Recent Advances of Natural Pentacyclic Triterpenoids as Bioactive Delivery System for Synergetic Biological Applications
Abstract
:1. Introduction
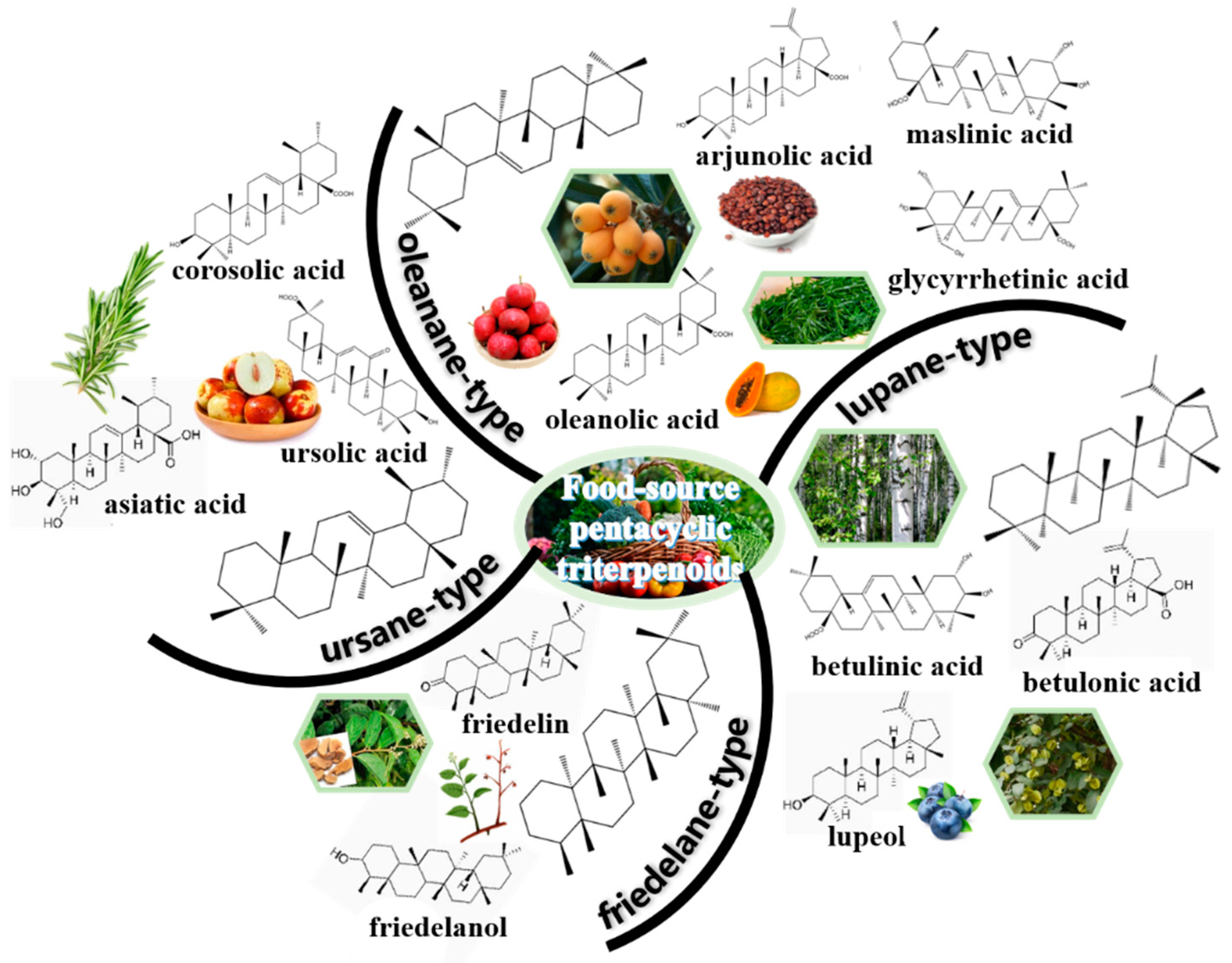
2. Distribution of Pentacyclic Triterpenoids in Nature
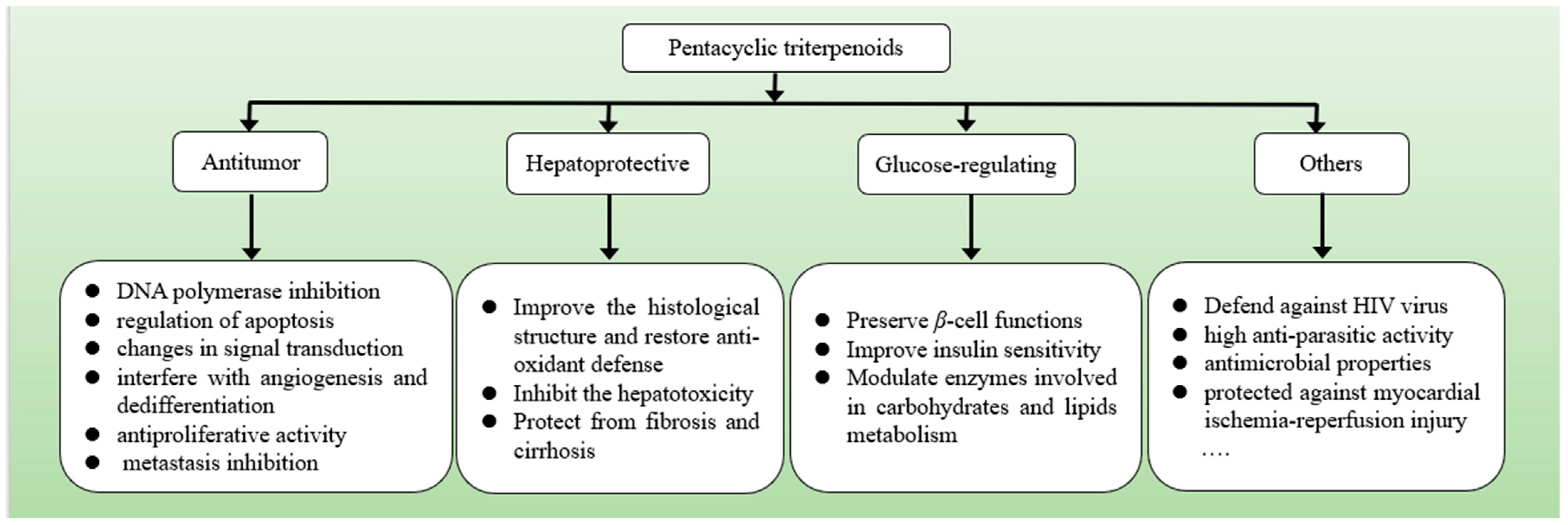
3. Biological Activities of Pentacyclic Triterpenoids
3.1. Anti-Tumor Activity
3.2. Hepatoprotective Activity
3.3. Glucose-Regulating Activity
3.4. Others
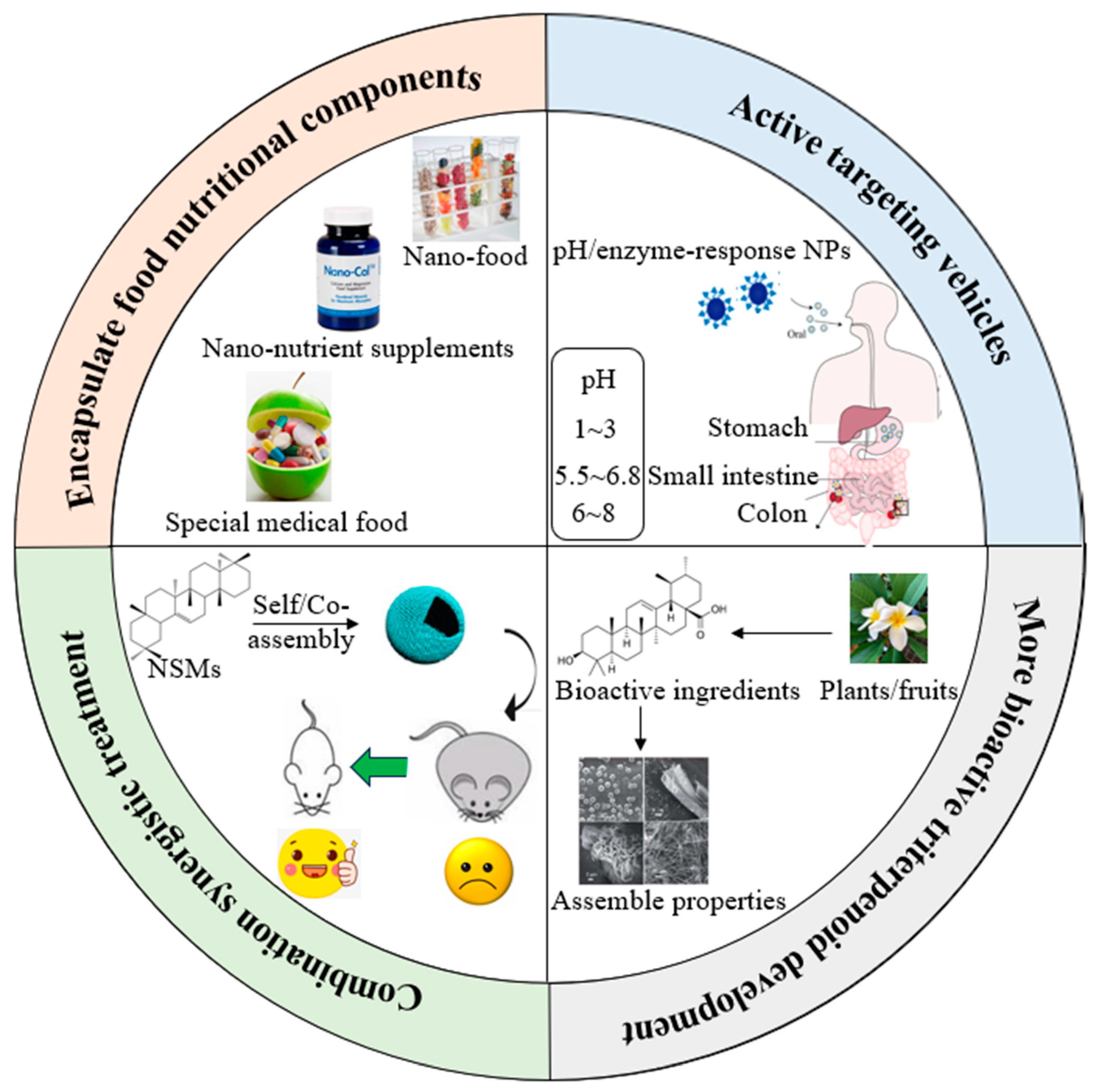
4. Self-Assembly and Co-Assembly Properties of Pentacyclic Triterpenoids
5. Applications of Pentacyclic Triterpenoids as Bioactive Delivery System
5.1. Directed Self-Assemblies
5.1.1. Oleanolic Acid
5.1.2. Ursolic Acid
5.1.3. Betulinic Acid
5.1.4. Betulonic Acid
5.1.5. Arjunolic Acid, Corosolic Acid and Maslinic Acid
5.2. Multi-Components Co-Assemblies
5.2.1. Oleanolic Acid-Glycyrrhetinic Acid/PTX
5.2.2. Carrier-Free Nanodelivery System
6. Conclusions and Further Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oh, Y.S. Bioactive compounds and their neuroprotective effects in diabetic complications. Nutrients 2016, 8, 472. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Bhattacharya, S. Multifarious facets of sugar-derived molecular gels: Molecular features, mechanisms of self-assembly and emerging applications. Chem. Soc. Rev. 2015, 44, 5596–5637. [Google Scholar] [CrossRef]
- Wani, T.A.; Shah, A.G.; Wani, S.M.; Wani, I.A.; Masoodi, F.A.; Nissar, N.; Shagoo, M.A. Suitability of different food grade materials for the encapsulation of some functional foods well reported for their advantages and susceptibility. Crit. Rev. Food Sci. Nutr. 2016, 56, 2431–2454. [Google Scholar] [CrossRef]
- Huang, L.; Huang, X.H.; Yang, X.; Hu, J.Q.; Zhu, Y.Z.; Yan, P.Y.; Xie, Y. Novel nano-drug delivery system for natural products and their application. Pharmacol. Res. 2024, 201, 107100. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Zhu, Y.; Yu, J.; Xu, X.; Gleeson, J.P.; Ryan, S.M.; Brayden, D.J. Oral delivery strategies for nutraceuticals: Delivery vehicles and absorption enhancers. Trends Food Sci. Technol. 2016, 53, 90–101. [Google Scholar]
- Furr, H.C.; Clark, R.M. Intestinal absorption and tissue distribution of carotenoids. J. Nutr. Biochem. 1997, 8, 364–377. [Google Scholar] [CrossRef]
- Teng, W.; Zhao, L.; Yang, S.; Zhang, C.; Liu, M.; Luo, J.; Jin, J.; Zhang, M.; Bao, C.; Li, D.; et al. The hepatic-targeted, resveratrol loaded nanoparticles for relief of high fat diet-induced nonalcoholic fatty liver disease. J. Control. Release 2019, 307, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Dangles, O.; Kopec, R.E. Fat-soluble vitamin and phytochemical metabolites: Production, gastrointestinal absorption, and health effects. Prog. Lipid Res. 2023, 90, 101220. [Google Scholar] [CrossRef]
- Gonnet, M.; Lethuaut, L.; Boury, F. New trends in encapsulation of liposoluble vitamins. J. Control. Release 2010, 146, 276–290. [Google Scholar] [CrossRef]
- Katouzian, I.; Jafari, S.M. Nano-encapsulation as a promising approach for targeted delivery and controlled release of vitamins. Trends Food Sci. Technol. 2016, 53, 34–48. [Google Scholar] [CrossRef]
- Guo, Y.; Qiao, D.; Zhao, S.; Zhang, B.; Xie, F. Starch-based materials encapsulating food ingredients: Recent advances in fabrication methods and applications. Carbohyd. Polym. 2021, 270, 118358. [Google Scholar] [CrossRef]
- Chen, S.; McClements, D.J.; Jian, L.; Han, Y.; Dai, L.; Mao, L.; Gao, Y. Core–shell biopolymer nanoparticles for co-delivery of curcumin and piperine: Sequential electrostatic deposition of hyaluronic acid and chitosan shells on the zein core. ACS Appl. Mater. 2019, 41, 38103–38115. [Google Scholar] [CrossRef]
- Huang, H.; Belwal, T.; Liu, S.; Duan, Z.; Luo, Z. Novel multi-phase nano-emulsion preparation for co-loading hydrophilic arbutin and hydrophobic coumaric acid using hydrocolloids. Food Hydrocoll. 2019, 93, 92–101. [Google Scholar] [CrossRef]
- Wei, Z.; Gao, Y. Physicochemical properties of β-carotene bilayer emulsions coated by milk proteins and chitosan-EGCG conjugates. Food Hydrocoll. 2016, 52, 590–599. [Google Scholar] [CrossRef]
- Kim, J.Y.; Huber, K.C. Preparation and characterization of corn starch β-carotene composites. Carbohyd. Polym. 2016, 136, 394–401. [Google Scholar] [CrossRef]
- Zhu, F. Encapsulation and delivery of food ingredients using starch based systems. Food Chem. 2017, 229, 542–552. [Google Scholar] [CrossRef]
- Cui, F.; Han, S.; Wang, J.; McClements, D.J.; Liu, X.; Liu, F. Co-delivery of curcumin and epigallocatechin gallate in W/O/W emulsions stabilized by protein fibril-cellulose complexes. Colloids Surf. B Biointerfaces 2023, 222, 113072. [Google Scholar] [CrossRef]
- Fu, S.; Yang, X. Recent advances in natural small molecules as drug delivery systems. J. Mater. Chem. B 2023, 11, 4584–4599. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wang, S.; Zhao, H.; Liu, Y.; Yang, X. Exploring the self-assembly mechanism and effective synergistic antitumor chemophototherapy of a biodegradable and glutathione responsive ursolic acid prodrug mediated photosensitive nanodrug. Biomater. Sci. 2021, 9, 3762–3775. [Google Scholar] [CrossRef]
- Lin, B.; Peng, X.; Cheng, J.; Wang, J. Natural gambogic acid-tuned self-assembly of nanodrugs towards synergistic chemophototherapy against breast cancer. J. Mater. Chem. B 2024, 12, 5940–5949. [Google Scholar] [CrossRef]
- Yang, X.; Ma, C.; Chen, Z.; Liu, J.; Liu, Y.; Xie, R.; Zhao, H.; Deng, G.; Chen, A.T.; Gong, N.B.; et al. Single small molecule-assembled nanoparticles mediate efficient oral drug delivery. Nano Res. 2019, 12, 2468–2476. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Qiao, W.; Cheng, J.; Han, Y.; Yang, X. Nanomedicine-cum-carrier by co-assembly of natural small products for synergistic enhanced antitumor with tissues protective actions. ACS Appl. Mater. 2020, 12, 42537–42550. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Ma, C.; Zhao, H.; Zhang, S.; Liu, J.; Liu, F.; Chen, Z.; Chen, A.T.; Yang, X.; Avery, J.; et al. Anti-edema and antioxidant combination therapy for ischemic stroke via glyburide-loaded betulinic acid nanoparticles. Theranostics 2019, 9, 6991–7002. [Google Scholar] [CrossRef] [PubMed]
- Ghante, M.H.; Jamkhande, P.G. Role of pentacyclic triterpenoids in chemoprevention and anticancer treatment: An overview on targets and underling mechanisms. J. Pharmacopunct. 2019, 22, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Li, L.; Song, W.; Li, M.; Hua, X.; Wang, Y.; Yuan, J.; Xue, Z. Natural products of pentacyclic triterpenoids: From discovery to heterologous biosynthesis. Nat. Prod. Rep. 2023, 40, 1303–1353. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Q.; Song, L.X.; Han, Z.Z.; Yang, Y.B.; Zhang, Y.; Gu, L.H.; Yang, L.; Chou, G.X.; Wang, Z.T. Pentacyclic triterpenoids from spikes of Prunella vulgaris L. with thyroid tumour cell cytostatic bioactivities. Nat. Prod. Res. 2023, 37, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Peng, B.; Chen, Z.; Yu, J.; Deng, G.; Bao, Y.; Ma, C.; Du, F.; Sheu, W.C.; Kimberly, W.T.; et al. Brain-targeting, acid-responsive antioxidant nanoparticles for stroke treatment and drug delivery. Bioact. Mater. 2022, 16, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, P.; Tian, S.; Xue, J.; Xu, L.; Li, H.; Wei, X. Bioactive pentacyclic triterpenoids from the leaves of Cleistocalyx operculatus. J. Nat. Prod. 2016, 79, 2912–2923. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Fazio, G.C.; Matsuda, S.P.T. On the origins of triterpenoid skeletal diversity. Phytochemistry 2004, 65, 261–291. [Google Scholar] [CrossRef]
- Wagle, A.; Seong, S.H.; Jung, H.A.; Choi, J.S. Identifying an isoflavone from the root of Pueraria lobata as a potent tyrosinase inhibitor. Food Chem. 2019, 15, 383–389. [Google Scholar] [CrossRef]
- Yang, Y.H.; Dai, S.Y.; Deng, F.H.; Peng, L.H.; Li, C.; Pei, Y.H. Recent advances in medicinal chemistry of oleanolic acid derivatives. Phytochemistry 2022, 203, 113397. [Google Scholar] [CrossRef]
- Guo, S.; Duan, J.; Tang, Y.; Qian, Y.; Zhao, J.; Qian, D.; Su, S.; Shang, E. Simultaneous qualitative and quantitative analysis of triterpenic acids, saponins and flavonoids in the leaves of two Ziziphus species by HPLC-PDA-MS/ELSD. J. Pharm. Biomed. Anal. 2011, 56, 264–270. [Google Scholar] [CrossRef]
- Laszczyk, M.N. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 2009, 75, 1549–1560. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Leal, A.S.; Valdeira, A.S.; Gonçalves, B.M.F.; Alho, D.P.S.; Figueiredo, S.A.C.; Silvestre, S.M.; Mendes, V.I.S. Oleanane-, ursane-, and quinone methide friedelane-type triterpenoid derivatives: Recent advances in cancer treatment. Eur. J. Med. Chem. 2017, 142, 95–130. [Google Scholar] [CrossRef] [PubMed]
- Yousef, B.A.; Hassan, H.M.; Zhang, L.Y.; Jiang, Z.Z. Anticancer potential and molecular targets of pristimerin: A mini-review. Curr. Cancer Drug Targets 2017, 17, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Qin, P.; Ji, M.; An, R.; Guo, H.; Shafi, J. Spinasterol, 22,23-Dihydrospinasterol and Fernenol from Citrullus colocynthis L. with Aphicidal Activity against Cabbage aphid Brevicoryne brassicae L. Molecules 2020, 25, 2184. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, X.; Feng, Y.; Wang, Y.; Guan, J.; Deng, B.; Chen, Q.; Wang, Y.; Chen, Y.; Wang, J.; et al. Cylindrin from Imperata cylindrica inhibits M2 macrophage formation and attenuates renal fibrosis by downregulating the LXR-α/PI3K/AKT pathway. Eur. J. Pharmacol. 2023, 950, 175771. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Lv, J.M.; Cao, Z.Q.; Wang, G.Q.; Lin, F.L.; Chen, G.D.; Qin, S.Y.; Hu, D.; Gao, H.; Yao, X.S. Biosynthetic characterization of the antifungal fernane-type triterpenoid polytolypin for generation of new analogues via combinatorial biosynthesis. Org. Biomol. Chem. 2023, 21, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, P.; Zhang, Y.; Li, J.; Tao, H.; Gu, Q.; Zhu, W. 2-Hydroxydiplopterol, a new cytotoxic pentacyclic triterpenoid from the halotolerant fungus Aspergillus variecolor B-17. Arch. Pharm. Res. 2009, 32, 1211–1214. [Google Scholar] [CrossRef]
- Carrero, J.J.; Ortiz, A.G.; Avesani, C.M.; Bakker, S.J.L.; Bellizzi, V.; Chauveau, P.; Clase, C.M.; Cupisti, A.; Cuevas, A.E.; Molina, P.; et al. Plant-based diets to manage the risks and complications of chronic kidney disease. Nat. Rev. Nephrol. 2020, 16, 525–542. [Google Scholar] [CrossRef]
- Ghosh, A.; Panda, C.K. Role of pentacyclic triterpenoid acids in the treatment of bladder cancer. Mini-Rev. Med. Chem. 2022, 22, 1331–1340. [Google Scholar] [CrossRef]
- Mandal, A.; Ghosh, S.; Bothra, A.K.; Nanda, A.K.; Ghosh, P. Synthesis of friedelan triterpenoid analogs with DNA topoisomerase IIα inhibitory activity and their molecular docking studies. Eur. J. Med. Chem. 2012, 54, 137–143. [Google Scholar] [CrossRef]
- Li, W.; Song, Y.; Zhang, P.; Zhu, H.; Chen, L.; Xiao, Y.; Xing, Y. Oleanolic acid inhibits cell survival and proliferation of prostate cancer cells in vitro and in vivo through the PI3K/Akt pathway. Tumor. Biol. 2016, 37, 7599–7613. [Google Scholar] [CrossRef]
- Shyu, M.H.; Kao, T.C.; Yen, G.C. Oleanolic acid and ursolic acid induce apoptosis in HuH7 human hepatocellular carcinoma cells through a mitochondrial-dependent pathway and downregulation of XIAP. J. Agric. Food. Chem. 2010, 58, 6110–6118. [Google Scholar] [CrossRef]
- Mlala, S.; Oyedeji, A.O.; Gondwe, M.; Oyedeji, O.O. Ursolic acid and its derivatives as bioactive agents. Molecules 2019, 24, 2751. [Google Scholar] [CrossRef]
- Zuco, V.; Supino, R.; Righetti, S.C.; Cleris, L.; Marchesi, E.; Gambacorti-Passerini, C.; Formelli, F. Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett. 2002, 175, 17–25. [Google Scholar] [CrossRef]
- Lin, C.; Wen, X.; Sun, H. Oleanolic acid derivatives for pharmaceutical use: A patent review. Expert Opin. Ther. Pat. 2016, 26, 643–655. [Google Scholar] [CrossRef]
- Wu, S.Y.; Wang, W.J.; Dou, J.H.; Gong, L.K. Research progress on the protective effects of licorice-derived 18β-glycyrrhetinic acid against liver injury. Acta Pharmacol. 2021, 42, 18–26. [Google Scholar] [CrossRef]
- Xiao, Y.; Xu, J.; Mao, C.; Jin, M.; Wu, Q.; Zou, J.; Gu, Q.; Zhang, Y.; Zhang, Y. 18β-glycyrrhetinic acid ameliorates acute Propionibacterium acnes-induced liver injury through inhibition of macrophage inflammatory protein-1alpha. J. Biol. Chem. 2010, 285, 1128–1137. [Google Scholar] [CrossRef]
- Pollier, J.; Goossens, A. Oleanolic acid. Phytochemistry 2012, 77, 10–15. [Google Scholar] [CrossRef]
- Aragón, S.M.; Heras, B.; Reus, M.I.S.; Benedi, J. Pharmacological modification of endogenous antioxidant enzymes by ursolic acid on tetrachloride-induced liver damage in rats and primary cultures of rat hepatocytes. Exp. Toxicol. Pathol. 2001, 53, 199–206. [Google Scholar] [CrossRef]
- Saravanan, R.; Viswanathan, P.; Pugalendi, K.V. Protective effect of ursolic acid on ethanol-mediated experimental liver damage in rats. Life Sci. 2006, 78, 713–718. [Google Scholar] [CrossRef]
- Manna, P.; Sil, P.C. Impaired redox signaling and mitochondrial uncoupling contributes vascular inflammation and cardiac dysfunction in type 1 diabetes: Protective role of arjunolic acid. Biochimie 2012, 94, 786–797. [Google Scholar] [CrossRef]
- Zheng, J.; He, J.; Ji, B.; Li, Y.; Zhang, X. Antihyperglycemic effects of platycodon grandiflorum (Jacq.) A. DC. extract on streptozotocin-induced diabetic mice. Plant Foods Hum. Nutr. 2007, 62, 7–11. [Google Scholar] [CrossRef]
- Lee, H.; Kang, R.; Kim, Y.S.; Chung, S.I.; Yoon, Y. Platycodin D inhibits adipogenesis of 3T3-L1 cells by modulating kruppel-like factor 2 and peroxisome proliferator-activated receptor γ. Phytother Res. 2010, 24, S161–S167. [Google Scholar] [CrossRef]
- Li, S.; Liao, X.; Meng, F.; Wang, Y.; Sun, Z.; Guo, F.; Li, X.; Meng, M.; Li, Y.; Sun, C. Therapeutic role of ursolic acid on ameliorating hepatic steatosis and improving metabolic disorders in high-fat diet-induced nonalcoholic fatty liver disease rats. PLoS ONE 2014, 9, e86724. [Google Scholar]
- Jung, S.H.; Ha, Y.J.; Shim, E.K.; Choi, S.Y.; Jin, J.L.; Yun-Choi, H.S.; Lee, J.R. Insulin-mimetic and insulin-sensitizing activities of a pentacyclic riterpenoid insulin receptor activator. Biochem. J. 2007, 403, 243–250. [Google Scholar] [CrossRef]
- Seo, D.Y.; Lee, S.R.; Heo, J.W.; No, M.H.; Rhee, B.D.; Ko, K.S.; Kwak, H.B.; Han, J. Ursolic acid in health and disease. Korean J. Physiol. Pharmacol. 2018, 22, 235–248. [Google Scholar] [CrossRef]
- Ajala-Lawal, R.A.; Aliyu, N.O.; Ajiboye, T.O. Betulinic acid improves insulin sensitivity, hyperglycemia, inflammation and oxidative stress in metabolic syndrome rats via PI3K/Akt pathways. Arch. Physiol. Biochem. 2020, 126, 107–115. [Google Scholar] [CrossRef]
- Melo, C.L.; Queiroz, M.G.R.; Filho, A.C.V.A.; Rodrigues, A.M.; Sousa, D.F.; Almeida, J.G.L.; Pessoa, O.D.L.; Silveira, E.R.; Menezes, D.B.; Melo, T.S.; et al. Betulinic acid, a natural pentacyclic triterpenoid, prevents abdominal fat accumulation in mice fed a high-fat diet. J. Agric. Food Chem. 2009, 57, 8776–8781. [Google Scholar] [CrossRef]
- Wu, H.F.; Natschke, S.L.M.; Xu, X.D.; Yang, M.H.; Cheng, Y.Y.; Yu, S.S.; Lee, K.H. Recent advances in natural anti-HIV triterpenoids and analogs. Med. Res. Rev. 2020, 40, 2339–2385. [Google Scholar] [CrossRef]
- Isah, M.B.; Ibrahim, M.A.; Mohammed, A.; Aliyu, A.B.; Masola, B.; Coetzer, T.H.T. A systematic review of pentacyclic triterpenes and their derivatives as chemotherapeutic agents against tropical parasitic diseases. J. Parasitol. 2016, 143, 1219–1231. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, M.; Xie, X.; Yang, H.; Wang, X.; Xiao, L.; Wang, N. Oleanolic acid ameliorates high glucose-induced endothelial dysfunction via PPAR delta activation. Sci. Rep. 2017, 7, 40237. [Google Scholar] [CrossRef]
- Jo, E.; Choi, M.H.; Kim, H.S.; Park, S.N.; Lim, Y.K.; Kang, C.K.; Kook, J.K. Antimicrobial effects of oleanolic acid, ursolic acid, and Sophoraflavanone G against enterococcus faecalis and propionibacterium acnes. Int. J. Oral Biol. 2014, 39, 75–79. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, L.; Xiao, S.; Chen, H.; Han, Y.; Niu, B. Ursolic acid, the main component of blueberry cuticular wax, inhibits Botrytis cinerea growth by damaging cell membrane integrity. Food Chem. 2023, 415, 135753. [Google Scholar] [CrossRef]
- Micota, B.; Sadowska, B.; Podsędek, A.; Redzynia, M.; Różalska, B. Leonurus cardiaca L. herb--a derived extract and an ursolic acid as the factors affecting the adhesion capacity of Staphylococcus aureus in the context of infective endocarditis. Acta Biochim. Pol. 2014, 61, 385–388. [Google Scholar] [CrossRef]
- Zhi, K.; Wang, J.; Zhao, H.; Yang, X. Self-assembled small molecule natural product gel for drug delivery: A breakthrough in new application of small molecule natural products. Acta Pharm. Sin. B 2020, 10, 913–927. [Google Scholar] [CrossRef]
- Yadav, S.; Sharma, A.K.; Kumar, P. Nanoscale self-assembly for therapeutic delivery. Front. Bioeng. Biotechnol. 2020, 8, 127. [Google Scholar] [CrossRef]
- Das, D.; Kar, T.; Das, P.K. Gel-nanocomposites: Materials with promising applications. Soft Matter 2012, 8, 2348–2365. [Google Scholar] [CrossRef]
- Bag, B.G.; Paul, K. Vesicular and fibrillar gels by self-assembly of nanosized oleanolic acid. Asian J. Org. Chem. 2012, 1, 150–154. [Google Scholar] [CrossRef]
- Bag, B.G.; Hasan, S.N.; Ghorai, S.; Panja, S.K. First self-assembly of dihydroxy triterpenoid maslinic acid yielding vesicles. ACS Omega 2019, 4, 7684–7690. [Google Scholar] [CrossRef]
- Bag, B.G.; Dash, S.S. First self-assembly study of betulinic acid, a renewable nano-sized, 6-6-6-6-5 pentacyclic monohydroxy triterpenic acid. Nanoscale 2011, 3, 4564–4566. [Google Scholar] [CrossRef] [PubMed]
- Zhi, K.; Zhao, H.; Yang, X.; Zhang, H.; Wang, J.; Wang, J.; Regenstein, J.M. Natural product gelators and a general method for obtaining them from organisms. Nanoscale 2018, 10, 3639–3643. [Google Scholar] [CrossRef] [PubMed]
- Bag, B.G.; Majumdar, R. Self-assembly of a renewable nano-sized triterpenoid 18β-glycyrrhetinic acid. RSC Adv. 2012, 2, 8623–8626. [Google Scholar] [CrossRef]
- Lu, J.; Wu, X.; Liu, L.; Chen, H.; Liang, Y. First organogelation study of ursolic acid, a natural ursane triterpenoid. Chem. Lett. 2016, 45, 860–862. [Google Scholar] [CrossRef]
- Bag, B.G.; Das, S.S.; Hasana, S.N.; Barai, A.C. Nanoarchitectures by hierarchical self-assembly of ursolic acid: Entrapment and release of fluorophores including anticancer drug doxorubicin. RSC Adv. 2017, 7, 18136–18143. [Google Scholar] [CrossRef]
- Hu, X.; Hou, B.; Xu, Z.; Saeed, M.; Sun, F.; Gao, Z.; Lai, Y.; Zhu, T.; Zhang, F.; Zhang, W.; et al. Supramolecular prodrug nanovectors for active tumor targeting and combination immunotherapy of colorectal cancer. Adv. Sci. 2020, 7, 1903332. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, P.P.; Krennrich, G. Bioavailability and potency of natural-source and all-racemic alpha-tocopherol in the human: A dispute. Eur. J. Nutr. 2000, 39, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cui, Y.X.; Feng, X.N.; Cheng, M.; Tang, A.N.; Kong, D.M. pH-controlled intracellular in situ reversible assembly of photothermal agent for smart chemo-photothermal synergetic therapy and ATP imaging. ACS Appl. Mater. 2019, 11, 39624–39632. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, J.; Fu, R.; Feng, H.; Chu, Y.; Huang, D.; Liu, H.; Li, C.; Ma, C.; El-Aty, A.M.A. Improved stability, epithelial permeability and cellular antioxidant activity of β-carotene via encapsulation by self-assembled α-lactalbumin micelles. Improved stability and aqueous solubility of β-carotene via encapsulation in self-assembled bioactive oleanolic acid nanoparticles. Food Chem. 2022, 373 Pt B, 131498. [Google Scholar]
- Wang, J.; Qiao, W.; Zhao, H.; Cheng, J.; Han, Y.; Yang, X. A highly atom-economical bioactive nanocarrier for synergistically enhanced antitumor with reduced liver injury. New J. Chem. 2020, 44, 16741–16751. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Zhi, K.; Yang, X. Exploration of the natural active small-molecule drug-loading process and highly efficient synergistic antitumor efficacy. ACS Appl. Mater. 2020, 12, 6827–6839. [Google Scholar] [CrossRef] [PubMed]
- Simard, J.M.; Chen, M.; Tarasov, K.V.; Bhatta, S.; Ivanova, S.; Melnitchenko, L.; Tsymbalyuk, N.; West, G.A.; Gerzanich, V. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat. Med. 2016, 12, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qiao, W.; Zhao, H.; Yang, X. Paclitaxel and betulonic acid synergistically enhance antitumor efficacy by forming co-assembled nanoparticles. Biochem. Pharmacol. 2020, 182, 114232. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhao, H.; Wang, J.; Han, Y. Bioactive natural small molecule-tuned coassembly of photosensitive drugs for highly efficient synergistic and enhanced Type I photochemotherapy. ACS Appl. Mater. 2020, 12, 43488–43500. [Google Scholar] [CrossRef]
- Bag, B.; Majumdar, R. Vesicular self-assembly of a natural triterpenoid arjunolic acid in aqueous medium: Study of entrapment properties and in situ generation of gel-gold nanoparticle hybrid material. RSC Adv. 2014, 4, 53327–53334. [Google Scholar] [CrossRef]
- Zheng, J.; Fan, R.; Wu, H.; Yao, H.; Yan, Y.; Liu, J.; Ran, L.; Sun, Z.; Yi, L.; Dang, L.; et al. Directed self-assembly of herbal small molecules into sustained release hydrogels for treating neural inflammation. Nat. Commun. 2019, 10, 1604. [Google Scholar] [CrossRef] [PubMed]
- Bag, B.G.; Garai, C.; Ghorai, S. Vesicular self-assembly of a natural ursane-type dihydroxy-triterpenoid corosolic acid. RSC Adv. 2019, 9, 15190–15195. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qiao, W.; Li, X.; Zhao, H.; Zhang, H.; Dong, A.; Yang, X. A directed co-assembly of herbal small molecules into carrier-free nanodrugs for enhanced synergistic antitumor efficacy. J. Mater. Chem. B 2021, 9, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Jiang, J.; Wu, P.; Zou, J.; Le, J.; Lin, J.; Li, C.; Luo, B.; Zhang, Y.; Huang, R.; et al. A smart dual-drug nanosystem based on co-assembly of plant and food-derived natural products for synergistic HCC immunotherapy. Acta Pharm. Sin. B 2021, 11, 246–257. [Google Scholar] [CrossRef]
- Kaps, A.; Gwiazdoń, P.; Chodurek, E. Nanoformulations for delivery of pentacyclic triterpenoids in anticancer therapies. Molecules 2021, 26, 1764–1782. [Google Scholar] [CrossRef]
- Garanti, T.; Stasik, A.; Burrow, A.J.; Alhnan, M.A.; Wan, K.W. Anti-glioma activity and the mechanism of cellular uptake of asiatic acid-loaded solid lipid nanoparticles. Int. J. Pharmaceut. 2016, 500, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ghosh, S.; De, A.K.; Bera, T. Oral delivery of ursolic acid-loaded nanostructured lipid carrier coated with chitosan oligosaccharides: Development, characterization, in vitro and in vivo assessment for the therapy of leishmaniasis. Int. J. Biol. Macromol. 2017, 102, 996–1008. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Cao, X.; Liu, K.; Li, C.; Zhang, G.; Deng, L.; Si, C.; He, J.; Lei, J. 2015. Self-assembled targeted folate-conjugated eight-arm-polyethylene glycol-betulinic acid nanoparticles for co-delivery of anticancer drugs. J. Mater. Chem. B 2015, 3, 3754–3766. [Google Scholar] [CrossRef] [PubMed]
- Saharkhiz, S.; Zarepour, A.; Zarrabi, A. A new theranostic pH-responsive niosome formulation for doxorubicin delivery and bio-imaging against breast cancer. Int. J. Pharm. 2023, 637, 122845. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ling, J.; Xu, X.; Ouyang, X.K.; Wang, N. Redox and pH dual sensitive carboxymethyl chitosan functionalized polydopamine nanoparticles loaded with doxorubicin for tumor chemo-photothermal therapy. Int. J. Biol. Macromol. 2023, 240, 124488. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Lu, X.; Wang, Y.; Brodelius, P.E. Comparison of the interaction between lactoferrin and isomeric drugs. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 173, 593–607. [Google Scholar] [CrossRef]
- Wu, Z.C.; Liu, X.Y.; Liu, J.Y.; Piao, J.S.; Piao, M.G. Preparation of betulinic acid galactosylated chitosan nanoparticles and their effect on liver fibrosis. Int. J. Nanomed. 2022, 17, 4195–4210. [Google Scholar] [CrossRef]
| Carrier | Loaded Compounds | LC&EE | Interaction Forces | Physicochemical Properties | Functional Activities | References |
|---|---|---|---|---|---|---|
| Oleanolic acid | Car | 32.6 ± 0.0%; 80.7 ± 0.1%. | Hydrogen bonding; hydrophobic interactions. | Increased aqueous solubility and protection against UV radiation ionic strength, and heat. Delayed release in simulated gastric conditions and controlled release in simulated intestinal conditions. | Enhanced hepatoprotective and antioxidant effects. | [80] |
| Oleanolic acid | PTX | 17.1 ± 0.0%; 62.6 ± 0.0%. | Hydrophobic interactions; hydrogen bonding. | Increased stability, slowed and sustained release under acidic conditions. | Tumor inhibition rate was 76.54 ± 0.66%, about 18% higher than that of PTX group. Reduced liver injury. | [81] |
| Ursolic acid | PTX | 23.12 ± 1.07%; 94.41 ± 4.28%, | Hydrophobic interactions; hydrogen bonding. | More stable in PBS for at least 15 days, in serum for 24 h and also under acidic conditions. Controlled release after entering the cells. | Prolonged plasma half-life. Tumor inhibition rate was 90.2%, 3.3 times higher than that of PTX group. Reduced liver injury. | [82] |
| Ursolic acid | Rho B/CF/DOX | Hydrogen bonding; Van der Waals interactions. | Controlled release of Rho B at physiological conditions. Slow release of DOX at physiological conditions and pH 6.6. | _ | [77] | |
| Betulinic acid | Glyburide | _ | _ | Increase the delivery to the brain. Controllably released over three days. | Synergetic effects for ischemic stroke by antioxidant and anti-edema. | [23] |
| Betulonic acid | PTX | _ | Hydrogen bonding; hydrophobic interaction. | Improved water solubility. More stable under acidic conditions. | Synergistic anti-tumor efficacy and minimize the side effects. | [83] |
| Betulonic acid | Ce6 | _ | π-π stacking; hydrophobic interactions. | Excellent water dispersity. Keep stable in water, cell cultural medium and PBS buffer (pH 7.4). Have better photostability. | prolonged blood circulation. Synergistic anticancer efficacy. | [84] |
| Arjunolic acid | Rho B/CF/DOX | Hydrogen bonding | Slow release of PTX at physiological pH (7.2). | _ | [85,86] | |
| Corosolic acid | Rho B/CF/DOX | Hydrogen bonding; Van der Waals interactions. | Triton X-100-triggered release of Rho-B. | _ | [87,88] | |
| Maslinic acid | Rho B/ CF/ DOX | Hydrogen bonding; lipophilic interactions. | Triton X-100-triggered release of DOX. | _ | [71] | |
| Carrier | Loaded Compounds | LC&EE | Interaction forces | Physicochemical Properties | Functional Activities | References |
|---|---|---|---|---|---|---|
| Oleanolic acid -glycyrrhetinic acid | PTX | 15.1 ± 0.4%, 98.8 ± 1.0% | Hydrogen bonding; hydrophobic interactions. | Stable under acidic conditions. Have good dispersion and chemical stability. | Tumor inhibition rate was 82.6%, 23.7% higher than the PTX group. Reduced liver damage and nanotoxicity. | [22] |
| Glycyrrhetinic acid—oleanolic acid | - | Hydrogen bonding; hydrophobic interactions. | - | Tumor inhibition rate was Enhanced from 50.5% to 69.5%. Reliable biosafety. | [89] | |
| Glycyrrhetinic acid-Liquidambaric acid | - | Hydrogen bonding; hydrophobic interactions. | - | Tumor inhibition rate was Enhanced from 37.3% to 82.9%. Reliable biosafety. | [89] | |
| Ursolic acid -EGCG-aptamer | 73.6% | Hydrophobic interactions; hydrogen bonding. | pH-responsive, released rapidly in acid conditions. | Synergistic anticancer effect. Enhanced tumor immune infiltration. | [90] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, W.; Zhou, Z.; Cao, J.; Guo, Q. Recent Advances of Natural Pentacyclic Triterpenoids as Bioactive Delivery System for Synergetic Biological Applications. Foods 2024, 13, 2226. https://doi.org/10.3390/foods13142226
Teng W, Zhou Z, Cao J, Guo Q. Recent Advances of Natural Pentacyclic Triterpenoids as Bioactive Delivery System for Synergetic Biological Applications. Foods. 2024; 13(14):2226. https://doi.org/10.3390/foods13142226
Chicago/Turabian StyleTeng, Wendi, Zixiao Zhou, Jinxuan Cao, and Qing Guo. 2024. "Recent Advances of Natural Pentacyclic Triterpenoids as Bioactive Delivery System for Synergetic Biological Applications" Foods 13, no. 14: 2226. https://doi.org/10.3390/foods13142226





