Recent Advances of Natural Pentacyclic Triterpenoids as Bioactive Delivery System for Synergetic Biological Applications
Abstract
1. Introduction
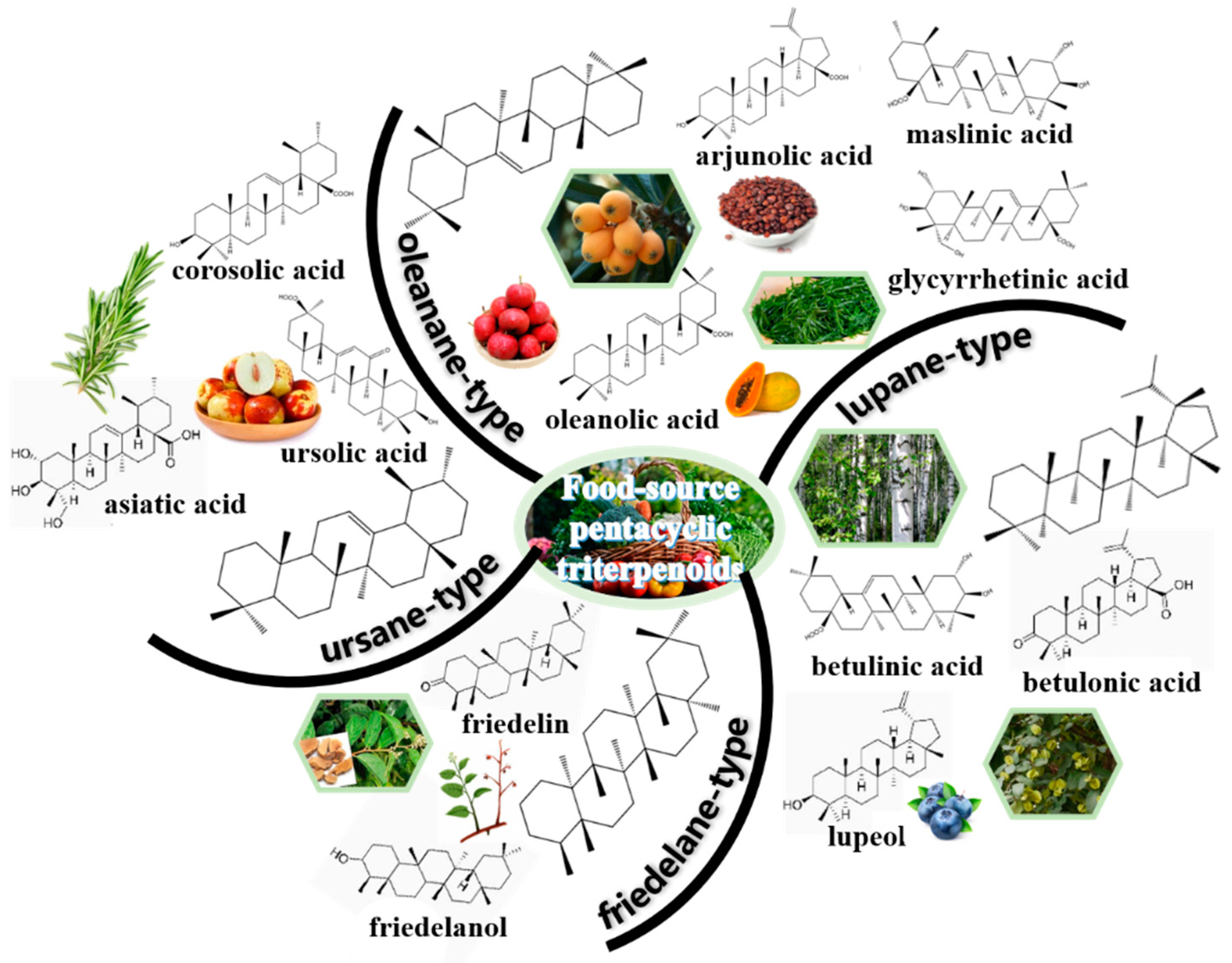
2. Distribution of Pentacyclic Triterpenoids in Nature
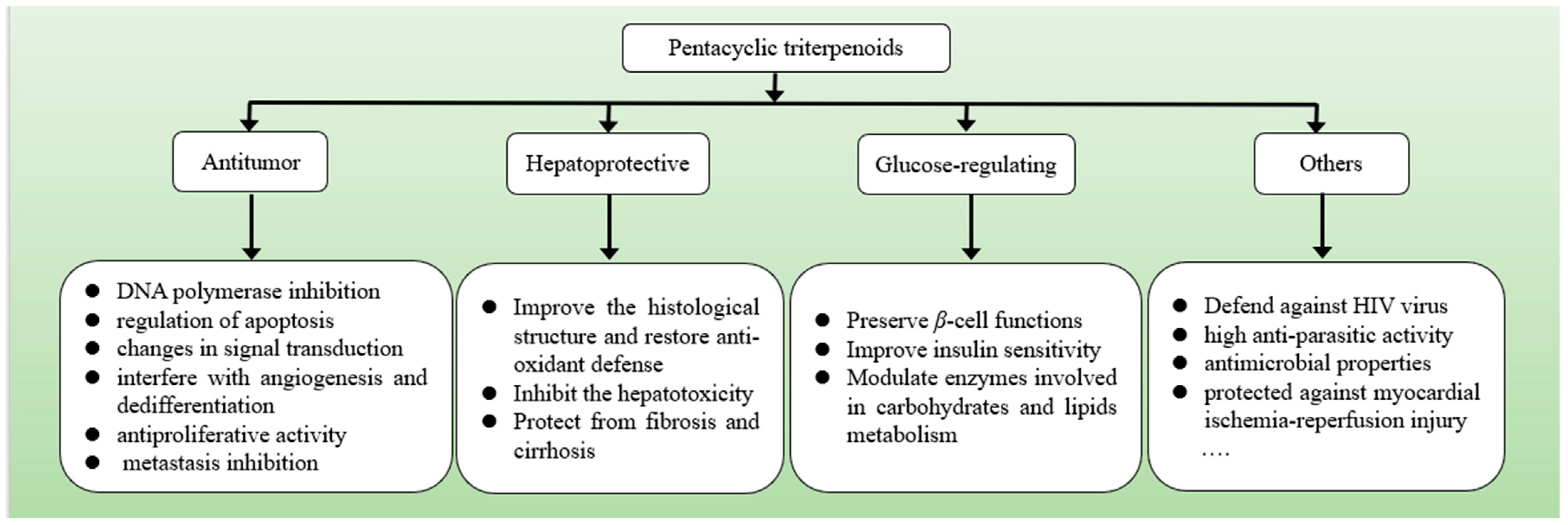
3. Biological Activities of Pentacyclic Triterpenoids
3.1. Anti-Tumor Activity
3.2. Hepatoprotective Activity
3.3. Glucose-Regulating Activity
3.4. Others
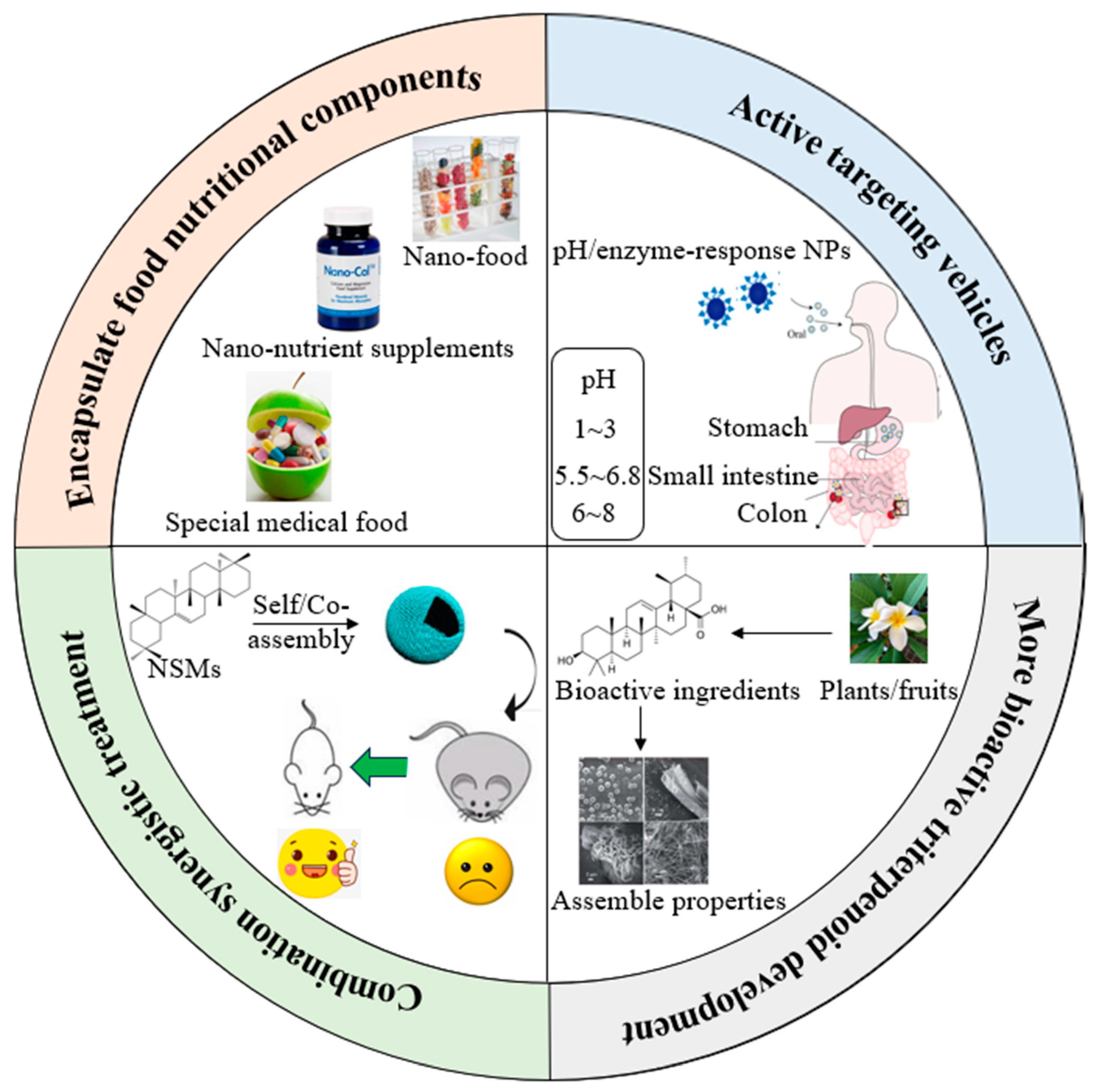
4. Self-Assembly and Co-Assembly Properties of Pentacyclic Triterpenoids
5. Applications of Pentacyclic Triterpenoids as Bioactive Delivery System
5.1. Directed Self-Assemblies
5.1.1. Oleanolic Acid
5.1.2. Ursolic Acid
5.1.3. Betulinic Acid
5.1.4. Betulonic Acid
5.1.5. Arjunolic Acid, Corosolic Acid and Maslinic Acid
5.2. Multi-Components Co-Assemblies
5.2.1. Oleanolic Acid-Glycyrrhetinic Acid/PTX
5.2.2. Carrier-Free Nanodelivery System
6. Conclusions and Further Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oh, Y.S. Bioactive compounds and their neuroprotective effects in diabetic complications. Nutrients 2016, 8, 472. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Bhattacharya, S. Multifarious facets of sugar-derived molecular gels: Molecular features, mechanisms of self-assembly and emerging applications. Chem. Soc. Rev. 2015, 44, 5596–5637. [Google Scholar] [CrossRef]
- Wani, T.A.; Shah, A.G.; Wani, S.M.; Wani, I.A.; Masoodi, F.A.; Nissar, N.; Shagoo, M.A. Suitability of different food grade materials for the encapsulation of some functional foods well reported for their advantages and susceptibility. Crit. Rev. Food Sci. Nutr. 2016, 56, 2431–2454. [Google Scholar] [CrossRef]
- Huang, L.; Huang, X.H.; Yang, X.; Hu, J.Q.; Zhu, Y.Z.; Yan, P.Y.; Xie, Y. Novel nano-drug delivery system for natural products and their application. Pharmacol. Res. 2024, 201, 107100. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Zhu, Y.; Yu, J.; Xu, X.; Gleeson, J.P.; Ryan, S.M.; Brayden, D.J. Oral delivery strategies for nutraceuticals: Delivery vehicles and absorption enhancers. Trends Food Sci. Technol. 2016, 53, 90–101. [Google Scholar]
- Furr, H.C.; Clark, R.M. Intestinal absorption and tissue distribution of carotenoids. J. Nutr. Biochem. 1997, 8, 364–377. [Google Scholar] [CrossRef]
- Teng, W.; Zhao, L.; Yang, S.; Zhang, C.; Liu, M.; Luo, J.; Jin, J.; Zhang, M.; Bao, C.; Li, D.; et al. The hepatic-targeted, resveratrol loaded nanoparticles for relief of high fat diet-induced nonalcoholic fatty liver disease. J. Control. Release 2019, 307, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Dangles, O.; Kopec, R.E. Fat-soluble vitamin and phytochemical metabolites: Production, gastrointestinal absorption, and health effects. Prog. Lipid Res. 2023, 90, 101220. [Google Scholar] [CrossRef]
- Gonnet, M.; Lethuaut, L.; Boury, F. New trends in encapsulation of liposoluble vitamins. J. Control. Release 2010, 146, 276–290. [Google Scholar] [CrossRef]
- Katouzian, I.; Jafari, S.M. Nano-encapsulation as a promising approach for targeted delivery and controlled release of vitamins. Trends Food Sci. Technol. 2016, 53, 34–48. [Google Scholar] [CrossRef]
- Guo, Y.; Qiao, D.; Zhao, S.; Zhang, B.; Xie, F. Starch-based materials encapsulating food ingredients: Recent advances in fabrication methods and applications. Carbohyd. Polym. 2021, 270, 118358. [Google Scholar] [CrossRef]
- Chen, S.; McClements, D.J.; Jian, L.; Han, Y.; Dai, L.; Mao, L.; Gao, Y. Core–shell biopolymer nanoparticles for co-delivery of curcumin and piperine: Sequential electrostatic deposition of hyaluronic acid and chitosan shells on the zein core. ACS Appl. Mater. 2019, 41, 38103–38115. [Google Scholar] [CrossRef]
- Huang, H.; Belwal, T.; Liu, S.; Duan, Z.; Luo, Z. Novel multi-phase nano-emulsion preparation for co-loading hydrophilic arbutin and hydrophobic coumaric acid using hydrocolloids. Food Hydrocoll. 2019, 93, 92–101. [Google Scholar] [CrossRef]
- Wei, Z.; Gao, Y. Physicochemical properties of β-carotene bilayer emulsions coated by milk proteins and chitosan-EGCG conjugates. Food Hydrocoll. 2016, 52, 590–599. [Google Scholar] [CrossRef]
- Kim, J.Y.; Huber, K.C. Preparation and characterization of corn starch β-carotene composites. Carbohyd. Polym. 2016, 136, 394–401. [Google Scholar] [CrossRef]
- Zhu, F. Encapsulation and delivery of food ingredients using starch based systems. Food Chem. 2017, 229, 542–552. [Google Scholar] [CrossRef]
- Cui, F.; Han, S.; Wang, J.; McClements, D.J.; Liu, X.; Liu, F. Co-delivery of curcumin and epigallocatechin gallate in W/O/W emulsions stabilized by protein fibril-cellulose complexes. Colloids Surf. B Biointerfaces 2023, 222, 113072. [Google Scholar] [CrossRef]
- Fu, S.; Yang, X. Recent advances in natural small molecules as drug delivery systems. J. Mater. Chem. B 2023, 11, 4584–4599. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wang, S.; Zhao, H.; Liu, Y.; Yang, X. Exploring the self-assembly mechanism and effective synergistic antitumor chemophototherapy of a biodegradable and glutathione responsive ursolic acid prodrug mediated photosensitive nanodrug. Biomater. Sci. 2021, 9, 3762–3775. [Google Scholar] [CrossRef]
- Lin, B.; Peng, X.; Cheng, J.; Wang, J. Natural gambogic acid-tuned self-assembly of nanodrugs towards synergistic chemophototherapy against breast cancer. J. Mater. Chem. B 2024, 12, 5940–5949. [Google Scholar] [CrossRef]
- Yang, X.; Ma, C.; Chen, Z.; Liu, J.; Liu, Y.; Xie, R.; Zhao, H.; Deng, G.; Chen, A.T.; Gong, N.B.; et al. Single small molecule-assembled nanoparticles mediate efficient oral drug delivery. Nano Res. 2019, 12, 2468–2476. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Qiao, W.; Cheng, J.; Han, Y.; Yang, X. Nanomedicine-cum-carrier by co-assembly of natural small products for synergistic enhanced antitumor with tissues protective actions. ACS Appl. Mater. 2020, 12, 42537–42550. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Ma, C.; Zhao, H.; Zhang, S.; Liu, J.; Liu, F.; Chen, Z.; Chen, A.T.; Yang, X.; Avery, J.; et al. Anti-edema and antioxidant combination therapy for ischemic stroke via glyburide-loaded betulinic acid nanoparticles. Theranostics 2019, 9, 6991–7002. [Google Scholar] [CrossRef] [PubMed]
- Ghante, M.H.; Jamkhande, P.G. Role of pentacyclic triterpenoids in chemoprevention and anticancer treatment: An overview on targets and underling mechanisms. J. Pharmacopunct. 2019, 22, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Li, L.; Song, W.; Li, M.; Hua, X.; Wang, Y.; Yuan, J.; Xue, Z. Natural products of pentacyclic triterpenoids: From discovery to heterologous biosynthesis. Nat. Prod. Rep. 2023, 40, 1303–1353. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Q.; Song, L.X.; Han, Z.Z.; Yang, Y.B.; Zhang, Y.; Gu, L.H.; Yang, L.; Chou, G.X.; Wang, Z.T. Pentacyclic triterpenoids from spikes of Prunella vulgaris L. with thyroid tumour cell cytostatic bioactivities. Nat. Prod. Res. 2023, 37, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Peng, B.; Chen, Z.; Yu, J.; Deng, G.; Bao, Y.; Ma, C.; Du, F.; Sheu, W.C.; Kimberly, W.T.; et al. Brain-targeting, acid-responsive antioxidant nanoparticles for stroke treatment and drug delivery. Bioact. Mater. 2022, 16, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, P.; Tian, S.; Xue, J.; Xu, L.; Li, H.; Wei, X. Bioactive pentacyclic triterpenoids from the leaves of Cleistocalyx operculatus. J. Nat. Prod. 2016, 79, 2912–2923. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Fazio, G.C.; Matsuda, S.P.T. On the origins of triterpenoid skeletal diversity. Phytochemistry 2004, 65, 261–291. [Google Scholar] [CrossRef]
- Wagle, A.; Seong, S.H.; Jung, H.A.; Choi, J.S. Identifying an isoflavone from the root of Pueraria lobata as a potent tyrosinase inhibitor. Food Chem. 2019, 15, 383–389. [Google Scholar] [CrossRef]
- Yang, Y.H.; Dai, S.Y.; Deng, F.H.; Peng, L.H.; Li, C.; Pei, Y.H. Recent advances in medicinal chemistry of oleanolic acid derivatives. Phytochemistry 2022, 203, 113397. [Google Scholar] [CrossRef]
- Guo, S.; Duan, J.; Tang, Y.; Qian, Y.; Zhao, J.; Qian, D.; Su, S.; Shang, E. Simultaneous qualitative and quantitative analysis of triterpenic acids, saponins and flavonoids in the leaves of two Ziziphus species by HPLC-PDA-MS/ELSD. J. Pharm. Biomed. Anal. 2011, 56, 264–270. [Google Scholar] [CrossRef]
- Laszczyk, M.N. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 2009, 75, 1549–1560. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Leal, A.S.; Valdeira, A.S.; Gonçalves, B.M.F.; Alho, D.P.S.; Figueiredo, S.A.C.; Silvestre, S.M.; Mendes, V.I.S. Oleanane-, ursane-, and quinone methide friedelane-type triterpenoid derivatives: Recent advances in cancer treatment. Eur. J. Med. Chem. 2017, 142, 95–130. [Google Scholar] [CrossRef] [PubMed]
- Yousef, B.A.; Hassan, H.M.; Zhang, L.Y.; Jiang, Z.Z. Anticancer potential and molecular targets of pristimerin: A mini-review. Curr. Cancer Drug Targets 2017, 17, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Qin, P.; Ji, M.; An, R.; Guo, H.; Shafi, J. Spinasterol, 22,23-Dihydrospinasterol and Fernenol from Citrullus colocynthis L. with Aphicidal Activity against Cabbage aphid Brevicoryne brassicae L. Molecules 2020, 25, 2184. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, X.; Feng, Y.; Wang, Y.; Guan, J.; Deng, B.; Chen, Q.; Wang, Y.; Chen, Y.; Wang, J.; et al. Cylindrin from Imperata cylindrica inhibits M2 macrophage formation and attenuates renal fibrosis by downregulating the LXR-α/PI3K/AKT pathway. Eur. J. Pharmacol. 2023, 950, 175771. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Lv, J.M.; Cao, Z.Q.; Wang, G.Q.; Lin, F.L.; Chen, G.D.; Qin, S.Y.; Hu, D.; Gao, H.; Yao, X.S. Biosynthetic characterization of the antifungal fernane-type triterpenoid polytolypin for generation of new analogues via combinatorial biosynthesis. Org. Biomol. Chem. 2023, 21, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, P.; Zhang, Y.; Li, J.; Tao, H.; Gu, Q.; Zhu, W. 2-Hydroxydiplopterol, a new cytotoxic pentacyclic triterpenoid from the halotolerant fungus Aspergillus variecolor B-17. Arch. Pharm. Res. 2009, 32, 1211–1214. [Google Scholar] [CrossRef]
- Carrero, J.J.; Ortiz, A.G.; Avesani, C.M.; Bakker, S.J.L.; Bellizzi, V.; Chauveau, P.; Clase, C.M.; Cupisti, A.; Cuevas, A.E.; Molina, P.; et al. Plant-based diets to manage the risks and complications of chronic kidney disease. Nat. Rev. Nephrol. 2020, 16, 525–542. [Google Scholar] [CrossRef]
- Ghosh, A.; Panda, C.K. Role of pentacyclic triterpenoid acids in the treatment of bladder cancer. Mini-Rev. Med. Chem. 2022, 22, 1331–1340. [Google Scholar] [CrossRef]
- Mandal, A.; Ghosh, S.; Bothra, A.K.; Nanda, A.K.; Ghosh, P. Synthesis of friedelan triterpenoid analogs with DNA topoisomerase IIα inhibitory activity and their molecular docking studies. Eur. J. Med. Chem. 2012, 54, 137–143. [Google Scholar] [CrossRef]
- Li, W.; Song, Y.; Zhang, P.; Zhu, H.; Chen, L.; Xiao, Y.; Xing, Y. Oleanolic acid inhibits cell survival and proliferation of prostate cancer cells in vitro and in vivo through the PI3K/Akt pathway. Tumor. Biol. 2016, 37, 7599–7613. [Google Scholar] [CrossRef]
- Shyu, M.H.; Kao, T.C.; Yen, G.C. Oleanolic acid and ursolic acid induce apoptosis in HuH7 human hepatocellular carcinoma cells through a mitochondrial-dependent pathway and downregulation of XIAP. J. Agric. Food. Chem. 2010, 58, 6110–6118. [Google Scholar] [CrossRef]
- Mlala, S.; Oyedeji, A.O.; Gondwe, M.; Oyedeji, O.O. Ursolic acid and its derivatives as bioactive agents. Molecules 2019, 24, 2751. [Google Scholar] [CrossRef]
- Zuco, V.; Supino, R.; Righetti, S.C.; Cleris, L.; Marchesi, E.; Gambacorti-Passerini, C.; Formelli, F. Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett. 2002, 175, 17–25. [Google Scholar] [CrossRef]
- Lin, C.; Wen, X.; Sun, H. Oleanolic acid derivatives for pharmaceutical use: A patent review. Expert Opin. Ther. Pat. 2016, 26, 643–655. [Google Scholar] [CrossRef]
- Wu, S.Y.; Wang, W.J.; Dou, J.H.; Gong, L.K. Research progress on the protective effects of licorice-derived 18β-glycyrrhetinic acid against liver injury. Acta Pharmacol. 2021, 42, 18–26. [Google Scholar] [CrossRef]
- Xiao, Y.; Xu, J.; Mao, C.; Jin, M.; Wu, Q.; Zou, J.; Gu, Q.; Zhang, Y.; Zhang, Y. 18β-glycyrrhetinic acid ameliorates acute Propionibacterium acnes-induced liver injury through inhibition of macrophage inflammatory protein-1alpha. J. Biol. Chem. 2010, 285, 1128–1137. [Google Scholar] [CrossRef]
- Pollier, J.; Goossens, A. Oleanolic acid. Phytochemistry 2012, 77, 10–15. [Google Scholar] [CrossRef]
- Aragón, S.M.; Heras, B.; Reus, M.I.S.; Benedi, J. Pharmacological modification of endogenous antioxidant enzymes by ursolic acid on tetrachloride-induced liver damage in rats and primary cultures of rat hepatocytes. Exp. Toxicol. Pathol. 2001, 53, 199–206. [Google Scholar] [CrossRef]
- Saravanan, R.; Viswanathan, P.; Pugalendi, K.V. Protective effect of ursolic acid on ethanol-mediated experimental liver damage in rats. Life Sci. 2006, 78, 713–718. [Google Scholar] [CrossRef]
- Manna, P.; Sil, P.C. Impaired redox signaling and mitochondrial uncoupling contributes vascular inflammation and cardiac dysfunction in type 1 diabetes: Protective role of arjunolic acid. Biochimie 2012, 94, 786–797. [Google Scholar] [CrossRef]
- Zheng, J.; He, J.; Ji, B.; Li, Y.; Zhang, X. Antihyperglycemic effects of platycodon grandiflorum (Jacq.) A. DC. extract on streptozotocin-induced diabetic mice. Plant Foods Hum. Nutr. 2007, 62, 7–11. [Google Scholar] [CrossRef]
- Lee, H.; Kang, R.; Kim, Y.S.; Chung, S.I.; Yoon, Y. Platycodin D inhibits adipogenesis of 3T3-L1 cells by modulating kruppel-like factor 2 and peroxisome proliferator-activated receptor γ. Phytother Res. 2010, 24, S161–S167. [Google Scholar] [CrossRef]
- Li, S.; Liao, X.; Meng, F.; Wang, Y.; Sun, Z.; Guo, F.; Li, X.; Meng, M.; Li, Y.; Sun, C. Therapeutic role of ursolic acid on ameliorating hepatic steatosis and improving metabolic disorders in high-fat diet-induced nonalcoholic fatty liver disease rats. PLoS ONE 2014, 9, e86724. [Google Scholar]
- Jung, S.H.; Ha, Y.J.; Shim, E.K.; Choi, S.Y.; Jin, J.L.; Yun-Choi, H.S.; Lee, J.R. Insulin-mimetic and insulin-sensitizing activities of a pentacyclic riterpenoid insulin receptor activator. Biochem. J. 2007, 403, 243–250. [Google Scholar] [CrossRef]
- Seo, D.Y.; Lee, S.R.; Heo, J.W.; No, M.H.; Rhee, B.D.; Ko, K.S.; Kwak, H.B.; Han, J. Ursolic acid in health and disease. Korean J. Physiol. Pharmacol. 2018, 22, 235–248. [Google Scholar] [CrossRef]
- Ajala-Lawal, R.A.; Aliyu, N.O.; Ajiboye, T.O. Betulinic acid improves insulin sensitivity, hyperglycemia, inflammation and oxidative stress in metabolic syndrome rats via PI3K/Akt pathways. Arch. Physiol. Biochem. 2020, 126, 107–115. [Google Scholar] [CrossRef]
- Melo, C.L.; Queiroz, M.G.R.; Filho, A.C.V.A.; Rodrigues, A.M.; Sousa, D.F.; Almeida, J.G.L.; Pessoa, O.D.L.; Silveira, E.R.; Menezes, D.B.; Melo, T.S.; et al. Betulinic acid, a natural pentacyclic triterpenoid, prevents abdominal fat accumulation in mice fed a high-fat diet. J. Agric. Food Chem. 2009, 57, 8776–8781. [Google Scholar] [CrossRef]
- Wu, H.F.; Natschke, S.L.M.; Xu, X.D.; Yang, M.H.; Cheng, Y.Y.; Yu, S.S.; Lee, K.H. Recent advances in natural anti-HIV triterpenoids and analogs. Med. Res. Rev. 2020, 40, 2339–2385. [Google Scholar] [CrossRef]
- Isah, M.B.; Ibrahim, M.A.; Mohammed, A.; Aliyu, A.B.; Masola, B.; Coetzer, T.H.T. A systematic review of pentacyclic triterpenes and their derivatives as chemotherapeutic agents against tropical parasitic diseases. J. Parasitol. 2016, 143, 1219–1231. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, M.; Xie, X.; Yang, H.; Wang, X.; Xiao, L.; Wang, N. Oleanolic acid ameliorates high glucose-induced endothelial dysfunction via PPAR delta activation. Sci. Rep. 2017, 7, 40237. [Google Scholar] [CrossRef]
- Jo, E.; Choi, M.H.; Kim, H.S.; Park, S.N.; Lim, Y.K.; Kang, C.K.; Kook, J.K. Antimicrobial effects of oleanolic acid, ursolic acid, and Sophoraflavanone G against enterococcus faecalis and propionibacterium acnes. Int. J. Oral Biol. 2014, 39, 75–79. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, L.; Xiao, S.; Chen, H.; Han, Y.; Niu, B. Ursolic acid, the main component of blueberry cuticular wax, inhibits Botrytis cinerea growth by damaging cell membrane integrity. Food Chem. 2023, 415, 135753. [Google Scholar] [CrossRef]
- Micota, B.; Sadowska, B.; Podsędek, A.; Redzynia, M.; Różalska, B. Leonurus cardiaca L. herb--a derived extract and an ursolic acid as the factors affecting the adhesion capacity of Staphylococcus aureus in the context of infective endocarditis. Acta Biochim. Pol. 2014, 61, 385–388. [Google Scholar] [CrossRef]
- Zhi, K.; Wang, J.; Zhao, H.; Yang, X. Self-assembled small molecule natural product gel for drug delivery: A breakthrough in new application of small molecule natural products. Acta Pharm. Sin. B 2020, 10, 913–927. [Google Scholar] [CrossRef]
- Yadav, S.; Sharma, A.K.; Kumar, P. Nanoscale self-assembly for therapeutic delivery. Front. Bioeng. Biotechnol. 2020, 8, 127. [Google Scholar] [CrossRef]
- Das, D.; Kar, T.; Das, P.K. Gel-nanocomposites: Materials with promising applications. Soft Matter 2012, 8, 2348–2365. [Google Scholar] [CrossRef]
- Bag, B.G.; Paul, K. Vesicular and fibrillar gels by self-assembly of nanosized oleanolic acid. Asian J. Org. Chem. 2012, 1, 150–154. [Google Scholar] [CrossRef]
- Bag, B.G.; Hasan, S.N.; Ghorai, S.; Panja, S.K. First self-assembly of dihydroxy triterpenoid maslinic acid yielding vesicles. ACS Omega 2019, 4, 7684–7690. [Google Scholar] [CrossRef]
- Bag, B.G.; Dash, S.S. First self-assembly study of betulinic acid, a renewable nano-sized, 6-6-6-6-5 pentacyclic monohydroxy triterpenic acid. Nanoscale 2011, 3, 4564–4566. [Google Scholar] [CrossRef] [PubMed]
- Zhi, K.; Zhao, H.; Yang, X.; Zhang, H.; Wang, J.; Wang, J.; Regenstein, J.M. Natural product gelators and a general method for obtaining them from organisms. Nanoscale 2018, 10, 3639–3643. [Google Scholar] [CrossRef] [PubMed]
- Bag, B.G.; Majumdar, R. Self-assembly of a renewable nano-sized triterpenoid 18β-glycyrrhetinic acid. RSC Adv. 2012, 2, 8623–8626. [Google Scholar] [CrossRef]
- Lu, J.; Wu, X.; Liu, L.; Chen, H.; Liang, Y. First organogelation study of ursolic acid, a natural ursane triterpenoid. Chem. Lett. 2016, 45, 860–862. [Google Scholar] [CrossRef]
- Bag, B.G.; Das, S.S.; Hasana, S.N.; Barai, A.C. Nanoarchitectures by hierarchical self-assembly of ursolic acid: Entrapment and release of fluorophores including anticancer drug doxorubicin. RSC Adv. 2017, 7, 18136–18143. [Google Scholar] [CrossRef]
- Hu, X.; Hou, B.; Xu, Z.; Saeed, M.; Sun, F.; Gao, Z.; Lai, Y.; Zhu, T.; Zhang, F.; Zhang, W.; et al. Supramolecular prodrug nanovectors for active tumor targeting and combination immunotherapy of colorectal cancer. Adv. Sci. 2020, 7, 1903332. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, P.P.; Krennrich, G. Bioavailability and potency of natural-source and all-racemic alpha-tocopherol in the human: A dispute. Eur. J. Nutr. 2000, 39, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cui, Y.X.; Feng, X.N.; Cheng, M.; Tang, A.N.; Kong, D.M. pH-controlled intracellular in situ reversible assembly of photothermal agent for smart chemo-photothermal synergetic therapy and ATP imaging. ACS Appl. Mater. 2019, 11, 39624–39632. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, J.; Fu, R.; Feng, H.; Chu, Y.; Huang, D.; Liu, H.; Li, C.; Ma, C.; El-Aty, A.M.A. Improved stability, epithelial permeability and cellular antioxidant activity of β-carotene via encapsulation by self-assembled α-lactalbumin micelles. Improved stability and aqueous solubility of β-carotene via encapsulation in self-assembled bioactive oleanolic acid nanoparticles. Food Chem. 2022, 373 Pt B, 131498. [Google Scholar]
- Wang, J.; Qiao, W.; Zhao, H.; Cheng, J.; Han, Y.; Yang, X. A highly atom-economical bioactive nanocarrier for synergistically enhanced antitumor with reduced liver injury. New J. Chem. 2020, 44, 16741–16751. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Zhi, K.; Yang, X. Exploration of the natural active small-molecule drug-loading process and highly efficient synergistic antitumor efficacy. ACS Appl. Mater. 2020, 12, 6827–6839. [Google Scholar] [CrossRef] [PubMed]
- Simard, J.M.; Chen, M.; Tarasov, K.V.; Bhatta, S.; Ivanova, S.; Melnitchenko, L.; Tsymbalyuk, N.; West, G.A.; Gerzanich, V. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat. Med. 2016, 12, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qiao, W.; Zhao, H.; Yang, X. Paclitaxel and betulonic acid synergistically enhance antitumor efficacy by forming co-assembled nanoparticles. Biochem. Pharmacol. 2020, 182, 114232. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhao, H.; Wang, J.; Han, Y. Bioactive natural small molecule-tuned coassembly of photosensitive drugs for highly efficient synergistic and enhanced Type I photochemotherapy. ACS Appl. Mater. 2020, 12, 43488–43500. [Google Scholar] [CrossRef]
- Bag, B.; Majumdar, R. Vesicular self-assembly of a natural triterpenoid arjunolic acid in aqueous medium: Study of entrapment properties and in situ generation of gel-gold nanoparticle hybrid material. RSC Adv. 2014, 4, 53327–53334. [Google Scholar] [CrossRef]
- Zheng, J.; Fan, R.; Wu, H.; Yao, H.; Yan, Y.; Liu, J.; Ran, L.; Sun, Z.; Yi, L.; Dang, L.; et al. Directed self-assembly of herbal small molecules into sustained release hydrogels for treating neural inflammation. Nat. Commun. 2019, 10, 1604. [Google Scholar] [CrossRef] [PubMed]
- Bag, B.G.; Garai, C.; Ghorai, S. Vesicular self-assembly of a natural ursane-type dihydroxy-triterpenoid corosolic acid. RSC Adv. 2019, 9, 15190–15195. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qiao, W.; Li, X.; Zhao, H.; Zhang, H.; Dong, A.; Yang, X. A directed co-assembly of herbal small molecules into carrier-free nanodrugs for enhanced synergistic antitumor efficacy. J. Mater. Chem. B 2021, 9, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Jiang, J.; Wu, P.; Zou, J.; Le, J.; Lin, J.; Li, C.; Luo, B.; Zhang, Y.; Huang, R.; et al. A smart dual-drug nanosystem based on co-assembly of plant and food-derived natural products for synergistic HCC immunotherapy. Acta Pharm. Sin. B 2021, 11, 246–257. [Google Scholar] [CrossRef]
- Kaps, A.; Gwiazdoń, P.; Chodurek, E. Nanoformulations for delivery of pentacyclic triterpenoids in anticancer therapies. Molecules 2021, 26, 1764–1782. [Google Scholar] [CrossRef]
- Garanti, T.; Stasik, A.; Burrow, A.J.; Alhnan, M.A.; Wan, K.W. Anti-glioma activity and the mechanism of cellular uptake of asiatic acid-loaded solid lipid nanoparticles. Int. J. Pharmaceut. 2016, 500, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ghosh, S.; De, A.K.; Bera, T. Oral delivery of ursolic acid-loaded nanostructured lipid carrier coated with chitosan oligosaccharides: Development, characterization, in vitro and in vivo assessment for the therapy of leishmaniasis. Int. J. Biol. Macromol. 2017, 102, 996–1008. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Cao, X.; Liu, K.; Li, C.; Zhang, G.; Deng, L.; Si, C.; He, J.; Lei, J. 2015. Self-assembled targeted folate-conjugated eight-arm-polyethylene glycol-betulinic acid nanoparticles for co-delivery of anticancer drugs. J. Mater. Chem. B 2015, 3, 3754–3766. [Google Scholar] [CrossRef] [PubMed]
- Saharkhiz, S.; Zarepour, A.; Zarrabi, A. A new theranostic pH-responsive niosome formulation for doxorubicin delivery and bio-imaging against breast cancer. Int. J. Pharm. 2023, 637, 122845. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ling, J.; Xu, X.; Ouyang, X.K.; Wang, N. Redox and pH dual sensitive carboxymethyl chitosan functionalized polydopamine nanoparticles loaded with doxorubicin for tumor chemo-photothermal therapy. Int. J. Biol. Macromol. 2023, 240, 124488. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Lu, X.; Wang, Y.; Brodelius, P.E. Comparison of the interaction between lactoferrin and isomeric drugs. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 173, 593–607. [Google Scholar] [CrossRef]
- Wu, Z.C.; Liu, X.Y.; Liu, J.Y.; Piao, J.S.; Piao, M.G. Preparation of betulinic acid galactosylated chitosan nanoparticles and their effect on liver fibrosis. Int. J. Nanomed. 2022, 17, 4195–4210. [Google Scholar] [CrossRef]
| Carrier | Loaded Compounds | LC&EE | Interaction Forces | Physicochemical Properties | Functional Activities | References |
|---|---|---|---|---|---|---|
| Oleanolic acid | Car | 32.6 ± 0.0%; 80.7 ± 0.1%. | Hydrogen bonding; hydrophobic interactions. | Increased aqueous solubility and protection against UV radiation ionic strength, and heat. Delayed release in simulated gastric conditions and controlled release in simulated intestinal conditions. | Enhanced hepatoprotective and antioxidant effects. | [80] |
| Oleanolic acid | PTX | 17.1 ± 0.0%; 62.6 ± 0.0%. | Hydrophobic interactions; hydrogen bonding. | Increased stability, slowed and sustained release under acidic conditions. | Tumor inhibition rate was 76.54 ± 0.66%, about 18% higher than that of PTX group. Reduced liver injury. | [81] |
| Ursolic acid | PTX | 23.12 ± 1.07%; 94.41 ± 4.28%, | Hydrophobic interactions; hydrogen bonding. | More stable in PBS for at least 15 days, in serum for 24 h and also under acidic conditions. Controlled release after entering the cells. | Prolonged plasma half-life. Tumor inhibition rate was 90.2%, 3.3 times higher than that of PTX group. Reduced liver injury. | [82] |
| Ursolic acid | Rho B/CF/DOX | Hydrogen bonding; Van der Waals interactions. | Controlled release of Rho B at physiological conditions. Slow release of DOX at physiological conditions and pH 6.6. | _ | [77] | |
| Betulinic acid | Glyburide | _ | _ | Increase the delivery to the brain. Controllably released over three days. | Synergetic effects for ischemic stroke by antioxidant and anti-edema. | [23] |
| Betulonic acid | PTX | _ | Hydrogen bonding; hydrophobic interaction. | Improved water solubility. More stable under acidic conditions. | Synergistic anti-tumor efficacy and minimize the side effects. | [83] |
| Betulonic acid | Ce6 | _ | π-π stacking; hydrophobic interactions. | Excellent water dispersity. Keep stable in water, cell cultural medium and PBS buffer (pH 7.4). Have better photostability. | prolonged blood circulation. Synergistic anticancer efficacy. | [84] |
| Arjunolic acid | Rho B/CF/DOX | Hydrogen bonding | Slow release of PTX at physiological pH (7.2). | _ | [85,86] | |
| Corosolic acid | Rho B/CF/DOX | Hydrogen bonding; Van der Waals interactions. | Triton X-100-triggered release of Rho-B. | _ | [87,88] | |
| Maslinic acid | Rho B/ CF/ DOX | Hydrogen bonding; lipophilic interactions. | Triton X-100-triggered release of DOX. | _ | [71] | |
| Carrier | Loaded Compounds | LC&EE | Interaction forces | Physicochemical Properties | Functional Activities | References |
|---|---|---|---|---|---|---|
| Oleanolic acid -glycyrrhetinic acid | PTX | 15.1 ± 0.4%, 98.8 ± 1.0% | Hydrogen bonding; hydrophobic interactions. | Stable under acidic conditions. Have good dispersion and chemical stability. | Tumor inhibition rate was 82.6%, 23.7% higher than the PTX group. Reduced liver damage and nanotoxicity. | [22] |
| Glycyrrhetinic acid—oleanolic acid | - | Hydrogen bonding; hydrophobic interactions. | - | Tumor inhibition rate was Enhanced from 50.5% to 69.5%. Reliable biosafety. | [89] | |
| Glycyrrhetinic acid-Liquidambaric acid | - | Hydrogen bonding; hydrophobic interactions. | - | Tumor inhibition rate was Enhanced from 37.3% to 82.9%. Reliable biosafety. | [89] | |
| Ursolic acid -EGCG-aptamer | 73.6% | Hydrophobic interactions; hydrogen bonding. | pH-responsive, released rapidly in acid conditions. | Synergistic anticancer effect. Enhanced tumor immune infiltration. | [90] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, W.; Zhou, Z.; Cao, J.; Guo, Q. Recent Advances of Natural Pentacyclic Triterpenoids as Bioactive Delivery System for Synergetic Biological Applications. Foods 2024, 13, 2226. https://doi.org/10.3390/foods13142226
Teng W, Zhou Z, Cao J, Guo Q. Recent Advances of Natural Pentacyclic Triterpenoids as Bioactive Delivery System for Synergetic Biological Applications. Foods. 2024; 13(14):2226. https://doi.org/10.3390/foods13142226
Chicago/Turabian StyleTeng, Wendi, Zixiao Zhou, Jinxuan Cao, and Qing Guo. 2024. "Recent Advances of Natural Pentacyclic Triterpenoids as Bioactive Delivery System for Synergetic Biological Applications" Foods 13, no. 14: 2226. https://doi.org/10.3390/foods13142226
APA StyleTeng, W., Zhou, Z., Cao, J., & Guo, Q. (2024). Recent Advances of Natural Pentacyclic Triterpenoids as Bioactive Delivery System for Synergetic Biological Applications. Foods, 13(14), 2226. https://doi.org/10.3390/foods13142226






