Recent Advances in Non-Contact Food Decontamination Technologies for Removing Mycotoxins and Fungal Contaminants
Abstract
:1. Introduction
2. Mycotoxins in Food Commodities
2.1. Current Status of Research on Mycotoxin Contamination of Grains and Cereals
2.2. Current Status of Research on Mycotoxin Contamination of Nut and Seed Products
2.3. Current Status of Research on Mycotoxin Contamination of Fruits, Vegetables, and Their Processed Products
2.4. Current Status of Research on Mycotoxin Contamination in Milk
3. Non-Contact Food Decontamination Technology for Removing Mycotoxins
3.1. Gamma Ray Irradiation
3.1.1. Principle
3.1.2. Applications of Gamma Ray Irradiation
3.1.3. Problems and Challenges
3.2. Ultraviolet Irradiation
3.2.1. Principle
3.2.2. Applications of Ultraviolet Irradiation
3.2.3. Problems and Challenges
3.3. Electron Beam Irradiation
3.3.1. Principle
3.3.2. Applications of Electron Beam Irradiation
3.3.3. Problems and Challenges
3.4. Microwave Irradiation
3.4.1. Principle
3.4.2. Applications of Microwave Irradiation
3.4.3. Problems and Challenges
3.5. Pulsed Light Irradiation
3.5.1. Principle
3.5.2. Applications of Pulsed Light Irradiation
3.5.3. Problems and Challenges
3.6. Pulsed Electric Field
3.6.1. Principle
3.6.2. Applications of Pulsed Electric Field
3.6.3. Problems and Challenges
3.7. Cold Plasma
3.7.1. Principle
3.7.2. Applications of Cold Plasma
3.7.3. Problems and Challenges
3.8. Ozone
3.8.1. Principle
3.8.2. Applications of Ozone
3.8.3. Problems and Challenges
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schaarschmidt, S.; Fauhl-Hassek, C. The fate of mycotoxins during secondary food processing of maize for human consumption. Compr. Rev. Food Sci. Food Saf. 2021, 20, 91–148. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Jiang, D.; Guan, E.; Bian, K.; Zhang, Y. Superheated steam processing of cereals and cereal products: A review. Com. Rev. Food Sci. Food Saf. 2023, 22, 1360–1386. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, S.Y.; Du, Z.L.; An, Y.; Zhang, C.C.; Yao, Y.P. Contamination status and control of mycotoxins in grain and oil crops. J. Agro-Environ. Sci. 2022, 41, 2680–2687. [Google Scholar]
- Gavahian, M.; Pallares, N.; Al Khawli, F.; Ferrer, E.; Barba, F.J. Recent advances in the application of innovative food processing technologies for mycotoxins and pesticide reduction in foods. Trends Food Sci. Technol. 2020, 106, 209–218. [Google Scholar] [CrossRef]
- Lee, H.J.; Ryu, D. Worldwide Occurrence of Mycotoxins in Cereals and Cereal-Derived Food Products: Public Health Perspectives of Their Co-occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef]
- CAC/RCP. Code of Practice for the Prevention and Reduction of Mycotoxin Contamination in Cereals. In International Food Standards; Codex Alimentarius Commission: Rome, Italy, 2017; Available online: www.codexalimentarius.org (accessed on 14 July 2024).
- García-Díaz, M.; Gil-Serna, J.; Vázquez, C.; Botia, M.N.; Patiño, B. A comprehensive study on the occurrence of mycotoxins and their producing fungi during the maize production cycle in Spain. Microorganisms 2020, 8, 141. [Google Scholar] [CrossRef]
- Nabwire, W.R.; Ombaka, J.; Dick, C.P.; Strickland, C.; Tang, L.; Xue, K.S.; Wang, J.-S. Aflatoxin in household maize for human consumption in Kenya, East Africa. Food Addit. Contam. Part B 2020, 13, 45–51. [Google Scholar] [CrossRef]
- Mahdjoubi, C.K.; Arroyo-Manzanares, N.; Hamini-Kadar, N.; García-Campaña, A.M.; Mebrouk, K.; Gámiz-Gracia, L. Multi-mycotoxin occurrence and exposure assessment approach in foodstuffs from algeria. Toxins 2020, 12, 194. [Google Scholar] [CrossRef]
- Stanciu, O.; Juan, C.; Berrada, H.; Miere, D.; Loghin, F.; Mañes, J. Study on trichothecene and zearalenone presence in Romanian wheat relative to weather conditions. Toxins 2019, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.J.; Yuan, J.J.; Shao, W.Q.; Peng, L.X.; Chen, W.L. Contamination status and health risk assessment of aflatoxin B1 in foodstuffs in Yunfu City, 2019–2022. South China J. Prev. Med. 2024, 50, 216–221+226. [Google Scholar]
- Aydin, A.; Aksu, H.; Gunsen, U. Mycotoxin levels and incidence of mould in Turkish rice. Environ. Monit. Assess. 2011, 178, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, I.; Usleber, E.; Klaffke, H.; Weber, R.; Majerus, P.; Otteneder, H.; Gareis, M.; Dietrich, R.; Märtlbauer, E. Fumonisin intake of the German consumer. Mycotoxin Res. 2008, 24, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Schwab, W.; Yu, P. Natural Occurrence and Co-Contamination of Twelve Mycotoxins in industry-submitted cool-season cereal grains grown under a low heat unit climate condition. Toxins 2019, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Golge, O.; Kabak, B. Occurrence of deoxynivalenol and zearalenone in cereals and cereal products from Turkey. Food Control 2020, 110, 106982. [Google Scholar] [CrossRef]
- Blankson, G.K.; Mills-Robertson, F.C.; Ofosu, I.W. Survey of occurrence levels of Aflatoxins in selected locally processed cereal-based foods for human consumption from Ghana. Food Control 2019, 95, 170–175. [Google Scholar] [CrossRef]
- Ji, X.; Xiao, Y.; Lyu, W.; Li, M.; Wang, W.; Tang, B.; Wang, X.; Yang, H. Probabilistic risk assessment of combined exposure to deoxynivalenol and emerging Alternaria toxins in cereal-based food products for infants and young children in China. Toxins 2022, 14, 509. [Google Scholar] [CrossRef]
- Hanvi, D.M.; Lawson-Evi, P.; De Boevre, M.; Goto, C.E.; De Saeger, S.; Eklu-Gadegbeku, K. Natural occurrence of mycotoxins in maize and sorghum in Togo. Mycotoxin Res. 2019, 35, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Badmos, F.O.; Muhammad, H.L.; Dabara, A.; Adefolalu, F.; Salubuyi, S.; Abdulkadir, A.; Oyetunji, V.T.; Apeh, D.O.; Muhammad, H.K.; Mwanza, M.; et al. Assessment of dietary exposure and levels of mycotoxins in sorghum from Niger State of Nigeria. Food Addit. Contam. Part A 2024, 41, 74–90. [Google Scholar] [CrossRef]
- Carballo, D.; Fernández-Franzón, M.; Ferrer, E.; Pallarés, N.; Berrada, H. Dietary Exposure to Mycotoxins through Alcoholic and Non-Alcoholic Beverages in Valencia, Spain. Toxins 2021, 13, 438. [Google Scholar] [CrossRef]
- Alasalvar, C.; Salvadó, J.-S.; Ros, E. Bioactives and health benefits of nuts and dried fruits. Food Chem. 2020, 314, 126192. [Google Scholar] [CrossRef]
- Bodoira, R.; Maestri, D. Phenolic compounds from nuts: Extraction, chemical profiles, and bioactivity. J. Agric. Food Chem. 2020, 68, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Beltran, A.; Moral, J.; Picot, A.; Puckett, R.D.; Cotty, P.J.; Michailides, T.J. Atoxigenic Aspergillus flavus isolates endemic to almond, fig, and pistachio orchards in california with potential to reduce aflatoxin contamination in these crops. Plant Dis. 2019, 103, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Atukwase, A.; Mutebi, R.; Acham, H.; Kaaya, A.N.; Wacoo, P.A. Aflatoxin exposure and risk assessment among peri-urban low income population in Kampala Capital City, Uganda. Meas. Food 2024, 13, 100122. [Google Scholar] [CrossRef]
- Boni, S.B.; Beed, F.; Kimanya, M.E.; Koyano, E.; Mponda, O.; Mamiro, D.; Kaoneka, B.; Bandyopadhyay, R.; Korie, S.; Mahuku, G. Aflatoxin contamination in Tanzania: Quantifying the problem in maize and groundnuts from rural households. World Mycotoxin J. 2021, 14, 553–564. [Google Scholar] [CrossRef]
- Mannani, N.; El Boujamaai, M.; Sifou, A.; Bennani, M.; El Adlouni, C.; Abdennebi, E.H.; Zinedine, A. Aflatoxins and Ochratoxin A in dried fruits from Morocco: Monitoring, regulatory aspects, and exposure assessment. Regul. Toxicol. Pharmacol. 2023, 145, 105503. [Google Scholar] [CrossRef]
- Oueslati, S.; Ben Yakhlef, S.; Vila-Donat, P.; Pallarés, N.; Ferrer, E.; Barba, F.J.; Berrada, H. Multi-mycotoxin determination in coffee beans marketed in Tunisia and the associated dietary exposure assessment. Food Control 2022, 140, 109127. [Google Scholar] [CrossRef]
- Songsermsakul, P.; Oeung, S.; Porasuphatana, S. Assessment of ochratoxin A exposure risk from the consumption of coffee beans in Phnom Penh, Cambodia. Food Addit. Contam. Part B 2022, 15, 71–77. [Google Scholar] [CrossRef]
- Vita, V.; Franchino, C.; Iammarino, M.; De Pace, R. Aflatoxins contamination in nuts for direct human consumption: Analytical findings from three years of official control in Italy. Int. J. Food Sci. Technol. 2022, 57, 7496–7504. [Google Scholar] [CrossRef]
- Singh, P.; Jaime, R.; Puckett, R.D.; Lake, J.; Papagelis, A.; Gabri, V.M.; Michailides, T.J. Ochratoxin A contamination of california pistachios and identification of causal agents. Plant Dis. 2024, 108, 1591–1601. [Google Scholar] [CrossRef]
- Kulahi, A.; Kabak, B. A preliminary assessment of dietary exposure of ochratoxin A in Central Anatolia Region, Turkey. Mycotoxin Res. 2020, 36, 327–337. [Google Scholar] [CrossRef]
- Alsharif, A.M.; Choo, Y.-M.; Tan, G.-H. Detection of five mycotoxins in different food matrices in the malaysian market by using validated liquid chromatography electrospray ionization triple quadrupole mass spectrometry. Toxins 2019, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Ngolong Ngea, G.L.; Qian, X.; Yang, Q.; Dhanasekaran, S.; Ianiri, G.; Ballester, A.R.; Zhang, X.; Castoria, R.; Zhang, H. Securing fruit production: Opportunities from the elucidation of the molecular mechanisms of postharvest fungal infections. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2508–2533. [Google Scholar] [CrossRef] [PubMed]
- Sanzani, S.M.; Reverberi, M.; Geisen, R. Mycotoxins in harvested fruits and vegetables: Insights in producing fungi, biological role, conducive conditions, and tools to manage postharvest contamination. Postharvest Biol. Technol. 2016, 122, 95–105. [Google Scholar] [CrossRef]
- Nan, M.; Xue, H.; Bi, Y. Contamination, detection and control of mycotoxins in fruits and vegetables. Toxins 2022, 14, 309. [Google Scholar] [CrossRef] [PubMed]
- Al Riachy, R.; Strub, C.; Durand, N.; Guibert, B.; Guichard, H.; Constancias, F.; Chochois, V.; Lopez-Lauri, F.; Fontana, A.; Schorr-Galindo, S. Microbiome status of cider-apples, from orchard to processing, with a special focus on Penicillium expansum occurrence and patulin contamination. J. Fungi 2021, 7, 244. [Google Scholar] [CrossRef] [PubMed]
- Sanzani, S.M.; Gallone, T.; Garganese, F.; Caruso, A.G.; Amenduni, M.; Ippolito, A. Contamination of fresh and dried tomato by Alternaria toxins in southern Italy. Food Addit. Contam. Part A 2019, 36, 789–799. [Google Scholar] [CrossRef]
- El Jai, A.; Juan, C.; Juan-García, A.; Mañes, J.; Zinedine, A. Multi-mycotoxin contamination of green tea infusion and dietary exposure assessment in Moroccan population. Food Res. Int. 2021, 140, 109958. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.J.; Iamanaka, B.T.; Fungaro, M.H.P.; Taniwaki, M.H. Aflatoxins in sugarcane production chain: What could be the source? Curr. Opin. Food Sci. 2019, 29, 94–98. [Google Scholar] [CrossRef]
- Fusco, V.; Chieffi, D.; Fanelli, F.; Logrieco, A.F.; Cho, G.S.; Kabisch, J.; Böhnlein, C.; Franz, C. Microbial quality and safety of milk and milk products in the 21st century. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2013–2049. [Google Scholar] [CrossRef]
- Xu, N.; Xiao, Y.P.; Xie, Q.G.; Li, Y.; Ye, J.A.; Ren, D. Occurrence of aflatoxin B1 in total mixed rations and aflatoxin M1 in raw and commercial dairy milk in northern China during winter season. Food Control 2021, 124, 107916. [Google Scholar] [CrossRef]
- Muaz, K.; Riaz, M.; Oliveira, C.A.F.d.; Akhtar, S.; Ali, S.W.; Nadeem, H.; Park, S.; Balasubramanian, B. Aflatoxin M1 in milk and dairy products: Global occurrence and potential decontamination strategies. Toxin Rev. 2022, 41, 588–605. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, Y.-C.; Bai, X.-L.; Liu, Y.-M.; Wu, G.-F.; Yang, F.-S.; Liao, X. Multi-mycotoxins analysis in liquid milk by UHPLC-Q-Exactive HRMS after magnetic solid-phase extraction based on PEGylated multi-walled carbon nanotubes. Food Chem. 2020, 305, 125429. [Google Scholar] [CrossRef] [PubMed]
- Daou, R.; Afif, C.; Joubrane, K.; Khabbaz, L.R.; Maroun, R.; Ismail, A.; Khoury, A.E. Occurrence of aflatoxin M1 in raw, pasteurized, UHT cows’ milk, and dairy products in Lebanon. Food Control 2020, 111, 107055. [Google Scholar] [CrossRef]
- Cammilleri, G.; Graci, S.; Collura, R.; Buscemi, M.D.; Vella, A.; Macaluso, A.; Giaccone, V.; Giangrosso, G.; Cicero, A.; Lo Dico, G.M.; et al. Aflatoxin M1 in cow, sheep, and donkey milk produced in Sicily, Southern Italy. Mycotoxin Res. 2019, 35, 47–53. [Google Scholar] [CrossRef]
- Xiong, J.; Wen, D.; Zhou, H.; Chen, R.; Wang, H.; Wang, C.; Wu, Z.; Qiu, Y.; Wu, L. Occurrence of aflatoxin M1 in yogurt and milk in central-eastern China and the risk of exposure in milk consumers. Food Control 2022, 137, 108928. [Google Scholar] [CrossRef]
- Hattimare, D.; Shakya, S.; Patyal, A.; Chandrakar, C.; Kumar, A. Occurrence and exposure assessment of Aflatoxin M1 in milk and milk products in India. J. Food Sci. Technol. 2022, 59, 2460–2468. [Google Scholar] [CrossRef] [PubMed]
- Marimón Sibaja, K.V.; Garcia, S.D.O.; Nogueira, W.V.; de Oliveira, F.K.; Badiale-Furlong, E.; Garda-Buffon, J. Dietary exposure assessment of aflatoxin M1 in milk and dairy products of Latin America. Food Rev. Int. 2022, 38, 669–682. [Google Scholar] [CrossRef]
- Sen, Y.; Onal-Ulusoy, B.; Mutlu, M. Detoxification of hazelnuts by different cold plasmas and gamma irradiation treatments. Innov. Food Sci. Emerg. Technol. 2019, 54, 252–259. [Google Scholar] [CrossRef]
- Hassanpour, M.; Rezaie, M.R.; Baghizadeh, A. Practical analysis of aflatoxin M1 reduction in pasteurized Milk using low dose gamma irradiation. J. Environ. Health Sci. Eng. 2019, 17, 863–872. [Google Scholar] [CrossRef]
- Ben Amara, A.; Mehrez, A.; Ragoubi, C.; Romero-González, R.; Garrido Frenich, A.; Landoulsi, A.; Maatouk, I. Fungal mycotoxins reduction by gamma irradiation in naturally contaminated sorghum. J. Food Process. Preserv. 2022, 46, e16345. [Google Scholar] [CrossRef]
- Patil, H.; Shah, N.; Hajare, S.; Gautam, S.; Kumar, G. Combination of microwave and gamma irradiation for reduction of aflatoxin B1 and microbiological contamination in peanuts (Arachis hypogaea L.). World Mycotoxin J. 2019, 12, 269–280. [Google Scholar] [CrossRef]
- Shanakhat, H.; Sorrentino, A.; Raiola, A.; Reverberi, M.; Salustri, M.; Masi, P.; Cavella, S. Technological properties of durum wheat semolina treated by heating and UV irradiation for reduction of mycotoxin content. J. Food Process. Eng. 2019, 43, e13006. [Google Scholar] [CrossRef]
- Udovicki, B.; Stankovic, S.; Tomic, N.; Djekic, I.; Smigic, N.; Trifunovic, B.S.; Milicevic, D.; Rajkovic, A. Evaluation of ultraviolet irradiation effects on Aspergillus flavus and Aflatoxin B1 in maize and peanut using innovative vibrating decontamination equipment. Food Control 2022, 134, 108691. [Google Scholar] [CrossRef]
- Jiang, N.; Li, Z.; Wang, L.; Li, H.; Zhu, X.; Feng, X.; Wang, M. Effects of ultraviolet-c treatment on growth and mycotoxin production by Alternaria strains isolated from tomato fruits. Int. J. Food Microbiol. 2019, 311, 108333. [Google Scholar] [CrossRef] [PubMed]
- Kurup, A.H.; Patras, A.; Pendyala, B.; Vergne, M.J.; Bansode, R.R. Evaluation of ultraviolet-light (UV-A) emitting diodes technology on the reduction of spiked aflatoxin B1 and aflatoxin M1 in whole milk. Food Bioprocess Technol. 2022, 15, 165–176. [Google Scholar] [CrossRef]
- Mo, Z.M.; Chen, N.Z.; Ning, R.; Wang, J.; Wang, H.B.; Wei, L.M. Degradation of aflatoxin B1 in peanut oil by ultraviolet LED cold-light technology. China Oils Fats 2019, 44, 83–88. [Google Scholar]
- Nguyen, T.; Palmer, J.; Loo, T.; Shilton, A.; Petcu, M.; Newson, H.L.; Flint, S. Investigation of UV light treatment (254 nm) on the reduction of aflatoxin M1 in skim milk and degradation products after treatment. Food Chem. 2022, 390, 133165. [Google Scholar] [CrossRef] [PubMed]
- Nicolau-Lapeña, I.; Rodríguez-Bencomo, J.J.; Colás-Medà, P.; Viñas, I.; Sanchis, V.; Alegre, I. Ultraviolet applications to control patulin produced by Penicillium expansum CMP-1 in apple products and study of further patulin degradation products formation and toxicity. Food Bioprocess Technol. 2023, 16, 804–823. [Google Scholar] [CrossRef]
- Woldemariam, H.W.; Kießling, M.; Emire, S.A.; Teshome, P.G.; Töpfl, S.; Aganovic, K. Influence of electron beam treatment on naturally contaminated red pepper (Capsicum annuum L.) powder: Kinetics of microbial inactivation and physicochemical quality changes. Innov. Food Sci. Emerg. Technol. 2021, 67, 102588. [Google Scholar] [CrossRef]
- Mohamed, A.B.; Chavez, R.A.; Wagacha, M.J.; Mutegi, C.K.; Muthomi, J.W.; Pillai, S.D.; Stasiewicz, M.J. Efficacy of electron beam irradiation in reduction of mycotoxin-producing fungi, aflatoxin, and fumonisin, in naturally contaminated maize slurry. Toxicon X 2022, 16, 100141. [Google Scholar] [CrossRef]
- Alkadi, H.; Altal, J. Effect of microwave oven processing treatments on reduction of Aflatoxin B1 and Ochratoxin A in maize flour. Eur. J. Med. Chem. 2019, 10, 224–227. [Google Scholar] [CrossRef]
- Jalili, M.S.; Selamat, J.; Rashidi, L. Effect of thermal processing and traditional flavouring mixture on mycotoxin reduction in pistachio. World Mycotoxin J. 2020, 13, 381–389. [Google Scholar] [CrossRef]
- Chen, D.; Chen, P.; Cheng, Y.; Peng, P.; Liu, J.; Ma, Y.; Liu, Y.; Ruan, R. Deoxynivalenol decontamination in raw and germinating barley treated by plasma-activated water and intense pulsed light. Food Bioprocess Technol. 2019, 12, 246–254. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Cai, R.; Ge, Q.; Zhao, Z.; Yue, T.; Yuan, Y.; Gao, Z.; Wang, Z. Detoxification of Ochratoxin A by pulsed light in grape juice and evaluation of its degradation products and safety. Innov. Food Sci. Emerg. Technol. 2022, 78, 103024. [Google Scholar] [CrossRef]
- Rodríguez-Bencomo, J.J.; Sanchis, V.; Viñas, I.; Martín-Belloso, O.; Soliva-Fortuny, R. Formation of patulin-glutathione conjugates induced by pulsed light: A tentative strategy for patulin degradation in apple juices. Food Chem. 2020, 315, 126283. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, R.; Fu, C.; Qi, L.; Yuan, Y.; Yue, T.; Ge, Q.; Zhao, Z.; Wang, Z. Degradation of patulin in apple juice by pulsed light and its effect on the quality. Food Bioprocess Technol. 2023, 16, 870–880. [Google Scholar] [CrossRef]
- Qi, L.; Ma, Y.; Cai, R.; Li, Y.; Wang, R.; Yue, T.; Yuan, Y.; Gao, Z.; Wang, Z. Degradation of aflatoxins in apple juice by pulsed light and the analysis of their degradation products. Food Control 2023, 148, 109648. [Google Scholar] [CrossRef]
- Bulut, N.; Atmaca, B.; Akdemir Evrendilek, G.; Uzuner, S. Potential of pulsed electric field to control Aspergillus parasiticus, aflatoxin and mutagenicity levels: Sesame seed quality. J. Food Saf. 2020, 40, e12855. [Google Scholar] [CrossRef]
- Pallarés, N.; Barba, F.J.; Berrada, H.; Tolosa, J.; Ferrer, E. Pulsed electric fields (PEF) to mitigate emerging mycotoxins in juices and smoothies. Appl. Sci. 2020, 10, 698. [Google Scholar] [CrossRef]
- Pallarés, N.; Berrada, H.; Tolosa, J.; Ferrer, E. Effect of high hydrostatic pressure (HPP) and pulsed electric field (PEF) technologies on reduction of aflatoxins in fruit juices. LWT 2021, 142, 111000. [Google Scholar] [CrossRef]
- Akdemir Evrendilek, G.; Bulut, N.; Atmaca, B.; Uzuner, S. Prediction of Aspergillus parasiticus inhibition and aflatoxin mitigation in red pepper flakes treated by pulsed electric field treatment using machine learning and neural networks. Food Res. Int. 2022, 162, 111954. [Google Scholar] [CrossRef]
- Stranska, M.; Prusova, N.; Behner, A.; Dzuman, Z.; Lazarek, M.; Tobolkova, A.; Chrpova, J.; Hajslova, J. Influence of pulsed electric field treatment on the fate of Fusarium and Alternaria mycotoxins present in malting barley. Food Control 2023, 145, 109440. [Google Scholar] [CrossRef]
- Kiš, M.; Milošević, S.; Vulić, A.; Herceg, Z.; Vukušić, T.; Pleadin, J. Efficacy of low pressure DBD plasma in the reduction of T-2 and HT-2 toxin in oat flour. Food Chem. 2020, 316, 126372. [Google Scholar] [CrossRef]
- Zhang, M.; Ye, Z.; Xing, C.; Chen, H.; Zhang, J.; Yan, W. Degradation of deoxynivalenol in wheat by double dielectric barrier discharge cold plasma: Identification and pathway of degradation products. J. Sci. Food Agric. 2023, 103, 2347–2356. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; He, Z.; Ma, C.; Li, W.; Wang, J.; Lin, F.; Liu, X.; Li, L. Evaluation of cold plasma for decontamination of molds and mycotoxins in rice grain. Food Chem. 2023, 402, 134159. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Palmer, J.; Phan, N.; Shi, H.; Keener, K.; Flint, S. Control of aflatoxin M1 in skim milk by high voltage atmospheric cold plasma. Food Chem. 2022, 386, 132814. [Google Scholar] [CrossRef]
- Torlak, E. Use of gaseous ozone for reduction of ochratoxin A and fungal populations on sultanas. Aust. J. Grape Wine Res. 2019, 25, 25–29. [Google Scholar] [CrossRef]
- Alexandre, A.P.S.; Castanha, N.; Costa, N.S.; Santos, A.S.; Badiale-Furlong, E.; Augusto, P.E.D.; Calori-Domingues, M.A. Ozone technology to reduce zearalenone contamination in whole maize flour: Degradation kinetics and impact on quality. J. Sci. Food Agric. 2019, 99, 6814–6821. [Google Scholar] [CrossRef] [PubMed]
- da Luz, S.R.; Almeida Villanova, F.; Tuchtenhagen Rockembach, C.; Dietrich Ferreira, C.; José Dallagnol, L.; Luis Fernandes Monks, J.; de Oliveira, M. Reduced of mycotoxin levels in parboiled rice by using ozone and its effects on technological and chemical properties. Food Chem. 2022, 372, 131174. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.F.; Faroni, L.R.D.A.; Pimentel, M.A.G.; Prates, L.H.F.; Heleno, F.F.; De Alencar, E.R. Ozone as a fungicidal and detoxifying agent to maize contaminated with fumonisins. Ozone Sci. Eng. 2022, 44, 38–49. [Google Scholar] [CrossRef]
- Horikiri, S.; Harada, M.; Asada, R.; Tsuchido, T.; Furuta, M. Gamma-irradiated Aspergillus conidia show a growth curve with a reproductive death phase. J. Radiat. Res. 2024, 65, 28–35. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, Y.; Ai, Z.; Pandiselvam, R.; Guo, J.; Kothakota, A.; Liu, Y. Current physical techniques for the degradation of aflatoxins in food and feed: Safety evaluation methods, degradation mechanisms and products. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4030–4052. [Google Scholar] [CrossRef] [PubMed]
- Calado, T.; Venâncio, A.; Abrunhosa, L. Irradiation for Mold and Mycotoxin Control: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1049–1061. [Google Scholar] [CrossRef]
- Calado, T.; Fernández-Cruz, M.L.; Cabo Verde, S.; Venâncio, A.; Abrunhosa, L. Gamma irradiation effects on ochratoxin A: Degradation, cytotoxicity and application in food. Food Chem. 2018, 240, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Domijan, A.-M.; Marjanović Čermak, A.M.; Vulić, A.; Tartaro Bujak, I.; Pavičić, I.; Pleadin, J.; Markov, K.; Mihaljević, B. Cytotoxicity of gamma irradiated aflatoxin B1 and ochratoxin A. J. Environ. Sci. Health Part B 2019, 54, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.; Uthairatanakij, A.; Laohakunjit, N.; Jitareerat, P.; Rattanakreetakul, C.; Boonsirichai, K.; Kaisangsri, N. Oil characterization and aflatoxin profile of peanut kernel subjected to gamma irradiation. Int. J. Food Eng. 2020, 6, 1–5. [Google Scholar] [CrossRef]
- Calado, T.; Abrunhosa, L.; Cabo Verde, S.; Alté, L.; Venâncio, A.; Fernández-Cruz, M.L. Effect of gamma-radiation on zearalenone-degradation, cytotoxicity and estrogenicity. Foods 2020, 9, 1687. [Google Scholar] [CrossRef] [PubMed]
- Jalili, M.; Jinap, S.; Noranizan, A. Effect of gamma radiation on reduction of mycotoxins in black pepper. Food Control 2010, 21, 1388–1393. [Google Scholar] [CrossRef]
- Fanaro, G.B.; Duarte, R.C.; Araújo, M.M.; Purgatto, E.; Villavicencio, A.L.C.H. Evaluation of γ-radiation on green tea odor volatiles. Radiat. Phys. Chem. 2011, 80, 85–88. [Google Scholar] [CrossRef]
- Sun, P.W.; Liu, Y.N.; Gao, H.; Fan, X.Z.; Yin, Z.M.; Shen, W.Y.; Wang, Z.; Chen, S.L.; Shi, D.F. The effects of γ-ray irradiation on edible quality of rice and physicochemical properties of rice starch. Food Sci. 2023, 1–17. [Google Scholar]
- Bliznyuk, U.A.; Avdyukhina, V.M.; Borshchegovskaya, P.Y.; Bolotnik, T.A.; Ipatova, V.S.; Rodin, I.A.; Ikhalainen, Y.A.; Studenikin, F.R.; Chernyaev, A.P.; Shinkarev, O.V.; et al. Determination of chemical and microbiological characteristics of meat products treated by radiation. Inorg. Mater. 2022, 58, 1422–1428. [Google Scholar] [CrossRef]
- Jin, R.; Li, M.M.; Wan, X.L.; Liu, Y.X.; Guan, R.Q.; Wang, R.H.; Bian, K. Advances in physical degradation of deoxynivalenol in cereals. Food Ferment. Ind. 2024, 1–10. [Google Scholar] [CrossRef]
- He, J.; Evans, N.M.; Liu, H.; Zhu, Y.; Zhou, T.; Shao, S. UV treatment for degradation of chemical contaminants in food: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1857–1886. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Wang, Y.-C. Mitigating fungal contamination of cereals: The efficacy of microplasma-based far-UVC lamps against Aspergillus flavus and Fusarium graminearum. Food Res. Int. 2024, 190, 114550. [Google Scholar] [CrossRef] [PubMed]
- Akhila, P.P.; Sunooj, K.V.; Aaliya, B.; Navaf, M.; Sudheesh, C.; Sabu, S.; Sasidharan, A.; Mir, S.A.; George, J.; Mousavi Khaneghah, A. Application of electromagnetic radiations for decontamination of fungi and mycotoxins in food products: A comprehensive review. Trends Food Sci. Technol. 2021, 114, 399–409. [Google Scholar] [CrossRef]
- Mao, J.; He, B.; Zhang, L.; Li, P.; Zhang, Q.; Ding, X.; Zhang, W. A structure identification and toxicity assessment of the degradation products of aflatoxin B1 in peanut oil under UV irradiation. Toxins 2016, 8, 332. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, F.; Liu, H.; Neugart, S. Nutritional and physiological effects of postharvest UV radiation on vegetables: A review. J. Agric. Food Chem. 2023, 71, 9951–9972. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.; Patras, A.; Pendyala, B.; Vergne, M.J.; Bansode, R.R. Performance of a UV-A LED system for degradation of aflatoxins B1 and M1 in pure water: Kinetics and cytotoxicity study. Sci. Rep. 2020, 10, 13473. [Google Scholar] [CrossRef]
- Byun, K.-H.; Park, S.Y.; Lee, D.U.; Chun, H.S.; Ha, S.-D. Effect of UV-C irradiation on inactivation of Aspergillus flavus and Aspergillus parasiticus and quality parameters of roasted coffee bean (Coffea arabica L.). Food Addit. Contam. Part A 2020, 37, 507–518. [Google Scholar] [CrossRef]
- Martin, N.; Carey, N.; Murphy, S.; Kent, D.; Bang, J.; Stubbs, T.; Wiedmann, M.; Dando, R. Exposure of fluid milk to LED light negatively affects consumer perception and alters underlying sensory properties. J. Dairy Sci. 2016, 99, 4309–4324. [Google Scholar] [CrossRef]
- Soro, A.B.; Shokri, S.; Nicolau-Lapeña, I.; Ekhlas, D.; Burgess, C.M.; Whyte, P.; Bolton, D.J.; Bourke, P.; Tiwari, B.K. Current challenges in the application of the UV-LED technology for food decontamination. Trends Food Sci. Technol. 2023, 131, 264–276. [Google Scholar] [CrossRef]
- Fan, L.; Liu, X.; Dong, X.; Dong, S.; Xiang, Q.; Bai, Y. Effects of UVC light-emitting diodes on microbial safety and quality attributes of raw tuna fillets. LWT 2021, 139, 110553. [Google Scholar] [CrossRef]
- Zhai, Y.; Tian, J.; Ping, R.; Yu, X.; Wang, Z.; Shen, R. Effects of UVC light-emitting diodes on inactivation of Escherichia coli O157:H7 and quality attributes of fresh-cut white pitaya. J. Food Meas. Charact. 2021, 15, 2637–2644. [Google Scholar] [CrossRef]
- Riganakos, K.A.; Karabagias, I.K.; Gertzou, I.; Stahl, M. Comparison of UV-C and thermal treatments for the preservation of carrot juice. Innov. Food Sci. Emerg. Technol. 2017, 42, 165–172. [Google Scholar] [CrossRef]
- Dong, T.; Gao, P.; Wang, H.Y.; Li, H.; Wang, D.; Chen, H. Research progress on the effects of electron beam irradiation on quality of fruits and vegetables. North. Hortic. 2020, 463, 133–138. [Google Scholar]
- Yousefi, M.; Mohammadi, M.A.; Khajavi, M.Z.; Ehsani, A.; Scholtz, V. Application of novel non-thermal physical technologies to degrade mycotoxins. J. Fungi 2021, 7, 395. [Google Scholar] [CrossRef]
- Rogovschi, V.D.; Aquino, S.; Zorzete, P.; Reis, T.A.; Villavicencio, A.L.C.H. Use of gamma radiation and electron beam treatment on decontamination of coconut agar medium used in the production of aflatoxins. In Proceedings of the 8th International Topical Meeting on Nuclear Applications and Utilization of Accelerators, ACCAPP’07, Pocatello, ID, USA, 29 July–2 August 2007; pp. 1026–1027. [Google Scholar]
- Liu, R.; Wang, R.; Lu, J.; Chang, M.; Jin, Q.; Du, Z.; Wang, S.; Li, Q.; Wang, X. Degradation of AFB1 in aqueous medium by electron beam irradiation: Kinetics, pathway and toxicology. Food Control 2016, 66, 151–157. [Google Scholar] [CrossRef]
- Mousavi Khaneghah, A.; Hashemi Moosavi, M.; Oliveira, C.A.F.; Vanin, F.; Sant’Ana, A.S. Electron beam irradiation to reduce the mycotoxin and microbial contaminations of cereal-based products: An overview. Food Chem. Toxicol. 2020, 143, 111557. [Google Scholar] [CrossRef]
- Li, K.; Pan, L.H.; Luo, X.H.; Wang, L.; Wang, R.; Du, Z.H.; Li, C.M.; Chen, Z.X. Degradation of zearalenone and deoxyniva-lenol in corn by electron beam irradiation. Food Ferment. Ind. 2019, 45, 73–78. [Google Scholar] [CrossRef]
- Luo, X.; Qi, L.; Liu, Y.; Wang, R.; Yang, D.; Li, K.; Wang, L.; Li, Y.; Zhang, Y.; Chen, Z. Effects of electron beam irradiation on zearalenone and ochratoxin A in naturally contaminated corn and corn quality parameters. Toxins 2017, 9, 84. [Google Scholar] [CrossRef]
- Luo, X.; Zhai, Y.; Qi, L.; Pan, L.; Wang, J.; Xing, J.; Wang, R.; Wang, L.; Zhang, Q.; Yang, K.; et al. Influences of electron beam irradiation on the physical and chemical properties of zearalenone- and ochratoxin A-contaminated corn and in vivo toxicity assessment. Foods 2020, 9, 376. [Google Scholar] [CrossRef]
- Liu, R.; Lu, M.; Wang, R.; Wang, S.; Chang, M.; Jin, Q.; Wang, X. Degradation of aflatoxin B1 in peanut meal by electron beam irradiation. Int. J. Food Prop. 2018, 21, 892–901. [Google Scholar] [CrossRef]
- Yang, K.; Li, K.; Pan, L.; Luo, X.; Xing, J.; Wang, J.; Wang, L.; Wang, R.; Zhai, Y.; Chen, Z. Effect of ozone and electron beam irradiation on degradation of zearalenone and ochratoxin A. Toxins 2020, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Castell-Perez, E.; Moreno, M.; Rodriguez, O.; Moreira, R.G. Electron beam irradiation treatment of cantaloupes: Effect on product quality. Food Sci. Technol. Int. 2004, 10, 383–390. [Google Scholar] [CrossRef]
- Abedi, A.-S.; Khaksar, R.; Ferdousi, R.; Komeilifanood, R.; Azadnia, E.; Eskandari, S. Influence of radiation processing of cooked beef sausage on its lipids. J. Am. Oil Chem. Soc. 2014, 91, 421–427. [Google Scholar] [CrossRef]
- Arshad, M.S.; Kwon, J.-H.; Ahmad, R.S.; Ameer, K.; Ahmad, S.; Jo, Y. Influence of E-beam irradiation on microbiological and physicochemical properties and fatty acid profile of frozen duck meat. Food Sci. Nutr. 2020, 8, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Pankaj, S.K.; Shi, H.; Keener, K.M. A review of novel physical and chemical decontamination technologies for aflatoxin in food. Trends Food Sci. Technol. 2018, 71, 73–83. [Google Scholar] [CrossRef]
- Chandravarnan, P.; Agyei, D.; Ali, A. Green and sustainable technologies for the decontamination of fungi and mycotoxins in rice: A review. Trends Food Sci. Technol. 2022, 124, 278–295. [Google Scholar] [CrossRef]
- Guo, C.; Wang, Y.; Luan, D. Study the synergism of microwave thermal and non-thermal effects on microbial inactivation and fatty acid quality of salmon fillet during pasteurization process. LWT 2021, 152, 112280. [Google Scholar] [CrossRef]
- Cao, Y.R.; Wu, R.Z.; Rao, J.Y.; Yang, M.J.; Han, G.Q. Advances in the application of microwave and combined sterilization technology in foods. J. Microbiol. 2023, 43, 113–120. [Google Scholar]
- Cao, H.; Wang, X.; Liu, J.; Sun, Z.; Yu, Z.; Battino, M.; El-Seedi, H.; Guan, X. Mechanistic insights into the changes of enzyme activity in food processing under microwave irradiation. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2465–2487. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Liu, Y.; Guan, E.; Bian, K. Degradation of aflatoxin B1 by water-assisted microwave irradiation: Kinetics, products, and pathways. LWT 2021, 152, 112310. [Google Scholar] [CrossRef]
- Jin, Z.Q.; Wang, S.X. Synergistic effects of microwave, ultraviolet and ozone combination on mold spores and aflatoxin. J. Northwest A F Univ. (Nat. Sci. Ed.) 2018, 46, 147–154. [Google Scholar]
- Knox, O.G.G.; McHugh, M.J.; Fountaine, J.M.; Havis, N.D. Effects of microwaves on fungal pathogens of wheat seed. Crop Prot. 2013, 50, 12–16. [Google Scholar] [CrossRef]
- Popelářová, E.; Vlková, E.; Švejstil, R.; Kouřimská, L. The effect of microwave irradiation on the representation and growth of moulds in nuts and almonds. Foods 2022, 11, 221. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.C.; Li, S.F. Multi-feed microwave heating temperature control system based on numerical simulation. IEEE J. Microw. 2019, 35, 92–96. [Google Scholar] [CrossRef]
- Zheng, X.Z.; Zhang, Y.H.; Liu, C.H.; Shen, L.Y.; Zhao, X.L.; Xue, L.L. Research progress in the microwave technologies for food stuffs and agricultural products. TCSAE 2024, 40, 14–28. [Google Scholar]
- Dong, W.; Cheng, K.; Hu, R.; Chu, Z.; Zhao, J.; Long, Y. Effect of microwave vacuum drying on the drying characteristics, color, microstructure, and antioxidant activity of green coffee beans. Molecules 2018, 23, 1146. [Google Scholar] [CrossRef]
- Li, R.; Lin, H.B. Research progress on application of new food sterilization technology. Modern Food 2022, 28, 63–67. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Yang, K. Research progress on application of pulsed light in food sterilization. J. Chin. Inst. Food Sci. Technol. 2021, 21, 397–408. [Google Scholar] [CrossRef]
- Deng, L.Z.; Sutar, P.P.; Mujumdar, A.S.; Tao, Y.; Pan, Z.; Liu, Y.H.; Xiao, H.W. Thermal decontamination technologies for microorganisms and mycotoxins in low-moisture foods. Annu. Rev. Food Sci. Technol. 2021, 12, 287–305. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Zhou, A.; Neng, J.; Wu, D.; Li Shen, X.; Lou, X.; Yang, K. Transcriptomic analysis reveals the inhibition mechanism of pulsed light on fungal growth and ochratoxin A biosynthesis in Aspergillus carbonarius. Food Res. Int. 2023, 165, 112501. [Google Scholar] [CrossRef] [PubMed]
- Abuagela, M.O.; Iqdiam, B.M.; Mostafa, H.; Gu, L.; Smith, M.E.; Sarnoski, P.J. Assessing pulsed light treatment on the reduction of aflatoxins in peanuts with and without skin. Int. J. Food Sci. Technol. 2018, 53, 2567–2575. [Google Scholar] [CrossRef]
- Zenklusen, M.H.; Coronel, M.B.; Castro, M.Á.; Alzamora, S.M.; González, H.H.L. Inactivation of Aspergillus carbonarius and Aspergillus flavus in malting barley by pulsed light and impact on germination capacity and microstructure. Innov. Food Sci. Emerg. Technol. 2018, 45, 161–168. [Google Scholar] [CrossRef]
- Romero Bernal, A.R.; Contigiani, E.V.; González, H.H.L.; Alzamora, S.M.; Gómez, P.L.; Raffellini, S. Botrytis cinerea response to pulsed light: Cultivability, physiological state, ultrastructure and growth ability on strawberry fruit. Int. J. Food Microbiol. 2019, 309, 108311. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Mahoney, N.E.; Pan, Z.; Khir, R.; Wu, B.; Ma, H.; Zhao, L. Effectiveness of pulsed light treatment for degradation and detoxification of aflatoxin B-1 and B-2 in rough rice and rice bran. Food Control 2016, 59, 461–467. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Shang, J.; Wu, D.; Zhou, A.; Cai, M.; Gao, H.; Yang, K. Pulsed light reduces postharvest losses of Chinese bayberries by affecting fungal microbiota during cold storage. Food Control 2023, 146, 109524. [Google Scholar] [CrossRef]
- Mahendran, R.; Ramanan, K.R.; Barba, F.J.; Lorenzo, J.M.; López-Fernández, O.; Munekata, P.E.S.; Roohinejad, S.; Sant’Ana, A.S.; Tiwari, B.K. Recent advances in the application of pulsed light processing for improving food safety and increasing shelf life. Trends Food Sci. Technol. 2019, 88, 67–79. [Google Scholar] [CrossRef]
- Tomasevic, I.; Rajkovic, A. The sensory quality of meat, game, poultry, seafood and meat products as affected by intense light pulses: A systematic review. Procedia Food Sci. 2015, 5, 285–288. [Google Scholar] [CrossRef]
- Adebo, O.A.; Molelekoa, T.; Makhuvele, R.; Adebiyi, J.A.; Oyedeji, A.B.; Gbashi, S.; Adefisoye, M.A.; Ogundele, O.M.; Njobeh, P.B. A review on novel non-thermal food processing techniques for mycotoxin reduction. Int. J. Food Sci. Technol. 2021, 56, 13–27. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, B.; Yang, R.; Zhao, W. Recent developments in the preservation of raw fresh food by pulsed electric field. Food Rev. Int. 2022, 38, 247–265. [Google Scholar] [CrossRef]
- Kulawik, P.; Rathod, N.B.; Ozogul, Y.; Ozogul, F.; Zhang, W. Recent developments in the use of cold plasma, high hydrostatic pressure, and pulsed electric fields on microorganisms and viruses in seafood. Crit. Rev. Food Sci. Nutr. 2023, 63, 9716–9730. [Google Scholar] [CrossRef] [PubMed]
- Demir, E.; Tappi, S.; Dymek, K.; Rocculi, P.; Gómez Galindo, F. Reversible electroporation caused by pulsed electric field—Opportunities and challenges for the food sector. Trends Food Sci. Technol. 2023, 139, 104120. [Google Scholar] [CrossRef]
- Yuan, J.L.; Lin, M.; Zhou, J.; Jiao, B.N.; Ma, Y.Q. Application and research progress of pulsed electric field in fruit and vegeta-ble juice processing. Food Ferment. Ind. 2023, 1–10. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in occurrence, importance, and mycotoxin control strategies: Prevention and detoxification in foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Chizoba Ekezie, F.-G.; Sun, D.-W.; Cheng, J.-H. A review on recent advances in cold plasma technology for the food industry: Current applications and future trends. Trends Food Sci. Technol. 2017, 69, 46–58. [Google Scholar] [CrossRef]
- Devi, Y.; Thirumdas, R.; Sarangapani, C.; Deshmukh, R.R.; Annapure, U.S. Influence of cold plasma on fungal growth and aflatoxins production on groundnuts. Food Control 2017, 77, 187–191. [Google Scholar] [CrossRef]
- Ten Bosch, L.; Pfohl, K.; Avramidis, G.; Wieneke, S.; Viöl, W.; Karlovsky, P. Plasma-based degradation of mycotoxins produced by Fusarium, Aspergillus and Alternaria species. Toxins 2017, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Gavahian, M.; Cullen, P.J. Cold plasma as an emerging technique for mycotoxin-free food: Efficacy, mechanisms, and trends. Food Rev. Int. 2020, 36, 193–214. [Google Scholar] [CrossRef]
- Hojnik, N.; Modic, M.; Walsh, J.L.; Žigon, D.; Javornik, U.; Plavec, J.; Žegura, B.; Filipič, M.; Cvelbar, U. Unravelling the pathways of air plasma induced aflatoxin B1 degradation and detoxification. J. Hazard. Mater. 2021, 403, 123593. [Google Scholar] [CrossRef]
- Nikmaram, N.; Brückner, L.; Cramer, B.; Humpf, H.-U.; Keener, K. Degradation products of aflatoxin M1 (AFM1) formed by high voltage atmospheric cold plasma (HVACP) treatment. Toxicon 2023, 230, 107160. [Google Scholar] [CrossRef]
- Nishimwe, K.; Agbemafle, I.; Reddy, M.B.; Keener, K.; Maier, D.E. Cytotoxicity assessment of Aflatoxin B1 after high voltage atmospheric cold plasma treatment. Toxicon 2021, 194, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Ott, L.C.; Appleton, H.J.; Shi, H.; Keener, K.; Mellata, M. High voltage atmospheric cold plasma treatment inactivates Aspergillus flavus spores and deoxynivalenol toxin. Food Microbiol. 2021, 95, 103669. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ileleji, K.; Stroshine, R.L.; Keener, K.; Jensen, J.L. Reduction of aflatoxin in corn by high voltage atmospheric cold plasma. Food Bioprocess Technol. 2017, 10, 1042–1052. [Google Scholar] [CrossRef]
- Hojnik, N.; Modic, M.; Tavčar-Kalcher, G.; Babič, J.; Walsh, J.L.; Cvelbar, U. Mycotoxin decontamination efficacy of atmospheric pressure air plasma. Toxins 2019, 11, 219. [Google Scholar] [CrossRef]
- Du, Y.; Mi, S.; Wang, H.; Yang, F.; Yu, H.; Xie, Y.; Guo, Y.; Cheng, Y.; Yao, W. Inactivation mechanism of Alternaria alternata by dielectric barrier discharge plasma and its quality control on fresh wolfberries. Food Control 2023, 148, 109620. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Shang, H.; Ma, R.; Zhu, Y.; Yang, X.; Ju, S.; Zhao, W.; Sun, H.; Zhuang, J.; et al. Effective inhibition of fungal growth, deoxynivalenol biosynthesis and pathogenicity in cereal pathogen Fusarium spp. by cold atmospheric plasma. Chem. Eng. J. 2022, 437, 135307. [Google Scholar] [CrossRef]
- Wielogorska, E.; Ahmed, Y.; Meneely, J.; Graham, W.G.; Elliott, C.T.; Gilmore, B.F. A holistic study to understand the detoxification of mycotoxins in maize and impact on its molecular integrity using cold atmospheric plasma treatment. Food Chem. 2019, 301, 125281. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.I.; Kim, H.-J.; Park, S.; Alahakoon, A.U.; Kim, K.; Choe, W.; Jo, C. Evaluation of pathogen inactivation on sliced cheese induced by encapsulated atmospheric pressure dielectric barrier discharge plasma. Food Microbiol. 2015, 46, 46–50. [Google Scholar] [CrossRef]
- Liu, C.C.; Wang, J.M.; Chen, G.; Sang, X.H.; Cai, Z.C. Research progress of lipids oxidation of animal-derived food by cold plasma. J. Food Saf. Qual. 2022, 13, 6535–6544. [Google Scholar] [CrossRef]
- Kim, H.-J.; Yong, H.I.; Park, S.; Choe, W.; Jo, C. Effects of dielectric barrier discharge plasma on pathogen inactivation and the physicochemical and sensory characteristics of pork loin. Curr. Appl. Phys. 2013, 13, 1420–1425. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Kim, H.J.; Yong, H.I.; Park, S.; Kim, K.; Choe, W.; Jo, C. Flexible thin-layer dielectric barrier discharge plasma treatment of pork butt and beef loin: Effects on pathogen inactivation and meat-quality attributes. Food Microbiol. 2015, 46, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Drishya, C.; Yoha, K.S.; Perumal, A.B.; Moses, J.A.; Anandharamakrishnan, C.; Balasubramaniam, V.M. Impact of nonthermal food processing techniques on mycotoxins and their producing fungi. Int. J. Food Sci. Technol. 2022, 57, 2140–2148. [Google Scholar] [CrossRef]
- Brodowska, A.J.; Nowak, A.; Śmigielski, K. Ozone in the food industry: Principles of ozone treatment, mechanisms of action, and applications: An overview. Crit. Rev. Food Sci. Nutr. 2018, 58, 2176–2201. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.M.; de Alba, M.; Sun, D.W.; Tiwari, B. Principles and recent applications of novel non-thermal processing technologies for the fish industry-a review. Crit. Rev. Food Sci. Nutr. 2019, 59, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Yang, J.; Wang, Y.; Ding, G.; Guo, L.; Qin, J. Mechanisms and transformed products of aflatoxin B1 degradation under multiple treatments: A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 2263–2275. [Google Scholar] [CrossRef] [PubMed]
- Premjit, Y.; Sruthi, N.U.; Pandiselvam, R.; Kothakota, A. Aqueous ozone: Chemistry, physiochemical properties, microbial inactivation, factors influencing antimicrobial effectiveness, and application in food. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1054–1085. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.-Z.; Tao, Y.; Mujumdar, A.S.; Pan, Z.; Chen, C.; Yang, X.-H.; Liu, Z.-L.; Wang, H.; Xiao, H.-W. Recent advances in non-thermal decontamination technologies for microorganisms and mycotoxins in low-moisture foods. Trends Food Sci. Technol. 2020, 106, 104–112. [Google Scholar] [CrossRef]
- Guo, B.R.; Xie, Y.F.; Yu, H.; Yao, W.R. Progress in the removal of pesticide residues from fruits andvegetables by ozone technology. Sci. Technol. Food Ind. 2022, 43, 383–390. [Google Scholar] [CrossRef]
- Flores, P.; Hernández, V.; Fenoll, J.; Hellín, P. Pre-harvest application of ozonated water on broccoli crops: Effect on head quality. J. Food Compos. Anal. 2019, 83, 103260. [Google Scholar] [CrossRef]
- Diao, E.; Ren, D.; Liu, T.; Zhang, J.; Hu, W.; Hou, H. Ozone detoxification of patulin in aqueous solution and cytotoxic evaluation using human hepatic carcinoma cells. Toxicon 2018, 155, 21–26. [Google Scholar] [CrossRef]
- Afsah-Hejri, L.; Hajeb, P.; Ehsani, R.J. Application of ozone for degradation of mycotoxins in food: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1777–1808. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.M.; de Alencar, E.R.; Blum, L.E.B.; de Souza Ferreira, W.F.; Botelho, S.d.C.C.; Racanicci, A.M.C.; Santos Leandro, E.d.; Mendonça, M.A.; Moscon, E.S.; Bizerra, L.V.A.d.S.; et al. Ozonation of Brazil nuts: Decomposition kinetics, control of Aspergillus flavus and the effect on color and on raw oil quality. LWT 2020, 123, 109106. [Google Scholar] [CrossRef]
- Li, H.; Xiong, Z.; Gui, D.; Pan, Y.; Xu, M.; Guo, Y.; Leng, J.; Li, X. Effect of ozonation and UV irradiation on aflatoxin degradation of peanuts. J. Food Process. Preserv. 2019, 43, e13914. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Subhashini, S.; Banuu Priya, E.P.; Kothakota, A.; Ramesh, S.V.; Shahir, S. Ozone based food preservation: A promising green technology for enhanced food safety. Ozone: Sci. Eng. 2019, 41, 17–34. [Google Scholar] [CrossRef]
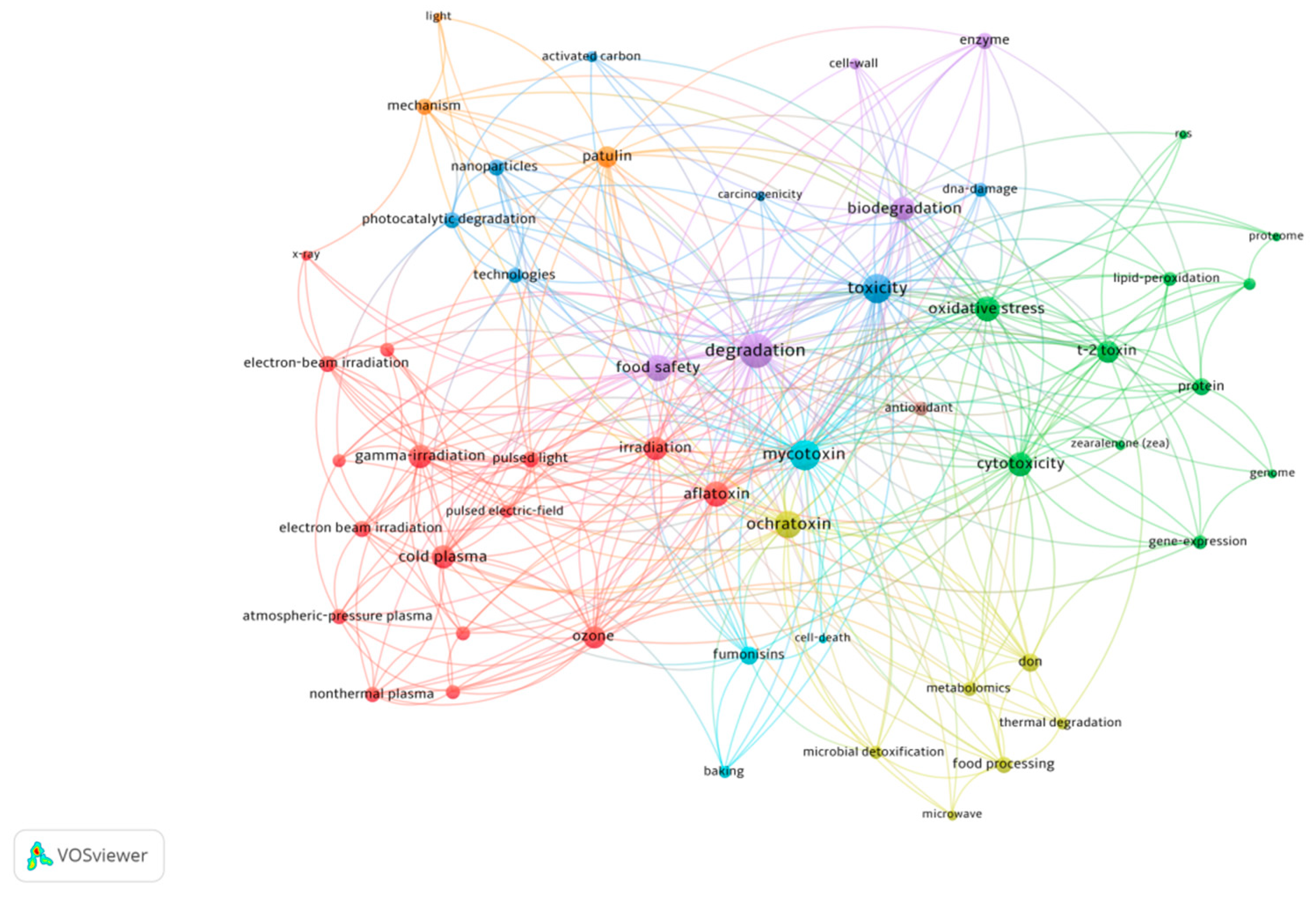
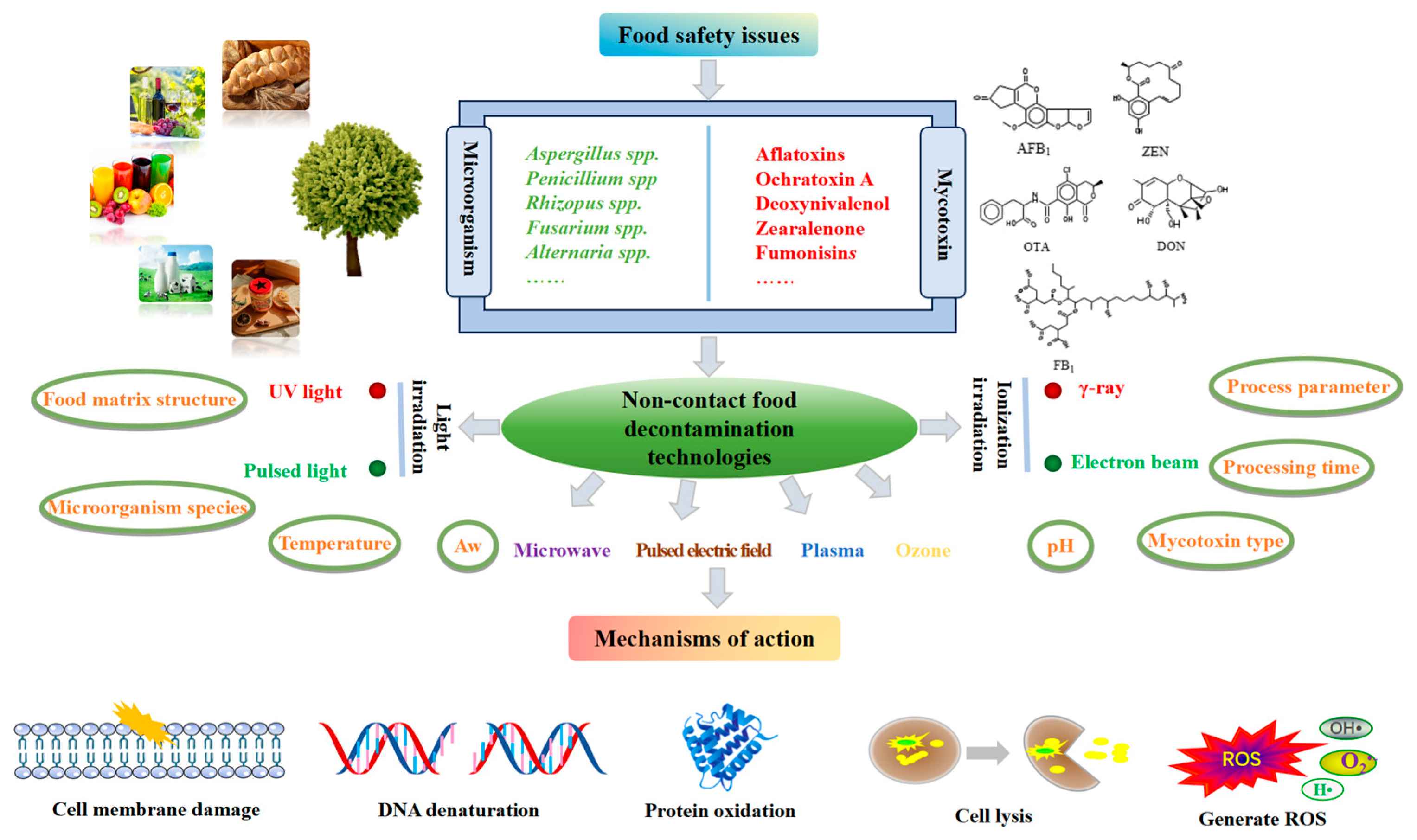

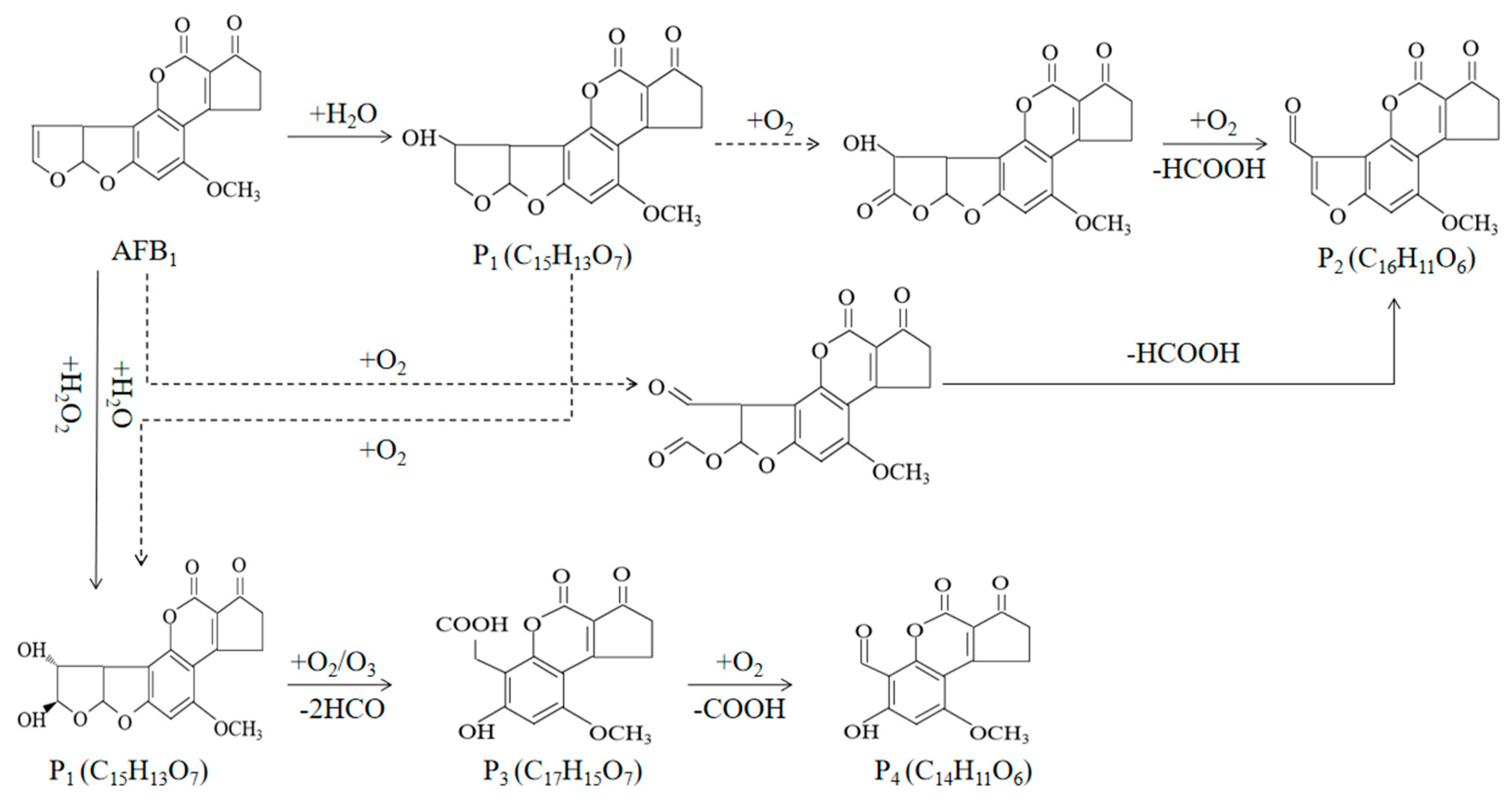
| Product | Type of Mycotoxin | Detection Rate (%) | Contamination Value (μg/kg) | Country | Reference |
|---|---|---|---|---|---|
| Corn | FB1 FB2 | 40.7 37.0 | average 1559.00 average 278.00 | Spain | [7] |
| AFB1 | 100.0 | 1.69~403.00 | Kenya | [8] | |
| FB1 T-2 ZEN | 96.7 100.0 23.3 | 289.00~4243.00 24.60~25.70 20.40~579.00 | Algeria | [9] | |
| Wheat | DON ZEN | 59.8 18.6 | ND~955.00 ND~300.00 | Romania | [10] |
| T-2 DON ZEN | 100.0 90.0 63.3 | 16.60~47.20 68.30~1363.00 9.60~295.00 | Algeria | [9] | |
| Rice | AFB1 | 4.8 | ND~6.28 | China | [11] |
| AFs OTA | 56.0 72.0 | 0.05~21.40 0.03~80.70 | Turkey | [12] | |
| OTA CIT | 6.3 13.3 | 8.00–25.00 49.00–92.00 | India | [13] | |
| Barley | DON AFB1 ZEN OTA T-2 | 44.4 1.4 2.8 8.3 8.3 | ND~6880.00 average 26.50 ND~962.00 ND~127.00 ND~56.80 | Canada | [14] |
| DON | 20.0 | 138.00~973.00 | Turkey | [15] | |
| Cereals | AFs | 72.0 | 0.18~25.93 | Ghana | [16] |
| DON | 56.4 | average 6.18 maximum 912.29 | China | [17] | |
| Sorghum | FUMs | 25.0 | 6.00–16.00 | Togo | [18] |
| AFs FUMs OTA | 100.0 50.0 72.0 | average 29.97 average 3269.80 average 37.50 | Niger State of Nigeria | [19] | |
| Beer | DON AFB1 | 80.0 60.0 | 0.87–10.60 8.44–11.82 | Spain | [20] |
| Product | Type of Mycotoxin | Detection Rate (%) | Contamination Value (μg/kg) | Country | Reference |
|---|---|---|---|---|---|
| Peanuts | AFs | 76.0 | average 37.94 | Uganda | [24] |
| AFB1 | 92.0~100.0 | average 6.37 | Tanzania | [25] | |
| AFB1 AFB2 AFG1 AFG2 | 13.3 | 1.90~6.50 1.80~2.90 9.60~23.80 5.50~13.20 | Morocco | [26] | |
| Coffee beans | AFB2 | 28.3 | 1.40~87.90 | Tunisia | [27] |
| AFG1 | 73.6 | 18.30~145.00 | |||
| AFG2 | 66.0 | 28.70~218.20 | |||
| OTA | 13.2 | 10.70~122.60 | |||
| ZEA | 51.0 | 25.40~231.70 | |||
| AOH | 22.6 | 109.70~321.00 | |||
| TENT | 98.0 | 11.20~286.00 | |||
| OTA | 7.5 | 0.19~1.12 | Cambodia | [28] | |
| Almond | AFB1 | 18.0 | 1.00~15.50 | Italy | [29] |
| AFs | 22.0 | 1.20~15.30 | |||
| Pistachios | OTA | 18.0 | >5.00 | California | [30] |
| OTA | 4.0 | 0.20~0.85 | Turkey | [31] | |
| AFB1 | 45.6 | 1.00~47.70 | Italy | [29] | |
| AFB1 AFB2 AFG1 AFG2 | 40.0 30.0 40.0 20.0 | 5.30~10.15 1.46~3.47 1.90~3.31 0.81~0.90 | Malaysia | [32] |
| Product | Type of Mycotoxin | Detection Rate (%) | Contamination Value (μg/kg or μg/L) | Country | Reference |
|---|---|---|---|---|---|
| Apple | PAT | 56.0 | 24.00~356.00 | France | [36] |
| Tomato | TeA | 100.0 | 11.00~4560.00 | Italy | [37] |
| Green tea | AFB2 AFG1 ZEN ENB AOH TENT | 2.0 2.0 35.0 2.0 40.0 1.0 | 3.66~7.40 1.35~1.60 14.32~45.80 0.10~0.30 1.70~5.90 0.31~4.60 | Morocco | [38] |
| Sugarcane juice | AFs | 22.2 | 0.50~6.50 | India | [39] |
| Wine | OTA AOH PAT | 47.0 52.0 32.0 | 0.57~2.28 0.61~26.86 15.35~88.24 | Spain | [20] |
| Cava and cider | OTA PAT | 26.0 40.0 | 0.77~2.44 14.73~41.93 | Spain | [20] |
| Product | Type of Mycotoxin | Detection Rate (%) | Contamination Value (μg/kg or μg/L) | Country | Reference |
|---|---|---|---|---|---|
| Liquid milk | AFM1 | 20.0 | 0.026~0.039 | China | [43] |
| Raw milk | AFM1 | 58.8 | 0.01~0.44 | Lebanon | [44] |
| AFM1 | 6.0 | 0.008~0.15 | Italy | [45] | |
| Pasteurized milk | AFM1 | 91.0 | 5.30~85.20 | China | [46] |
| AFM1 | 40.0 | 0.00~1.21 | India | [47] | |
| Yoghurt | AFM1 | 59.0 | 10.00~66.70 | China | [46] |
| AFM1 | 64.3 | 0.015~0.545 | Lebanon | [44] | |
| AFM1 | 30.0 | 0.00~0.30 | India | [47] | |
| UHT milk | AFM1 | 53.7 | 5.10~46.60 | China | [46] |
| AFM1 | 41.7 | 0.00~1.52 | India | [47] | |
| Milk | AFM1 | 67.0 | 0.001~23.10 | Latin America | [48] |
| Target Mycotoxin | Treated Sample | Treatment Parameters | Degradation Effect | References |
|---|---|---|---|---|
| Gamma ray irradiation | ||||
| AFB1 | Hazelnut | 10 kGy 10 min | AFB1: 47.0% | [49] |
| AFM1 | Milk | 0.39 mGy per day | 4 d: 51.5% 8 d: 99.0% | [50] |
| AFB1 OTA | Sorghum | 10 kGy | AFB1: 59.0% OTA: 32.0% | [51] |
| AFB1 | Peanut | 5~9 kGy | 20.0~43.0% | [52] |
| UV irradiation | ||||
| AFB1 OTA FB2 | Wheat flour | 30 W, 15 min | AFB1:100.0% OTA:100.0% FB2:100.0% | [53] |
| AFB1 | Corn Peanut | 10 d: 8.37 J·cm−2 | Corn AFB1: 17.0~43.0% Peanut AFB1: 14.0~51.0% | [54] |
| AOH AME TeA | Tomato | 2.5 J·cm−2 | AOH: 44.5% AME: 37.1% TeA: 34.5% | [55] |
| AFB1 AFM1 | Whole milk | 0.836 J·cm−2 0.857 J·cm−2 | AFB1: 78.2% AFM1: 65.7% | [56] |
| AFB1 | Peanut oil | 3500 μW·cm−2 120 s | AFB1: >95.0% | [57] |
| AFM1 | Milk | UV-C254 nm, 5~20 min | 20 min AFM1: 50.0% | [58] |
| PAT | Apple juice | 45.06 J·cm−2 | PAT: >98.0% | [59] |
| Electron beam irradiation | ||||
| OTA | Red pepper | 30 kGy | OTA: 25.0% | [60] |
| AFs | Corn syrup | 20 kGy | AFs decreases by 0.3 log (ng·g−1) on average | [61] |
| Microwave irradiation | ||||
| AFB1 OTA | Maize flour | 2450 MHz 100% power | AFB1: 50.58% OTA: 46.97% | [62] |
| AFB1 | Peanut | 360 W, 6 min 480 W, 5 min 600 W, 3 min | AFB1: 59% AFB1: 67% AFB1: 62% | [52] |
| AFB1 AFB2 AFG1 AFG2 OTA | Pistachio | 2450 MHz 100% power 10 min | AFB1: 34.6% AFB2: 23.3% AFG1: 29.3% AFG2: 36.6% OTA: 34.2% | [63] |
| Pulsed light | ||||
| DON | Barley | 180 pulses in 60 s | DON: 69.1% | [64] |
| OTA | Grape juice | 39 J·cm−2 | OTA: 95.18% | [65] |
| PAT | Apple juice | 24 J·cm−2 | PAT: 74% | [66] |
| PAT | Apple juice | 40.5 J·cm−2 | PAT: >95.44% | [67] |
| AFB1 AFB2 AFG1 AFG2 | Apple juice | 40 flashes | AFB1: 71.96% AFB2: 73.32% AFG1: 54.04% AFG2: 69.58% | [68] |
| Pulsed electric field | ||||
| AFB1 AFB2 AFG1 AFG2 | Sesame seed | 17.28 J | AFB1: 86.9% AFB2: 98.7% AFG1: 94.7% AFG2: 92.7% | [69] |
| ENs BEA | Juices and Smoothies | 30 kV, 3 kV·cm−1, 500 kJ·kg−1 | 43~70% | [70] |
| AFB1 AFB2 AFG1 AFG2 | Grape juice | 30 kV, 3 kV·cm−1, 500 kJ·kg−1 | AFB1: 25% AFB2: 72% AFG1: 84% AFG2: 24% | [71] |
| AFB1 AFB2 AFG1 AFG2 | Red pepper | 17.28 J | AFB1: 97.75% AFB2:99.58% AFG1:99.88% AFG2:99.47% | [72] |
| DON T-2 | Barley | Less intensive: 100 bipolar pulses, 10 Hz, 9 kV·cm−1 Intensive: 500 bipolar pulses, 10 Hz, 9 kV·cm−1 | Less intensive: DON: 14% T-2: 18% Intensive: DON: 31% T-2: 24% | [73] |
| Plasma | ||||
| T-2 HT-2 | Oat flour | Nitrogen: 100 Pa 30 min | T-2: 43.25% HT-2: 38.54% | [74] |
| DON | Wheat | 100 V, 20 Hz, 15 min | DON: 61% | [75] |
| DON OTA | Rice grain | 25 kV, 8 min | DON: 61.25% OTA: 55.64% | [76] |
| AFM1 | Skim milk | 200 W, 60 Hz, 20 min | AFM1: 78.9% | [77] |
| Ozone | ||||
| OTA | Sultanas | 12.8 mg/L, 120/240 min | 120 min:60.2% 240 min:82.5% | [78] |
| ZEN | Whole corn flour | 51.5 mg/L 5, 60 min | 5 min: 37.9% 60 min: 62.3% | [79] |
| AFB1 AFB2 AFG1 AFG2 DON OTA ZEN | Parboiled rice | 5 L/min, 5 h | AFB1: 80.9% AFB2: 59.2% AFG1: 61.8% AFG2: 47.6% DON: 56.0% OTA: 87.9% ZEN: 75.9% | [80] |
| FB1 FB2 | Corn kernel | 13.5 mg·L−1, 1.0·L min−1, 24 h | FB1: 81.2% FB2: 86.2% | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhou, A.; Yu, B.; Sun, X. Recent Advances in Non-Contact Food Decontamination Technologies for Removing Mycotoxins and Fungal Contaminants. Foods 2024, 13, 2244. https://doi.org/10.3390/foods13142244
Wang Y, Zhou A, Yu B, Sun X. Recent Advances in Non-Contact Food Decontamination Technologies for Removing Mycotoxins and Fungal Contaminants. Foods. 2024; 13(14):2244. https://doi.org/10.3390/foods13142244
Chicago/Turabian StyleWang, Yan, Aiyun Zhou, Bei Yu, and Xiulan Sun. 2024. "Recent Advances in Non-Contact Food Decontamination Technologies for Removing Mycotoxins and Fungal Contaminants" Foods 13, no. 14: 2244. https://doi.org/10.3390/foods13142244






