Ratanjot (Alkanna tinctoria L.) Root Extract, Rich in Antioxidants, Exhibits Strong Antimicrobial Activity against Foodborne Pathogens and Is a Potential Food Preservative
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Media
2.2. Bacterial Cultures and Growth Conditions
2.3. Preparation of Ethanol and Microwave-Assisted Hot Water Extracts of Ratanjot (Alkanna tinctoria L.) Root and Characterization
2.4. Antioxidant Activity Analysis
2.4.1. Total Phenolic Content
2.4.2. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Assay
2.4.3. 2,2-Azino-Bis (3-Ethylbenzothiazoline-6-Sulfonic Acid) (ABTS.+) Radical Cation Decolorization Assay
2.4.4. Identification of Phytochemicals Using Gas Chromatography–Mass Spectrometry (GC–MS/MS)
2.5. Antimicrobial Activity Assays
2.5.1. Agar Well Diffusion Assay
2.5.2. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.5.3. Dose and Time-Dependent In-Vitro Growth Kinetics of S. aureus and L. monocytogenes Treated with RRP Extract
2.5.4. Stability Assessment
2.6. Effect of RRP on Lactobacilli Growth
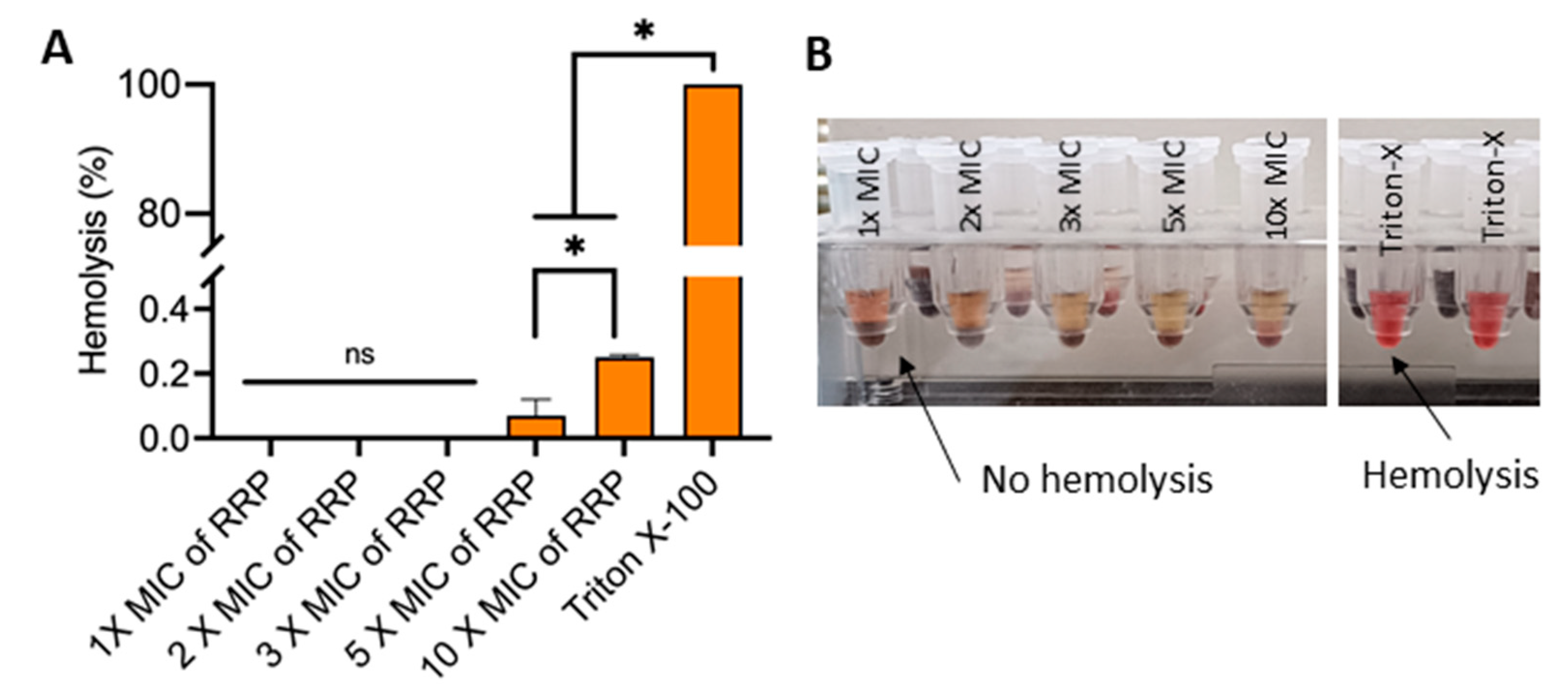
2.7. Effect of RRP on Red Blood Cells
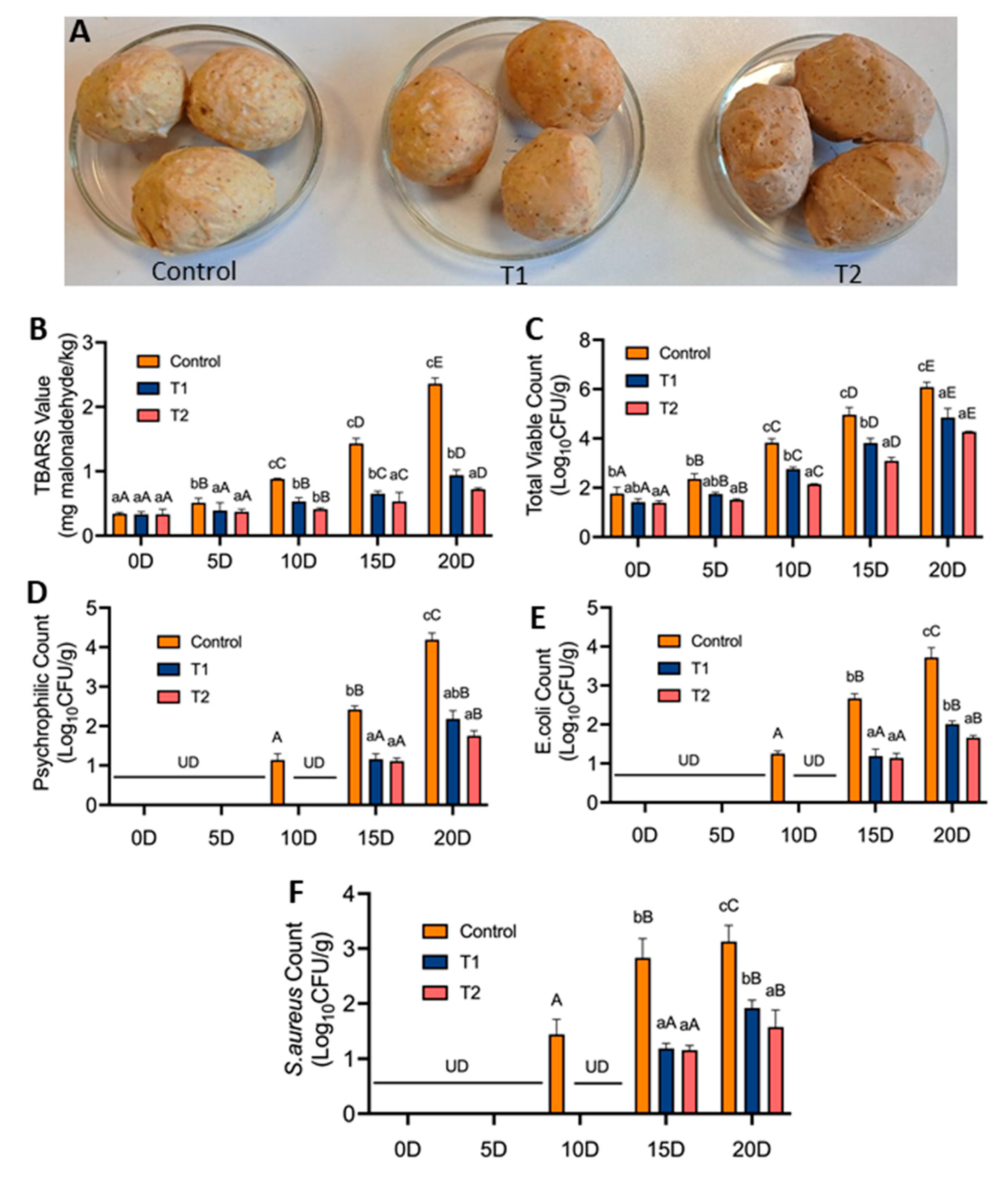
2.8. Application of RRP in Meat-Model System
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Ethanol and Water Extracts of Ratanjot (A. tinctoria L.) Root
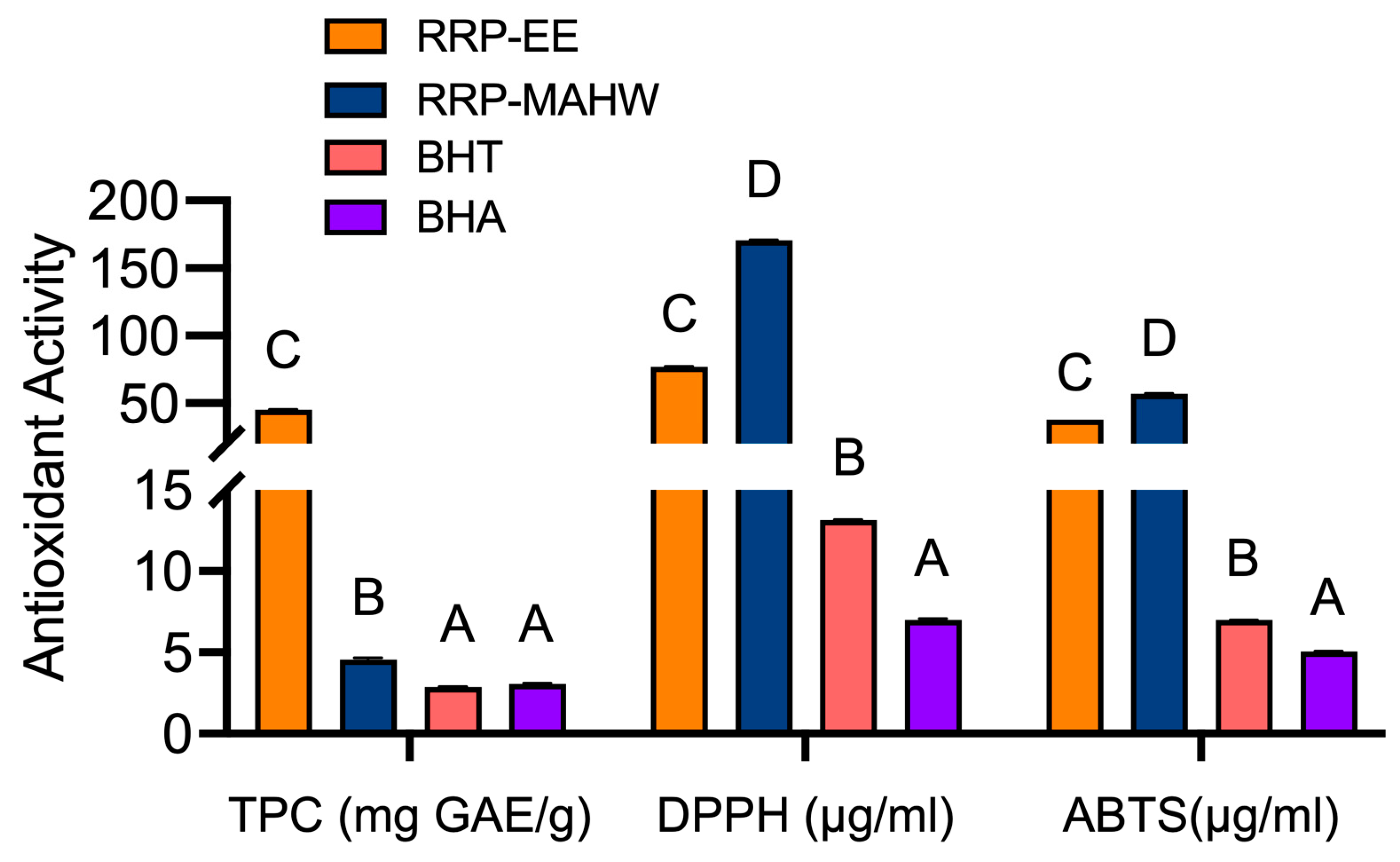
3.2. Antioxidant Activity of RRP
3.2.1. Total Phenolic Content
3.2.2. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Assay
3.2.3. 2.2-Azino-Bis (3-Ethylbenzothiazoline-6-Sulfonic Acid) (ABTS.+) Radical Cation Decolorization Assay
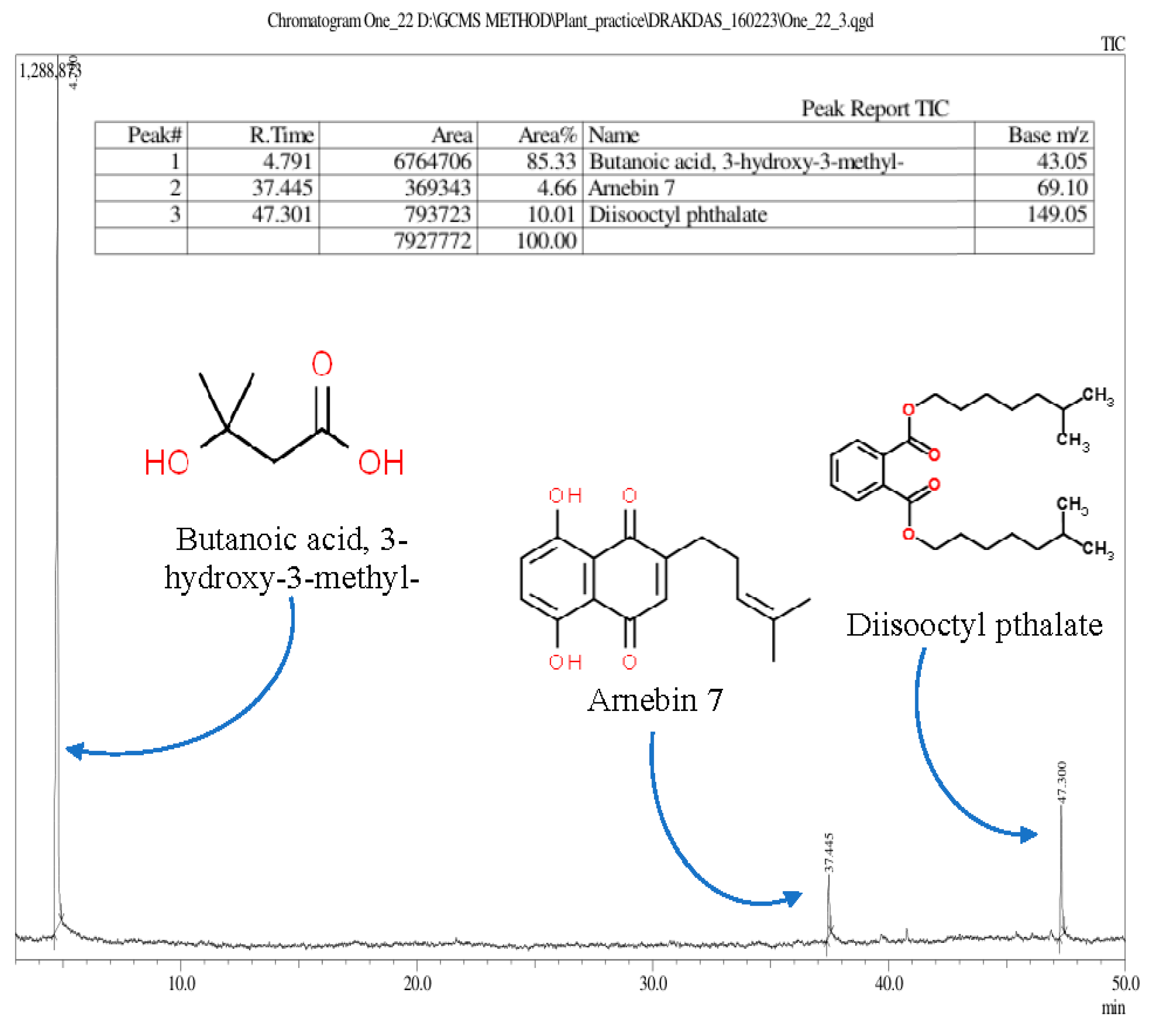
3.2.4. Identification of Phytochemicals Using Gas Chromatography–Mass Spectrometry (GC–MS/MS)
3.3. Antimicrobial Activity of RRP
3.3.1. Antimicrobial Activity Spectrum of RRP
3.3.2. Determination of MIC and MBC
3.3.3. Effect of RRP Extract on Growth Kinetics of S. aureus and L. monocytogenes
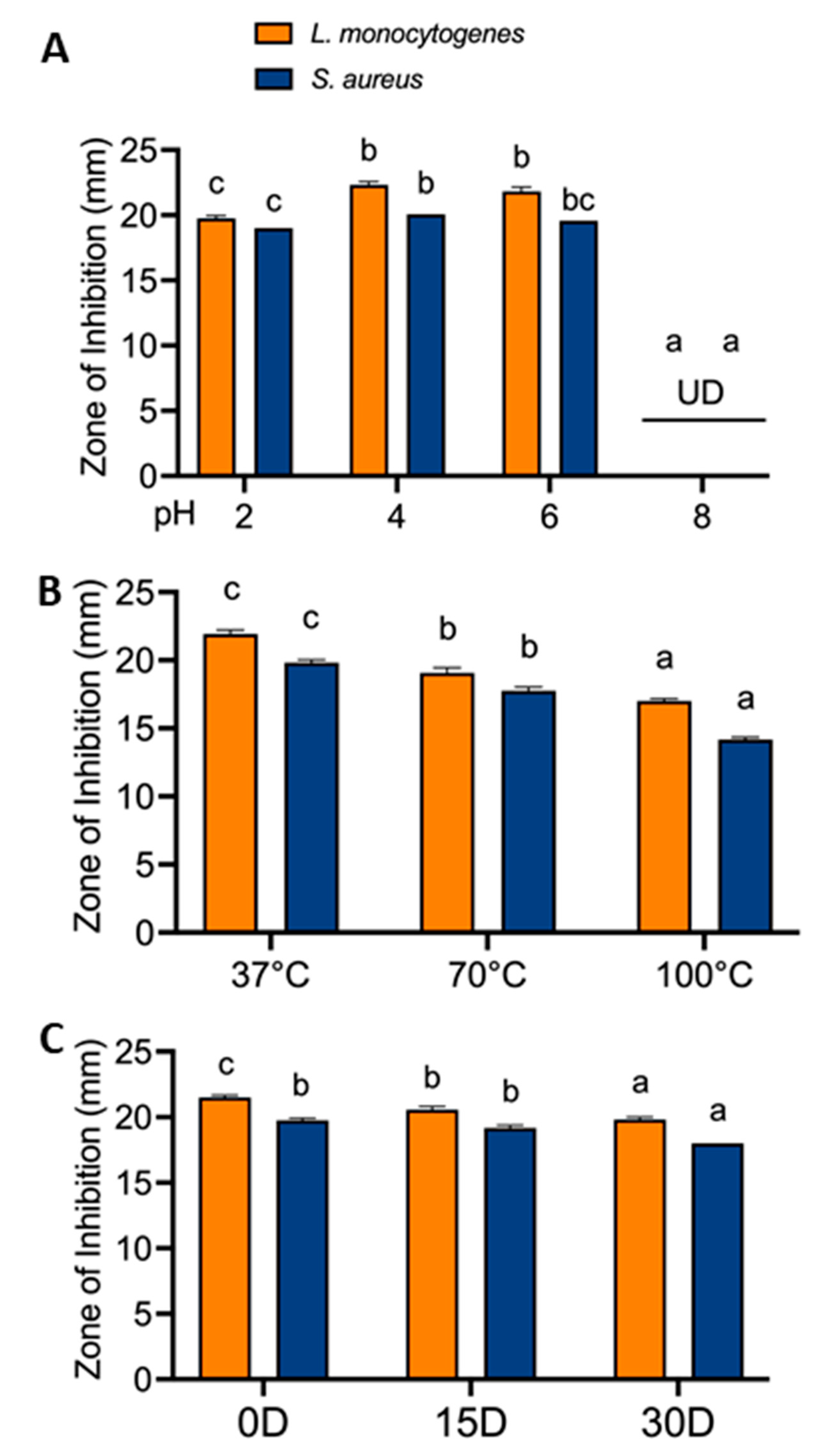
3.3.4. Stability of RRP Extract under a Wide Range of Conditions
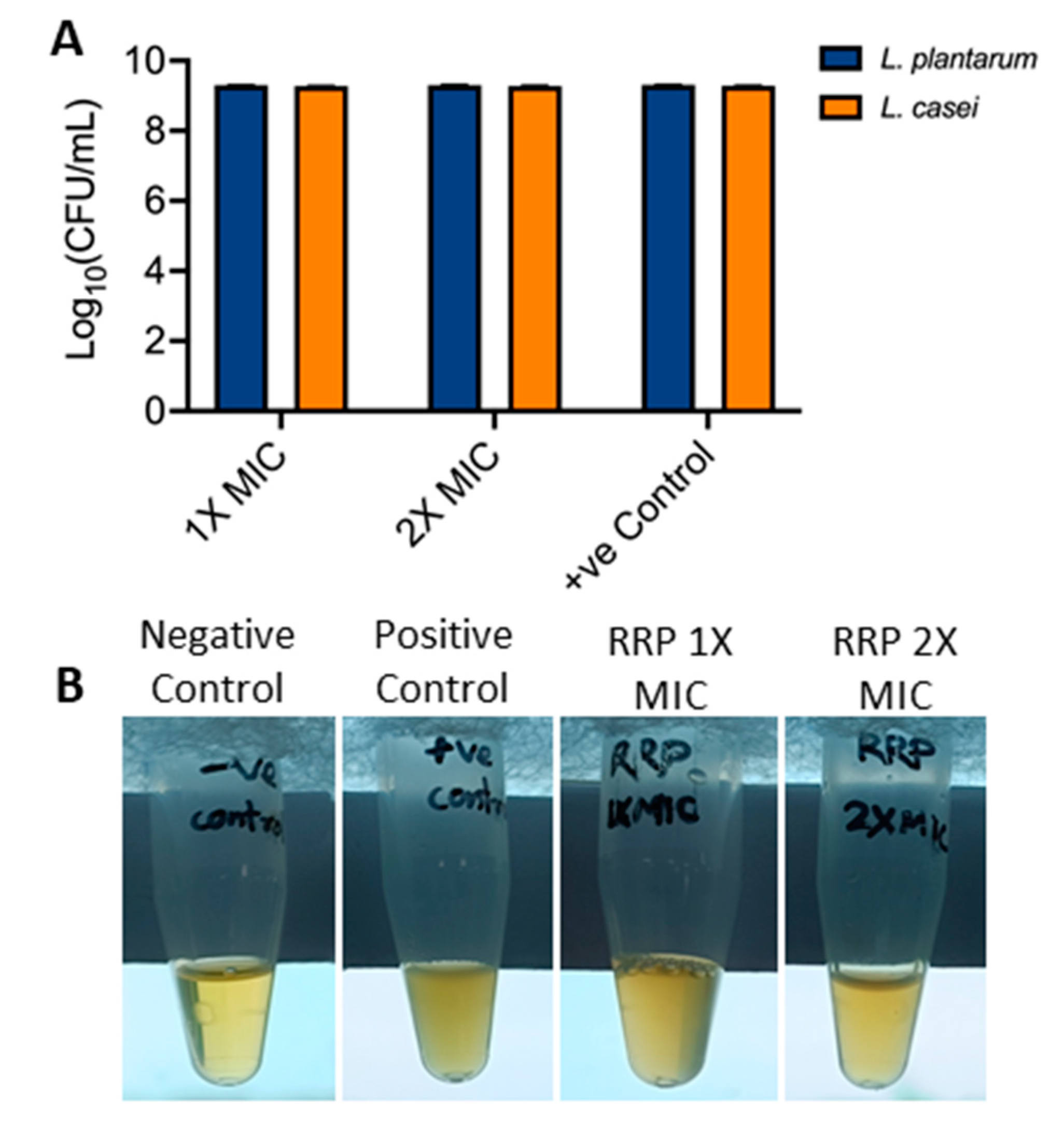
3.4. Effect of RRP on Lactobacillus Growth
3.5. Effect of RRP on Red Blood Cells
3.6. Application of RRP as Food Preservative in Meat-Model System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dey, S.; Nagababu, B.H. Applications of Food Color and Bio-Preservatives in the Food and Its Effect on the Human Health. Food Chem. Adv. 2022, 1, 100019. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, A.; Sharma, D.K.; Kishore, K. Toxicity of Food Additives. In Food Safety and Human Health; Academic Press: Cambridge, MA, USA, 2019; pp. 67–98. [Google Scholar] [CrossRef]
- Das, J.K.; Chatterjee, N.; Nanda, P.K.; Das, A.; Nath, S.; Pal, S.; Dhar, P.; Bandyopadhyay, S.; Verma, A.K.; Sen, A.; et al. Encapsulation and Delivery of Clove Essential Oil Using Nanoemulsions: Impact on the Physicochemical, Microbial, and Sensory Properties of Chicken Meatballs. Food Biophys. 2024, 19. [Google Scholar] [CrossRef]
- Das, A.; Bhattacharya, D.; Nanda, P.K.; Nath, S.; Das, A.K. Biopreservation in Meat and Meat Products. In Novel Approaches in Biopreservation for Food and Clinical Purposes; CRC Press: Boca Raton, FL, USA, 2024; pp. 66–97. [Google Scholar] [CrossRef]
- Pinto, L.; Tapia-Rodríguez, M.R.; Baruzzi, F.; Ayala-Zavala, J.F. Plant Antimicrobials for Food Quality and Safety: Recent Views and Future Challenges. Foods 2023, 12, 2315. [Google Scholar] [CrossRef] [PubMed]
- Endale, H.; Mathewos, M.; Abdeta, D. Potential Causes of Spread of Antimicrobial Resistance and Preventive Measures in One Health Perspective-A Review. Infect. Drug Resist. 2023, 16, 7515–7545. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Patani, A.; Patel, A.; Prajapati, D.; Khare, N.; Singh, S. Various Agriculture Crop Plant-Based Bioactive Compounds and Their Use in Nanomaterial Synthesis and Applications; Springer Nature: Singapore, 2023; pp. 223–241. [Google Scholar] [CrossRef]
- McClements, D.J.; Das, A.K.; Dhar, P.; Nanda, P.K.; Chatterjee, N. Nanoemulsion-Based Technologies for Delivering Natural Plant-Based Antimicrobials in Foods. Front. Sustain. Food Syst. 2021, 5, 208. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Ora, A.; Häkkinen, S.T.; Ritala, A.; Räisänen, R.; Kallioinen-Mänttäri, M.; Melin, K. Innovative Extraction Technologies of Bioactive Compounds from Plant By-Products for Textile Colorants and Antimicrobial Agents. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Yang, X.; Lan, W.; Sun, X. Antibacterial and Antioxidant Properties of Phenolic Acid Grafted Chitosan and Its Application in Food Preservation: A Review. Food Chem. 2023, 428, 136788. [Google Scholar] [CrossRef] [PubMed]
- Gooderham, N.J.; Cohen, S.M.; Eisenbrand, G.; Fukushima, S.; Guengerich, F.P.; Hecht, S.S.; Rietjens, I.M.C.M.; Rosol, T.J.; Davidsen, J.M.; Harman, C.L.; et al. FEMA GRAS Assessment of Natural Flavor Complexes: Clove, Cinnamon Leaf and West Indian Bay Leaf-Derived Flavoring Ingredients. Food Chem. Toxicol. 2020, 145, 111585. [Google Scholar] [CrossRef]
- Singh, S.; Kumar Sharma, P.; Chaturvedi, S.; Kumar, P.; Deepak Nannaware, A.; Kalra, A.; Kumar Rout, P. Biocatalyst for the Synthesis of Natural Flavouring Compounds as Food Additives: Bridging the Gap for a More Sustainable Industrial Future. Food Chem. 2024, 435, 137217. [Google Scholar] [CrossRef]
- Burdock, G.A. Assessment of Black Cumin (Nigella sativa L.) as a Food Ingredient and Putative Therapeutic Agent. Regul. Toxicol. Pharmacol. 2022, 128, 105088. [Google Scholar] [CrossRef]
- Aliyu-Amoo, H.; Isa, H.I. Antimicrobial, Antioxidant and Anti-Inflammatory Activities of the Root Extracts and Fractions of Terminalia Avicennioides Guill. and Perr. Bull. Natl. Res. Cent. 2023, 47, 137. [Google Scholar] [CrossRef]
- Akbar, S. Alkanna tinctoria (L.) Tausch (Boraginaceae). In Handbook of 200 Medicinal Plants; Springer International Publishing: Cham, Switzerland, 2020; pp. 135–138. [Google Scholar]
- Adeel, S.; Kiran, S.; Alam, M.; Farooq, T.; Amin, N.; Gulzar, T. Alkanna tinctoria-Based Sustainable Alkanin Natural Colorant for Eco-Dyeing of Wool. Environ. Sci. Pollut. Res. 2023, 30, 27073–27080. [Google Scholar] [CrossRef]
- Alshamar, H.A.; Dapson, R.W. Using Extract from Alkanet (Alkanna tinctoria) as a Source of Both a Red Lipid Stain and a Blue Counterstain for Histology. Biotech. Histochem. 2023, 98, 554–560. [Google Scholar] [CrossRef]
- Gerardi, C.; Mita, G.; Grillo, E.; Giovinazzo, G.; Miceli, A.; De Leo, P. Alkanna tinctoria T. (Alkanets): In Vitro Culture and the Production of Alkannin and Other Secondary Metabolites. In Medicinal and Aromatic Plants X. Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1998; Volume 41, pp. 14–27. [Google Scholar]
- Jaradat, N.A.; Zaid, A.N.; Hussen, F.; Issa, L.; Altamimi, M.; Fuqaha, B.; Nawahda, A.; Assadi, M. Phytoconstituents, Antioxidant, Sun Protection and Skin Anti-Wrinkle Effects Using Four Solvents Fractions of the Root Bark of the Traditional Plant Alkanna tinctoria (L.). Eur. J. Integr. Med. 2018, 21, 88–93. [Google Scholar] [CrossRef]
- Kusculu, N.; Eser, F. Applicability of Alkanet (Alkanna tinctoria) Extract for the Histological Staining of Liver Tissue. J. Indian Chem. Soc. 2022, 99, 100409. [Google Scholar] [CrossRef]
- Michalak, M. Plant Extracts as Skin Care and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 15444. [Google Scholar] [CrossRef]
- Adeel, S.; Abrar, S.; Ozomay, M.; Fazal-ur-Rehman; Hussaan, M.; Batool, F. Evolving Role of Plant Pigments in the Cosmetic Industry. In Renewable Dyes and Pigments; Elsevier: Amsterdam, The Netherlands, 2024; pp. 307–319. [Google Scholar]
- Rana, S.; Chauhan, P. Spices That Heal: Review on Untapped Potential of Lesser-Known Spices as Immunity Booster during COVID-19 Pandemic. Ann. Phytomed. Int. J. 2022, 11, 7–11. [Google Scholar] [CrossRef]
- Zannou, O.; Koca, I. Optimization and Stabilization of the Antioxidant Properties from Alkanet (Alkanna tinctoria) with Natural Deep Eutectic Solvents. Arab. J. Chem. 2020, 13, 6437–6450. [Google Scholar] [CrossRef]
- Nikolova, M.; Aneva, I.; Zhelev, P.; Semerdjieva, I.; Zheljazkov, V.D.; Vladimirov, V.; Stoyanov, S.; Berkov, S.; Yankova-Tsvetkova, E. Metabolic Profiles, Genetic Diversity, and Genome Size of Bulgarian Population of Alkanna tinctoria. Plants 2022, 12, 111. [Google Scholar] [CrossRef]
- Mercimek Takci, H.A.; Turkmen, F.U.; Anlas, F.C.; Ustun Alkan, F.; Bakirhan, P.; Demir, C.; Sekeroglu, N. Antimicrobial Activity and Cytotoxicity of Alkanna tinctoria (L.) Tausch Root Extracts. Karadeniz Fen Bilim. Derg. 2019, 9, 176–185. [Google Scholar] [CrossRef]
- Rani, V.; Revanasiddappa, B.C.; Mahendra, G.S.; Deshpande, S.; Venkatesan, J.; Appana Dalavi, P.; Prabhu, A. Cytotoxic and Apoptotic Efficacy of Alkanna tinctoria on Glioma Cells. Nat. Prod. Res. 2023, 37, 3873–3877. [Google Scholar] [CrossRef]
- Sachan, A.K.; Kumar, S.; Kumari, K.; Singh, D.; Anupam Kr Sachan, C. Medicinal Uses of Spices Used in Our Traditional Culture: World Wide. J. Med. Plants Stud. 2018, 6, 116–122. [Google Scholar]
- Malik, S.; Brudzyńska, P.; Khan, M.R.; Sytar, O.; Makhzoum, A.; Sionkowska, A. Natural Plant-Derived Compounds in Food and Cosmetics: A Paradigm of Shikonin and Its Derivatives. Materials 2023, 16, 4377. [Google Scholar] [CrossRef]
- Khan, U.A.; Rahman, H.; Qasim, M.; Hussain, A.; Azizllah, A.; Murad, W.; Khan, Z.; Anees, M.; Adnan, M. Alkanna tinctoria Leaves Extracts: A Prospective Remedy against Multidrug Resistant Human Pathogenic Bacteria. BMC Complement. Altern. Med. 2015, 15, 127. [Google Scholar] [CrossRef]
- Ngoc, P.C.; Leclercq, L.; Rossi, J.C.; Desvignes, I.; Hertzog, J.; Fabiano-Tixier, A.S.; Chemat, F.; Schmitt-Kopplin, P.; Cottet, H. Optimizing Water-Based Extraction of Bioactive Principles of Hawthorn: From Experimental Laboratory Research to Homemade Preparations. Molecules 2019, 24, 4420. [Google Scholar] [CrossRef]
- Nawaz, H.; Shad, M.A.; Rehman, N.; Andaleeb, H.; Ullah, N. Effect of Solvent Polarity on Extraction Yield and Antioxidant Properties of Phytochemicals from Bean (Phaseolus vulgaris) Seeds. Braz. J. Pharm. Sci. 2020, 56, e17129. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; pp. 152–178. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Nicoletta, P.; Anna, P.; Ananth, P.; Min, Y.; Catherine, R.-E. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Al-Owaisi, M.; Al-Hadiwi, N.; Khan, S.A. GC-MS Analysis, Determination of Total Phenolics, Flavonoid Content and Free Radical Scavenging Activities of Various Crude Extracts of Moringa Peregrina (Forssk.) Fiori Leaves. Asian Pac. J. Trop. Biomed. 2014, 4, 964–970. [Google Scholar] [CrossRef]
- Perez, C.; Pauli, M.; Bazerque, P. Antibiotic Assay by Agarwell Diffusion Method. Acta Biol. Med. Exp. 1990, 15, 113–115. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, CLSI Supplement M100, 28th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA; Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 15 August 2023).
- Olajuyigbe, O.O.; Afolayan, A.J. In Vitro Antibacterial and Time-Kill Evaluation of the Erythrina Caffra Thunb. Extract against Bacteria Associated with Diarrhoea. Sci. World J. 2012, 738314. [Google Scholar] [CrossRef]
- Gonzalez, N.; Sevillano, D.; Alou, L.; Cafini, F.; Gimenez, M.J.; Gomez-Lus, M.L.; Prieto, J.; Aguilar, L. Influence of the MBC/MIC Ratio on the Antibacterial Activity of Vancomycin versus Linezolid against Methicillin-Resistant Staphylococcus Aureus Isolates in a Pharmacodynamic Model Simulating Serum and Soft Tissue Interstitial Fluid Concentrations Reported. J. Antimicrob. Chemother. 2013, 68, 2291–2295. [Google Scholar] [CrossRef]
- Das, A.; Datta, S.; Mukherjee, S.; Bose, S.; Ghosh, S.; Dhar, P. Evaluation of Antioxidative, Antibacterial and Probiotic Growth Stimulatory Activities of Sesamum Indicum Honey Containing Phenolic Compounds and Lignans. LWT 2015, 61, 244–250. [Google Scholar] [CrossRef]
- Ebbensgaard, A.; Mordhorst, H.; Overgaard, M.T.; Nielsen, C.G.; Aarestrup, F.M.; Hansen, E.B. Comparative Evaluation of the Antimicrobial Activity of Different Antimicrobial Peptides against a Range of Pathogenic Bacteria. PLoS ONE 2015, 10, e0144611. [Google Scholar] [CrossRef]
- Das, J.K.; Chatterjee, N.; Pal, S.; Nanda, P.K.; Das, A.; Das, L.; Dhar, P.; Das, A.K. Effect of Bamboo Essential Oil on the Oxidative Stability, Microbial Attributes and Sensory Quality of Chicken Meatballs. Foods 2023, 12, 218. [Google Scholar] [CrossRef]
- Koniecko, E.K. Handbook for Meat Chemists; Avery Publishing Group Inc.: Wayne, NJ, USA, 1979; p. 55. [Google Scholar]
- Witte, V.C.; Krause, G.F.; Bailey, M.F. A New Extraction Method for Determining 2-Thiobarbituric Acid Values of Pork and Beef during Storage. J. Food Sci. 1970, 35, 582–585. [Google Scholar] [CrossRef]
- APHA. Compendium of Methods for the Microbiological Examination of Foods; APHA: Washington, DC, USA, 2015. [Google Scholar] [CrossRef]
- Guemmaz, T.; Arrar, L.; Baghiani, A. Total Phenolic Contents and Antioxidant Properties of Algerian Alkanna tinctoria Aerial Part Extracts. J. Drug Deliv. Ther. 2020, 10, 39–44. [Google Scholar] [CrossRef]
- Gao, H.; Shupe, T.F.; Eberhardt, T.L.; Hse, C.Y. Antioxidant Activity of Extracts from the Wood and Bark of Port Orford Cedar. J. Wood Sci. 2007, 53, 147–152. [Google Scholar] [CrossRef]
- Ngo, V.T.; Scarlett, C.J.; Bowyer, M.C.; Ngo, P.D.; Vuong, V.Q. Impact of Different Extraction Solvents on Bioactive Compounds and Antioxidant Capacity from the Root of Salacia chinensis L. J. Food Qual. 2017, 9305047. [Google Scholar] [CrossRef]
- Kainama, H.; Fatmawati, S.; Santoso, M.; Papilaya, P.M.; Ersam, T. The Relationship of Free Radical Scavenging and Total Phenolic and Flavonoid Contents of Garcinia Lasoar PAM. Pharm. Chem. J. 2020, 53, 1151–1157. [Google Scholar] [CrossRef]
- Kozłowska, M.; Ścibisz, I.; Przybył, J.L.; Laudy, A.E.; Majewska, E.; Tarnowska, K.; Małajowicz, J.; Ziarno, M. Antioxidant and Antibacterial Activity of Extracts from Selected Plant Material. Appl. Sci. 2022, 12, 9871. [Google Scholar] [CrossRef]
- Goyeneche, R.; Roura, S.; Ponce, A.; Vega-Gálvez, A.; Quispe-Fuentes, I.; Uribe, E.; Di Scala, K. Chemical Characterization and Antioxidant Capacity of Red Radish (Raphanus sativus L.) Leaves and Roots. J. Funct. Foods 2015, 16, 256–264. [Google Scholar] [CrossRef]
- Das, A.; Biswas, S.; Nanda, P.K.; Chatterjee, N.; Pal, S.; Dhar, P.; Verma, A.K.; Bhattacharya, D.; Koshy, R.; Das, A.K. Moringa Pod Derived Antioxidant Dietary Fibre as a Quality Enhancer in Goat Meat Nuggets. Sustain. Food Technol. 2024, 2, 232–242. [Google Scholar] [CrossRef]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and Antioxidant Assays of Polyphenols: A Review. J. Future Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Yamauchi, M.; Kitamura, Y.; Nagano, H.; Kawatsu, J.; Gotoh, H. DPPH Measurements and Structure—Activity Relationship Studies on the Antioxidant Capacity of Phenols. Antioxidants 2024, 13, 309. [Google Scholar] [CrossRef]
- Sundaram, V.; Sadhasivam, S.; Chandrasekaran, S.; Nanjian, R.; Pandian, A. Strobilanthes Heyneanus Root Extract as a Potential Source for Antioxidant and Antimicrobial Activity. Future J. Pharm. Sci. 2021, 7, 91. [Google Scholar] [CrossRef]
- Ganos, C.; Zengin, G.; Chinou, I.; Aligiannis, N.; Graikou, K. Phytochemical Profiling and Biological Assessment of the Aerial Parts from Three Mediterranean Alkanna Species (A. orientalis, A. tinctoria, A. kotschyana) in the Boraginaceae Family. Plants 2024, 13, 278. [Google Scholar] [CrossRef]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the Antioxidant Activity of Plant Extracts: Analysis of Sensitivity and Hierarchization Based on the Method Used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef]
- Angeloni, S.; Scortichini, S.; Fiorini, D.; Sagratini, G.; Vittori, S.; Neiens, S.D.; Steinhaus, M.; Zheljazkov, V.D.; Maggi, F.; Caprioli, G. Characterization of Odor-Active Compounds, Polyphenols, and Fatty Acids in Coffee Silverskin. Molecules 2020, 25, 2993. [Google Scholar] [CrossRef]
- Albak, F.; Tekin, A.R. Variation of Total Aroma and Polyphenol Content of Dark Chocolate during Three Phase of Conching. J. Food Sci. Technol. 2016, 53, 848–855. [Google Scholar] [CrossRef]
- Zamora-Gasga, V.M.; Álvarez-Vidal, C.; Montalvo-González, E.; Loarca-Piña, G.; Vázquez-Landaverde, P.A.; Bello-Pérez, L.A.; Tovar, J.; Sáyago-Ayerdi, S.G. Gut Metabolites Associated with pH and Antioxidant Capacity during in Vitro Colonic Fermentation of Mexican Corn Products. Cereal Chem. 2018, 95, 399–410. [Google Scholar] [CrossRef]
- Papageorgiou, V.; Assimopoulou, A.; Samanidou, V.; Papadoyannis, I. Analytical Methods for the Determination of Alkannins and Shikonins. Curr. Org. Chem. 2006, 10, 583–622. [Google Scholar] [CrossRef]
- Alwahibi, M.S.; Perveen, K. Chemical Analysis by GC-MS and in Vitro Antibacterial Activity of Alkanna tinctoria Extracts against Skin Infection Causing Bacteria. Biomed. Res. 2017, 28, 7946–7949. [Google Scholar]
- Sastry, V.M.V.S.; Rao, G.R.K. Dioctyl Phthalate, and Antibacterial Compound from the Marine Brown Alga ?Sargassum Wightii. J. Appl. Phycol. 1995, 7, 185–186. [Google Scholar] [CrossRef]
- Banda, G.C.; Monjerezi, M.; Sumani, J.; Mpeketula, P.M.G. Phytoconstituents and Antimycobacterial Activities of Root Extracts and Fractions from Vernonia Glabra, (Steetz) Vatke. J. Chem. 2022, 2022, 7003809. [Google Scholar] [CrossRef]
- Tung, N.H.; Du, G.-J.; Yuan, C.-S.; Shoyama, Y.; Wang, C.-Z. Isolation and Chemopreventive Evaluation of Novel Naphthoquinone Compounds from Alkanna tinctoria. Anti-Cancer Drugs 2013, 24, 1058–1068. [Google Scholar] [CrossRef]
- Malik, E.; Dennison, S.R.; Harris, F.; Phoenix, D.A. pH Dependent Antimicrobial Peptides and Proteins, Their Mechanisms of Action and Potential as Therapeutic Agents. Pharmaceuticals 2016, 9, 67. [Google Scholar] [CrossRef]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial Properties and Mechanism of Action of Some Plant Extracts against Food Pathogens and Spoilage Microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Drolia, R.; Amalaradjou, M.A.R.; Ryan, V.; Tenguria, S.; Liu, D.; Bai, X.; Xu, L.; Singh, A.K.; Cox, A.D.; Bernal-Crespo, V. Receptor-Targeted Engineered Probiotics Mitigate Lethal Listeria Infection. Nat. Commun. 2020, 11, 6344. [Google Scholar] [CrossRef]
- Banerjee, P.; Merkel, G.J.; Bhunia, A.K. Lactobacillus delbrueckii ssp. Bulgaricus B-30892 Can Inhibit Cytotoxic Effects and Adhesion of Pathogenic Clostridium Difficile to Caco-2 Cells. Gut Pathog. 2009, 1, 8. [Google Scholar] [CrossRef]
- Burkholder, K.M.; Bhunia, A.K. Salmonella Enterica Serovar Typhimurium Adhesion and Cytotoxicity during Epithelial Cell Stress Is Reduced by Lactobacillus rhamnosus GG. Gut Pathog. 2009, 1, 14. [Google Scholar] [CrossRef]
- Frassinetti, S.; Gabriele, M.; Moccia, E.; Longo, V.; Di Gioia, D. Antimicrobial and Antibiofilm Activity of Cannabis Sativa L. Seeds Extract against Staphylococcus Aureus and Growth Effects on Probiotic Lactobacillus spp. LWT 2020, 124, 109149. [Google Scholar] [CrossRef]
- Milutinović, M.; Dimitrijević-Branković, S.; Rajilić-Stojanović, M. Plant Extracts Rich in Polyphenols as Potent Modulators in the Growth of Probiotic and Pathogenic Intestinal Microorganisms. Front. Nutr. 2021, 8, 688843. [Google Scholar] [CrossRef]
- Holkem, A.T.; Silva, d.M.P.; Favaro-Trindade, C.S. Probiotics and Plant Extracts: A Promising Synergy and Delivery Systems. Crit. Rev. Food Sci. Nutr. 2023, 63, 9561–9579. [Google Scholar] [CrossRef]
- Ziarno, M.; Kozłowska, M.; Ścibisz, I.; Kowalczyk, M.; Pawelec, S.; Stochmal, A.; Szleszyński, B. The Effect of Selected Herbal Extracts on Lactic Acid Bacteria Activity. Appl. Sci. 2021, 11, 3898. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, S.; Yang, G.; Zhao, W.; Li, H. Development and Evaluation of a Herbal Formulation with Anti-Pathogenic Activities and Probiotics Stimulatory Effects. J. Integr. Agric. 2016, 15, 1103–1111. [Google Scholar] [CrossRef]
- Gkalpinos, V.K.; Anagnostou, V.A.; Mitropoulou, G.; Kompoura, V.; Karapantzou, I.; Fasoulis, C.K.; Vasdekis, E.P.; Kourkoutas, Y.; Tzakos, A.G. Aloysia Citrodora Extracts Cultivated in Greece as Antioxidants and Potent Regulators of Food Microbiota. Appl. Sci. 2023, 13, 3663. [Google Scholar] [CrossRef]
- China, R.; Mukherjee, S.; Sen, S.; Bose, S.; Datta, S.; Koley, H.; Ghosh, S.; Dhar, P. Antimicrobial Activity of Sesbania Grandiflora Flower Polyphenol Extracts on Some Pathogenic Bacteria and Growth Stimulatory Effect on the Probiotic Organism Lactobacillus Acidophilus. Microbiol. Res. 2012, 167, 500–506. [Google Scholar] [CrossRef]
- Selin, C.; Stietz, M.S.; Blanchard, J.E.; Gehrke, S.S.; Bernard, S.; Hall, D.G.; Brown, E.D.; Cardona, S.T. A Pipeline for Screening Small Molecules with Growth Inhibitory Activity against Burkholderia Cenocepacia. PLoS ONE 2015, 10, e0128587. [Google Scholar] [CrossRef]
- Bowes, J.; Brown, A.J.; Hamon, J.; Jarolimek, W.; Sridhar, A.; Waldron, G.; Whitebread, S. Reducing Safety-Related Drug Attrition: The Use of in Vitro Pharmacological Profiling. Nat. Rev. Drug Discov. 2012, 11, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Rodi, P.M.; Gianello, M.B.; Corregido, M.C.; Gennaro, A.M. Comparative Study of the Interaction of CHAPS and Triton X-100 with the Erythrocyte Membrane. Biochim. Biophys. Acta (BBA)-Biomembr. 2014, 1838, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Afsar, T.; Razak, S.; Khan, M.R.; Mawash, S.; Almajwal, A.; Shabir, M.; Haq, I.U. Evaluation of Antioxidant, Anti-Hemolytic and Anticancer Activity of Various Solvent Extracts of Acacia Hydaspica R. Parker Aerial Parts. BMC Complement. Altern. Med. 2016, 16, 258. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, S.; Ahmad, S.; Rehman Khan, K.; Tabassum, F.; Khursheed, A.; Zaman, Q.; Bukhari, N.; Alfagham, A.; Hatamleh, A.; Chen, Y. Phytochemical Profiling, Antioxidant, Anti-Inflammatory, Thrombolytic, Hemolytic Activity In Vitro and In Silico Potential of Portulacaria Afra. Molecules 2022, 27, 2377. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Tasnim, H.; Arshad, L.; Haque, M.A.; Tareq, S.M.; Kamal, A.T.M.M.; Rahman, M.M.; Reza, A.S.M.A.; Chowdhury, K.A.A.; Tareq, A.M. Stem Extract of Albizia Richardiana Exhibits Potent Antioxidant, Cytotoxic, Antimicrobial, Anti-Inflammatory and Thrombolytic Effects through in Vitro Approach. Clin. Phytosci. 2020, 6, 60. [Google Scholar] [CrossRef]
- Karim, M.A.; Islam, M.A.; Islam, M.M.; Rahman, M.S.; Sultana, S.; Biswas, S.; Hosen, M.J.; Mazumder, K.; Rahman, M.M.; Hasan, M.N. Evaluation of Antioxidant, Anti-Hemolytic, Cytotoxic Effects and Anti-Bacterial Activity of Selected Mangrove Plants (Bruguiera gymnorrhiza and Heritiera littoralis) in Bangladesh. Clin. Phytosci. 2020, 6, 8. [Google Scholar] [CrossRef]
- Fssai Chapter 2, Food Product Standards, Meat And Meat Products 2023. Available online: https://www.fssai.gov.in/upload/uploadfiles/files/6_%20Chapter%202_5%20(Meat%20and%20Meat%20products).pdf (accessed on 5 February 2024).
- Taormina, P.J. Purposes and Principles of Shelf Life Determination. In Food Safety and Quality-Based Shelf Life of Perishable Foods; Taormina, P.J., Hardin, M.D., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–26. ISBN 978-3-030-54374-7. [Google Scholar]
- Das, A.K.; Nanda, P.K.; Madane, P.; Biswas, S.; Das, A.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Antioxidant Dietary Fibre Enriched Meat-Based Functional Foods. Trends Food Sci. Technol. 2020, 99, 323–336. [Google Scholar] [CrossRef]
- Chauhan, P.; Pradhan, S.R.; Das, A.; Nanda, P.K.; Bandyopadhyay, S.; Das, A.K. Inhibition of Lipid and Protein Oxidation in Raw Ground Pork by Terminalia Arjuna Fruit Extract during Refrigerated Storage. Asian-Australas. J. Anim. Sci. 2019, 32, 265–273. [Google Scholar] [CrossRef]
- Madane, P.; Das, A.K.; Nanda, P.K.; Bandyopadhyay, S.; Jagtap, P.; Shewalkar, A.; Maity, B. Dragon Fruit (Hylocereus undatus) Peel as Antioxidant Dietary Fibre on Quality and Lipid Oxidation of Chicken Nuggets. J. Food Sci. Technol. 2020, 57, 1449–1461. [Google Scholar] [CrossRef]
- Zwolan, A.; Pietrzak, D.; Adamczak, L.; Chmiel, M.; Kalisz, S.; Wirkowska-Wojdyła, M.; Florowski, T.; Oszmiański, J. Effects of Nigella sativa L. Seed Extracts on Lipid Oxidation and Color of Chicken Meatballs during Refrigerated Storage. LWT 2020, 130, 109718. [Google Scholar] [CrossRef]


| Culture | Source |
|---|---|
| Staphylococcus aureus ATCC 25923 | American Type Culture Collection (ATCC), Manasas, VA, USA |
| Listeria monocytogenes ATCC 19111 | ATCC, Manasas, VA, USA |
| Listeria monocytogenes ATCC 13932 | ATCC, Manasas, VA, USA |
| Salmonella enterica serovar Typhimurium ATCC 14028 | ATCC, Manasas, VA, USA |
| Escherichia coli ATCC 25922 | ATCC, Manasas, VA, USA |
| Lactiplantibacillus plantarum MTCC 2621 | Microbial Type Culture Collection and Gene Bank (MTCC), Chandigarh, India |
| Lacticaseibacillus casei MTCC 1423 | MTCC, Chandigarh, India |
| Staphylococcus aureus strain MZP/SM/05 | Swine meat from Mirzapur, Uttar Pradesh, India |
| Staphylococcus aureus MZP/PM/38 | Poultry meat from Mirzapur, Uttar Pradesh, India |
| Staphylococcus aureus MZP/GM/02 | Goat meat from Mirzapur, Uttar Pradesh, India |
| Staphylococcus aureus ALD/PM/22 | Poultry meat from Allahabad, Uttar Pradesh, India |
| Staphylococcus aureus ALD/PM/23 | Poultry meat from Allahabad, Uttar Pradesh, India |
| Staphylococcus aureus LKO/GM/14 | Goat meat from Lucknow, Uttar Pradesh, India |
| Staphylococcus aureus LKO/PM/04 | Poultry meat from Lucknow, Uttar Pradesh, India |
| Staphylococcus aureus LKO/PM/08 | Poultry meat from Lucknow, Uttar Pradesh, India |
| Staphylococcus aureus LKO/GM/11 | Goat meat from Lucknow, Uttar Pradesh, India |
| Parameter | Ethanol Extract ± SE * | MAHW Extract ± SE * |
|---|---|---|
| Total Phenolic Content (TPC)% | 18.27 ± 0.69 b | 6.29 ± 0.30 a |
| pH | 2.92 ± 0.02 a | 3.15 ± 0.01 b |
| CIELAB color parameters | ||
| L* | 25.07 ± 0.97 a | 56.55 ± 0.79 b |
| a* | 45.13 ± 0.44 b | 12.02 ± 0.76 a |
| b* | 63.35 ± 0.53 | 62.20 ± 0.65 |
| H°ab | 2.61 ± 0.08 a | 41.70 ± 0.31 b |
| C*ab | 77.97 ± 0.61 | 63.72 ± 0.38 |
| Bacteria | Zone of Inhibition (mm) (Mean ± SE *) | |
|---|---|---|
| Ethanol Extract | Hot Water Extract | |
| Staphylococcus aureus ATCC 25923 | 19.75 ± 0.11 ef | 0.00 ± 0.00 |
| Listeria monocytogenes ATCC 13932 | 21.17 ± 0.28 gh | 0.00 ± 0.00 |
| L. monocytogenes ATCC 19111 | 21.92 ± 0.33 h | 0.00 ± 0.00 |
| Salmonella Typhimurium ATCC 14028 | 9.92 ± 0.15 b | 0.00 ± 0.00 |
| Escherichia coli ATCC 25922 | 8.58 ± 0.24 a | 0.00 ± 0.00 |
| S. aureus MZP/SM/05 | 21.17 ± 0.40 gh | 0.00 ± 0.00 |
| S. aureus MZP/PM/38 | 18.25 ± 0.11 d | 0.00 ± 0.00 |
| S. aureus MZP/GM/02 | 20.08 ±0.27 fg | 0.00 ± 0.00 |
| S. aureus ALD/PM/22 | 19.50 ± 0.26 ef | 0.00 ± 0.00 |
| S. aureus ALD/PM/23 | 18.67 ± 0.25 de | 0.00 ± 0.00 |
| S. aureus LKO/GM/14 | 27.50 ± 0.22 i | 0.00 ± 0.00 |
| S. aureus LKO/PM/04 | 16.00 ± 0.13 c | 0.00 ± 0.00 |
| S. aureus LKO/PM/08 | 20.00 ±0.37 fg | 0.00 ± 0.00 |
| S. aureus LKO/GM/11 | 27.83 ± 0.21 i | 0.00 ± 0.00 |
| Bacteria | RRP Ethanol Extract (mg/mL) * | Ciprofloxacin (µg/mL) * |
|---|---|---|
| MIC | ||
| Salmonella Typhimurium ATCC 14028 | 25.00 ± 0.00 c | 0.125 ± 0.00 a |
| Escherichia coli ATCC 25922 | 25.00 ± 0.00 c | 0.125 ± 0.00 a |
| Staphylococcus aureus ATCC 25923 | 0.049 ± 0.00 a | 0.250 ± 0.00 b |
| Listeria monocytogenes ATCC 19111 | 0.098 ± 0.00 b | 0.250 ± 0.00 b |
| MBC | ||
| Salmonella Typhimurium ATCC 14028 | 25.00 ± 0.00 c | 0.25 ± 0.00 a |
| Escherichia coli ATCC 25922 | 25.00 ± 0.00 c | 0.25 ± 0.00 a |
| Staphylococcus aureus ATCC 25923 | 0.098 ± 0.00 a | 0.50 ± 0.00 b |
| Listeria monocytogenes ATCC 19111 | 0.195 ± 0.00 b | 0.50 ± 0.00 b |
| Tolerance | ||
| Salmonella Typhimurium ATCC 14028 | 1.00 ± 0.00 a | 2.00 ± 0.00 |
| Escherichia coli ATCC 25922 | 1.00 ± 0.00 a | 2.00 ± 0.00 |
| Staphylococcus aureus ATCC 25923 | 2.00 ± 0.00 b | 2.00 ± 0.00 |
| Listeria monocytogenes ATCC 19111 | 2.00 ± 0.00 b | 2.00 ± 0.00 |
| Sample | Refrigerated Storage Days * | ||||
|---|---|---|---|---|---|
| Day 0 | Day 5 | Day 10 | Day 15 | Day 20 | |
| Color and Appearance | |||||
| Control | 6.39 ± 0.07 aA | 6.28 ± 0.04 aB | 5.86 ± 0.06 aC | 5.44 ± 0.12 aD | 5.27 ± 0.14 aE |
| T1 | 7.41 ± 0.05 bA | 7.33 ± 0.09 bB | 7.16 ± 0.05 bC | 6.94 ± 0.09 bD | 6.38 ± 0.21 bE |
| T2 | 7.42 ± 0.04 bA | 7.37 ± 0.04 cB | 7. 24 ± 0.11 cC | 7.01 ± 0.05 cD | 6.63 ± 0.13 cE |
| Flavor | |||||
| Control | 6.37 ± 0.09 aA | 6.30 ± 0.13 aB | 5.86 ± 0.06 aC | 4.98 ± 0.10 aD | 4.04 ± 0.06 aE |
| T1 | 6.52 ± 0.04 bA | 6.50 ± 0.20 bA | 6.26 ± 0.02 bC | 6.07 ± 0.17 bD | 5.63 ± 0.03 bE |
| T2 | 6.76 ± 0.06 cA | 6.74 ± 0.21 cA | 6.33 ± 0.04 cB | 6.19 ± 0.11 cC | 5.81 ± 0.04 cD |
| Texture and Tenderness | |||||
| Control | 6.76 ± 0.18 aA | 6.68 ± 0.13 aB | 6.21 ± 0.07 aC | 5.69 ± 0.05 aD | 5.46 ± 0.21 aE |
| T1 | 6.82 ± 0.05 bA | 6.80 ± 0.27 bA | 6.64 ± 0.06 bB | 6.23 ± 0.11 bC | 6.09 ± 0.18 bD |
| T2 | 6.84 ± 0.06 bA | 6.82 ± 0.12 bA | 6.73 ± 0.09 cB | 6.44 ± 0.04 cC | 6.15 ± 0.21 cD |
| Juiciness | |||||
| Control | 6.25 ± 0.10 aA | 6.21 ± 0.17 aB | 5.69 ± 0.38 aC | 5.20 ± 0.07 aD | 5.04 ± 0.04 aE |
| T1 | 6.28 ± 0.12 bA | 6.26 ± 0.15 abA | 5.86 ± 0.24 bB | 5.41 ± 0.10 bC | 5.16 ± 0.11 bD |
| T2 | 6.30 ± 0.17 bA | 6.29 ± 0.04 bA | 5.98 ± 0.21 cB | 5.67 ± 0.10 cC | 5.23 ± 0.10 cD |
| Overall Acceptability | |||||
| Control | 6.84 ± 0.09 aA | 6.80 ± 0.04 aB | 5.68 ± 0.21 aC | 4.98 ± 0.38 aD | 4.02 ± 0.50 aE |
| T1 | 6.89 ± 0.04 bA | 6.86 ± 0.08 bA | 6.04 ± 0.18 bB | 5.65 ± 0.17 bC | 5.06 ± 0.26 bD |
| T2 | 6.92 ± 0.07 bA | 6.91 ± 0.10 cA | 6.17 ± 0.23 cB | 5.84 ± 0.15 cC | 5.13 ± 0.14 cD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, A.; Biswas, S.; Satyaprakash, K.; Bhattacharya, D.; Nanda, P.K.; Patra, G.; Moirangthem, S.; Nath, S.; Dhar, P.; Verma, A.K.; et al. Ratanjot (Alkanna tinctoria L.) Root Extract, Rich in Antioxidants, Exhibits Strong Antimicrobial Activity against Foodborne Pathogens and Is a Potential Food Preservative. Foods 2024, 13, 2254. https://doi.org/10.3390/foods13142254
Das A, Biswas S, Satyaprakash K, Bhattacharya D, Nanda PK, Patra G, Moirangthem S, Nath S, Dhar P, Verma AK, et al. Ratanjot (Alkanna tinctoria L.) Root Extract, Rich in Antioxidants, Exhibits Strong Antimicrobial Activity against Foodborne Pathogens and Is a Potential Food Preservative. Foods. 2024; 13(14):2254. https://doi.org/10.3390/foods13142254
Chicago/Turabian StyleDas, Annada, Subhasish Biswas, Kaushik Satyaprakash, Dipanwita Bhattacharya, Pramod Kumar Nanda, Gopal Patra, Sushmita Moirangthem, Santanu Nath, Pubali Dhar, Arun K. Verma, and et al. 2024. "Ratanjot (Alkanna tinctoria L.) Root Extract, Rich in Antioxidants, Exhibits Strong Antimicrobial Activity against Foodborne Pathogens and Is a Potential Food Preservative" Foods 13, no. 14: 2254. https://doi.org/10.3390/foods13142254
APA StyleDas, A., Biswas, S., Satyaprakash, K., Bhattacharya, D., Nanda, P. K., Patra, G., Moirangthem, S., Nath, S., Dhar, P., Verma, A. K., Biswas, O., Tardi, N. I., Bhunia, A. K., & Das, A. K. (2024). Ratanjot (Alkanna tinctoria L.) Root Extract, Rich in Antioxidants, Exhibits Strong Antimicrobial Activity against Foodborne Pathogens and Is a Potential Food Preservative. Foods, 13(14), 2254. https://doi.org/10.3390/foods13142254







