Metabolomics and Biochemical Benefits of Multivitamin and Multimineral Supplementation in Healthy Individuals: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Participants
2.2. Study Product
2.3. Blood Analyses
2.4. Liquid Chromatography–Mass Spectrometry Methods
2.4.1. Metabolite Extraction
2.4.2. Liquid Chromatography–Mass Spectrometry Settings
2.4.3. Metabolite Identification by Tandem Mass Spectrometry
2.5. Data Processing and Statistical Analysis
3. Results
3.1. Blood Analyses
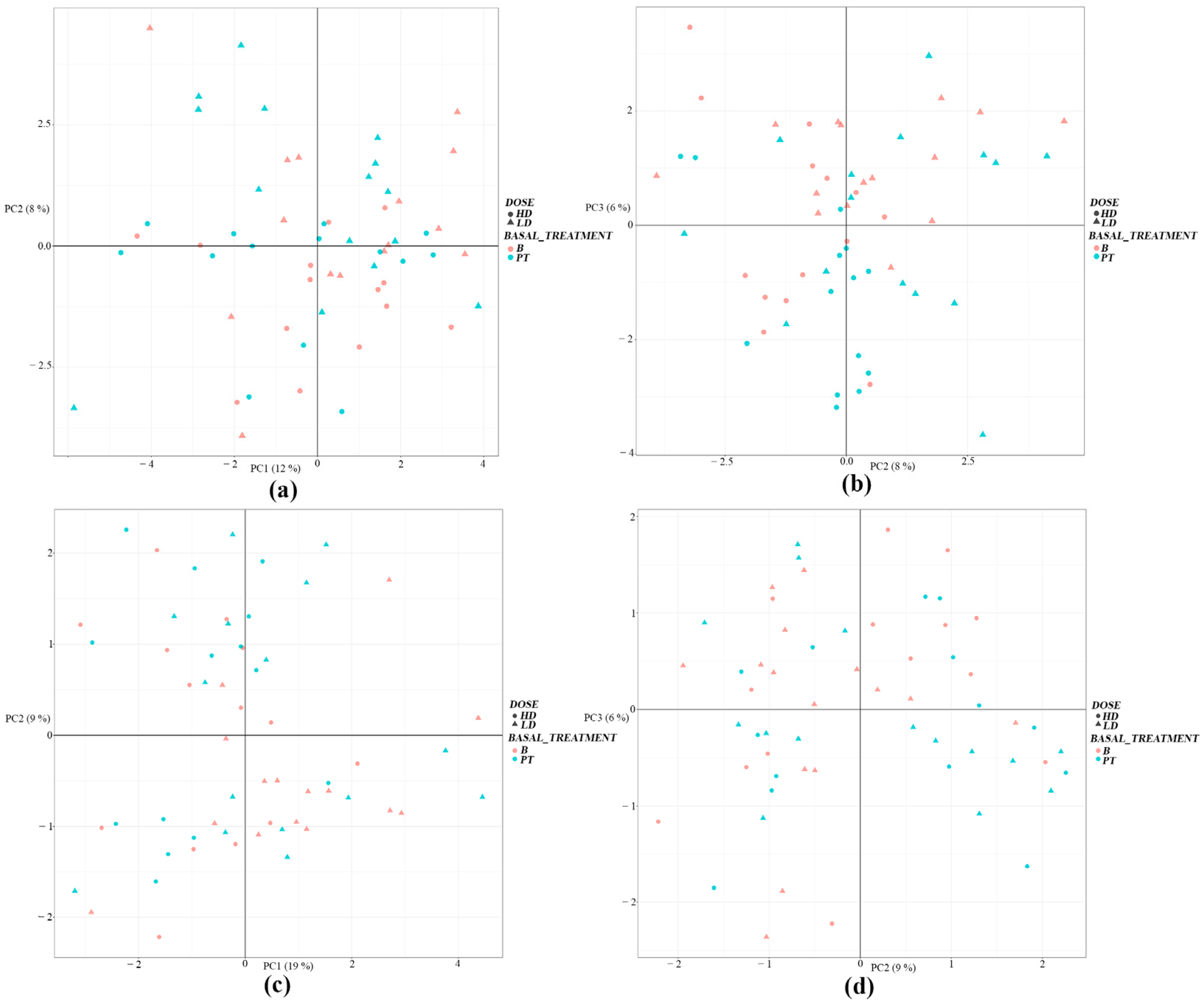
3.2. Liquid Chromatography–Mass Spectrometry Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldstein, D.S. How does homeostasis happen? Integrative physiological, systems biological, and evolutionary perspectives. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 316, R301–R317. [Google Scholar] [CrossRef] [PubMed]
- Billman, G.E. Homeostasis: The Underappreciated and Far Too Often Ignored Central Organizing Principle of Physiology. Front. Physiol. 2020, 11, 200. [Google Scholar] [CrossRef]
- Evans, P.; Halliwell, B. Micronutrients: Oxidant/antioxidant status. Br. J. Nutr. 2001, 85 (Suppl. S2), S67–S74. [Google Scholar] [CrossRef]
- Haryanto, B.; Suksmasari, T.; Wintergerst, E.S.; Maggini, S. Multivitamin Supplementation Supports Immune Function and Ameliorates Conditions Triggered by Reduced Air Quality. Vitam. Miner 2015, 4, 128. [Google Scholar]
- Morine, M.J.; Monteiro, J.P.; Wise, C.; Teitel, C.; Pence, L.; Williams, A.; Ning, B.; McCabe-Sellers, B.; Champagne, C.; Turner, J.; et al. Genetic associations with micronutrient levels identified in immune and gastrointestinal networks. Genes Nutr. 2014, 9, 408. [Google Scholar] [CrossRef] [PubMed]
- van Ommen, B.; Fairweather-Tait, S.; Freidig, A.; Kardinaal, A.; Scalbert, A.; Wopereis, S. A network biology model of micronutrient related health. Br. J. Nutr. 2008, 99 (Suppl. S3), S72–S80. [Google Scholar] [CrossRef] [PubMed]
- Ofoedu, C.E.; Iwouno, J.O.; Ofoedu, E.O.; Ogueke, C.C.; Igwe, V.S.; Agunwah, I.M.; Ofoedum, A.F.; Chacha, J.S.; Muobike, O.P.; Agunbiade, A.O.; et al. Revisiting food-sourced vitamins for consumer diet and health needs: A perspective review, from vitamin classification, metabolic functions, absorption, utilization, to balancing nutritional requirements. PeerJ 2021, 9, e11940. [Google Scholar] [CrossRef] [PubMed]
- Raiten, D.J.; Combs, G.F.; Steiber, A.L.; Bremer, A.A. Perspective: Nutritional Status as a Biological Variable (NABV): Integrating Nutrition Science into Basic and Clinical Research and Care. Adv. Nutr. 2021, 12, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, M.; Li, C.; Jiang, X.; Su, Y.; Zhang, Y. Benefits of Vitamins in the Treatment of Parkinson’s Disease. Oxid. Med. Cell Longev. 2019, 2019, 9426867. [Google Scholar] [CrossRef]
- Chen, Y.; Michalak, M.; Agellon, L.B. Importance of Nutrients and Nutrient Metabolism on Human Health. Yale J. Biol. Med. 2018, 91, 95–103. [Google Scholar]
- Institute of Medicine Committee on Micronutrient Deficiencies. Prevention of Micronutrient Deficiencies: Tools for Policymakers and Public Health Workers; Howson, C.P., Kennedy, E.T., Horwitz, A., Eds.; National Academies Press (US): Washington, DC, USA, 1998. [Google Scholar]
- Oakley, G.P., Jr.; Bell, K.N.; Weber, M.B. Recommendations for accelerating global action to prevent folic acid-preventable birth defects and other folate-deficiency diseases: Meeting of experts on preventing folic acid-preventable neural tube defects. Birth Defects Res. A Clin. Mol. Teratol. 2004, 70, 835–837. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaram, P. Micronutrient malnutrition, infection, and immunity: An overview. Nutr. Rev. 2002, 60, S40–S45. [Google Scholar] [CrossRef] [PubMed]
- Black, R. Micronutrient deficiency--an underlying cause of morbidity and mortality. Bull. World Health Organ 2003, 81, 79. [Google Scholar] [PubMed]
- Singhal, N.; Austin, J. A clinical review of micronutrients in HIV infection. J. Int. Assoc. Physicians AIDS Care 2002, 1, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef]
- Shenkin, A. Micronutrients in health and disease. Postgrad. Med. J. 2006, 82, 559–567. [Google Scholar] [CrossRef]
- Bielik, V.; Kolisek, M. Bioaccessibility and Bioavailability of Minerals in Relation to a Healthy Gut Microbiome. Int. J. Mol. Sci. 2021, 22, 6803. [Google Scholar] [CrossRef]
- van den Broek, T.J.; Kremer, B.H.A.; Marcondes Rezende, M.; Hoevenaars, F.P.M.; Weber, P.; Hoeller, U.; van Ommen, B.; Wopereis, S. The impact of micronutrient status on health: Correlation network analysis to understand the role of micronutrients in metabolic-inflammatory processes regulating homeostasis and phenotypic flexibility. Genes. Nutr. 2017, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Dainin, K.; Ide, R.; Maeda, A.; Suyama, K.; Akagawa, M. Pyridoxamine scavenges protein carbonyls and inhibits protein aggregation in oxidative stress-induced human HepG2 hepatocytes. Biochem. Biophys. Res. Commun. 2017, 486, 845–851. [Google Scholar] [CrossRef]
- Burton, G.W.; Traber, M.G.; Acuff, R.V.; Walters, D.N.; Kayden, H.; Hughes, L.; Ingold, K.U. Human plasma and tissue alpha-tocopherol concentrations in response to supplementation with deuterated natural and synthetic vitamin E. Am. J. Clin. Nutr. 1998, 67, 669–684. [Google Scholar] [CrossRef]
- Damiani, E.; Belaid, C.; Carloni, P.; Greci, L. Comparison of antioxidant activity between aromatic indolinonic nitroxides and natural and synthetic antioxidants. Free Radic. Res. 2003, 37, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, S.; Meganathan, K.; Wagh, V.; Winkler, J.; Hescheler, J.; Sachinidis, A. Chemoprotective mechanism of the natural compounds, epigallocatechin-3-O-gallate, quercetin and curcumin against cancer and cardiovascular diseases. Curr. Med. Chem. 2009, 16, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Lindschinger, M.; Tatzber, F.; Schimetta, W.; Schmid, I.; Lindschinger, B.; Cvirn, G.; Stanger, O.; Lamont, E.; Wonisch, W. A Randomized Pilot Trial to Evaluate the Bioavailability of Natural versus Synthetic Vitamin B Complexes in Healthy Humans and Their Effects on Homocysteine, Oxidative Stress, and Antioxidant Levels. Oxid. Med. Cell Longev. 2019, 2019, 6082613. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Bozonet, S.M.; Pullar, J.M.; Simcock, J.W.; Vissers, M.C. A randomized steady-state bioavailability study of synthetic versus natural (kiwifruit-derived) vitamin C. Nutrients 2013, 5, 3684–3695. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Issa, O.M.; Roberts, R.; Mark, D.B.; Boineau, R.; Goertz, C.; Rosenberg, Y.; Lewis, E.F.; Guarneri, E.; Drisko, J.; Magaziner, A.; et al. Effect of high-dose oral multivitamins and minerals in participants not treated with statins in the randomized Trial to Assess Chelation Therapy (TACT). Am. Heart J. 2018, 195, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Spence, J.D.; Giovannucci, E.L.; Kim, Y.I.; Josse, R.G.; Vieth, R.; Sahye-Pudaruth, S.; Paquette, M.; Patel, D.; Blanco Mejia, S.; et al. Supplemental Vitamins and Minerals for Cardiovascular Disease Prevention and Treatment: JACC Focus Seminar. J. Am. Coll. Cardiol. 2021, 77, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Yu, J.; Xu, H.; Zhou, Y.; Li, H.; Yin, S.; Xu, D.; Wang, Y.; Xia, H.; Liao, W.; et al. Comparative effects of vitamin and mineral supplements in the management of type 2 diabetes in primary care: A systematic review and network meta-analysis of randomized controlled trials. Pharmacol. Res. 2023, 188, 106647. [Google Scholar] [CrossRef] [PubMed]
- Isakov, V.A.; Bogdanova, A.A.; Bessonov, V.V.; Sentsova, T.B.; Tutelyan, V.A.; Lin, Y.; Kazlova, V.; Hong, J.; Velliquette, R.A. Effects of Multivitamin, Multimineral and Phytonutrient Supplementation on Nutrient Status and Biomarkers of Heart Health Risk in a Russian Population: A Randomized, Double Blind, Placebo Controlled Study. Nutrients 2018, 10, 120. [Google Scholar] [CrossRef]
- Andersen, M.B.; Rinnan, A.; Manach, C.; Poulsen, S.K.; Pujos-Guillot, E.; Larsen, T.M.; Astrup, A.; Dragsted, L.O. Untargeted metabolomics as a screening tool for estimating compliance to a dietary pattern. J. Proteome Res. 2014, 13, 1405–1418. [Google Scholar] [CrossRef]
- Reinke, S.N.; Broadhurst, D.L.; Sykes, B.D.; Baker, G.B.; Catz, I.; Warren, K.G.; Power, C. Metabolomic profiling in multiple sclerosis: Insights into biomarkers and pathogenesis. Mult. Scler. 2014, 20, 1396–1400. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Piccolo, M.; Vannini, L.; Siragusa, S.; De Giacomo, A.; Serrazzanetti, D.I.; Cristofori, F.; Guerzoni, M.E.; Gobbetti, M.; Francavilla, R. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS ONE 2013, 8, e76993. [Google Scholar] [CrossRef] [PubMed]
- Pieragostino, D.; D’Alessandro, M.; di Ioia, M.; Rossi, C.; Zucchelli, M.; Urbani, A.; Di Ilio, C.; Lugaresi, A.; Sacchetta, P.; Del Boccio, P. An integrated metabolomics approach for the research of new cerebrospinal fluid biomarkers of multiple sclerosis. Mol. Biosyst. 2015, 11, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Qiu, Y.; Zou, X.; Chen, T.; Xie, G.; Cheng, Y.; Dong, T.; Zhao, L.; Feng, B.; Hu, X.; et al. Metabonomics identifies serum metabolite markers of colorectal cancer. J. Proteome Res. 2013, 12, 3000–3009. [Google Scholar] [CrossRef] [PubMed]
- Armitage, E.G.; Barbas, C. Metabolomics in cancer biomarker discovery: Current trends and future perspectives. J. Pharm. Biomed. Anal. 2014, 87, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cornago, A.; Brennan, L.; Ibero-Baraibar, I.; Hermsdorff, H.H.; O’Gorman, A.; Zulet, M.A.; Martinez, J.A. Metabolomics identifies changes in fatty acid and amino acid profiles in serum of overweight older adults following a weight loss intervention. J. Physiol. Biochem. 2014, 70, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Quinones, M.P.; Kaddurah-Daouk, R. Metabolomics tools for identifying biomarkers for neuropsychiatric diseases. Neurobiol. Dis. 2009, 35, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Vinayavekhin, N.; Homan, E.A.; Saghatelian, A. Exploring disease through metabolomics. ACS Chem. Biol. 2010, 5, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Gine, R.; Capellades, J.; Badia, J.M.; Vughs, D.; Schwaiger-Haber, M.; Alexandrov, T.; Vinaixa, M.; Brunner, A.M.; Patti, G.J.; Yanes, O. HERMES: A molecular-formula-oriented method to target the metabolome. Nat. Methods 2021, 18, 1370–1376. [Google Scholar] [CrossRef]
- Heusschen, L.; Berendsen, A.A.M.; Cooiman, M.I.; Deden, L.N.; Hazebroek, E.J.; Aarts, E.O. Optimizing Multivitamin Supplementation for Sleeve Gastrectomy Patients. Obes. Surg. 2021, 31, 2520–2528. [Google Scholar] [CrossRef]
- Clarke, R.; Armitage, J. Vitamin supplements and cardiovascular risk: Review of the randomized trials of homocysteine-lowering vitamin supplements. Semin. Thromb. Hemost. 2000, 26, 341–348. [Google Scholar] [CrossRef] [PubMed]
- McIver, D.J.; Grizales, A.M.; Brownstein, J.S.; Goldfine, A.B. Risk of Type 2 Diabetes Is Lower in US Adults Taking Chromium-Containing Supplements. J. Nutr. 2015, 145, 2675–2682. [Google Scholar] [CrossRef]
- Huang, H.Y.; Caballero, B.; Chang, S.; Alberg, A.J.; Semba, R.D.; Schneyer, C.R.; Wilson, R.F.; Cheng, T.Y.; Vassy, J.; Prokopowicz, G.; et al. The efficacy and safety of multivitamin and mineral supplement use to prevent cancer and chronic disease in adults: A systematic review for a National Institutes of Health state-of-the-science conference. Ann. Intern. Med. 2006, 145, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.D.; Martin, J.J. Homocysteine. Int. J. Biochem. Cell Biol. 2000, 32, 385–389. [Google Scholar] [CrossRef]
- Zaric, B.L.; Obradovic, M.; Bajic, V.; Haidara, M.A.; Jovanovic, M.; Isenovic, E.R. Homocysteine and Hyperhomocysteinaemia. Curr. Med. Chem. 2019, 26, 2948–2961. [Google Scholar] [CrossRef] [PubMed]
- Faeh, D.; Chiolero, A.; Paccaud, F. Homocysteine as a risk factor for cardiovascular disease: Should we (still) worry about? Swiss Med. Wkly. 2006, 136, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Hankey, G.J.; Eikelboom, J.W. Homocysteine and vascular disease. Lancet 1999, 354, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Lanyau-Dominguez, Y.; Macias-Matos, C.; Jesus, J.; Maria, G.; Suarez-Medina, R.; Eugenia, M.; Noriega-Fernandez, L.; Guerra-Hernandez, M.; Calvo-Rodriguez, M.; Sanchez-Gil, Y.; et al. Levels of Vitamins and Homocysteine in Older Adults with Alzheimer Disease or Mild Cognitive Impairment in Cuba. MEDICC Rev. 2020, 22, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, V.; Mehendale, V.; Prabhu, K.; Shetty, R.; Rao, P. Correlation of serum homocysteine levels with the severity of coronary artery disease. Indian. J. Clin. Biochem. 2014, 29, 339–344. [Google Scholar] [CrossRef]
- Pintó Sala, X. La homocisteína como factor de riesgo cardiovascular. Med. Integral. 2000, 36, 179–185. [Google Scholar]
- Bailey, L.B.; Stover, P.J.; McNulty, H.; Fenech, M.F.; Gregory, J.F., 3rd; Mills, J.L.; Pfeiffer, C.M.; Fazili, Z.; Zhang, M.; Ueland, P.M.; et al. Biomarkers of Nutrition for Development-Folate Review. J. Nutr. 2015, 145, 1636S–1680S. [Google Scholar] [CrossRef]
- Serbina, N.V.; Jia, T.; Hohl, T.M.; Pamer, E.G. Monocyte-mediated defense against microbial pathogens. Annu. Rev. Immunol. 2008, 26, 421–452. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.L.; Tai, J.J.; Wong, W.C.; Han, H.; Sem, X.; Yeap, W.H.; Kourilsky, P.; Wong, S.C. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood 2011, 118, e16–e31. [Google Scholar] [CrossRef] [PubMed]
- Ozanska, A.; Szymczak, D.; Rybka, J. Pattern of human monocyte subpopulations in health and disease. Scand. J. Immunol. 2020, 92, e12883. [Google Scholar] [CrossRef] [PubMed]
- Tolouei Semnani, R.; Moore, V.; Bennuru, S.; McDonald-Fleming, R.; Ganesan, S.; Cotton, R.; Anuradha, R.; Babu, S.; Nutman, T.B. Human monocyte subsets at homeostasis and their perturbation in numbers and function in filarial infection. Infect. Immun. 2014, 82, 4438–4446. [Google Scholar] [CrossRef] [PubMed]
- Auffray, C.; Fogg, D.; Garfa, M.; Elain, G.; Join-Lambert, O.; Kayal, S.; Sarnacki, S.; Cumano, A.; Lauvau, G.; Geissmann, F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 2007, 317, 666–670. [Google Scholar] [CrossRef]
- Carlin, L.M.; Stamatiades, E.G.; Auffray, C.; Hanna, R.N.; Glover, L.; Vizcay-Barrena, G.; Hedrick, C.C.; Cook, H.T.; Diebold, S.; Geissmann, F. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell 2013, 153, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Cros, J.; Cagnard, N.; Woollard, K.; Patey, N.; Zhang, S.Y.; Senechal, B.; Puel, A.; Biswas, S.K.; Moshous, D.; Picard, C.; et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 2010, 33, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Geissmann, F.; Jung, S.; Littman, D.R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003, 19, 71–82. [Google Scholar] [CrossRef]
- Quintar, A.; McArdle, S.; Wolf, D.; Marki, A.; Ehinger, E.; Vassallo, M.; Miller, J.; Mikulski, Z.; Ley, K.; Buscher, K. Endothelial Protective Monocyte Patrolling in Large Arteries Intensified by Western Diet and Atherosclerosis. Circ. Res. 2017, 120, 1789–1799. [Google Scholar] [CrossRef]
- Ke, G.; Huang, J.; Zhu, Y.; Yang, J.; Zhang, Y.; Chen, L.; Hu, J.; Tao, S.; Hu, Y.; Yang, D.; et al. Effect of Ascorbic Acid on Mineral and Bone Disorders in Hemodialysis Patients: A Systematic Review and Meta-Analysis. Kidney Blood Press. Res. 2018, 43, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J.; Richardson, D.R. The active role of vitamin C in mammalian iron metabolism: Much more than just enhanced iron absorption! Free Radic. Biol. Med. 2014, 75, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Pitts, M.W.; Hoffmann, P.R. Endoplasmic reticulum-resident selenoproteins as regulators of calcium signaling and homeostasis. Cell Calcium 2018, 70, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, I. Molecular mechanisms and regulation of urinary acidification. Compr. Physiol. 2014, 4, 1737–1774. [Google Scholar] [CrossRef] [PubMed]
- Eaton, S. Control of mitochondrial beta-oxidation flux. Prog. Lipid Res. 2002, 41, 197–239. [Google Scholar] [CrossRef] [PubMed]
- Kerner, J.; Hoppel, C. Fatty acid import into mitochondria. Biochim. Biophys. Acta 2000, 1486, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.E.; Evans, A.M. Long-chain acylcarnitine deficiency in patients with chronic fatigue syndrome. Potential involvement of altered carnitine palmitoyltransferase-I activity. J. Intern. Med. 2011, 270, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Turer, A.T. Using metabolomics to assess myocardial metabolism and energetics in heart failure. J. Mol. Cell Cardiol. 2013, 55, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Kolwicz, S.C., Jr.; Purohit, S.; Tian, R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ. Res. 2013, 113, 603–616. [Google Scholar] [CrossRef]
- McGarrah, R.W.; Crown, S.B.; Zhang, G.F.; Shah, S.H.; Newgard, C.B. Cardiovascular Metabolomics. Circ. Res. 2018, 122, 1238–1258. [Google Scholar] [CrossRef]
- Oliveira, P.J.; Rolo, A.P.; Palmeira, C.M.; Moreno, A.J. Carvedilol reduces mitochondrial damage induced by hypoxanthine/xanthine oxidase: Relevance to hypoxia/reoxygenation injury. Cardiovasc. Toxicol. 2001, 1, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Bavaresco, C.S.; Chiarani, F.; Kolling, J.; Netto, C.A.; de Souza Wyse, A.T. Biochemical effects of pretreatment with vitamins E and C in rats submitted to intrastriatal hypoxanthine administration. Neurochem. Int. 2008, 52, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y. Bile acid regulation of hepatic physiology: III. Bile acids and nuclear receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G349–G356. [Google Scholar] [CrossRef] [PubMed]
- Claudel, T.; Staels, B.; Kuipers, F. The Farnesoid X receptor: A molecular link between bile acid and lipid and glucose metabolism. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2020–2030. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.A.; Miyake, J.H.; Hui, T.Y.; Spann, N.J. Regulation of cholesterol-7alpha-hydroxylase: BAREly missing a SHP. J. Lipid Res. 2002, 43, 533–543. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, M.V.; Kullak-Ublick, G.A.; Hagenbuch, B.; Meier, P.J. Transport of bile acids in hepatic and non-hepatic tissues. J. Exp. Biol. 2001, 204, 1673–1686. [Google Scholar] [CrossRef] [PubMed]
- Alkam, T.; Nitta, A.; Furukawa-Hibi, Y.; Niwa, M.; Mizoguchi, H.; Yamada, K.; Nabeshima, T. Oral supplementation with Leu-Ile, a hydrophobic dipeptide, prevents the impairment of memory induced by amyloid beta in mice via restraining the hyperphosphorylation of extracellular signal-regulated kinase. Behav. Brain Res. 2010, 210, 184–190. [Google Scholar] [CrossRef]
- Furukawa-Hibi, Y.; Nitta, A.; Ikeda, T.; Morishita, K.; Liu, W.; Ibi, D.; Alkam, T.; Nabeshima, T.; Yamada, K. The hydrophobic dipeptide Leu-Ile inhibits immobility induced by repeated forced swimming via the induction of BDNF. Behav. Brain Res. 2011, 220, 271–280. [Google Scholar] [CrossRef]


| Components | For 1 Tablet |
|---|---|
| Powdered acerola berry juice | 248 mg |
| Vegetable extract concentrate with a quantified vitamin and mineral content | 220 mg |
| Provides: | Quantity/NRV * |
| Vitamin B1 | 0.65 mg/59% |
| Vitamin B2 | 0.83 mg/59% |
| Vitamin B3 | 5.5 mg/34% |
| Vitamin B5 | 5.5 mg/92% |
| Vitamin B6 | 4.2 mg/300% |
| Vitamin B8 | 28.0 µg/55% |
| Vitamin B9 | 110.0 µg/55% |
| Vitamin C | 72.0 mg/89% |
| Vitamin E | 15.0 mg/123% |
| Iron | 5.0 mg/35% |
| Selenium | 19.0 µg/35% |
| Zinc | 2.1 mg/21% |
| Manganese | 0.5 mg/26% |
| Chromium | 32.0 µg/80% |
| Single-Dose (LD) Group (n = 15) | Double-Dose (HD) Group (n = 15) | p-Value | |
|---|---|---|---|
| Age (years) | 24.3 (11.1) | 21.5 (1.8) | 0.978 |
| Weight (kg) | 64.7 (8.6) | 61.8 (12.4) | 0.741 |
| Height (m) | 1.7 (0.1) | 1.7 (0.7) | 0.544 |
| BMI | 22.6 (2.4) | 22.2 (3.5) | 0.066 |
| Serum Parameters (Units) | N | Mean (SD) | Reference Value | p-Value | |
|---|---|---|---|---|---|
| Baseline | After Treatment | ||||
| Red blood cells (×106/mm3) | 15 | 4.55 (0.24) | 4.64 (0.30) | 3.9–5.2 | 0.039 * |
| Hemoglobin (g/dL) | 15 | 14.07 (0.62) | 14.19 (0.82) | 12–15.6 | 0.337 |
| Hematocrit (%) | 15 | 41.08 (1.85) | 41.88 (2.55) | 35.5–45.5 | 0.034 * |
| MCV (fL) | 15 | 90.40 (2.50) | 90.27 (2.66) | 78–99 | 0.709 |
| MCH (pg) | 15 | 31.01 (1.10) | 30.58 (0.92) | 26–33.5 | 0.018 ** |
| MCHC (g/dL) | 15 | 34.26 (0.83) | 33.90 (0.49) | 31.5–36 | 0.131 |
| RDW (%) | 15 | 13.03 (0.78) | 13.22 (0.78) | 11.5–15.5 | 0.093 |
| Leukocytes (×103/mm3) | 15 | 5.94 (1.68) | 5.83 (1.23) | 3.9–10.5 | 0.739 |
| Neutrophils (%) | 15 | 56.03 (8.38) | 55.05 (7.37) | 42–77 | 0.439 |
| Lymphocytes (%) | 15 | 33.30 (8.14) | 33.34 (6.95) | 20–44 | 0.973 |
| Monocytes (%) | 15 | 7.78 (1.74) | 8.49 (2.21) | 1.5–9.5 | 0.025 ** |
| Eosinophils (%) | 15 | 2.11 (1.21) | 2.49 (1.85) | 0.5–5.5 | 0.218 |
| Basophils (%) | 15 | 0.77 (0.29) | 0.63 (0.25) | 0–1.75 | 0.075 |
| Neutrophils (×103/mm3) | 15 | 3.41 (1.42) | 3.29 (1.12) | 1.5–7.7 | 0.595 |
| Lymphocytes (×103/mm3) | 15 | 1.89 (0.41) | 1.89 (0.25) | 1.1–4.5 | 1.000 |
| Monocytes (×103/mm3) | 15 | 0.87 (1.56) | 0.49 (0.12) | 0.1–0.95 | 0.377 |
| Eosinophils (×103/mm3) | 15 | 0.20 (0.24) | 0.15 (0.12) | 0.02–0.5 | 0.513 |
| Basophils (×103/mm3) | 15 | 0.03 (0.05) | 0.01 (0.04) | 0–0.2 | 0.334 |
| Platelets (×103/mm3) | 15 | 227.87 (49.91) | 238.73 (41.69) | 150–370 | 0.127 |
| MPV (fL) | 15 | 8.97 (0.92) | 9.09 (0.85) | 7–12 | 0.207 |
| Glucose (mg/dL) | 15 | 83.80 (3.91) | 83.87 (6.12) | 74–106 | 0.958 |
| Total cholesterol (mg/dL) | 15 | 170.13 (35.84) | 174.47 (32.84) | <200 | 0.390 |
| HDL cholesterol (mg/dL) | 15 | 64.73 (15.64) | 63.20 (17.38) | >50 | 0.366 |
| LDL cholesterol (mg/dL) | 15 | 95.13 (28.77) | 99.60 (23.78) | 0–130 | 0.346 |
| Triglycerides (mg/dL) | 15 | 51.73 (19.46) | 58.20 (24.62) | <150 | 0.242 |
| Total proteins (g/L) | 15 | 69.87 (3.66) | 71.13 (2.26) | 64–83 | 0.055 |
| Albumin (g/L) | 15 | 46.60 (2.72) | 47.00 (2.24) | 35–52 | 0.320 |
| Creatinine (mg/dL) | 15 | 0.74 (0.12) | 0.77 (0.13) | 0.55–1.02 | 0.138 |
| Urea (mg/dL) | 15 | 28.40 (6.45) | 28.53 (5.29) | 17–49 | 0.934 |
| Sodium (mEq/L) | 15 | 138.13 (2.07) | 138.47 (1.85) | 136–146 | 0.582 |
| Potassium (mEq/L) | 15 | 4.35 (0.30) | 4.36 (0.24) | 3.5–5.1 | 0.887 |
| Chloride (mEq/L) | 15 | 104.93 (2.02) | 105.40 (1.60) | 99–109 | 0.513 |
| AST/GOT (U/L) | 15 | 29.53 (21.65) | 26.73 (6.10) | 0–31 | 0.616 |
| ALT/GPT (U/L) | 15 | 25.60 (13.75) | 26.93 (17.55) | 0–34 | 0.789 |
| GGT (U/L) | 15 | 13.00 (11.33) | 13.20 (8.02) | 0–38 | 0.876 |
| Iron (µg/dL) | 15 | 95.33 (33.15) | 104.07 (36.38) | 50–170 | 0.477 |
| Ferritin (ng/dL) | 15 | 67.40 (59.16) | 67.07 (56.42) | 11–307 | 0.938 |
| Phosphate (mg/dL) | 15 | 3.67 (0.41) | 3.70 (0.46) | 2.5–4.5 | 0.747 |
| Total calcium (mg/dL) | 15 | 9.53 (0.47) | 10.02 (0.32) | 8.6–10.2 | 0.002 ** |
| Homocysteine (µmol/L) | 15 | 13.93 (3.85) | 10.33 (1.76) | 4.44–13.56 | 0.000 *** |
| Serum Parameters (Units) | N | Mean (SD) | Reference Value | p-Value | |
|---|---|---|---|---|---|
| Baseline | After Treatment | ||||
| Red blood cells (×106/mm3) | 14 | 4.68 (0.43) | 4.69 (0.44) | 3.9–5.2 | 0.875 |
| Hemoglobin (g/dL) | 14 | 14.02 (1.27) | 13.94 (1.06) | 12–15.6 | 0.650 |
| Hematocrit (%) | 14 | 41.76 (3.45) | 41.60 (2.89) | 35.5–45.5 | 0.746 |
| MCV (fL) | 14 | 89.71 (6.41) | 89.14 (5.50) | 78–99 | 0.120 |
| MCH (pg) | 14 | 30.06 (2.41) | 29.84 (2.09) | 26–33.5 | 0.179 |
| MCHC (g/dL) | 14 | 33.56 (0.73) | 33.50 (0.48) | 31.5–36 | 0.736 |
| RDW (%) | 14 | 13.86 (1.35) | 14.31 (2.43) | 11.5–15.5 | 0.213 |
| Leukocytes (×103/mm3) | 14 | 6.01 (1.26) | 6.31 (1.12) | 3.9–10.5 | 0.475 |
| Neutrophils (%) | 14 | 62.29 (8.40) | 63.35 (7.05) | 42–77 | 0.613 |
| Lymphocytes (%) | 14 | 29.33 (7.75) | 27.92 (6.71) | 20–44 | 0.444 |
| Monocytes (%) | 14 | 6.71 (1.71) | 7.34 (1.81) | 1.5–9.5 | 0.040 * |
| Eosinophils (%) | 14 | 1.04 (0.90) | 0.82 (0.67) | 0.5–5.5 | 0.220 |
| Basophils (%) | 14 | 0.64 (0.23) | 0.56 (0.22) | 0–1.75 | 0.146 |
| Neutrophils (×103/mm3) | 14 | 3.80 (1.13) | 4.03 (0.97) | 1.5–7.7 | 0.544 |
| Lymphocytes (×103/mm3) | 14 | 1.74 (0.42) | 1.74 (0.47) | 1.1–4.5 | 1.000 |
| Monocytes (×103/mm3) | 14 | 0.40 (0.15) | 0.46 (0.14) | 0.1–0.95 | 0.055 |
| Eosinophils (×103/mm3) | 14 | 0.05 (0.07) | 0.05 (0.08) | 0.02–0.5 | 1.000 |
| Basophils (×103/mm3) | 14 | 0.01 (0.04) | 0.00 (0.00) | 0–0.2 | 0.165 |
| Platelets (×103/mm3) | 14 | 235.43 (57.95) | 243.71 (43.59) | 150–370 | 0.202 |
| MPV (fL) | 14 | 8.99 (0.74) | 8.88 (0.75) | 7–12 | 0.182 |
| Glucose (mg/dL) | 14 | 82.64 (6.71) | 82.93 (4.68) | 74–106 | 0.863 |
| Total cholesterol (mg/dL) | 14 | 171.57 (21.26) | 169.36 (21.57) | <200 | 0.601 |
| HDL cholesterol (mg/dL) | 14 | 64.86 (13.74) | 62.43 (14.17) | >50 | 0.081 |
| LDL cholesterol (mg/dL) | 14 | 94.07 (18.02) | 94.14 (14.75) | 0–130 | 0.983 |
| Triglycerides (mg/dL) | 14 | 64.00 (28.34) | 63.79 (18.43) | <150 | 0.972 |
| Total proteins (g/L) | 14 | 70.57 (3.69) | 71.36 (3.97) | 64–83 | 0.394 |
| Albumin (g/L) | 14 | 47.00 (2.18) | 47.21 (2.89) | 35–52 | 0.793 |
| Creatinine (mg/dL) | 14 | 0.79 (0.14) | 0.80 (0.14) | 0.55–1.02 | 0.598 |
| Urea (mg/dL) | 14 | 29.29 (6.13) | 30.57 (8.39) | 17–49 | 0.505 |
| Sodium (mEq/L) | 14 | 139.07 (2.02) | 138.86 (2.21) | 136–146 | 0.787 |
| Potassium (mEq/L) | 14 | 4.51 (0.28) | 4.42 (0.31) | 3.5–5.1 | 0.411 |
| Chloride (mEq/L) | 14 | 105.07 (1.54) | 104.57 (2.17) | 99–109 | 0.446 |
| AST/GOT (U/L) | 14 | 30.64 (30.82) | 29.00 (8.19) | 0–31 | 0.827 |
| ALT/GPT (U/L) | 14 | 22.21 (13.86) | 24.86 (7.59) | 0–34 | 0.387 |
| GGT (U/L) | 14 | 14.29 (5.11) | 14.21 (6.28) | 0–38 | 0.947 |
| Iron (µg/dL) | 14 | 78.86 (39.72) | 101.00 (45.75) | 50–170 | 0.053 |
| Ferritin (ng/dL) | 14 | 33.21 (23.33) | 38.79 (26.22) | 11–307 | 0.169 |
| Phosphate (mg/dL) | 14 | 3.78 (0.43) | 3.69 (0.48) | 2.5–4.5 | 0.616 |
| Total calcium (mg/dL) | 14 | 9.74 (0.39) | 10.04 (0.32) | 8.6–10.2 | 0.015 ** |
| Homocysteine (µmol/L) | 14 | 13.62 (2.53) | 9.94 (1.55) | 4.44–13.56 | 0.000 *** |
| Single-Dose (LD) | |||||
|---|---|---|---|---|---|
| Ion Mode | SOI Code | Formula | Mass | p-Value (FDR) | ID |
| Positive | 567 | [C6H16NO6]+ | 198.09721 | 0.0422 * | Hexose sugar; maybe Tagatose |
| Positive | 666 | [C12H25N2O3]+ | 245.18597 | 0.0167 ** | Leu-Ile dipeptide |
| Positive | 932 | [C25H46NO4]+ | 424.34213 | 0.0188 ** | Linoleoylcarnitine |
| Negative | 1137 | [C24H39O4]- | 391.28538 | 0.026 ** | Deoxycholic acid |
| Double-Dose (HD) | |||||
| Ion Mode | SOI | Formula | Mass | p-Value (FDR) | ID |
| Positive | 213;221 | [C5H5N4O]+ | 137.04579 | 0.0256 ** | Hypoxanthine |
| Positive | 413 | [C8H9N2O2]+ | 165.06585 | 0.0256 ** | 8-Amino-2H-1,4-benzoxazin-3(4H)-one (Benzoxazine) |
| Positive | 523 | [C8H10NO4]+ | 184.06043 | 8.00 × 10−4 *** | Pyridoxic acid |
| Positive | 706;707;708 | [C10H13N4O5]+ | 269.08805 | 0.0444 *** | Inosine M + H |
| Positive | 763;764 | [C10H12N4NaO5]+ | 291.06999 | 0.0448 * | Inosine M + Na |
| Negative | 312 | [C8H8NO4]- | 182.04588 | 0.0024 ** | Pyridoxic acid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, M.C.; Herráiz, A.; Ciudad, M.J.; Arias, M.; Alonso, R.; Doblas, C.; Llama-Palacios, A.; Collado, L. Metabolomics and Biochemical Benefits of Multivitamin and Multimineral Supplementation in Healthy Individuals: A Pilot Study. Foods 2024, 13, 2207. https://doi.org/10.3390/foods13142207
Sánchez MC, Herráiz A, Ciudad MJ, Arias M, Alonso R, Doblas C, Llama-Palacios A, Collado L. Metabolomics and Biochemical Benefits of Multivitamin and Multimineral Supplementation in Healthy Individuals: A Pilot Study. Foods. 2024; 13(14):2207. https://doi.org/10.3390/foods13142207
Chicago/Turabian StyleSánchez, María C., Ana Herráiz, María J. Ciudad, Marta Arias, Raquel Alonso, Carmen Doblas, Arancha Llama-Palacios, and Luis Collado. 2024. "Metabolomics and Biochemical Benefits of Multivitamin and Multimineral Supplementation in Healthy Individuals: A Pilot Study" Foods 13, no. 14: 2207. https://doi.org/10.3390/foods13142207





