, 144, 112351. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Gu, Y.; Sun, Y.; Cui, H. Characterization of chrysanthemum essential oil triple-layer liposomes and its application against Campylobacter jejuni on chicken. LWT Food Sci. Technol. 2019, 107, 16–24. [Google Scholar] [CrossRef]
- Sebaaly, C.; Jraij, A.; Fessi, H.; Charcosset, C.; Greige-Gerges, H. Preparation and characterization of clove essential oil-loaded liposomes. Food Chem. 2015, 178, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Mendes, J.F.; Norcino, L.B.; Corrêa, T.Q.; Barbosa, T.V.; Paschoalin, R.T.; Mattoso, L.H.C. Obtaining poly (lactic acid) nanofibers encapsulated with peppermint essential oil as potential packaging via solution-blow-spinning. Int. J. Biol. Macromol. 2023, 230, 123424. [Google Scholar] [CrossRef]
- Fonseca, L.M.; Radünz, M.; dos Santos Hackbart, H.C.; da Silva, F.T.; Camargo, T.M.; Bruni, G.P.; Dias, A.R. Electrospun potato starch nanofibers for thyme essential oil encapsulation: Antioxidant activity and thermal resistance. J. Sci. Food Agric. 2020, 100, 4263–4271. [Google Scholar] [CrossRef]
- Castro, J.C.; Pante, G.C.; de Souza, D.S.; Pires, T.Y.; Miyoshi, J.H.; Garcia, F.P.; Matioli, G. Molecular inclusion of Cymbopogon martinii essential oil with β-cyclodextrin as a strategy to stabilize and increase its bioactivity. Food Hydrocoll. Health 2022, 2, 100066. [Google Scholar] [CrossRef]
- Luo, X.; Zeng, L.; Li, Q.; Wang, Z.; Kong, F.; Bi, Y. β-cyclodextrin inclusion complex containing essential oil from wampee [Clausena lansium (lour.) Skeels] fruit pericarp: Synthesis, characterization, and evaluation of antioxidant activity. J. Mol. Struct. 2022, 1266, 133525. [Google Scholar] [CrossRef]
- Xi, X.; Huang, J.; Zhang, S.; Lu, Q.; Fang, Z.; Li, C.; Hu, B. Preparation and characterization of inclusion complex of Myristica fragrans Houtt. (nutmeg) essential oil with 2-hydroxypropyl-β-cyclodextrin. Food Chem. 2023, 423, 136316. [Google Scholar] [CrossRef] [PubMed]
- Veiga, R.D.S.D.; Aparecida Da Silva-Buzanello, R.; Corso, M.P.; Canan, C. Essential oils microencapsulated obtained by spray drying: A review. J. Essent. Oil Res. 2019, 31, 457–473. [Google Scholar] [CrossRef]
- Emerenciano, D.P.; Baracho, B.B.; Medeiros, M.L.D.; Rocha, H.A.; Xavier, F.H.; Veiga, V.F.D.; Maciel, M.A.M. Physicochemical characterizations and antioxidant property of copaiba oil loaded into SNEDDS systems. J. Braz. Chem. Soc. 2019, 30, 234–246. [Google Scholar] [CrossRef]
- Augustin, M.A.; Sanguansri, L.; Rusli, J.K.; Shen, Z.; Cheng, L.J.; Keogh, J.; Clifton, P. Digestion of microencapsulated oil powders: In vitro lipolysis and in vivo absorption from a food matrix. Food Funct. 2014, 5, 2905–2912. [Google Scholar] [CrossRef] [PubMed]
- Bornhorst, G.M.; Paul Singh, R. Gastric digestion in vivo and in vitro: How the structural aspects of food influence the digestion process. Annu. Rev. Food Sci. Technol. 2014, 5, 111–132. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Adhikari, R.; Barrow, C.J.; Adhikari, B. Digestion behaviour of chia seed oil encapsulated in chia seed protein-gum complex coacervates. Food Hydrocoll. 2017, 66, 71–81. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Vongsvivut, J.; Adhikari, R.; Adhikari, B. Physicochemical and thermal characteristics of Australian chia seed oil. Food Chem. 2017, 228, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Leyva, J.D.; Bello-Pérez, L.A.; Alvarez-Ramirez, J.; Garcia, H.S. Microencapsulation using starch as wall material: A review. Food Rev. Int. 2018, 34, 148–161. [Google Scholar] [CrossRef]
- Song, Y.; Fan, J.B.; Wang, S. Recent progress in interfacial polymerization. Mat. Chem. Front. 2017, 1, 1028–1040. [Google Scholar] [CrossRef]
- Lages, M.; Nicolas, J. In situ encapsulation of biologically active ingredients into polymer particles by polymerization in dispersed media. Prog. Polym. Sci. 2023, 137, 101637. [Google Scholar] [CrossRef]
- Wang, X.; Amason, A.C.; Miceli, R.T.; He, P.; Lei, Y.; Gabbard, R.; Wieland, J.A.; Lindardt, R.J.; Corr, D.T.; Gross, R.A. Biobased diglycidyl ether diphenolates: Effect of the ester moiety on fragrance oil microencapsulation by interfacial polymerization. Colloids Surf. A Physicochem. Eng. Aspects. 2022, 648, 129243. [Google Scholar] [CrossRef]
- Lam, P.L.; Gambari, R. Advanced progress of microencapsulation technologies: In vivo and in vitro models for studying oral and transdermal drug deliveries. J. Control. Release 2014, 178, 25–45. [Google Scholar] [CrossRef] [PubMed]
- Ach, D.; Briançon, S.; Broze, G.; Puel, F.; Rivoire, A.; Galvan, J.M.; Chevalier, Y. Formation of microcapsules by complex coacervation. Can. J. Chem. Eng. 2015, 93, 183–191. [Google Scholar] [CrossRef]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of oils: A comprehensive review of benefits, techniques, and applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef] [PubMed]
- Kurozawa, L.E.; Hubinger, M.D. Hydrophilic food compounds encapsulation by ionic gelation. Curr. Opin. Food Sci. 2017, 15, 50–55. [Google Scholar] [CrossRef]
- Bagheri, R.; Ariaii, P.; Motamedzadegan, A. Characterization, antioxidant and antibacterial activities of chitosan nanoparticles loaded with nettle essential oil. J. Food Meas. Charact. 2021, 15, 1395–1402. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with chitosan by spray drying for industry applications–A review. Trends Food Sci. Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- Hammoud, Z.; Gharib, R.; Fourmentin, S.; Elaissari, A.; Greige-Gerges, H. Drug-in-hydroxypropyl-β-cyclodextrin-in-lipoid S100/cholesterol liposomes: Effect of the characteristics of essential oil components on their encapsulation and release. Int. J. Pharm. 2020, 579, 119151. [Google Scholar] [CrossRef]
- Nasr, G.; Greige-Gerges, H.; Elaissari, A.; Khreich, N. Liposome Permeability to Essential Oil Components: A Focus on Cholesterol Content. J. Membr. Biol. 2021, 254, 381–395. [Google Scholar] [CrossRef]
- Rather, A.H.; Wani, T.U.; Khan, R.S.; Pant, B.; Park, M.; Sheikh, F.A. Prospects of polymeric nanofibers loaded with essential oils for biomedical and food-packaging applications. Int. J. Mol. Sci. 2021, 22, 4017. [Google Scholar] [CrossRef]
- Anal, A.K.; Singh, H. Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci. Technol. 2007, 18, 240–251. [Google Scholar] [CrossRef]
- Peng, Q.; Luo, X.; Su, J.; Bi, Y.; Kong, F.; Wang, Z.; Tan, S.; Zhang, J. Microencapsulation of star anise essential oil: Preparation, characterization, in vitro digestion, and biological activity. Colloids Surf. A Physicochem. Eng. Asp. 2024, 696, 134358. [Google Scholar] [CrossRef]
- Soutelino, M.E.M.; Silva, A.C.O.; Rocha, R.S. Natural Antimicrobials in Dairy Products: Benefits, Challenges, and Future Trends. Antibiotics 2024, 13, 415. [Google Scholar] [CrossRef]
- Van, C.K.; Nguyen, P.T.N.; Nguyen, T.-T.T.; Bach, L.G. Microencapsulation of Citrus latifolia peel essential oil by spray-drying using maltodextrin: Characterization, antimicrobial activities, and release profile. LWT Food Sci. Technol. 2024, 197, 115825. [Google Scholar] [CrossRef]
- Pan, K.; Chen, H.; Davidson, P.M.; Zhong, Q.J. Thymol nanoencapsulated by sodium caseinate: Physical and antilisterial properties. J. Agric. Food Chem. 2014, 62, 1649–1657. [Google Scholar] [CrossRef]
- Locali-Pereira, A.R.; Lopes, N.A.; Menis-Henrique, M.E.C.; Janzantti, N.S.; Nicoletti, V.R. Modulation of volatile release and antimicrobial properties of pink pepper essential oil by microencapsulation in single- and double-layer structured matrices. Int. J. Food Microbiol. 2020, 335, 108890. [Google Scholar] [CrossRef]
- Melo, A.M.D.; Turola Barbi, R.C.; Souza, W.F.C.D.; Luna, L.C.; de Souza, H.J.B.; Lucena, G.L.; de Sousa, S. Microencapsulated lemongrass (Cymbopogon flexuosus) essential oil: A new source of natural additive applied to Coalho cheese. J. Food Process. Preserv. 2020, 44, e14783. [Google Scholar] [CrossRef]
- Fernandes, R.V.D.B.; Guimarães, I.C.; Ferreira, C.L.R.; Botrel, D.A.; Borges, S.V.; de Souza, A.U. Microencapsulated rosemary (Rosmarinus officinalis) essential oil as a biopreservative in minas frescal cheese. J. Food Process. Preserv. 2017, 41, e12759. [Google Scholar] [CrossRef]
- Bedoya-Serna, C.M.; Dacanal, G.C.; Fernandes, A.M.; Pinho, S.C. Antifungal activity of nanoemulsions encapsulating oregano (Origanum vulgare) essential oil: In vitro study and application in Minas Padrão cheese. Braz. J. Microbiol. 2018, 49, 929–935. [Google Scholar] [CrossRef]
- Fernandes, R.; Botrel, D.; Borges, S.; Souza, A.; Monteiro, P.; Mendes, L. Microencapsulated oregano essential oil in grated Parmesan cheese conservation. Int. Food Res. J. 2018, 25, 661. [Google Scholar]
- Artiga-Artigas, M.; Acevedo-Fani, A.; Martín-Belloso, O. Improving the shelf life of low-fat cut cheese using nanoemulsion-based edible coatings containing oregano essential oil and mandarin fiber. Food Control. 2017, 76, 1–12. [Google Scholar] [CrossRef]
- Nazari, M.; Ghanbarzadeh, B.; Kafil, H.S.; Zeinali, M.; Hamishehkar, H. Garlic essential oil nanophytosomes as a natural food preservative: Its application in yogurt as food model. Colloid Interface Sci. Commun. 2019, 30, 100176. [Google Scholar] [CrossRef]
- Smigic, N.; Djekic, I.; Tomasevic, I.; Miocinovic, J.; Gvozdenovic, R. Implication of food safety measures on microbiological quality of raw and pasteurized milk. Food Control. 2012, 25, 728–731. [Google Scholar]
- Oliver, S.P.; Boor, K.J.; Murphy, S.C.; Murinda, E.S. Review food safety hazard associated with consumption of raw milk. J. Dairy Sci. 2009, 85, 112–121. [Google Scholar]
- Godefay, B.; Molla, B. Bacteriological quality of raw cow’s milk from four dairy farms and a milk collection centre in and around Addis Ababa. Berl. Munch. Tierarztl. Wochenschr. 2000, 113, 276–278. [Google Scholar] [PubMed]
- Selim, S. Antimicrobial activity of essential oils against vancomycin-resistant enterococci (vre) and Escherichia coli o157: h7 in feta soft cheese and minced beef meat. Braz. J. Microbiol. 2011, 42, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Sassi, G.; Shankar, S.; Jaiswal, L.; Salmieri, S.; Karboune, S.; Lacroix, M. Nanoemulsion-based spray-dried formulation of essential oils, whey protein isolate, and maltodextrin: An approach for antifungal preservation of grated mozzarella cheese. Int. Dairy J. 2024, 154, 105919. [Google Scholar] [CrossRef]
- Bakry, A.M.; Chen, Y.Q.; Liang, L. Developing a mint yogurt enriched with omega-3 oil: Physiochemical, microbiological, rheological, and sensorial characteristics. J. Food Process. Preserv. 2019, 43, e14287. [Google Scholar] [CrossRef]
- Thabet, H.M.; Nogaim, Q.A.; Qasha, A.S.; Abdoalaziz, O.; Alnsheme, N. Evaluation of the effects of some plant derived essential oils on shelf life extension of Labneh. Merit Res. J. Food Sci. Technol. 2014, 2, 8–14. [Google Scholar]
- Nanakali, N.M. Fabrication of nano-encapsulated angelica (Heracleum persicum) essential oil for enriching dairy dessert: Physicochemical, rheological and sensorial properties. IET Nanobiotechnology 2023, 17, 171–181. [Google Scholar] [CrossRef]
- Adinepour, F.; Pouramin, S.; Rashidinejad, A.; Jafari, S.M. Fortification/enrichment of milk and dairy products by encapsulated bioactive ingredients. Food Res. Int. 2022, 157, 111212. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, H.; Alizadeh, M.; Hassanzadeh, R.; Ghanbarzadeh, B. Garlic essential oil-based nanoemulsion carrier: Release and stability kinetics of volatile components. Food Sci. Nutr. 2022, 5, 1613–1625. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, F.; Oyarzun-Ampuero, F.; Matiacevich, S.; Ortiz-Viedma, J.; Lemus-Mondaca, R.; Char, C. Use of whey protein concentrate to encapsulate hydrophobic natural antimicrobials to improve their incorporation into high moisture foods enhancing their antimicrobial activity. Innov. Food Sci. Emerg. Technol. 2024, 94, 103687. [Google Scholar] [CrossRef]
- Nedeljkovic, S.K.; Nikoli, N.C.; Radan, M.; Milivojevic, D.; Stevic, T.; Pljevljakusic, D.; Nikodinovic-Runic, J.; Bigovic, D.; Savikin, K.; Filipic, B. Microencapsulation of Origanum heracleoticum L. and Thymus vulgaris L. essential oils—Novel strategy to combat multi-resistant Acinetobacter baumannii. Ind. Crops Prod. 2024, 216, 118762. [Google Scholar]
- Ashaq, B.; Rasool, K.; Habib, S.; Bashir, I.; Nisar, N.; Mustafa, S.; Ayaz, Q.; Nayik, G.A.; Uddin, J.; Ramniwas, S.; et al. Insights into chemistry, extraction and industrial application of lemon grass essential oil—A review of recent advances. Food Chem. X 2024, 22, 101521. [Google Scholar] [CrossRef]



| Common Name | Scientific Name | Compounds with Antimicrobial Action | References |
|---|---|---|---|
| Rosemary | Rosmarinus officinalis | α-pinene, camphor, and 1,8 cineole | [50,51] |
| Garlic | Allium sativum | Diallyl disulfide, diallyl trisulfide, and diallyl tetrasulfide | [52,53] |
| Peppermint | Mentha × piperita L. | Menthone, menthol, 1,8-cineole, and limonene | [13] |
| Angelica | Angelica archangelica | E-3-butylidene phthalide, (Z)-ligustilide, (Z)-β-ocimene, and γ-terpinene | [54,55] |
| Barije | Ferula gummosa | α-pinene and β-pinene | [56,57] |
| Basil | Ocimum basilicum | γ-cadinene, α-bergamotene, eugenol, and linalool | [45] |
| Cinnamon | Cinnamomum zeylanycum | Cinnamaldehyde and trans-cinnamaldehyde | [58,59] |
| Lemon grass | Cymbopogon citratus | Neryl acetate, eraniale, geraniol, neral, α-myrcene, linalool, and verbenol | [60,61] |
| Citronella | Cymbopogon winterianus | Citronellol, citronellal, elemol, and linalool | [62,63] |
| Coriander | Coriandrum sativum | Geranyl acetate, camphor, γ-terpinene, linalool, and α-pinene | [64,65] |
| Clove | Syzygium aromaticum | α-humulene, α- karyophylene, β-karyophylene, and eugenol | [38,45] |
| Black cumin | Nigella sativa | Nigellicin, thymoquinone, thymol, α-Thujene, p-cymene, and thymohydroquinone | [66,67] |
| Jamaica pepper | Pimenta dioica (L.) Merr. | Eugenol and β-Myrcene | [38] |
| Lavender | Lavandula sp. | Linalyl acetate, camphor, eucalyptol, and linalool | [68,69] |
| Oregano | Origanum vulgare | Carvacrol, p-cymene, gamma-terpinene, and thymol | [70,71] |
| Allspice | Pimenta dioica (L.) Merr. | β-myrcene and eugenol | [72,73] |
| Pitanga | Eugenia uniflora | Germacrene B, Seline-1,3,7(11)-trien-8-one, and seline epoxide-1,3,7(11)-trien-8-one | [74,75] |
| Sage | Salvia officinalis | Camphor, eucalyptol, α-thujone, and borneol | [76] |
| Wise | Salvia officinalis | Borneol, camphor, eucalyptol, and α-thujone | [76,77] |
| Thyme | Thymus vulgaris | Thymol, carvacrol, γ-terpinene, α-pinene, and p-cymene | [78,79] |
| Encapsulation Technique | Active Material | Wall Materials | Main Results | References |
|---|---|---|---|---|
| Microencapsulation | ||||
| Interfacial polymerization | Clove essential oil (Syzygium aromatum) | Polyurethane and poly(urea-formaldehyde) (PU and PUF) | - The manufactured microcapsules had great antibacterial activities against the bacteria Vibrio coralliilyticus, Escherichia coli, and Exiguobacterium aestuarii. - Particle size: 102.2 μm. - The microencapsulated essential oils showed a controlled release rate by adjusting the amount of PU reagents and the duration of PUF deposition time. | [114] |
| Essential oil of oregano (Origanum vulgare) and sage (Salvia officinalis) | Polyurea | - Antimicrobial activity against Penicillium citrinum, Rhizopus oryzae, Salmonella enterica, and Escherichia coli (>90% reduction in activity). - Particle size: 100 µm (oregano) and 25 µm (sage). | [115] | |
| Complex coacervation | Sichuan pepper essential oil (Zanthoxylum L.) (SPEO) | Soluble fiber extracted from Sichuan pepper seeds (SDF) and soy protein isolate (SPI) | - The microcapsules showed good dispersion in water. - High encapsulation efficiency: 91.33%. - Particle size: 6.79 μm. - The antibacterial activity of SPEO was improved after microencapsulation. - Microcapsules provided SPEO with better thermal stability and slow-release property. | [116] |
| Rose essential oil (Rosa damascena) (REO) | Mung bean protein isolate (MBPI) and apricot peel pectin (APP) | - Encapsulation efficiency of freeze-dried microcapsules: 89.91%. - REO microcapsules showed substantially higher thermal stability compared to free REO. - The coacervate shell of the MBPI-APP complex was also stable in the oral and gastric phases of in vitro digestion. - 65.5% of REO was delivered to the intestinal phase. - Particle size: 10.23 μm. | [117] | |
| Citronella essential oil (Cymbopogon citratus) (CEO) | Gelatin extracted from chrome-tanned leather and sodium alginate | - The best condition was represented by 4% gelatin and 10% CEO, resulting in 83.5% microencapsulation yield. - Encapsulation efficiency: 73.7%. - Particle size: 434.06 μm. | [118] | |
| Ionic gelling | Marjoram essential oil (Origanum majorana L.) | Sodium Alginate and Whey Protein Isolate (WPI) | - Encapsulation efficiency ranged from 45.6 to 66%. - Lower concentrations of alginate and WPI resulted in higher encapsulation efficiency. - Particle size: 1.10 to 1.73 μm. | [119] |
| Thyme essential oil (Thymus vulgaris) | Calcium Alginate | - The best encapsulation conditions were obtained with 2% v/v of thyme essential oil with a high degree of dispersion (18,000 rpm/5 min). - The microcapsules showed a significant antimicrobial effect, especially on Gram-positive bacteria (Staphylococcus aureus). - Particle size: 890 μm. | [120] | |
| Flaxseed essential oil (Linum usitatissimum) (FEO) | Pectin | - The encapsulation efficiency of the oil was 97%. - The oxidative stability of the encapsulated FEO was 13 times greater than that of the oil in its free form. - Particle size: 862 to 1463 μm. | [121] | |
| Spray drying | Cinnamon essential oil (Cinnamomum zeylanicum) (CEO) | Whey protein isolate (WPI), maltodextrin (MD), and sodium alginate | - The ideal formulation consisted of 70% wall material (WPI/MD/sodium alginate = 1:3:0.01 (w/w)) 30% CEO. - The useful life of the microcapsules was 1032 days at 25 °C. - Particle size: 178 to 347 nm. | [122] |
| Juniper essential oil (Juniperus communis L.) | Gum arabic (GA), maltodextrin (MD), sodium alginate (ALG), and whey protein concentrate (WPC) | - The combination of GA/MD (1:1) as OZ carrier produced microcapsules with the highest encapsulation efficiency (70.07%). - The GA/MD formulation achieved complete and prolonged release of from microcapsules in an oily food matrix. - Particle size: 3.97 to 9.59 μm. | [123] | |
| Mint essential oil (Mentha piperita) | Inulin and gum arabic | - The ideal condition was 35% solid wall, 4% essential oil concentration, and inlet temperature of 110 °C. - The Peppas–Sahlin model was found to be the best approach for SEO launch profiling across four food models. - The optimized spray-dried powder showed faster and greater release in a 50% ethanol medium. | [124] | |
| Chavir essential oil (Ferulago angulata) (CO) | Low- and medium-molecular-weight chitosan | - The microcapsules presented uniform particle size and encapsulation efficiency greater than 70%. - Particle size: 1–3 μm. - Oil stability has been improved by microencapsulation along with antibacterial antioxidant activity. - The release of CO from microcapsules revealed a rapid rate during the initial 5 h and a subsequent delayed release for up to 17 h. - Shelf life: 4 months at 25 °C. | [125] | |
| Nanoencapsulation | ||||
| Nanoliposomes | Lentis essential oil (Pistacia lentiscus L.) | Soy lecithin | - Nanovesicles were considered ideal for treating skin wounds. - Nanovesicles promote the accumulation of bioactive in the dermis, neutralizing damage induced by oxidative processes. - Particle size: 118 nm. | [126] |
| Chrysanthemum essential oil (Chrysanthemum morifolium) | Soy lecithin, chitosan, and pectin | - Single-layer liposomes (soy lecithin), double-layer liposomes (chitosan layer), triple-layer liposomes (chitosan-pectin layer) were prepared. - Triple-layer liposomes were more stable than other types (p < 0.05) and had high antibacterial activity against Campylobacter jejuni in chicken during 14 days of storage (4–37 °C), with no impact on chicken quality. - Particle size: 132.4 nm to 2148.4 nm. | [127] | |
| Barije essential oil (Ferula gummosa) (BEO) | Soy lecithin/cholesterol | - The nanoliposomal system containing BEO showed greater antimicrobial activity against Escherichia coli O157:H7 than the free form of BEO. - Particle size: 74.27 to 99.93 nm. - There was a gradual release of EO from the liposomes, which continued throughout the 24 h after inoculation. | [57] | |
| Clove essential oil (Syzygium aromaticum L.) | Saturated and unsaturated soy phospholipids/cholesterol | - Liposomes protected eugenol from degradation induced by UV exposure and maintained its elimination activity by DPPH. - Liposome formulations demonstrated stability after 2 months of storage at 4 °C. | [128] | |
| Nanofibers | Mint essential oil (Mentha piperita) (MEO) | Poly (lactic acid)/polyethylene glycol (PLA/PEG) | - All nanofibers showed high thermal stability (278–345 °C). - Nanofibers with 20% MEO extended the shelf life of strawberries at 25 °C, showing the release of oil over time. - Particle size: 139–192 nm. | [129] |
| Angelica essential oil (Angelica sinensis (Oliv.)) | Gelatin | - The microcapsules showed an inhibitory effect against E. coli and S. aureus in a manner dependent on the gelatin concentration. - Particle size: 330.50 to 377.38 nm. | [54] | |
| Thyme essential oil (Thymus vulgaris L.) | Starch (50% w/v) and formic acid (75% v/v) | - Starch nanofibers showed high encapsulation efficiency (99.1% to 99.8%). - Free oil showed initial degradation from 62.1 °C while encapsulated oil started at 269.2 °C. - Particle size: 87.4 to 117.7 nm. | [130] | |
| Molecular inclusion | Cymbopogon essential oil (Cymbopogon martinii) | β-cyclodextrin (CD) | - The inclusion complexes provided greater stability and bioavailability of the oil during storage. - The complexes showed better antifungal activity against A. flavus and F. verticillioides. - The complexes showed better activity against HT-29 cells when compared to HeLa cells. Free β-CD did not show antitumor activity in the assays. | [131] |
| Wampee essential oil (Clausena lansium) | β-cyclodextrin | - The water solubility of the oil was clearly increased by 14 times after complexation. - Inclusion complexes preserved the antioxidant activity of the oil and improved its thermal stability. | [132] | |
| Nutmeg essential oil (Myristica fragrans Houtt.) | 2-hydroxypropyl-β-cyclodextrin | - The ideal condition was inclusion temperature of 36 °C, time of 247 min, stirring speed of 520 r/min, and wall-to-core ratio of 12:1, resulting in a recovery of 80.63%. - The release of oil from the inclusion complex was controlled by regulating temperature and humidity. - There was an improvement in the thermal stability, antioxidant activities, and nitrite elimination of the oil after encapsulation. | [133] | |
| Product | Microbes | Natural Antimicrobial | Encapsulating Material | Outcomes | References |
|---|---|---|---|---|---|
| Milk | Listeria monocytogenes, Salmonella enteritidis, Staphylococcus aureus, and Escherichia coli | Nisin and garlic | Liposomes | A 1–4 log CFU/mL microbial load difference was observed between free and encapsulated nisin-GE. | [21] |
| Escherichia coli and Listeria monocytogenes | Eugenol | Whey protein isolate and maltodextrin | Free and nanodispersed eugenol demonstrated the same antimicrobial characteristics, being more effective against Gram-negative Escherichia coli than Gram-positive Listeria monocytogenes. | [20] | |
| Listeria monocytogenes | Thymus vulgaris (Thymol) | Sodium caseinate | In skim milk, encapsulated thymol has slightly better antilisterial activity (ca. 1 log CFU/mL) than free thymol in the first 48 h, while no difference was observed at 72 or 168 h. The inactivation of L. monocytogenes was slower at a higher fat level and the encapsulated thymol consistently reduced the L. monocytogenes population to a lower level in a shorter time than free thymol. With 1.14% and 1.33% fat, both free and encapsulated thymol reduced L. monocytogenes to below the detection limit of 1 log CFU/mL in 168 h. With 1.49% fat, L. monocytogenes was reduced to about 2.5 log CFU/mL in 168 h by free and. | [157] | |
| Staphylococcus aureus, Bacillus subtilis, Listeria monocytogenes and Listeria innocua | Schinus terebinthifolia (Pink pepper) | Soy protein isolate and high methoxyl pectin | Reduction in the population below the detection limits of S. aureus and L. monocytogenes, between 4 and 6 Log CFU/mL. For L. monocytogenes, there was a reduction in the bacterial population of 2 Log CFU/mL. | [158] | |
| Cheese | Total coliforms and Staphylococcus | Cymbopogon Citratus (lemongrass) | Arabic gum and maltodextrin | Microencapsulated oil reduced the growth count of coliforms at 45 °C and Staphylococcus aureus, corroborating with an increase in shelf life of 21 days. | [159] |
| Mesophilic bacteria | Rosmarinus offcinalis (Rosemary) | Whey protein isolate and inulin | Addition of 0.5% microencapsulated essential oil reduced the mesophilic bacterial count 1.36 log cycles after three days and 0.73 log cycles after 15 days of storage. | [160] | |
| Fusarium sp., Penicillium sp. and Cladosporium sp. | Origanum vulgare (Oregano) | Sunflower oil, surfactants, and deionized water | Oregano essential oil encapsulated in nanoemulsions showed antifungal activity against the growth of Cladosporium sp., Fusarium sp. and Penicillium sp. Penicillium sp. showed greater resistance to the antifungal effect of oregano essential oil than Cladosporium sp. and Fusarium sp. | [161] | |
| Filamentous fungi and yeast | Origanum vulgare (Oregano) | Whey protein isolate | Microencapsulated oregano oil was effective in inhibiting the growth of fungi and yeast during 45 days of storage of grated cheese. Only the treatment containing 0.5% microencapsulated oil still had an undetectable count, being considered the most effective treatment in controlling filamentous fungi and yeast growth in grated Parmesan cheese. | [162] | |
| Aerobic mesophilic bacteria, molds and yeasts, and total coliforms | Opuntia oligacantha (Xoconostle) | Microcapsules: maltodextrin and gum arabic; nanoemulsion: soy lecithin and orange essential oil | Total coliforms decreased in all samples from the first days of storage (Control: 4.23 ± 0.12, Micro: 3.27 ± 0.02, and Nano: 2.68 ± 0.08 Log10 CFU), as well as aerobic mesophiles and mold–yeast counts. | [3] | |
| Staphylococcus aureus, Psychrophilic bacteria, molds and yeast | Origanum vulgare (Oregano) | Tween 80, sodium alginate, and mandarin fiber | The microbial population decreased by 1.4 and 1.5 log CFU/g in coated cheese pieces containing 2.0% or 2.5% w/w of oregano, respectively, during 15 days of refrigerated storage. However, coatings with a oregano concentration of 1.5% w/w were not effective in reducing Staphylococcus aureus population. Coated-cheese pieces containing 2.5% (w/w) oregano inhibited psychrophilic bacteria or molds and yeasts growth during 6 or 24 days of storage, respectively. However, a concentration of 1.5% w/w of oregano was not enough to inhibit the development of neither psychrophilic bacteria nor molds and yeast in cheese pieces. | [163] | |
| Yogurt | Escherichia coli and Staphylococcus aureus | Nepeta crispa | Pectin, whey protein concentrate | The decrease in Escherichia coli and Staphylococcus aureus bacteria at 40 and 60 days of storage. | [1] |
| Escherichia coli and Staphylococcus aureus | Garlic | composed of soy phosphatidylcholine | The inhibitory effect Escherichia coli than Staphylococcus aureus (minimum inhibitory concentration = 3.75 and 7.5 mg/mL, respectively). | [164] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, H.F.d.; Santos, F.R.d.; Cunha, J.S.; Pacheco, F.C.; Pacheco, A.F.C.; Soutelino, M.E.M.; Martins, C.C.N.; Andressa, I.; Rocha, R.d.S.; Cruz, A.G.d.; et al. Microencapsulation to Harness the Antimicrobial Potential of Essential Oils and Their Applicability in Dairy Products: A Comprehensive Review of the Literature. Foods 2024, 13, 2197. https://doi.org/10.3390/foods13142197
Souza HFd, Santos FRd, Cunha JS, Pacheco FC, Pacheco AFC, Soutelino MEM, Martins CCN, Andressa I, Rocha RdS, Cruz AGd, et al. Microencapsulation to Harness the Antimicrobial Potential of Essential Oils and Their Applicability in Dairy Products: A Comprehensive Review of the Literature. Foods. 2024; 13(14):2197. https://doi.org/10.3390/foods13142197
Chicago/Turabian StyleSouza, Handray Fernandes de, Fabio Ribeiro dos Santos, Jeferson Silva Cunha, Flaviana Coelho Pacheco, Ana Flávia Coelho Pacheco, Maria Eduarda Marques Soutelino, Caio Cesar Nemer Martins, Irene Andressa, Ramon da Silva Rocha, Adriano Gomes da Cruz, and et al. 2024. "Microencapsulation to Harness the Antimicrobial Potential of Essential Oils and Their Applicability in Dairy Products: A Comprehensive Review of the Literature" Foods 13, no. 14: 2197. https://doi.org/10.3390/foods13142197
APA StyleSouza, H. F. d., Santos, F. R. d., Cunha, J. S., Pacheco, F. C., Pacheco, A. F. C., Soutelino, M. E. M., Martins, C. C. N., Andressa, I., Rocha, R. d. S., Cruz, A. G. d., Paiva, P. H. C., Brandi, I. V., & Kamimura, E. S. (2024). Microencapsulation to Harness the Antimicrobial Potential of Essential Oils and Their Applicability in Dairy Products: A Comprehensive Review of the Literature. Foods, 13(14), 2197. https://doi.org/10.3390/foods13142197







