Revitalising Riboflavin: Unveiling Its Timeless Significance in Human Physiology and Health
Abstract
1. Introduction
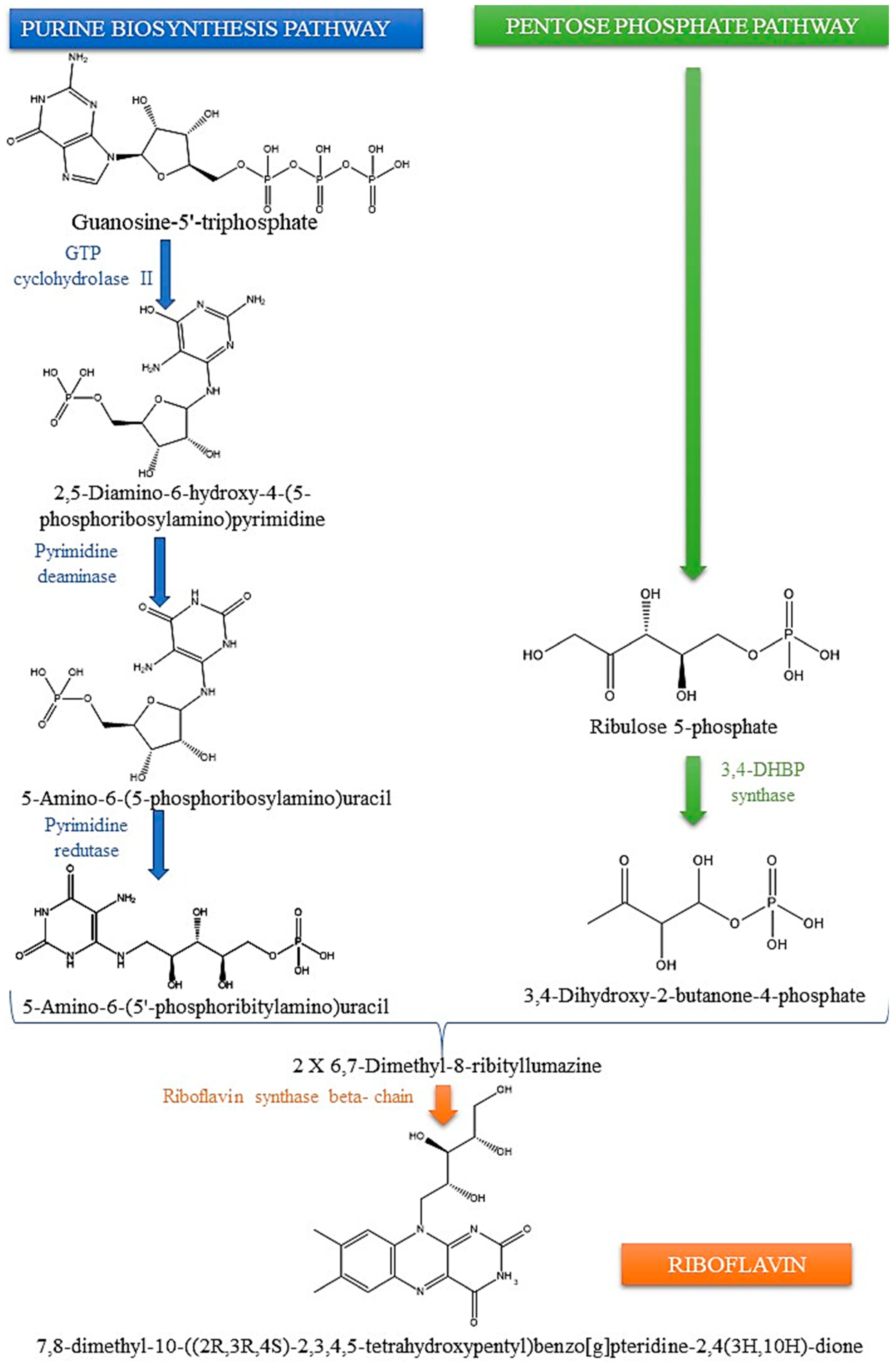
2. Insights into the Biosynthesis of Riboflavin: Microbial and Plant Pathways
3. The Vital Role of Riboflavin in Human Health
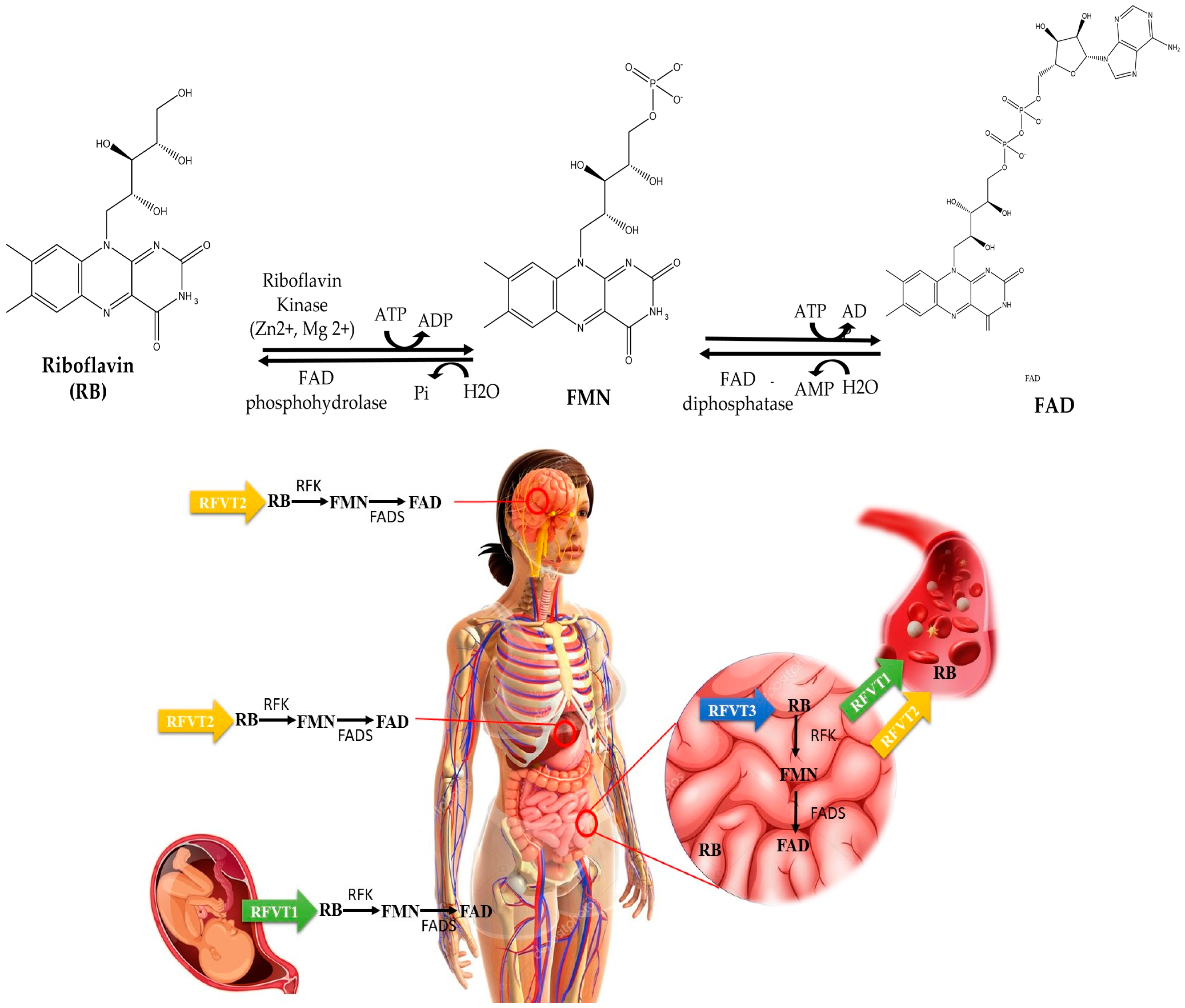
4. Deciphering the Riboflavin Journey: Absorption, Transport, and Metabolism
5. Metabolic Role of Riboflavin
6. Disorders in Riboflavin Metabolism: Malfunctions in Transportation
7. Primary Dysfunction in Riboflavin Coenzyme Pathways
8. Malfunctions within the Human Flavoproteome
9. Riboflavin’s Interactions with Other Nutrients
10. Implications of Riboflavin in Diseases
10.1. Deficiency Signs
10.2. Anaemia
10.3. Migraine
10.4. Diabetes Mellitus
10.5. Cataracts
10.6. Childhood Neuropathy
10.7. Hypertension
10.8. Cancer
11. Measurable Indicators of Riboflavin Concentrations
11.1. Erythrocyte Glutathione Reductase Activation Coefficient
11.2. Direct Riboflavin Biomarkers
11.3. Excretion of Riboflavin in Urine
12. Conclusions and Final Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uribe, N.G.; García-Galbis, M.R.; Espinosa, R.M.M. New Advances about the Effect of Vitamins on Human Health: Vitamins Supplements and Nutritional Aspects. In Functional Food-Improve Health through Adequate Food; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Bacher, A.; Eberhardt, S.; Fischer, M.; Kis, K.; Richter, G. Biosynthesis of vitamin B2 (riboflavin). Annu. Rev. Nutr. 2000, 20, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Bacher, A.; Eberhardt, S.; Eisenreich, W.; Fischer, M.; Herz, S.; Illarionov, B.; Kis, K.; Richter, G. Biosynthesis of riboflavin. Vitam. Horm. 2001, 69, 1–49. [Google Scholar] [CrossRef]
- Cassier-Chauvat, C.; Marceau, F.; Farci, S.; Ouchane, S.; Chauvat, F. The Glutathione System: A Journey from Cyanobacteria to Higher Eukaryotes. Antioxidants 2023, 12, 1199. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.R.; Iwata, M.; von Andrian, U.H. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Saedisomeolia, A.; Ashoori, M. Riboflavin in Human Health: A Review of Current Evidences. Adv. Food Nutr. Res. 2018, 83, 57–81. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Cunningham-Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Macheroux, P.; Kappes, B.; Ealick, S.E. Flavogenomics—A genomic and structural view of flavin-dependent proteins. FEBS J. 2011, 278, 2625–2634. [Google Scholar] [CrossRef]
- Martínez-Limón, A.; Alriquet, M.; Lang, W.-H.; Calloni, G.; Wittig, I.; Vabulas, R.M. Recognition of enzymes lacking bound cofactor by protein quality control. Proc. Natl. Acad. Sci. USA 2016, 113, 12156–12161. [Google Scholar] [CrossRef]
- Northrop-Clewes, C.A.; Thurnham, D.I. The Discovery and Characterization of Riboflavin. Ann. Nutr. Metab. 2012, 61, 224–230. [Google Scholar] [CrossRef]
- Cimino, J.A.; Jhangiani, S.; Schwartz, E.; Cooperman, J.M. Riboflavin Metabolism in the Hypothyroid Human Adult. Exp. Biol. Med. 1987, 184, 151–153. [Google Scholar] [CrossRef]
- Powers, H.J. Riboflavin (vitamin B-2) and health. Am. J. Clin. Nutr. 2003, 77, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.; Carmo, A.M. CD6 as a Therapeutic Target in Autoimmune Diseases: Successes and Challenges. BioDrugs 2013, 27, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Bishayi, B. Riboflavin along with antibiotics balances reactive oxygen species and inflammatory cytokines and controls Staphylococcus aureus infection by boosting murine macrophage function and regulates inflammation. J. Inflamm. 2016, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Farah, N.; Chin, V.K.; Chong, P.P.; Lim, W.F.; Lim, C.W.; Basir, R.; Chang, S.K.; Lee, T.Y. Riboflavin as a promising antimicrobial agent? A multi-perspective review. Curr. Res. Microb. Sci. 2022, 3, 100111. [Google Scholar] [CrossRef]
- Alam, M.M.; Iqbal, S.; Naseem, I. Ameliorative effect of riboflavin on hyperglycemia, oxidative stress and DNA damage in type-2 diabetic mice: Mechanistic and therapeutic strategies. Arch. Biochem. Biophys. 2015, 584, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, M.; Sato, Y.; Okubo, T.; Todo, H.; Hasegawa, T.; Sugibayashi, K. Conversion of FAD to FMN and Riboflavin in Plasma: Effects of Measuring Method. Biol. Pharm. 2006, 28, 1779–1782. Available online: https://www.jstage.jst.go.jp/article/bpb/29/8/29_8_1779/_pdf (accessed on 2 May 2024). [CrossRef] [PubMed][Green Version]
- McNulty, H.; Pentieva, K.; Ward, M. Causes and Clinical Sequelae of Riboflavin Deficiency. Annu. Rev. Nutr. 2023, 43, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M. A Review on Different Dietary Sources of Important Vitamins and Electrolytes. Int. J. Res. Publ. Rev. 2023, 4, 731–736. [Google Scholar] [CrossRef]
- Olfat, N.; Ashoori, M.; Saedisomeolia, A. Riboflavin is an antioxidant: A review update. Br. J. Nutr. 2022, 128, 1887–1895. [Google Scholar] [CrossRef]
- Fischer, M.; Bacher, A. Biosynthesis of vitamin B 2 in plants. Physiol. Plant. 2006, 126, 304–318. [Google Scholar] [CrossRef]
- Revuelta, J.L.; Ledesma-Amaro, R.; Lozano-Martinez, P.; Díaz-Fernández, D.; Buey, R.M.; Jiménez, A. Bioproduction of riboflavin: A bright yellow history. J. Ind. Microbiol. Biotechnol. 2017, 44, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Sarkar, D.; Ray, N.; Talukdar, A. Understanding the riboflavin biosynthesis pathway for the development of antimicrobial agents. Med. Res. Rev. 2019, 39, 1338–1371. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.; McMurtrey, C.; Sorensen, M.L.; Huber, M.E.; Kurapova, R.; Coleman, F.T.; Mizgerd, J.P.; Hildebrand, W.; Kronenberg, M.; Lewinsohn, D.M.; et al. Riboflavin Metabolism Variation among Clinical Isolates of Streptococcus pneumoniae Results in Differential Activation of Mucosal-associated Invariant T Cells. Am. J. Respir. Cell Mol. Biol. 2018, 58, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Lyzak, O.O.; Ledesma-Amaro, R.; Dmytruk, K.V.; Sibirny, A.A.; Revuelta, J.L. Molecular Studies of the Flavinogenic Fungus Ashbya gossypii and the Flavinogenic Yeast Candida famata. In Biotechnology of Yeasts and Filamentous Fungi; Springer International Publishing: Cham, Switzerland, 2017; pp. 281–296. [Google Scholar] [CrossRef]
- Hasnain, G.; Frelin, O.; Roje, S.; Ellens, K.W.; Ali, K.; Guan, J.C.; Garrett, T.J.; de Crécy-Lagard, V.; Gregory, J.F., III; McCarty, D.R.; et al. Identification and characterization of the missing pyrimidine reductase in the plant riboflavin biosynthesis pathway. Plant Physiol. 2013, 161, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Gambhir, G.; Dass, A.; Tripathi, A.K.; Singh, A.; Jha, A.K.; Yadava, P.; Choudhary, M.; Rakshit, S. Genetically modified crops: Current status and future prospects. Planta 2020, 251, 91. [Google Scholar] [CrossRef] [PubMed]
- Giancaspero, T.A.; Busco, G.; Panebianco, C.; Carmone, C.; Miccolis, A.; Liuzzi, G.M.; Colella, M.; Barile, M. FAD Synthesis and Degradation in the Nucleus Create a Local Flavin Cofactor Pool. J. Biol. Chem. 2013, 288, 29069–29080. [Google Scholar] [CrossRef] [PubMed]
- Kirschning, A. On the evolution of coenzyme biosynthesis. Nat. Prod. Rep. 2022, 39, 2175–2199. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, G.; Bacher, A. Biosynthesis of riboflavin. An aliphatic intermediate in the formation of 6,7-dimethyl-8-ribityllumazine from pentose phosphate. Biochem. Biophys. Res. Commun. 1985, 127, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Mosegaard, S.; Dipace, G.; Bross, P.; Carlsen, J.; Gregersen, N.; Olsen, R.K.J. Riboflavin Deficiency—Implications for General Human Health and Inborn Errors of Metabolism. Int. J. Mol. Sci. 2020, 21, 3847. [Google Scholar] [CrossRef]
- Yonezawa, A.; Inui, K. Novel riboflavin transporter family RFVT/SLC52: Identification, nomenclature, functional characterization and genetic diseases of RFVT/SLC52. Mol. Asp. Med. 2013, 34, 693–701. [Google Scholar] [CrossRef]
- Kennedy, D. B Vitamins and the Brain: Mechanisms, Dose and Efficacy—A Review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Marashly, E.T.; Bohlega, S.A. Riboflavin Has Neuroprotective Potential: Focus on Parkinson’s Disease and Migraine. Front. Neurol. 2017, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Laiño, J.E.; Del Valle, M.J.; Vannini, V.V.; van Sinderen, D.; Taranto, M.P.; de Valdez, G.F.; de Giori, G.S.; Sesma, F. B-Group vitamin production by lactic acid bacteria—Current knowledge and potential applications. J. Appl. Microbiol. 2011, 111, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Tardy, A.-L.; Pouteau, E.; Marquez, D.; Yilmaz, C.; Scholey, A. Vitamins and Minerals for Energy, Fatigue and Cognition: A Narrative Review of the Biochemical and Clinical Evidence. Nutrients 2020, 12, 228. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.S.; Subramanya, S.B.; Ghosal, A.; Said, H.M. Chronic alcohol feeding inhibits physiological and molecular parameters of intestinal and renal riboflavin transport. Am. J. Physiol.-Cell Physiol. 2013, 305, C539–C546. [Google Scholar] [CrossRef] [PubMed]
- Plantone, D.; Pardini, M.; Rinaldi, G. Riboflavin in Neurological Diseases: A Narrative Review. Clin. Drug Investig. 2021, 41, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Mensink, G.B.M.; Fletcher, R.; Gurinovic, M.; Huybrechts, I.; Lafay, L.; Serra-Majem, L.; Szponar, L.; Tetens, I.; Verkaik-Kloosterman, J.; Baka, A.; et al. Mapping low intake of micronutrients across Europe. Br. J. Nutr. 2013, 110, 755–773. [Google Scholar] [CrossRef] [PubMed]
- Fabian, E.; Majchrzak, D.; Dieminger, B.; Meyer, E.; Elmadfa, I. Influence of Probiotic and Conventional Yoghurt on the Status of Vitamins. Ann. Nutr. Metab. 2008, 52, 29–36. [Google Scholar] [CrossRef]
- Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A. Dietary Reference Values for riboflavin. EFSA J. 2017, 15, e04919. [Google Scholar] [CrossRef]
- Kehoe, L.; Walton, J.; Hopkins, S.M.; McNulty, B.A.; Nugent, A.P.; McNulty, H.; Ward, M.; Flynn, A. Intake, status and dietary sources of riboflavin in a representative sample of Irish adults aged 18–90 years. Proc. Nutr. Soc. 2018, 77, E66. [Google Scholar] [CrossRef]
- Flynn, A.; Moreiras, O.; Stehle, P.; Fletcher, R.J.; Müller, D.J.G.; Rolland, V. Vitamins and minerals: A model for safe addition to foods. Eur. J. Nutr. 2003, 42, 118–130. [Google Scholar] [CrossRef]
- Balasubramaniam, S.; Christodoulou, J.; Rahman, S. Disorders of riboflavin metabolism. J. Inherit. Metab. Dis. 2019, 42, 608–619. [Google Scholar] [CrossRef]
- Hovdenak, N.; Haram, K. Influence of mineral and vitamin supplements on pregnancy outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 164, 127–132. [Google Scholar] [CrossRef]
- Smith, L.D.; Garg, U. Disorders of vitamins and cofactors. In Biomarkers in Inborn Errors of Metabolism; Elsevier: Amsterdam, The Netherlands, 2017; pp. 361–397. [Google Scholar] [CrossRef]
- Barile, M.; Giancaspero, T.A.; Leone, P.; Galluccio, M.; Indiveri, C. Riboflavin transport and metabolism in humans. J. Inherit. Metab. Dis. 2016, 39, 545–557. [Google Scholar] [CrossRef]
- Pires, L.; González-Paramás, A.M.; Heleno, S.A.; Calhelha, R.C. The Role of Gut Microbiota in the Etiopathogenesis of Multiple Chronic Diseases. Antibiotics 2024, 13, 392. [Google Scholar] [CrossRef]
- Golbach, J.L.; Ricke, S.C.; O’Bryan, C.A.; Crandall, P.G. Riboflavin in Nutrition, Food Processing, and Analysis—A Review. J. Food Res. 2014, 3, 23. [Google Scholar] [CrossRef]
- McCormick, D.B. Vitamin/mineral supplements: Of questionable benefit for the general population. Nutr. Rev. 2010, 68, 207–213. [Google Scholar] [CrossRef]
- König, B.; Kümmel, S.; Svobodová, E.; Cibulka, R. Flavin photocatalysis. Phys. Sci. Rev. 2018, 3, 20170168. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Zhou, Q.; Mseeh, F.; Grishin, N.V.; Osterman, A.L.; Zhang, H. Crystal Structure of Human Riboflavin Kinase Reveals a Barrel Fold and a Novel Active Site Arch. Structure 2003, 11, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Brizio, C.; Galluccio, M.; Wait, R.; Torchetti, E.M.; Bafunno, V.; Accardi, R.; Gianazza, E.; Indiveri, C.; Barile, M. Over-expression in Escherichia coli and characterization of two recombinant isoforms of human FAD synthetase. Biochem. Biophys. Res. Commun. 2006, 344, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Torchetti, E.M.; Brizio, C.; Colella, M.; Galluccio, M.; Giancaspero, T.A.; Indiveri, C.; Roberti, M.; Barile, M. Mitochondrial localization of human FAD synthetase isoform 1. Mitochondrion 2010, 10, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.K.J.; Koňaříková, E.; Giancaspero, T.A.; Mosegaard, S.; Boczonadi, V.; Mataković, L.; Veauville-Merllié, A.; Terrile, C.; Schwarzmayr, T.; Haack, T.B.; et al. Riboflavin-Responsive and -Non-responsive Mutations in FAD Synthase Cause Multiple Acyl-CoA Dehydrogenase and Combined Respiratory-Chain Deficiency. Am. J. Hum. Genet. 2016, 98, 1130–1145. [Google Scholar] [CrossRef] [PubMed]
- Giancaspero, T.A.; Colella, M.; Brizio, C.; Difonzo, G.; Fiorino, G.M.; Leone, P.; Brandsch, R.; Bonomi, F.; Iametti, S.; Barile, M. Remaining challenges in cellular flavin cofactor homeostasis and flavoprotein biogenesis. Front. Chem. 2015, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, S.; Yaplito-Lee, J. Riboflavin metabolism: Role in mitochondrial function. J. Transl. Genet. Genom. 2020, 4, 285–306. [Google Scholar] [CrossRef]
- Leone, P.; Galluccio, M.; Barbiroli, A.; Eberini, I.; Tolomeo, M.; Vrenna, F.; Gianazza, E.; Iametti, S.; Bonomi, F.; Indiveri, C.; et al. Bacterial Production, Characterization and Protein Modeling of a Novel Monofuctional Isoform of FAD Synthase in Humans: An Emergency Protein? Molecules 2018, 23, 116. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, T.; Ohnishi, S.T.; Salerno, J.C. Five decades of research on mitochondrial NADH-quinone oxidoreductase (complex I). Biol. Chem. 2018, 399, 1249–1264. [Google Scholar] [CrossRef] [PubMed]
- Santhiago, M.R.; Randleman, J.B. The biology of corneal cross-linking derived from ultraviolet light and riboflavin. Exp. Eye Res. 2021, 202, 108355. [Google Scholar] [CrossRef] [PubMed]
- Thakur, K.; Tomar, S.K.; Singh, A.K.; Mandal, S.; Arora, S. Riboflavin and health: A review of recent human research. Crit. Rev. Food Sci. Nutr. 2017, 57, 3650–3660. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.D.; Chen, L.; Liu, Y.; Feng, L.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhou, X.Q. Impact and consequences of dietary riboflavin deficiency treatment on flesh quality loss in on-growing grass carp. Food Funct. 2019, 10, 3396–3409. [Google Scholar] [CrossRef]
- Bajic, Z.; Sobot, T.; Skrbic, R.; Stojiljkovic, M.P.; Ponorac, N.; Matavulj, A.; Djuric, D.M. Homocysteine, Vitamins B6 and Folic Acid in Experimental Models of Myocardial Infarction and Heart Failure—How Strong Is That Link? Biomolecules 2022, 12, 536. [Google Scholar] [CrossRef]
- Huang, R.; Choe, E.; Min, D.B. Kinetics for Singlet Oxygen Formation by Riboflavin Photosensitization and the Reaction between Riboflavin and Singlet Oxygen. J. Food Sci. 2004, 69, C726–C732. [Google Scholar] [CrossRef]
- Cardoso, D.R.; Olsen, K.; Skibsted, L.H. Mechanism of Deactivation of Triplet-Excited Riboflavin by Ascorbate, Carotenoids, and Tocopherols in Homogeneous and Heterogeneous Aqueous Food Model Systems. J. Agric. Food Chem. 2007, 55, 6285–6291. [Google Scholar] [CrossRef] [PubMed]
- Kutta, R.J.; Archipowa, N.; Johannissen, L.O.; Jones, A.R.; Scrutton, N.S. Vertebrate Cryptochromes are Vestigial Flavoproteins. Sci. Rep. 2017, 7, 44906. [Google Scholar] [CrossRef] [PubMed]
- Belarbi, S.; Makri Mokrane, S. Une double discordance cardiaque décompensée suite à une aspergillose pulmonaire, à propos d’un cas et revue de la littérature. J. Faculté Médecine Oran 2022, 6. [Google Scholar] [CrossRef]
- Jin, C.; Yonezawa, A. Recent advances in riboflavin transporter RFVT and its genetic disease. Pharmacol. Ther. 2022, 233, 108023. [Google Scholar] [CrossRef] [PubMed]
- Colasuonno, F.; Marioli, C.; Tartaglia, M.; Bertini, E.; Compagnucci, C.; Moreno, S. New Insights into the Neurodegeneration Mechanisms Underlying Riboflavin Transporter Deficiency (RTD): Involvement of Energy Dysmetabolism and Cytoskeletal Derangement. Biomedicines 2022, 10, 1329. [Google Scholar] [CrossRef] [PubMed]
- Chiong, M.A.; Sim, K.G.; Carpenter, K.; Rhead, W.; Ho, G.; Olsen, R.K.J.; Christodoulou, J. Transient multiple acyl-CoA dehydrogenation deficiency in a newborn female caused by maternal riboflavin deficiency. Mol. Genet. Metab. 2007, 92, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Murgia, C.; Dehlia, A.; Guthridge, M.A. New insights into the nutritional genomics of adult-onset riboflavin-responsive diseases. Nutr. Metab. 2023, 20, 42. [Google Scholar] [CrossRef] [PubMed]
- Hellebrekers, D.M.E.I.; Sallevelt, S.C.; Theunissen, T.E.; Hendrickx, A.; Gottschalk, R.W.; Hoeijmakers, J.G.; Habets, D.D.; Bierau, J.; Schoonderwoerd, K.G.; Smeets, H.J. Novel SLC25A32 mutation in a patient with a severe neuromuscular phenotype. Eur. J. Hum. Genet. 2017, 25, 886–888. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.W.; Pyle, A.; Griffin, H.; Blakely, E.L.; Duff, J.; He, L.; Smertenko, T.; Alston, C.L.; Neeve, V.C.; Best, A.; et al. Use of Whole-Exome Sequencing to Determine the Genetic Basis of Multiple Mitochondrial Respiratory Chain Complex Deficiencies. JAMA 2014, 312, 68. [Google Scholar] [CrossRef]
- Yıldız, Y.; Olsen, R.K.J.; Sivri, H.S.; Akçören, Z.; Nygaard, H.H.; Tokatlı, A. Post-mortem detection of FLAD1 mutations in 2 Turkish siblings with hypotonia in early infancy. Neuromuscul. Disord. 2018, 28, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Ryder, B.; Tolomeo, M.; Nochi, Z.; Colella, M.; Barile, M.; Olsen, R.K.; Inbar-Feigenberg, M. A Novel Truncating FLAD1 Variant, Causing Multiple Acyl-CoA Dehydrogenase Deficiency (MADD) in an 8-Year-Old Boy. JIMD Rep. 2018, 45, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Auranen, M.; Paetau, A.; Piirilä, P.; Pohju, A.; Salmi, T.; Lamminen, A.; Löfberg, M.; Mosegaard, S.; Olsen, R.K.; Tyni, T. Patient with multiple acyl-CoA dehydrogenation deficiency disease and FLAD1 mutations benefits from riboflavin therapy. Neuromuscul. Disord. 2017, 27, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Tolomeo, M.; Nisco, A.; Leone, P.; Barile, M. Development of Novel Experimental Models to Study Flavoproteome Alterations in Human Neuromuscular Diseases: The Effect of Rf Therapy. Int. J. Mol. Sci. 2020, 21, 5310. [Google Scholar] [CrossRef]
- Rahman, S. Leigh syndrome. Handb. Clin. Neurol. 2023, 194, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Grünert, S.C. Clinical and genetical heterogeneity of late-onset multiple acyl-coenzyme A dehydrogenase deficiency. Orphanet. J. Rare Dis. 2014, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Hargreaves, I.P. Efficacy and Safety of Coenzyme Q10 Supplementation in Neonates, Infants and Children: An Overview. Antioxidants 2024, 13, 530. [Google Scholar] [CrossRef]
- Nouws, J.; Brinke, H.T.; Nijtmans, L.G.; Houten, S.M. ACAD9, a complex I assembly factor with a moonlighting function in fatty acid oxidation deficiencies. Hum. Mol. Genet. 2014, 23, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Collet, M.; Assouline, Z.; Bonnet, D.; Rio, M.; Iserin, F.; Sidi, D.; Goldenberg, A.; Lardennois, C.; Metodiev, M.D.; Haberberger, B.; et al. High incidence and variable clinical outcome of cardiac hypertrophy due to ACAD9 mutations in childhood. Eur. J. Hum. Genet. 2016, 24, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Repp, B.M.; Mastantuono, E.; Alston, C.L.; Schiff, M.; Haack, T.B.; Rötig, A.; Ardissone, A.; Lombès, A.; Catarino, C.B.; Diodato, D.; et al. Clinical, biochemical and genetic spectrum of 70 patients with ACAD9 deficiency: Is riboflavin supplementation effective? Orphanet. J. Rare Dis. 2018, 13, 120. [Google Scholar] [CrossRef]
- Pithukpakorn, M. Disorders of pyruvate metabolism and the tricarboxylic acid cycle. Mol. Genet. Metab. 2005, 85, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Goetz, V.; Yang, D.D.; Lacaille, F.; Pelosi, M.; Angoulvant, F.; Brassier, A.; Arnoux, J.B.; Schiff, M.; Heilbronner, C.; Salvador, E.; et al. What are the clues for an inherited metabolic disorder in Reye syndrome? A single Centre study of 58 children. Mol. Genet. Metab. 2022, 135, 320–326. [Google Scholar] [CrossRef]
- Vabulas, R.M. Ferroptosis-Related Flavoproteins: Their Function and Stability. Int. J. Mol. Sci. 2021, 22, 430. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.J.; Crameri, J.J.; Thorburn, D.R.; Frazier, A.E.; Stojanovski, D. Mitochondrial biology and dysfunction in secondary mitochondrial disease. Open Biol. 2022, 12, 220274. [Google Scholar] [CrossRef]
- Wolthers, K.R.; Scrutton, N.S. Cobalamin uptake and reactivation occurs through specific protein interactions in the methionine synthase–methionine synthase reductase complex. FEBS J. 2009, 276, 1942–1951. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.B.; Stover, P.J.; McNulty, H.; Fenech, M.F.; Gregory, J.F., III; Mills, J.L.; Pfeiffer, C.M.; Fazili, Z.; Zhang, M.; Ueland, P.M.; et al. Biomarkers of Nutrition for Development—Folate Review. J. Nutr. 2015, 145, 1636S–1680S. [Google Scholar] [CrossRef]
- Madigan, S.M.; Tracey, F.; McNulty, H.; Eaton-Evans, J.; Coulter, J.; McCartney, H.; Strain, J.J. Riboflavin and vitamin B-6 intakes and status and biochemical response to riboflavin supplementation in free-living elderly people. Am. J. Clin. Nutr. 1998, 68, 389–395. [Google Scholar] [CrossRef]
- Esquivel, D.G.; Ramírez-Ortega, D.; Pineda, B.; Castro, N.; Ríos, C.; de la Cruz, V.P. Kynurenine pathway metabolites and enzymes involved in redox reactions. Neuropharmacology 2017, 112, 331–345. [Google Scholar] [CrossRef]
- Bou-Abdallah, F.; Paliakkara, J.; Melman, G.; Melman, A. Reductive Mobilization of Iron from Intact Ferritin: Mechanisms and Physiological Implication. Pharmaceuticals 2018, 11, 120. [Google Scholar] [CrossRef]
- Ward, M.; Hughes, C.F.; Strain, J.J.; Reilly, R.; Cunningham, C.; Molloy, A.M.; Horigan, G.; Casey, M.; McCarroll, K.; O’Kane, M.; et al. Impact of the common MTHFR 677C→T polymorphism on blood pressure in adulthood and role of riboflavin in modifying the genetic risk of hypertension: Evidence from the JINGO project. BMC Med. 2020, 18, 318. [Google Scholar] [CrossRef]
- Khan, S.; Rayis, M.; Rizvi, A.; Alam, M.M.; Rizvi, M.; Naseem, I. ROS mediated antibacterial activity of photoilluminated riboflavin: A photodynamic mechanism against nosocomial infections. Toxicol. Rep. 2019, 6, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Jungert, A.; McNulty, H.; Hoey, L.; Ward, M.; Strain, J.J.; Hughes, C.F.; McAnena, L.; Neuhäuser-Berthold, M.; Pentieva, K. Riboflavin Is an Important Determinant of Vitamin B-6 Status in Healthy Adults. J. Nutr. 2020, 150, 2699–2706. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Chen, Z.; Rozen, R.; Matthews, R.G. Effects of common polymorphisms on the properties of recombinant human methylenetetrahydrofolate reductase. Proc. Natl. Acad. Sci. USA 2001, 98, 14853–14858. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, S. Brown-Vialetto-Van Laere syndrome. Orphanet. J. Rare Dis. 2008, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.M.; Jia, J.; Mao, L.N.; Men, C.; Tang, K.T.; Li, Y.Y.; Ding, H.X.; Zhan, Y.Y. Methylenetetrahydrofolate reductase C677T gene polymorphism and essential hypertension: A meta-analysis of 10,415 subjects. Biomed. Rep. 2014, 2, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Wilcken, B. Geographical and ethnic variation of the 677C>T allele of 5,10 methylenetetrahydrofolate reductase (MTHFR): Findings from over 7000 newborns from 16 areas world wide. J. Med. Genet. 2003, 40, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Tan, Y.; Zhu, L. Dietary vitamin B2 intake and breast cancer risk: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2017, 295, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Aljaadi, A.M.; Devlin, A.M.; Green, T.J. Riboflavin intake and status and relationship to anemia. Nutr. Rev. 2022, 81, 114–132. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Powers, H.J.; Minski, M.J.; Whitehead, J.; Downes, R. Riboflavin Deficiency and Iron Absorption in Adult Gambian Men. Ann. Nutr. Metab. 1992, 36, 34–40. [Google Scholar] [CrossRef]
- Shi, Z.; Zhen, S.; Wittert, G.A.; Yuan, B.; Zuo, H.; Taylor, A.W. Inadequate Riboflavin Intake and Anemia Risk in a Chinese Population: Five-Year Follow Up of the Jiangsu Nutrition Study. PLoS ONE 2014, 9, e88862. [Google Scholar] [CrossRef]
- Powers, H.J.; Hill, M.H.; Mushtaq, S.; Dainty, J.R.; Majsak-Newman, G.; Williams, E.A. Correcting a marginal riboflavin deficiency improves hematologic status in young women in the United Kingdom (RIBOFEM). Am. J. Clin. Nutr. 2011, 93, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Varkey, T.; Farhat, J.; Tobin, J. B2 or Not B2: A Systematic Literature Review on Migraine Headaches and Riboflavin Deficiency. Headache Med. 2023, 14, 193–202. [Google Scholar] [CrossRef]
- Foley, A.R.; Menezes, M.P.; Pandraud, A.; Gonzalez, M.A.; Al-Odaib, A.; Abrams, A.J.; Sugano, K.; Yonezawa, A.; Manzur, A.Y.; Burns, J.; et al. Treatable childhood neuronopathy caused by mutations in riboflavin transporter RFVT2. Brain 2014, 137, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Condò, M.; Posar, A.; Arbizzani, A.; Parmeggiani, A. Riboflavin prophylaxis in pediatric and adolescent migraine. J. Headache Pain 2009, 10, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M. Reported evidence of vitamin E protection against cataract and glaucoma. Free Radic. Biol. Med. 2021, 177, 100–119. [Google Scholar] [CrossRef] [PubMed]
- Ashoori, M.; Saedisomeolia, A. Riboflavin (vitamin B) and oxidative stress: A review. Br. J. Nutr. 2014, 111, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.E. Epidemiology of Chronic Disease: Global Perspectives; Jones & Bartlett Learning: Burlington, MA, USA, 2013. [Google Scholar]
- McNulty, H.; Strain, J.J.; Hughes, C.F.; Ward, M. Riboflavin, MTHFR genotype and blood pressure: A personalized approach to prevention and treatment of hypertension. Mol. Asp. Med. 2017, 53, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Darguzyte, M.; Drude, N.; Lammers, T.; Kiessling, F. Riboflavin-Targeted Drug Delivery. Cancers 2020, 12, 295. [Google Scholar] [CrossRef]
- Pinto, J.T.; Zempleni, J. Riboflavin. Adv. Nutr. 2016, 7, 973–975. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, W.; Obeid, R. Vitamins in the Prevention of Human Diseases; DE GRUYTER: Berlin, Germany, 2010. [Google Scholar] [CrossRef]
- Mamede, A.C.; Tavares, S.D.; Abrantes, A.M.; Trindade, J.; Maia, J.M.; Botelho, M.F. The Role of Vitamins in Cancer: A Review. Nutr. Cancer 2011, 63, 479–494. [Google Scholar] [CrossRef]
- Hoey, L.; McNulty, H.; Strain, J. Studies of biomarker responses to intervention with riboflavin: A systematic review. Am. J. Clin. Nutr. 2009, 89, 1960S–1980S. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, H.; McNulty, H.; Hughes, C.F.; Pentieva, K.; Strain, J.J.; McCann, A.; McAnena, L.; Cunningham, C.; Molloy, A.M.; Flynn, A.; et al. Vitamin B-6 and riboflavin, their metabolic interaction, and relationship with MTHFR genotype in adults aged 18–102 years. Am. J. Clin. Nutr. 2022, 116, 1767–1778. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.M.; Peerson, J.M.; Haskell, M.J.; Shrestha, R.K.; Brown, K.H.; Allen, L.H. Erythrocyte Riboflavin for the Detection of Riboflavin Deficiency in Pregnant Nepali Women. Clin. Chem. 2005, 51, 2162–2165. [Google Scholar] [CrossRef]
- Jaeger, B.; Bosch, A.M. Clinical presentation and outcome of riboflavin transporter deficiency: Mini review after five years of experience. J. Inherit. Metab. Dis. 2016, 39, 559–564. [Google Scholar] [CrossRef] [PubMed]
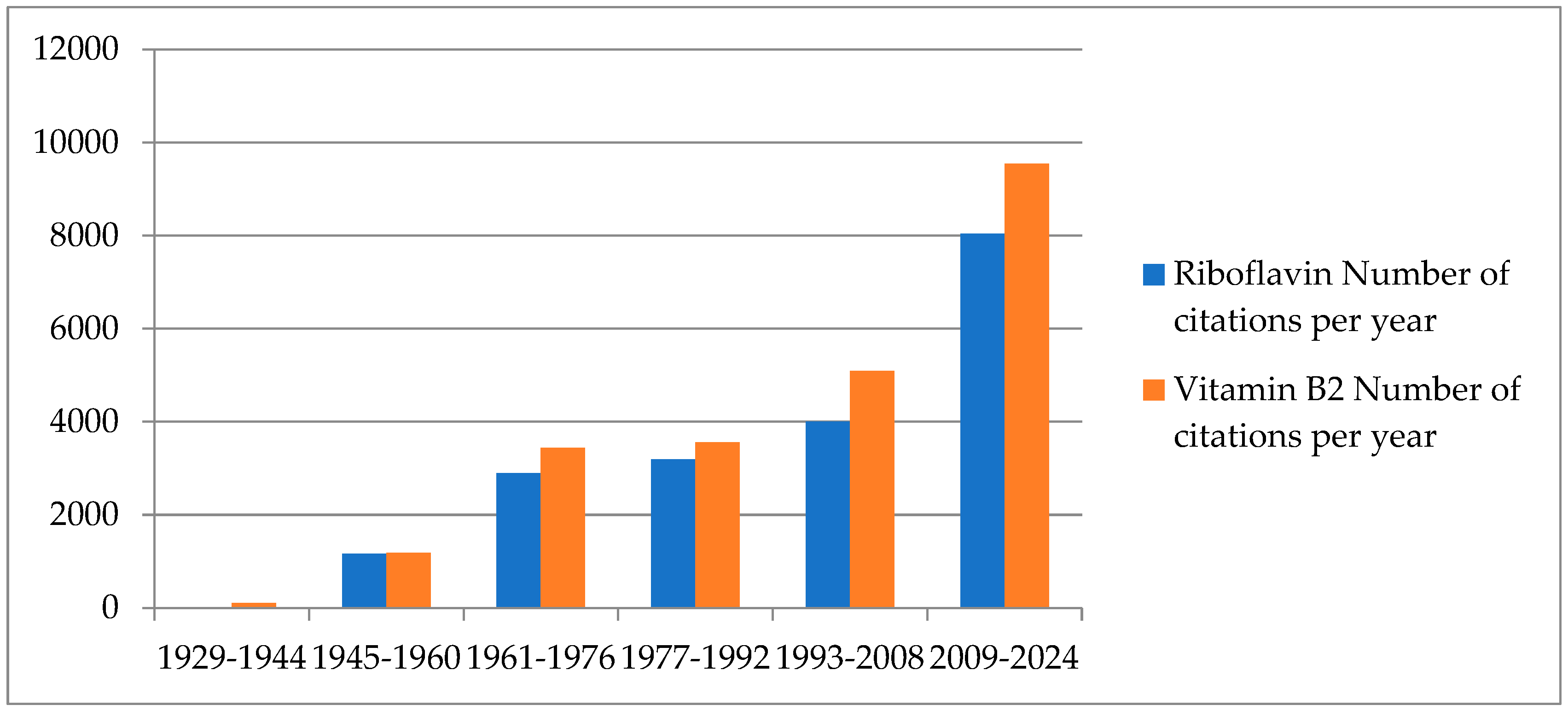

| Age | Average Requirement (mg/Day) | Population Reference Intake (mg/Day) a |
|---|---|---|
| 7–11 months | - | 0.4 b |
| 1–3 years | 0.5 | 0.6 |
| 4–6 years | 0.6 | 0.7 |
| 7–10 years | 0.8 | 1.0 |
| 11–14 years | 1.1 | 1.4 |
| 15–17 years | 1.4 | 1.6 |
| ≥18 years | 1.3 | 1.6 |
| Pregnancy | 1.5 | 1.9 |
| Lactation | 1.7 | 2.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragão, M.Â.; Pires, L.; Santos-Buelga, C.; Barros, L.; Calhelha, R.C. Revitalising Riboflavin: Unveiling Its Timeless Significance in Human Physiology and Health. Foods 2024, 13, 2255. https://doi.org/10.3390/foods13142255
Aragão MÂ, Pires L, Santos-Buelga C, Barros L, Calhelha RC. Revitalising Riboflavin: Unveiling Its Timeless Significance in Human Physiology and Health. Foods. 2024; 13(14):2255. https://doi.org/10.3390/foods13142255
Chicago/Turabian StyleAragão, M. Ângela, Lara Pires, Celestino Santos-Buelga, Lillian Barros, and Ricardo C. Calhelha. 2024. "Revitalising Riboflavin: Unveiling Its Timeless Significance in Human Physiology and Health" Foods 13, no. 14: 2255. https://doi.org/10.3390/foods13142255
APA StyleAragão, M. Â., Pires, L., Santos-Buelga, C., Barros, L., & Calhelha, R. C. (2024). Revitalising Riboflavin: Unveiling Its Timeless Significance in Human Physiology and Health. Foods, 13(14), 2255. https://doi.org/10.3390/foods13142255










