Anticancer Potential of Flavonoids: Their Role in Cancer Prevention and Health Benefits
Abstract
:1. Introduction
2. Methods
2.1. Eligibility Criteria
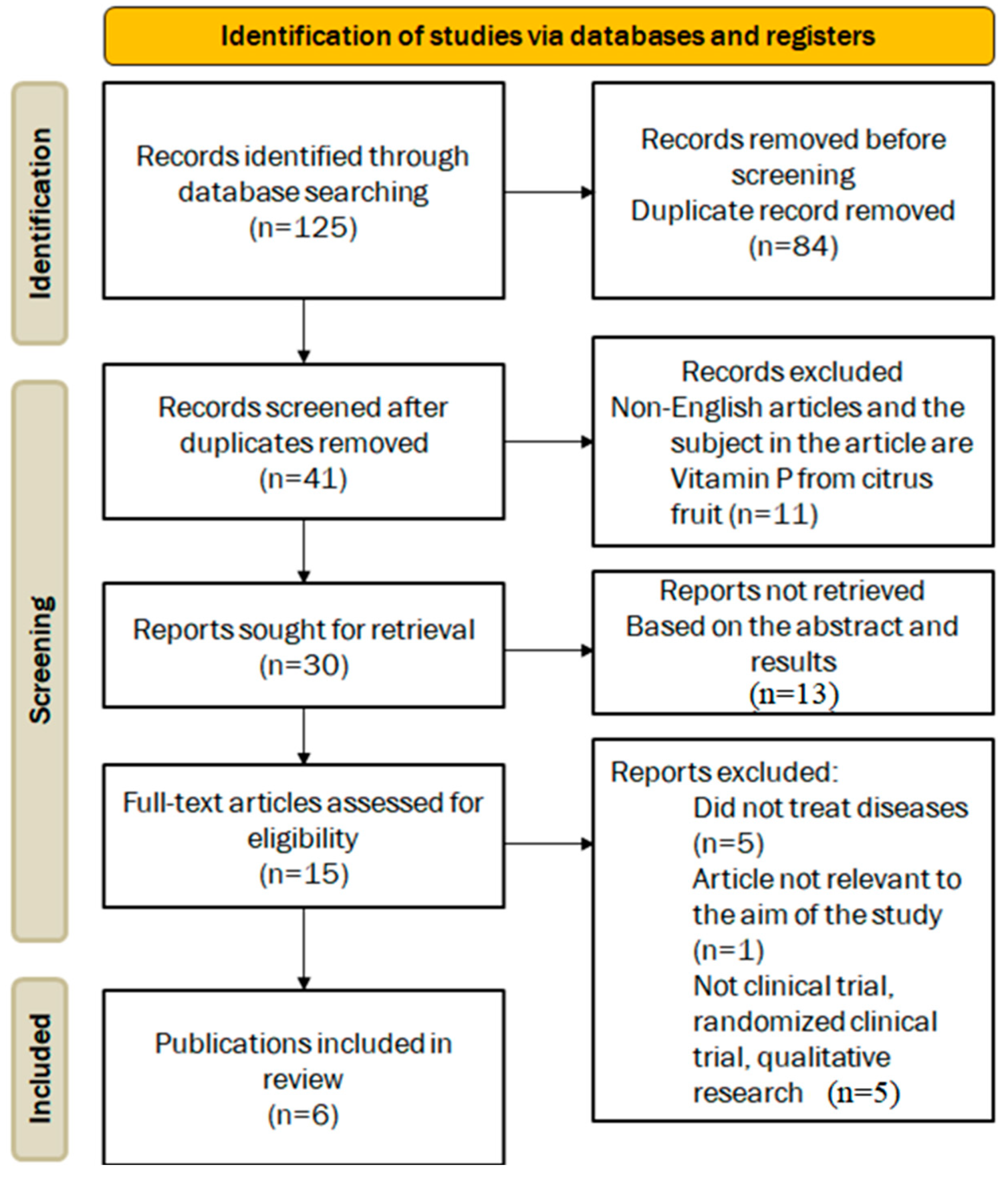
2.2. Screening and Data Extraction
2.3. Study Selection and Data Extraction
3. Dietary Sources of Flavonoid
| Dietary Source | Vitamin P Content | Anticancer Properties | Reference |
|---|---|---|---|
| Tomato | Average content of 20 mg 100 g−1 14–20 mg 100 g−1 on a dry basis | Flavonoids, lycopene and carotenoids protect against cellular damage and prevent prostate cancer and cardiovascular disease | [30] |
| Mulberry fruit | Average content is 0.14 ± 0.050% DW | Strengthens capillaries, reduces high blood pressure and decreases vascular permeability, has an anti-edema effect, reduces the risk of atherosclerosis, has antioxidant activity | [31] |
| Amazon grape | The rutin content in the peel is 155.45 ± 2.06 mg kg−1 Flesh 2.64 ± 1.21 mg kg−1 fresh weight | Cytotoxic effects in cancer cell lines | [32] |
| Apple | 12.136~483.89 µg/g | Antioxidant activity and activity similar to that of ascorbic acid | [33] |
| Citrus fruits | 326.59 mg/100 g | Health-promoting effects of citrus-fruit extracts (grapefruit extract, pomelo extract, naringin, etc.) and flavonoid biological systems | [34] |
| Sweet cherry | 1092.56 μg/g (MAE) 646.03 μg/g (CSE) | Various bioactive compounds, rutin, quercetin, quinic acid, and kaemferol-3-rutinoside Suppresses cardiovascular disease, diabetes, degenerative diseases, and cancer | [71] |
| Hackberry | 0.55 µg/g | Possesses phenolic compounds in addition to flavonoids, which have antibacterial and antifungal properties | [72] |
4. Flavonoid and Apoptosis Promotion
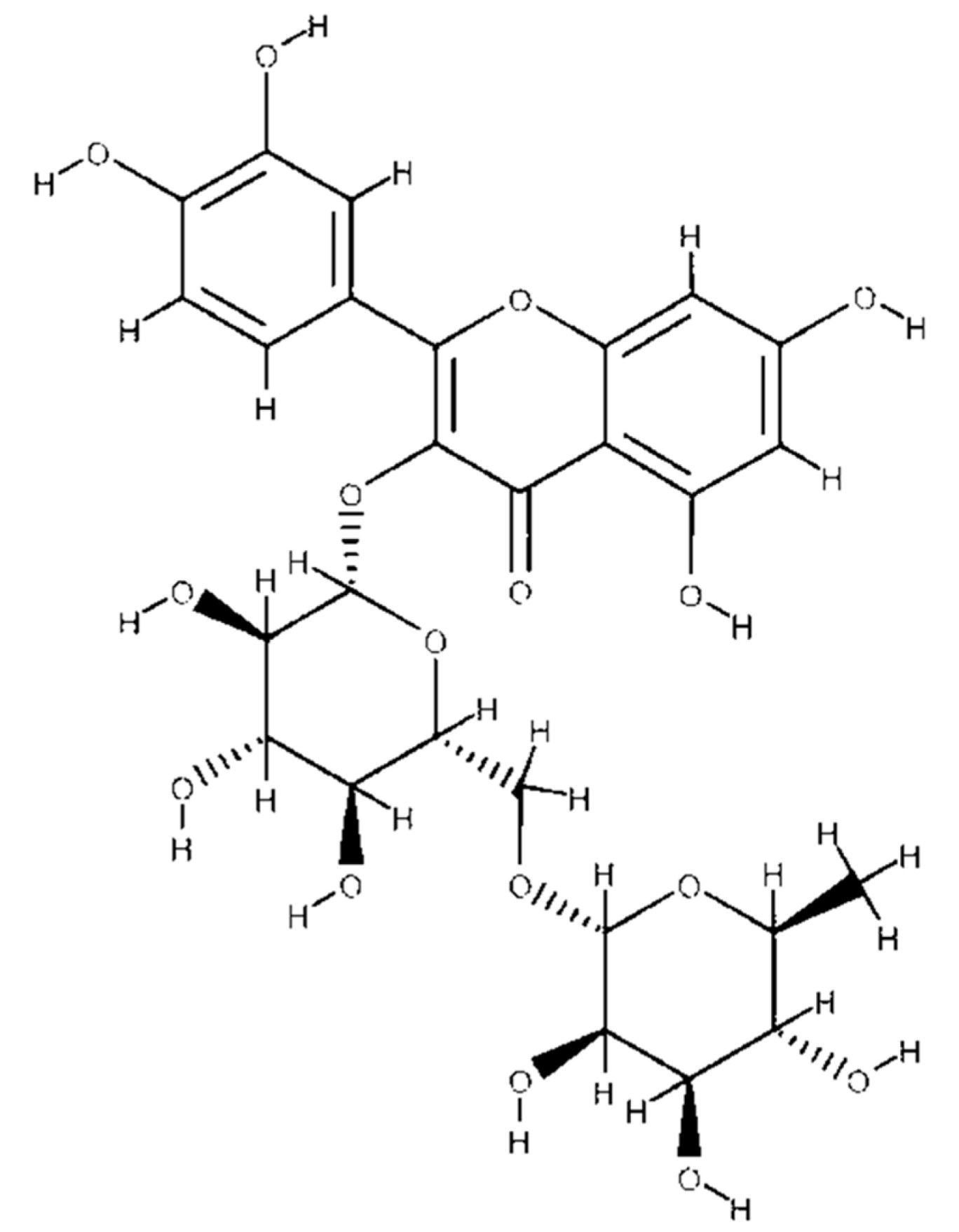
4.1. Chemical Structure of Flavonoids
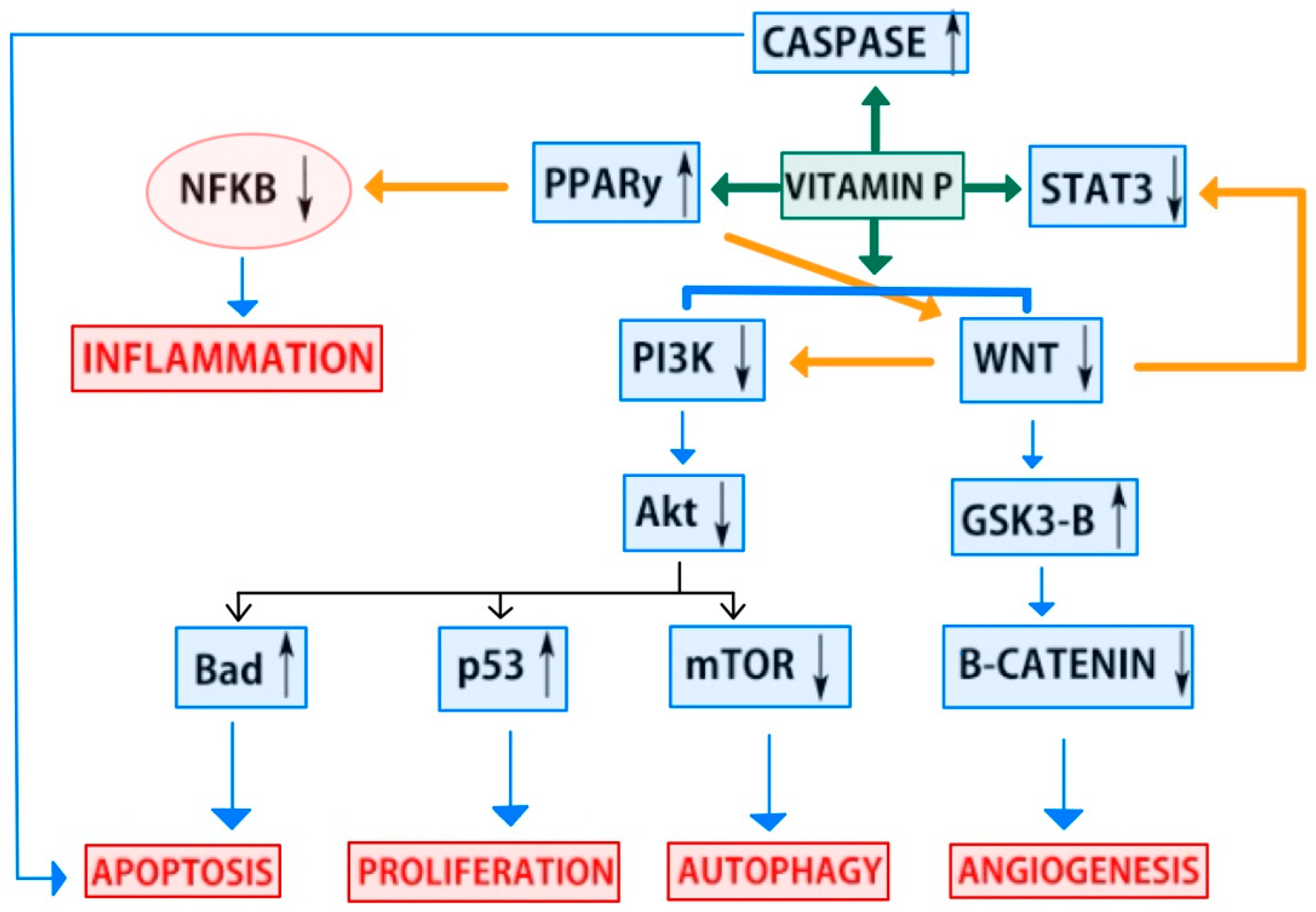
4.2. Anticancer Pathways of Flavonoids
5. Flavonoid-Rich Fruits
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wang, C.; Wang, X.; Zhang, Y.; Tang, Y.; Yang, Y.; Wang, B.; Wei, S.; Wang, Z.; Sun, G. Ionic liquid-based carbon dots as highly biocompatible and sensitive fluorescent probe for the determination of vitamin P in fruit samples. Food Chem. 2023, 406, 134898. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Doaei, S.; Mardi, A.; Zare, M. Role of micronutrients in the modulation of immune system and platelet activating factor in patients with COVID-19; a narrative review. Front. Nutr. 2023, 10, 1207237. [Google Scholar] [CrossRef]
- Mozos, I.; Flangea, C.; Vlad, D.C.; Gug, C.; Mozos, C.; Stoian, D.; Luca, C.T.; Horbańczuk, J.O.; Horbańczuk, O.K.; Atanasov, A.G. Effects of Anthocyanins on Vascular Health. Biomolecules 2021, 11, 811. [Google Scholar] [CrossRef]
- Rai, S.N.; Singh, P.; Steinbusch, H.W.M.; Vamanu, E.; Ashraf, G.; Singh, M.P. The Role of Vitamins in Neurodegenerative Disease: An Update. Biomedicines 2021, 9, 1284. [Google Scholar] [CrossRef]
- Choi, K.S.; Kundu, J.K.; Chun, K.S.; Na, H.K.; Surh, Y.J. Rutin inhibits UVB radiation-induced expression of COX-2 and iNOS in hairless mouse skin: p38 MAP kinase and JNK as potential targets. Arch. Biochem. Biophys. 2014, 559, 38–45. [Google Scholar] [CrossRef]
- Gutiérrez-Hoya, A.; Soto-Cruz, I. Role of the JAK/STAT Pathway in Cervical Cancer: Its Relationship with HPV E6/E7 Oncoproteins. Cells 2020, 9, 2297. [Google Scholar] [CrossRef]
- Qureshy, Z.; Johnson, D.E.; Grandis, J.R. Targeting the JAK/STAT pathway in solid tumors. J. Cancer Metastasis Treat. 2020, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Waheed Janabi, A.H.; Kamboh, A.A.; Saeed, M.; Xiaoyu, L.; BiBi, J.; Majeed, F.; Naveed, M.; Mughal, M.J.; Korejo, N.A.; Kamboh, R.; et al. Flavonoid-rich foods (FRF): A promising nutraceutical approach against lifespan-shortening diseases. Iran. J. Basic Med. Sci. 2020, 23, 140–153. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Almatroudi, A.; Allemailem, K.S.; Alwanian, W.M.; Alharbi, B.F.; Alrumaihi, F.; Khan, A.A.; Almatroodi, S.A. Myricetin: A Significant Emphasis on Its Anticancer Potential via the Modulation of Inflammation and Signal Transduction Pathways. Int. J. Mol. Sci. 2023, 24, 9665. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Belka, M.; Papierska, K. Targeting STAT3 and NF-κB Signaling Pathways in Cancer Prevention and Treatment: The Role of Chalcones. Cancers 2024, 16, 1092. [Google Scholar] [CrossRef]
- Oh, H.; Park, S.H.; Kang, M.K.; Kim, Y.H.; Lee, E.J.; Kim, D.Y.; Kim, S.I.; Oh, S.; Lim, S.S.; Kang, Y.H. Asaronic Acid Attenuates Macrophage Activation toward M1 Phenotype through Inhibition of NF-κB Pathway and JAK-STAT Signaling in Glucose-Loaded Murine Macrophages. J. Agric. Food Chem. 2019, 67, 10069–10078. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Qari, H.A.; Oves, M. Rutin (Bioflavonoid) as Cell Signaling Pathway Modulator: Prospects in Treatment and Chemoprevention. Pharmaceuticals 2021, 14, 1069. [Google Scholar] [CrossRef]
- Park, S.; Chapuis, N.; Tamburini, J.; Bardet, V.; Cornillet-Lefebvre, P.; Willems, L.; Green, A.; Mayeux, P.; Lacombe, C.; Bouscary, D. Role of the PI3K/AKT and mTOR signaling pathways in acute myeloid leukemia. Haematologica 2010, 95, 819–828. [Google Scholar] [CrossRef]
- Sharma, V.R.; Gupta, G.K.; Sharma, A.K.; Batra, N.; Sharma, D.K.; Joshi, A.; Sharma, A.K. PI3K/Akt/mTOR Intracellular Pathway and Breast Cancer: Factors, Mechanism and Regulation. Curr. Pharm. Des. 2017, 23, 1633–1638. [Google Scholar] [CrossRef]
- Fattahi, S.; Amjadi-Moheb, F.; Tabaripour, R.; Ashrafi, G.H.; Akhavan-Niaki, H. PI3K/AKT/mTOR signaling in gastric cancer: Epigenetics and beyond. Life Sci. 2020, 262, 118513. [Google Scholar] [CrossRef]
- Hosseinzade, A.; Sadeghi, O.; Naghdipour Biregani, A.; Soukhtehzari, S.; Brandt, G.S.; Esmaillzadeh, A. Immunomodulatory Effects of Flavonoids: Possible Induction of T CD4+ Regulatory Cells Through Suppression of mTOR Pathway Signaling Activity. Front. Immunol. 2019, 10, 51. [Google Scholar] [CrossRef]
- Shakya, G.; Balasubramanian, S.; Hoda, M.; Rajagopalan, R. Inhibition of metastasis and angiogenesis in Hep-2 cells by wheatgrass extract—An in vitro and in silico approach. Toxicol. Mech. Methods 2018, 28, 205–218. [Google Scholar] [CrossRef]
- Banerjee, S.; Katiyar, P.; Kumar, V.; Waghmode, B.; Nathani, S.; Krishnan, V.; Sircar, D.; Roy, P. Wheatgrass inhibits the lipopolysaccharide-stimulated inflammatory effect in RAW 264.7 macrophages. Curr. Res. Toxicol. 2021, 2, 116–127. [Google Scholar] [CrossRef]
- Zhao, B.; Xiong, Y.; Zhang, Y.; Jia, L.; Zhang, W.; Xu, X. Rutin promotes osteogenic differentiation of periodontal ligament stem cells through the GPR30-mediated PI3K/AKT/mTOR signaling pathway. Exp. Biol. Med. 2020, 245, 552–561. [Google Scholar] [CrossRef]
- Sun, J.; Wang, H.; Liu, B.; Shi, W.; Shi, J.; Zhang, Z.; Xing, J. Rutin attenuates H2O2-induced oxidation damage and apoptosis in Leydig cells by activating PI3K/Akt signal pathways. Biomed. Pharmacother. 2017, 88, 500–506. [Google Scholar] [CrossRef]
- Talebi, H.; Farahpour, M.R.; Hamishehkar, H. The effectiveness of Rutin for prevention of surgical induced endometriosis development in a rat model. Sci. Rep. 2021, 11, 7180. [Google Scholar] [CrossRef]
- Nouri, Z.; Fakhri, S.; Nouri, K.; Wallace, C.E.; Farzaei, M.H.; Bishayee, A. Targeting Multiple Signaling Pathways in Cancer: The Rutin Therapeutic Approach. Cancers 2020, 12, 2276. [Google Scholar] [CrossRef]
- Mohan, C.D.; Rangappa, S.; Preetham, H.D.; Chandra Nayaka, S.; Gupta, V.K.; Basappa, S.; Sethi, G.; Rangappa, K.S. Targeting STAT3 signaling pathway in cancer by agents derived from Mother Nature. Semin. Cancer Biol. 2022, 80, 157–182. [Google Scholar] [CrossRef]
- Kim, M.; Baek, M.; Kim, D.J. Protein Tyrosine Signaling and its Potential Therapeutic Implications in Carcinogenesis. Curr Pharm Des. 2017, 23, 4226–4246. [Google Scholar] [CrossRef]
- Chen, W.; Wang, S.; Wu, Y.; Shen, X.; Xu, S.; Guo, Z.; Xing, D. The physiologic activity and mechanism of quercetin-like natural plant flavonoids. Curr. Pharm. Biotechnol. 2020, 21, 654–658. [Google Scholar] [CrossRef]
- Morgacheva, N.V.; Zakharov, V.L.; Petrisheva, T.Y.; Sotnikova, E.B. Vitamin value assessment of fruits and berries in the Central Black Earth Region (CBER) by the level of biologically active substances in the wild analogues. IOP Conf. Ser. Earth Environ. Sci. 2020, 548, 072024. [Google Scholar] [CrossRef]
- Azad, A.K.; Dayoob, M.; Zohera, F.T. Anticancer Activity of Flavonoids: Past, Present, and Future. In Harnessing Medicinal Plants in Cancer Prevention and Treatmen; IGI Global: Hershey, PA, USA, 2024; pp. 1–21. [Google Scholar] [CrossRef]
- Tovar-Pérez, E.G.; Aguilera-Aguirre, S.; López-García, U.; Valdez-Morales, M.; Ibarra-Zurita, A.K.; Chacón-López, A. Effect of ultrasound treatment on the quality and contents of polyphenols, lycopene and rutin in tomato fruits. Czech J. Food Sci. 2020, 38, 20–27. [Google Scholar] [CrossRef]
- Wang, R.S.; Dong, P.H.; Shuai, X.X.; Chen, M.S. Evaluation of Different Black Mulberry Fruits (Morus nigra L.) Based on Phenolic Compounds and Antioxidant Activity. Foods 2022, 11, 1252. [Google Scholar] [CrossRef]
- Lopes-Lutz, D.; Dettmann, J.; Nimalaratne, C.; Schieber, A. Characterization and Quantification of Polyphenols in Amazon Grape (Pourouma cecropiifolia Martius). Molecules 2010, 15, 8543–8552. [Google Scholar] [CrossRef]
- Shafi, W.; Mansoor, S.; Jan, S.; Singh, D.B.; Kazi, M.; Raish, M.; Alwadei, M.; Mir, J.I.; Ahmad, P. Variability in Catechin and Rutin Contents and Their Antioxidant Potential in Diverse Apple Genotypes. Molecules 2019, 24, 943. [Google Scholar] [CrossRef]
- Batra, P.; Sharma, A.K. Anti-cancer potential of flavonoids: Recent trends and future perspectives. 3 Biotech 2013, 3, 439–459. [Google Scholar] [CrossRef]
- Patel, K.; Patel, D.K. The beneficial role of rutin, a naturally occurring flavonoid in health promotion and disease prevention: A systematic review and update. Bioact. Food Diet. Interv. Arthritis Relat. Inflamm. Dis. 2019, 457–479. [Google Scholar] [CrossRef]
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of phytochemicals present in tomato. J. Food Sci. Technol. 2018, 55, 2833–2849. [Google Scholar] [CrossRef]
- Martí, R.; Roselló, S.; Cebolla-Cornejo, J. Tomato as a Source of Carotenoids and Polyphenols Targeted to Cancer Prevention. Cancers 2016, 8, 58. [Google Scholar] [CrossRef]
- Mazzucato, A.; Willems, D.; Bernini, R.; Picarella, M.E.; Santangelo, E.; Ruiu, F.; Tilesi, F.; Soressi, G.P. Novel phenotypes related to the breeding of purple-fruited tomatoes and effect of peel extracts on human cancer cell proliferation. Plant Physiol. Biochem. 2013, 72, 125–133. [Google Scholar] [CrossRef]
- de Araújo, M.E.; Moreira Franco, Y.E.; Alberto, T.G.; Sobreiro, M.A.; Conrado, M.A.; Priolli, D.G.; Frankland Sawaya, A.C.; Ruiz, A.L.; de Carvalho, J.E.; de Oliveira Carvalho, P. Enzymatic de-glycosylation of rutin improves its antioxidant and antiproliferative activities. Food Chem. 2013, 141, 266–273. [Google Scholar] [CrossRef]
- You, H.J.; Ahn, H.J.; Ji, G.E. Transformation of rutin to antiproliferative quercetin-3-glucoside by Aspergillus niger. J. Agric. Food Chem. 2010, 58, 10886–10892. [Google Scholar] [CrossRef]
- Yang, J.; Liu, R.H. Synergistic effect of apple extracts and quercetin 3-beta-d-glucoside combination on antiproliferative activity in MCF-7 human breast cancer cells in vitro. J. Agric. Food Chem. 2009, 57, 8581–8586. [Google Scholar] [CrossRef]
- Khatri, P.; Chen, L.; Rajcan, I.; Dhaubhadel, S. Functional characterization of Cinnamate 4-hydroxylase gene family in soybean (Glycine max). PLoS ONE 2023, 18, e0285698. [Google Scholar] [CrossRef]
- Davies, K.M. Genetic modification of plant metabolism for human health benefits. Mutat. Res. 2007, 622, 122–137. [Google Scholar] [CrossRef]
- Ai, J.; Bao, B.; Battino, M.; Giampieri, F.; Chen, C.; You, L.; Cespedes-Acuña, C.L.; Ognyanov, M.; Tian, L.; Bai, W. Recent advances on bioactive polysaccharides from mulberry. Food Funct. 2021, 12, 5219–5235. [Google Scholar] [CrossRef]
- Peng, C.H.; Lin, H.T.; Chung, D.J.; Huang, C.N.; Wang, C.J. Mulberry Leaf Extracts prevent obesity-induced NAFLD with regulating adipocytokines, inflammation and oxidative stress. J. Food Drug Anal. 2018, 26, 778–787. [Google Scholar] [CrossRef]
- Demir, S.; Turan, I.; Aliyazicioglu, Y.; Kilinc, K.; Yaman, S.O.; Ayazoglu Demir, E.; Arslan, A.; Mentese, A.; Deger, O. Morus Rubra Extract Induces Cell Cycle Arrest and Apoptosis in Human Colon Cancer Cells Through Endoplasmic Reticulum Stress and Telomerase. Nutr. Cancer 2017, 69, 74–83. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Zhang, H.J.; Tsang, S.W. Perspectives of Plant Natural Products in Inhibition of Cancer Invasion and Metastasis by Regulating Multiple Signaling Pathways. Curr. Med. Chem. 2018, 25, 5057–5087. [Google Scholar] [CrossRef]
- Shang, A.; Luo, M.; Gan, R.-Y.; Xu, X.-Y.; Xia, Y.; Guo, H.; Liu, Y.; Li, H.-B. Effects of Microwave-Assisted Extraction Conditions on Antioxidant Capacity of Sweet Tea (Lithocarpus polystachyus Rehd.). Antioxidants 2020, 9, 678. [Google Scholar] [CrossRef]
- Wu, T.-Y.; Liang, J.; Ai, J.-Y.; Cui, J.-L.; Huang, W.-D.; You, Y.-L.; Zhan, J.-C. Mulberry Ethanol Extract and Rutin Protect Alcohol-Damaged GES-1 Cells by Inhibiting the MAPK Pathway. Molecules 2022, 27, 4266. [Google Scholar] [CrossRef]
- Wu, T.; Yin, J.; Zhang, G.; Long, H.; Zheng, X. Mulberry and cherry anthocyanin consumption prevents oxidative stress and inflammation in diet-induced obese mice. Mol. Nutr. Food Res. 2016, 60, 687–694. [Google Scholar] [CrossRef]
- Choi, S.K.; Zhang, X.H.; Seo, J.S. Suppression of oxidative stress by grape seed supplementation in rats. Nutr. Res. Pract. 2012, 6, 3–8. [Google Scholar] [CrossRef]
- Cetin, A.; Kaynar, L.; Koçyiğit, I.; Hacioğlu, S.K.; Saraymen, R.; Oztürk, A.; Orhan, O.; Sağdiç, O. The effect of grape seed extract on radiation-induced oxidative stress in the rat liver. Turk. J. Gastroenterol. 2008, 19, 92–98. [Google Scholar] [PubMed]
- Khan, S.G.; Katiyar, S.K.; Agarwal, R.; Mukhtar, H. Enhancement of antioxidant and phase II enzymes by oral feeding of green tea polyphenols in drinking water to SKH-1 hairless mice: Possible role in cancer chemoprevention. Cancer Res. 1992, 52, 4050–4052. [Google Scholar] [PubMed]
- Swartz, H.M.; Mason, R.P.; Hogg, N.; Kalyanaraman, B.; Sarna, T.; Plonka, P.M.; Zareb, M.; Gutierrez, P.L.; Berliner, L.J. Free Radicals and Medicine. Biomed. EPR Part A Free Radic. Met. Med. Physiol. 2005, 23, 25–74. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Habib, H.M.; El-Fakharany, E.M.; Kheadr, E.; Ibrahim, W.H. Grape seed proanthocyanidin extract inhibits DNA and protein damage and labile iron, enzyme, and cancer cell activities. Sci. Rep. 2022, 12, 12393. [Google Scholar] [CrossRef]
- Singh, C.K.; Siddiqui, I.A.; El-Abd, S.; Mukhtar, H.; Ahmad, N. Combination chemoprevention with grape antioxidants. Mol. Nutr. Food Res. 2016, 60, 1406–1415. [Google Scholar] [CrossRef]
- Alberti, A.; Zielinski, A.A.; Zardo, D.M.; Demiate, I.M.; Nogueira, A.; Mafra, L.I. Optimisation of the extraction of phenolic compounds from apples using response surface methodology. Food Chem. 2014, 149, 151–158. [Google Scholar] [CrossRef]
- Perussello, C.A.; Zhang, Z.; Marzocchella, A.; Tiwari, B.K. Valorization of Apple Pomace by Extraction of Valuable Compounds. Compr. Rev. Food Sci. Food Saf. 2017, 16, 776–796. [Google Scholar] [CrossRef]
- Nile, A.; Nile, S.H.; Shin, J.; Park, G.; Oh, J.-W. Quercetin-3-Glucoside Extracted from Apple Pomace Induces Cell Cycle Arrest and Apoptosis by Increasing Intracellular ROS Levels. Int. J. Mol. Sci. 2021, 22, 10749. [Google Scholar] [CrossRef]
- Pandey, J.; Bastola, T.; Tripathi, J.; Tripathi, M.; Rokaya, R.K.; Dhakal, B.; Poudel, A. Estimation of total quercetin and rutin content in Malus domestica of Nepalese origin by HPLC method and determination of their antioxidative activity. J. Food Qual. 2020, 2020, 8853426. [Google Scholar] [CrossRef]
- Zhu, C.; Zhou, X.; Long, C.; Du, Y.; Li, J.; Yue, J.; Pan, S. Variations of Flavonoid Composition and Antioxidant Properties among Different Cultivars, Fruit Tissues and Developmental Stages of Citrus Fruits. Chem. Biodivers. 2020, 17, e1900690. [Google Scholar] [CrossRef]
- Addi, M.; Elbouzidi, A.; Abid, M.; Tungmunnithum, D.; Elamrani, A.; Hano, C. An Overview of Bioactive Flavonoids from Citrus Fruits. Appl. Sci. 2022, 12, 29. [Google Scholar] [CrossRef]
- Bento-Silva, A.; Koistinen, V.M.; Mena, P.; Bronze, M.R.; Hanhineva, K.; Sahlstrøm, S.; Kitrytė, V.; Moco, S.; Aura, A.M. Factors affecting intake, metabolism and health benefits of phenolic acids: Do we understand individual variability? Eur. J. Nutr. 2020, 59, 1275–1293. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Lin, M.T.; Zhu, Y.D.; Xu, H.L.; Zhao, Y.Z. Recent Trends in Potential Therapeutic Applications of the Dietary Flavonoid Didymin. Molecules 2018, 23, 2547. [Google Scholar] [CrossRef]
- Moustafa, A.M.Y.; Ahmed, S.H.; Nabil, Z.I.; Hussein, A.A.; Omran, M.A. Extraction and phytochemical investigation of Calotropis procera: Effect of plant extracts on the activity of diverse muscles. Pharm. Biol. 2010, 10, 1080–1190. [Google Scholar] [CrossRef]
- Afraz, M.T.; Xu, X.; Adil, M.; Manzoor, M.F.; Zeng, X.-A.; Han, Z.; Aadil, R.M. Subcritical and Supercritical Fluids to Valorize Industrial Fruit and Vegetable Waste. Foods 2023, 12, 2417. [Google Scholar] [CrossRef]
- Koolaji, N.; Shammugasamy, B.; Schindeler, A.; Dong, Q.; Dehghani, F.; Valtchev, P. Citrus Peel Flavonoids as Potential Cancer Prevention Agents. Curr. Dev. Nutr. 2020, 4, nzaa025. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J. Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.; Riar, C.S.; Jindal, N. Effect of extraction methods and simulated in vitro gastrointestinal digestion on phenolic compound profile, bio-accessibility, and antioxidant activity of Meghalayan cherry (Prunus nepalensis) pomace extracts. LWT 2022, 153, 112570. [Google Scholar] [CrossRef]
- Farhan, M.; Rizvi, A.; Aatif, M.; Ahmad, A. Current Understanding of Flavonoids in Cancer Therapy and Prevention. Metabolites 2023, 13, 481. [Google Scholar] [CrossRef] [PubMed]
- Safari, F.; Hassanpour, H.; Alijanpour, A. Evaluation of hackberry (Celtis australis L.) fruits as sources of bioactive compounds. Sci. Rep. 2023, 13, 12233. [Google Scholar] [CrossRef] [PubMed]
- Orlikowski, L.B. Plant extracts in the control of Phytophthora cryptogea. Meded. Rijksuniv. Gent. Fak. Landbouwkd. Toegep. Biol. Wet. 2001, 66, 83–89. [Google Scholar] [PubMed]
- Goyal, J.; Verma, P.K. An overview of biosynthetic pathway and therapeutic potential of rutin. Mini Rev. Med. Chem. 2023, 23, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Elancheziyan, M.; Ganesan, S.; Theyagarajan, K.; Duraisamy, M.; Thenmozhi, K.; Weng, C.H.; Ponnusamy, V.K. Novel biomass-derived porous-graphitic carbon coated iron oxide nanocomposite as an efficient electrocatalyst for the sensitive detection of rutin (vitamin P) in food and environmental samples. Environ. Res. 2022, 211, 113012. [Google Scholar] [CrossRef] [PubMed]
- Pivec, T.; Kargl, R.; Maver, U.; Bračič, M.; Elschner, T.; Žagar, E.; Gradišnik, L.; Stana Kleinschek, K. Chemical Structure–Antioxidant Activity Relationship of Water–Based Enzymatic Polymerized Rutin and Its Wound Healing Potential. Polymers 2019, 11, 1566. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kim, J.K.; Park, S.Y.; Zhao, S.; Kim, Y.B.; Lee, S.; Park, S.U. Comparative analysis of flavonoids and polar metabolite profiling of Tanno-original and Tanno-high rutin buckwheat. J. Agric. Food Chem. 2014, 62, 2701–2708. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tang, Y.; Ye, Y.; Zuo, M.; Lu, Q. Potential of natural flavonols and flavanones in the treatment of ulcerative colitis. Front. Pharmacol. 2023, 14, 1120616. [Google Scholar] [CrossRef]
- Imani, A.; Maleki, N.; Bohlouli, S.; Kouhsoltani, M.; Sharifi, S.; Maleki Dizaj, S. Molecular mechanisms of anticancer effect of rutin. Phytother. Res. 2021, 35, 2500–2513. [Google Scholar] [CrossRef]
- Sheu, J.R.; Hsiao, G.; Chou, P.H.; Shen, M.Y.; Chou, D.S. Mechanisms involved in the antiplatelet activity of rutin, a glycoside of the flavonol quercetin, in human platelets. J. Agric. Food Chem. 2004, 52, 4414–4418. [Google Scholar] [CrossRef]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Q.H.; Sui, Y.; Wang, Y.; Qiu, X. Rutin protects endothelial dysfunction by disturbing Nox4 and ROS-sensitive NLRP3 inflammasome. Biomed. Pharmacother. 2017, 86, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Ugusman, A.; Zakaria, Z.; Chua, K.H.; Nordin, N.A.; Abdullah Mahdy, Z. Role of rutin on nitric oxide synthesis in human umbilical vein endothelial cells. Sci. World J. 2014, 2014, 169370. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Maoqiang, L.; Fan, H.; Zhenyu, B.; Qifang, H.; Xuepeng, W.; Liulong, Z. Rutin attenuates neuroinflammation in spinal cord injury rats. J. Surg. Res. 2016, 203, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. Oxid. Med. Cell Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef] [PubMed]
- Budzynska, B.; Faggio, C.; Kruk-Slomka, M.; Samec, D.; Nabavi, S.F.; Sureda, A.; Devi, K.P.; Nabavi, S.M. Rutin as Neuroprotective Agent: From Bench to Bedside. Curr. Med. Chem. 2019, 26, 5152–5164. [Google Scholar] [CrossRef] [PubMed]
- Radwan, R.R.; Abdel Fattah, S.M. Mechanisms involved in the possible nephroprotective effect of rutin and low dose γ irradiation against cisplatin-induced nephropathy in rats. J. Photochem. Photobiol. B 2017, 169, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Dai, C.; Lang, F.; Hu, L.; Tang, Q.; Wang, H.; Zhang, Y.; Hao, Z. Rutin Attenuates Vancomycin-Induced Nephrotoxicity by Ameliorating Oxidative Stress, Apoptosis, and Inflammation in Rats. Antimicrob. Agents Chemother. 2018, 63, e01545-18. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Pan, R.; Ding, L.; Zhang, F.; Hu, L.; Ding, B.; Zhu, L.; Xia, Y.; Dou, X. Rutin exhibits hepatoprotective effects in a mouse model of non-alcoholic fatty liver disease by reducing hepatic lipid levels and mitigating lipid-induced oxidative injuries. Int. Immunopharmacol. 2017, 49, 132–141. [Google Scholar] [CrossRef]
- Munir, M.T.; Ponce, C.; Powell, C.A.; Tarafdar, K.; Yanagita, T.; Choudhury, M.; Gollahon, L.S.; Rahman, S.M. The contribution of cholesterol and epigenetic changes to the pathophysiology of breast cancer. J. Steroid Biochem. Mol. Biol. 2018, 183, 1–9. [Google Scholar] [CrossRef]
- Garcia-Estevez, L.; Moreno-Bueno, G. Updating the role of obesity and cholesterol in breast cancer. Breast Cancer Res. 2019, 21, 35. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Wang, C.; Yin, H.; Yu, J.; Chen, S.; Fang, J.; Guo, F. Leucine deprivation inhibits proliferation and induces apoptosis of human breast cancer cells via fatty acid synthase. Oncotarget 2016, 7, 63679–63689. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.; ElFayoumi, H.M.; Youns, M.; Barakat, W. Rutin and orlistat produce antitumor effects via antioxidant and apoptotic actions. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Li, M.; Yu, Y.; He, J.; Hu, S.; Pan, M.; Lu, S.; Liao, K.; Pan, Z.; Zhou, Y.; et al. Effects of harmaline on cell growth of human liver cancer through the p53/p21 and Fas/FasL signaling pathways. Oncol. Lett. 2018, 15, 1931–1936. [Google Scholar] [CrossRef] [PubMed]
- Keay, S.; Nallar, S.C.; Gade, P.; Zhang, C.O.; Kalvakolanu, D.V. Oncosuppressor protein p53 and cyclin-dependent kinase inhibitor p21 regulate interstitial cystitis associated gene expression. Cytokine 2018, 110, 110–115. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Satari, A.; Ghasemi, S.; Habtemariam, S.; Asgharian, S.; Lorigooini, Z. Rutin: A flavonoid as an effective sensitizer for anticancer therapy; insights into multifaceted mechanisms and applicability for combination therapy. Evid.-Based Complement. Altern. Med. 2021, 9913179. [Google Scholar] [CrossRef]
- Yeary, K.H.K.; Yu, H.; Kuliszewski, M.G.; Li, Q.; McCann, S.E.; Pratt, R.; Saad-Harfouche, F.G.; Wang, Z.; Clark, N.; Wang, C.; et al. Outcomes of a Dietary Intervention to Reduce Bladder Cancer Recurrence and Progression in Survivors of Non-Muscle-Invasive Bladder Cancer. J. Natl. Compr. Cancer Netw. 2024, 22, e237086. [Google Scholar] [CrossRef] [PubMed]
- Berrino, F.; Villarini, A.; Gargano, G.; Krogh, V.; Grioni, S.; Bellegotti, M.; Venturelli, E.; Raimondi, M.; Traina, A.; Zarcone, M.; et al. The Effect of Diet on Breast Cancer Recurrence: The DIANA-5 Randomized Trial. Clin. Cancer Res. 2024, 30, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, L.; Simkin, A.J.; George Priya Doss, C.; Siva, R. Fruit ripening: Dynamics and integrated analysis of carotenoids and anthocyanins. BMC Plant Biol. 2022, 22, 27. [Google Scholar] [CrossRef]
- Paur, I.; Lilleby, W.; Bøhn, S.K.; Hulander, E.; Klein, W.; Vlatkovic, L.; Axcrona, K.; Bolstad, N.; Bjøro, T.; Laake, P.; et al. Tomato-based randomized controlled trial in prostate cancer patients: Effect on PSA. Clin. Nutr. 2017, 36, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Burnell, M.; Gaba, F.; Sobocan, M.; Desai, R.; Sanderson, S.; Loggenberg, K.; Gessler, S.; Side, L.; Brady, A.F.; Dorkins, H.; et al. Randomised trial of population-based BRCA testing in Ashkenazi Jews: Long-term secondary lifestyle behavioural outcomes. BJOG 2022, 129, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Gouws, C.A.; McKune, A.; Tee, N.; Somerset, S.; Mortazavi, R. Prickly pear juice consumption after fat intake affects postprandial heart rate variability but not traditional risk factors of cardiovascular disease in healthy men. Nutrition 2022, 96, 111555. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Fernández, J.R.; Locher, J.L.; Demark-Wahnefried, W. Rural and Urban Differences in Vegetable and Fruit Consumption Among Older Cancer Survivors in the Deep South: An Exploratory Cross-Sectional Study. J. Acad. Nutr. Diet 2022, 122, 1717–1724.e4. [Google Scholar] [CrossRef]
- Amawi, H.; Ashby, C.R., Jr.; Tiwari, A.K. Cancer chemoprevention through dietary flavonoids: What’s limiting? Chin. J. Cancer 2017, 36, 50. [Google Scholar] [CrossRef]
| Subjects | Target Cancer | Setting | Findings | Reference |
|---|---|---|---|---|
| 49 NMIBC patients | Bladder cancer | Two-arm, double-blinded, randomized controlled trial A six-month intervention Daily consumption of fruits and vegetables | Significantly increased cruciferae intake and urinary ITC levels in NMIBC Survivors Improved bladder cancer outcomes and prognosis | [99] |
| 1542 patients with breast cancer | Breast cancer | Randomized controlled trial Randomly assigned to an active dietary intervention | 5 years of follow-up; 95 patients in the IG and 98 in the CG developed breast cancer recurrence. Self-reported diet at year 1 combined with IG and CG showed association of protection with higher degree of dietary change | [100] |
| 79 patients with prostate cancer | Prostate cancer | Randomized to a nutritional intervention Controlled diet containing tomato products for 3 weeks. | A three-week nutritional intervention with tomato products alone or in combination with selenium and n-3 fatty acids lowers PSA in patients with metastatic prostate cancer. | [102] |
| 1034 participants | Breast cancer | Two-arm randomized controlled trials | Similar long-term lifestyle influences include smoking, alcohol intake, dietary intake of fruit/vegetables/meat/vitamins, exercise, participation in breast cancer screening and reduced perception of cancer risk. | [103] |
| 17 male patients | Cardiovascular disease | Double-blind, randomized, placebo-controlled, crossover trial | May moderate traditional responses to cardiovascular risk Nutrient content is important | [104] |
| 731 medicare-eligible cancer survivors | Cancer prevention | A cross-sectional secondary analysis Kruskal−Wallis rank sum and post-hoc tests | Eating vegetables and fruits extends the life of cancer survivors. Gender, race, and cancer type affect survival. | [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyo, Y.; Kwon, K.H.; Jung, Y.J. Anticancer Potential of Flavonoids: Their Role in Cancer Prevention and Health Benefits. Foods 2024, 13, 2253. https://doi.org/10.3390/foods13142253
Pyo Y, Kwon KH, Jung YJ. Anticancer Potential of Flavonoids: Their Role in Cancer Prevention and Health Benefits. Foods. 2024; 13(14):2253. https://doi.org/10.3390/foods13142253
Chicago/Turabian StylePyo, Yeonhee, Ki Han Kwon, and Yeon Ja Jung. 2024. "Anticancer Potential of Flavonoids: Their Role in Cancer Prevention and Health Benefits" Foods 13, no. 14: 2253. https://doi.org/10.3390/foods13142253





