In Vivo Glycemic Response of Fruit-Based Mango (Mangifera indica) and Pineapple (Ananas comosus) Bars in In Vitro and In Silico Enzyme Inhibitory Effects Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Fruit Bars
2.2. Chemical Composition of Mango “Ataulfo” and Pineapple “Esmeralda” Bar
2.3. Glycemic Response In Vivo Study
2.4. Glycemic Index (GI) and Glycemic Load (GL)
2.5. Enzyme Inhibition
2.6. In Silico Assay for α-Amylase and α-Glucosidase
2.7. Statistical Analysis
3. Results
3.1. Chemical Composition of Mango and Pineapple Bars
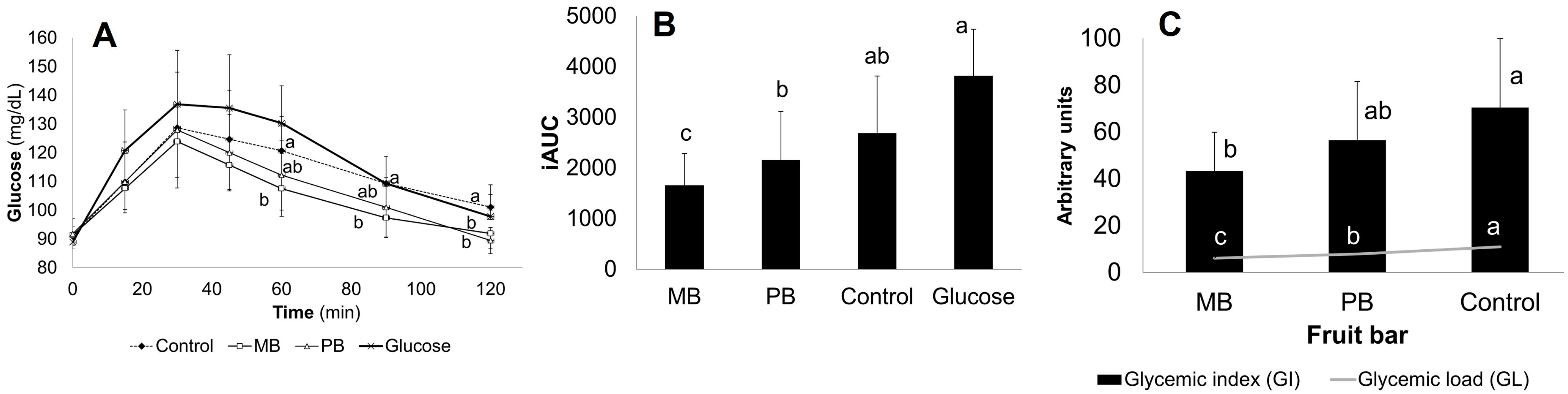
3.2. Glycemic Response In Vivo Assays: Glycemic Index (GI) and Load (GL)
3.3. Enzymatic Inhibition: In Vitro Assay
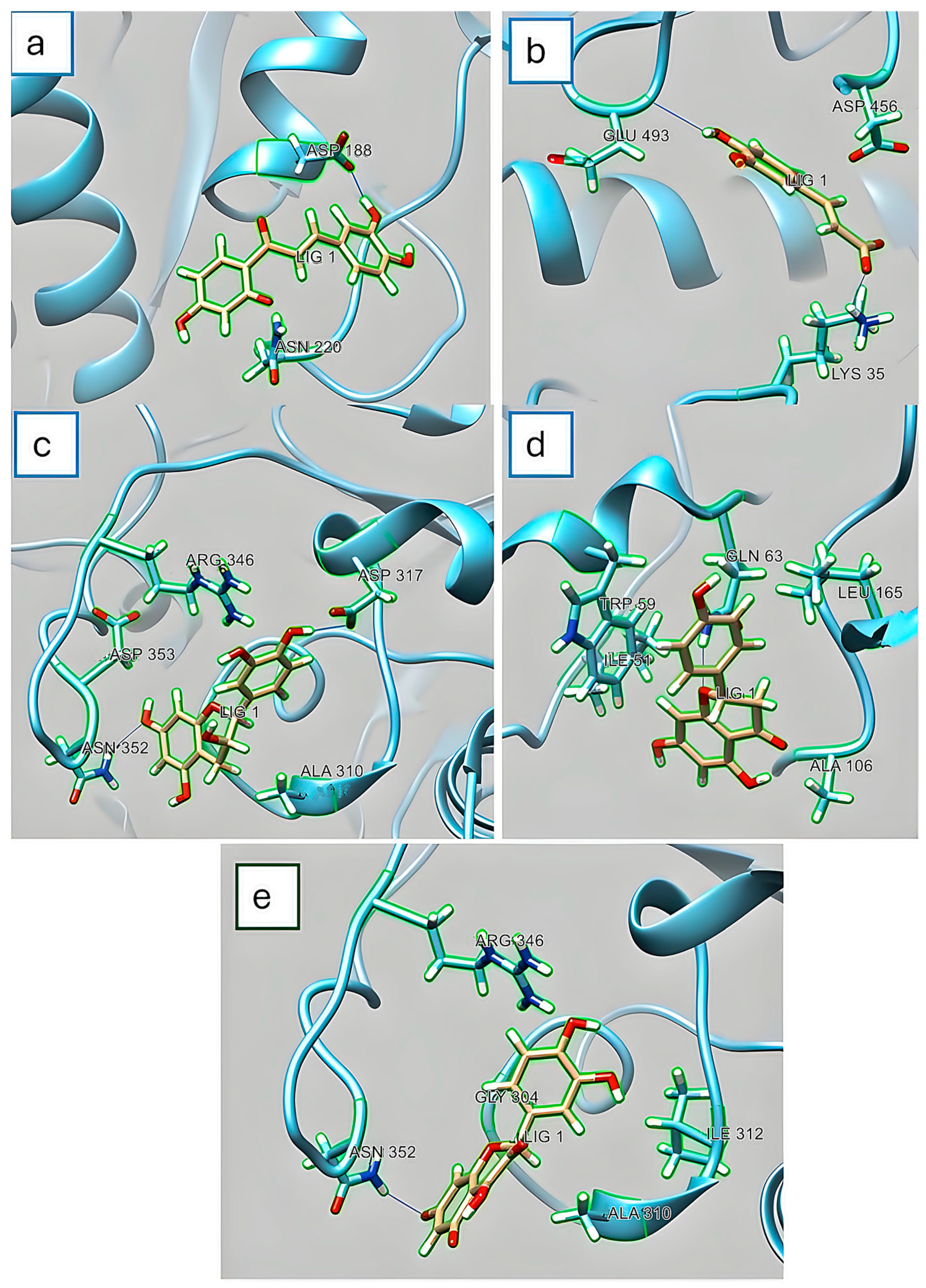
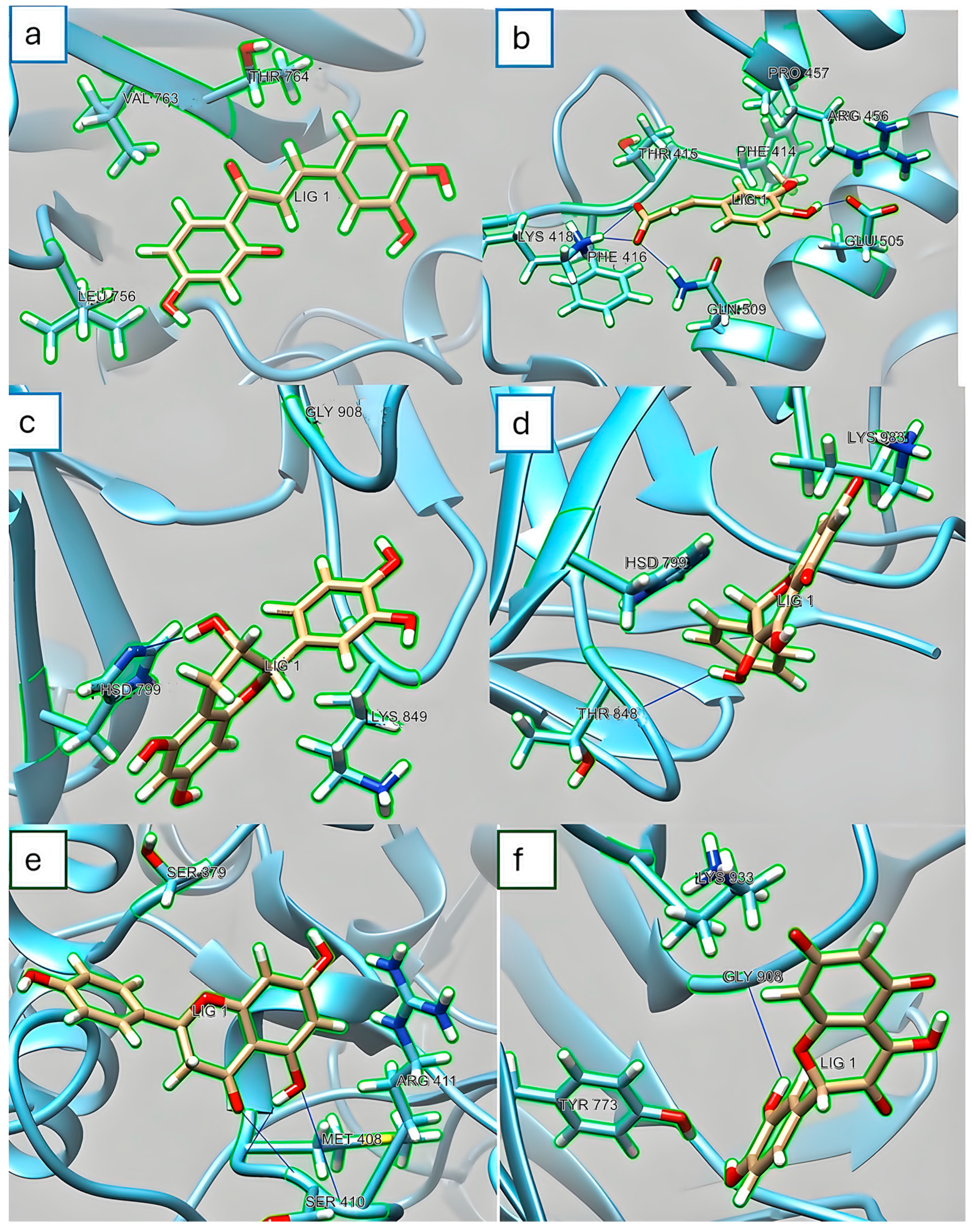
3.4. In Silico Assay for α-Amylase and α-Glucosidase
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chobot, M.; Kozłowska, M.; Ignaczak, A.; Kowalska, H. Development of Drying and Roasting Processes for the Production of Plant-Based pro-Healthy Snacks in the Light of Nutritional Trends and Sustainable Techniques. Trends Food Sci. Technol. 2024, 149, 104553. [Google Scholar] [CrossRef]
- Bakaloudi, D.R.; Jeyakumar, D.T.; Jayawardena, R.; Chourdakis, M. The Impact of COVID-19 Lockdown on Snacking Habits, Fast-Food and Alcohol Consumption: A Systematic Review of the Evidence. Clin. Nutr. 2022, 41, 3038–3045. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Van Der Horst, K.; Jacquier, E.F.; Afeiche, M.C.; Eldridge, A.L. Snacking Patterns in Children: A Comparison between Australia, China, Mexico, and the US. Nutrients 2018, 10, 198. [Google Scholar] [CrossRef] [PubMed]
- Schaan, C.W.; Cureau, F.V.; Salvo, D.; Kohl, H.W.; Schaan, B.D. Unhealthy Snack Intake Modifies the Association between Screen-Based Sedentary Time and Metabolic Syndrome in Brazilian Adolescents. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 115. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, F.; Martini, D.; Scaglioni, S.; Sculati, M.; Donini, L.M.; Leonardi, F.; Agostoni, C.; Castelnuovo, G.; Ferrara, N.; Ghiselli, A.; et al. Snacking in Nutrition and Health. Int. J. Food Sci. Nutr. 2019, 70, 909–923. [Google Scholar] [CrossRef] [PubMed]
- Chew, N.W.S.; Ng, C.H.; Tan, D.J.H.; Kong, G.; Lin, C.; Chin, Y.H.; Lim, W.H.; Huang, D.Q.; Quek, J.; Fu, C.E.; et al. The Global Burden of Metabolic Disease: Data from 2000 to 2019. Cell Metab. 2023, 35, 414–428.e3. [Google Scholar] [CrossRef] [PubMed]
- Hill-Briggs, F.; Adler, N.E.; Berkowitz, S.A.; Chin, M.H.; Gary-Webb, T.L.; Navas-Acien, A.; Thornton, P.L.; Haire-Joshu, D. Social Determinants of Health and Diabetes: A Scientific Review. Diabetes Care 2021, 44, 258–279. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Awana, M.; Mandal, S.; Pandit, K.; Singh, A.; Syeunda, C.O.; Thandapilly, S.J.; Krishnan, V. Functional Foods with a Tailored Glycemic Response Based on Food Matrix and Its Interactions: Can It Be a Reality? Food Chem. X 2024, 22, 101358. [Google Scholar] [CrossRef]
- Grand View Research. Diabetic Food Market Size, Share & Trends Analysis Report by Product (Confectionery, Snacks), by Distribution Channel (Supermarkets & Hypermarkets, Online), by Region, and Segment Forecasts, 2022–2030; 2020. Available online: https://www.grandviewresearch.com/industry-analysis/diabetic-food-market# (accessed on 23 November 2020).
- Burgos-Araiza, A.K.; Gaytán-Martínez, M.; Ramírez-Jiménez, A.K.; de la Luz Reyes-Vega, M. Sensory and Process Optimization of a Mango Bagasse-Based Beverage with High Fiber Content and Low Glycemic Index. J. Food Sci. Technol. 2022, 59, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Beltrán, Y.E.; Becerra-Verdín, E.M.; Sáyago-Ayerdi, S.G.; Rocha-Guzmán, N.E.; García-López, E.G.; Castañeda-Martínez, A.; Montalvo-González, R.; Rodríguez-Aguayo, C.; Montalvo-González, E. Nutritional Characteristics and Bioactive Compound Content of Guava Purees and Their Effect on Biochemical Markers of Hyperglycemic and Hypercholesterolemic Rats. J. Funct. Foods 2017, 35, 447–457. [Google Scholar] [CrossRef]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef] [PubMed]
- Sayago-Ayerdi, S.; García-Martínez, D.L.; Ramírez-Castillo, A.C.; Ramírez-Concepción, H.R.; Viuda-Martos, M. Tropical Fruits and Their Co-Products as Bioactive Compounds and Their Health Effects: A Review. Foods 2021, 10, 1952. [Google Scholar] [CrossRef] [PubMed]
- Striegel, L.; Kang, B.; Pilkenton, S.J.; Rychlik, M.; Apostolidis, E. Effect of Black Tea and Black Tea Pomace Polyphenols on α-Glucosidase and α-Amylase Inhibition, Relevant to Type 2 Diabetes Prevention. Front. Nutr. 2015, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of Dietary Polyphenols on Carbohydrate Metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef] [PubMed]
- Del juncal-Guzmán, D.; Hernández-Maldonado, L.M.; Sánchez-Burgos, J.A.; González-Aguilar, G.A.; Ruiz-Valdiviezo, V.M.; Tovar, J.; Sáyago-Ayerdi, S.G. In Vitro Gastrointestinal Digestion and Colonic Fermentation of Phenolic Compounds in UV-C Irradiated Pineapple (Ananas comosus) Snack-Bars. LWT-Food Sci. Technol. 2021, 138, 110636. [Google Scholar] [CrossRef]
- Hernández-Maldonado, L.M.; Blancas-Benítez, F.J.; Zamora-Gasga, V.M.; Cárdenas-Castro, A.P.; Tovar, J.; Sáyago-Ayerdi, S.G. In Vitro Gastrointestinal Digestion and Colonic Fermentation of High Dietary Fiber and Antioxidant-Rich Mango (Mangifera indica L.) “Ataulfo”-Based Fruit Bars. Nutrients 2019, 11, 1564. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of the AOAC, 15th ed.; Chemists, A., Ed.; Association of Official Analytical Chemist: Washington, DC, USA, 1990. [Google Scholar]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rerbers, P.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Mañas, E.; Saura-Calixto, F. Dietary Fibre Analysis: Methodological Error Sources. Eur. J. Clin. Nutr. 1995, 49, S158. [Google Scholar] [PubMed]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz- Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated Methodology to Determine Antioxidant Capacity in Plant Foods, Oils and Beverages: Extraction, Measurement and Expression of Results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Montreau, F. Sur Le Dosage Des Composés Phénoliques Totaux Dans Les Vins Par La Methode Folin–Ciocalteau. Connaiss Vigne Vinifera 1972, 24, 397–404. [Google Scholar]
- Bellmann, S.; Minekus, M.; Sanders, P.; Bosgra, S.; Havenaar, R. Human Glycemic Response Curves after Intake of Carbohydrate Foods Are Accurately Predicted by Combining in Vitro Gastrointestinal Digestion with in Silico Kinetic Modeling. Clin. Nutr. Exp. 2018, 17, 8–22. [Google Scholar] [CrossRef]
- Meng, H.; Matthan, N.R.; Ausman, L.M.; Lichtenstein, A.H. Effect of Macronutrients and Fiber on Postprandial Glycemic Responses and Meal Glycemic Index and Glycemic Load Value Determinations1, 2, 3. Am. J. Clin. Nutr. 2017, 105, 842–853. [Google Scholar] [CrossRef] [PubMed]
- ISO 26642-2010; Food Products–Determination of the Glycaemic Index (GI) and Recommendation for Food Classification. International Standards Organization: Geneva, Switzerland, 2010. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:26642:ed-1:v1:en (accessed on 9 February 2021).
- Wolever, T.M.S.; Jenkins, D.J.A. The Use of the Glycemie Index in Predicting the Blood Glucose Response to Mixed Meals. Am. J. Clin. Nutr. 1986, 43, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Gourineni, V.; Stewart, M.L.; Wilcox, M.L.; Maki, K.C. Nutritional Bar with Potato-Based Resistant Starch Attenuated Post-Prandial Glucose and Insulin Response in Healthy Adults. Foods 2020, 9, 1679. [Google Scholar] [CrossRef] [PubMed]
- Granfeldt, Y.; Björck, I.; Drews, A.; Tovar, J. An in Vitro Procedure Based on Chewing to Predict Metabolic Response to Starch in Cereal and Legume Products. Am. J. Clin. Nutr. 1994, 59, 777S. [Google Scholar] [CrossRef]
- Nair, S.S.; Kavrekar, V.; Mishra, A. In Vitro Studies on Alpha Amylase and Alpha Glucosidase Inhibitory Activities of Selected Plant Extracts. Eur. J. Exp. Biol. 2013, 3, 128–132. [Google Scholar]
- Bitencourt-Ferreira, G.; de Azevedo, W.F., Jr. Docking with SwissDock BT-Docking Screens for Drug Discovery; de Azevedo, W.F., Jr., Ed.; Springer: New York, NY, USA, 2019; pp. 189–202. [Google Scholar] [CrossRef]
- Chávez-Fumagalli, M.A.; Schneider, M.S.; LaChávez-Fumagalli, M.A.; Schneider, M.S.; Lage, D.P.; Tavares, G.S.V.; Mendonça, D.V.C.; Santos, T.T.O.; Pádua, R.M.; Machado-de-Ávila, R.A.; et al. A Computational Approach Using Bioinformatics to Screening Drug Targets for Leishmania infantum Species. Evid.-Based Complement. Altern. Med. 2018, 2018, 6813467. [Google Scholar] [CrossRef] [PubMed]
- Grosdidier, A.; Zoete, V.; Michielin, O. SwissDock, a Protein-Small Molecule Docking Web Service Based on EADock DSS. Nucleic Acids Res. 2011, 39, W270–W277. [Google Scholar] [CrossRef] [PubMed]
- Mohd Ali, M.; Hashim, N.; Abd Aziz, S.; Lasekan, O. Pineapple (Ananas comosus): A Comprehensive Review of Nutritional Values, Volatile Compounds, Health Benefits, and Potential Food Products. Food Res. Int. 2020, 137, 109675. [Google Scholar] [CrossRef] [PubMed]
- Martínez, R.; Torres, P.; Meneses, M.; Figueroa, J.G.; Pérez-Álvarez, J.; Viuda-Martos, M. Chemical, Technological and in Vitro Antioxidant Properties of Mango, Guava, Pineapple and Passion Fruit Dietary Fibre Concentrate. Food Chem. 2012, 135, 1520–1526. [Google Scholar] [CrossRef] [PubMed]
- Sadler, M.J.; Gibson, S.; Whelan, K.; Ha, M.A.; Lovegrove, J.; Higgs, J. Dried Fruit and Public Health-What Does the Evidence Tell Us? Int. J. Food Sci. Nutr. 2019, 70, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Sarmiento, W.; Sáyago-Ayerdi, S.G.; Goñi, I.; Gutiérrez-Miceli, F.A.; Abud-Archila, M.; Rejón-Orantes, J.D.C.; Rincón-Rosales, R.; Peña-Ocaña, B.A.; Ruíz-Valdiviezo, V.M. Changes in Intestinal Microbiota and Predicted Metabolic Pathways during Colonic Fermentation of Mango (Mangifera indica L.)—Based Bar Indigestible Fraction. Nutrients 2020, 12, 683. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, F.S.; Brand-Miller, J.C.; Foster-Powell, K.; Buyken, A.E.; Goletzke, J. International Tables of Glycemic Index and Glycemic Load Values 2021: A Systematic Review. Am. J. Clin. Nutr. 2021, 114, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Vega-López, S.; Venn, B.J.; Slavin, J.L. Relevance of the Glycemic Index and Glycemic Load for Body Weight, Diabetes, and Cardiovascular Disease. Nutrients 2018, 10, 1361. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Christopher, A.; Shetty, K. Phenolic Bioactives From Plant-Based Foods for Glycemic Control. Front. Endocrinol. 2022, 12, 727503. [Google Scholar] [CrossRef] [PubMed]
- Gorinstein, S.; Poovarodom, S.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Vearasilp, S.; Haruenkit, R.; Ruamsuke, P.; Katrich, E.; Tashma, Z. Antioxidant Properties and Bioactive Constituents of Some Rare Exotic Thai Fruits and Comparison with Conventional Fruits: In Vitro and In Vivo Studies. Food Res. Int. 2011, 44, 2222–2232. [Google Scholar] [CrossRef]
- Cao, Y.; Sheng, J.; Zhang, D.; Chen, L.; Jiang, Y.; Cheng, D.; Su, Y.; Yu, Y.; Jia, H.; He, P.; et al. The Role of Dietary Fiber on Preventing Gestational Diabetes Mellitus in an At-Risk Group of High Triglyceride-Glucose Index Women: A Randomized Controlled Trial. Endocrine 2023, 82, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Santoso, U.; Rubi, D.S.; Pramana, C.; Agus, A.; Huriyati, E.; Yasmine, N. Impacts of High-Fiber Snack on Satiety Hormonal Responses and Glucose Homeostasis in Healthy Volunteers. Curr. Nutr. Food Sci. 2023, 19, 291–299. [Google Scholar] [CrossRef]
- Xu, X.; Zhu, Y.; Li, J.; Wang, S. Dietary Fiber, Glycemic Index, Glycemic Load and Renal Cell Carcinoma Risk. Carcinogenesis 2019, 40, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Domínguez Avila, J.A.; Rodrigo García, J.; González Aguilar, G.A.; la Rosa, L.A. The Antidiabetic Mechanisms of Polyphenols Related to Increased Glucagon-Like Peptide-1 (GLP1) and Insulin Signaling. Molecules 2017, 22, 903. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T.; Szkudelska, K. Resveratrol and Diabetes: From Animal to Human Studies. Biochim. Biophys. Acta-Mol. Basis Dis. 2015, 1852, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Xu, Z.; Sheng, Z. Ability of Resveratrol to Inhibit Advanced Glycation End Product Formation and Carbohydrate-Hydrolyzing Enzyme Activity, and to Conjugate Methylglyoxal. Food Chem. 2017, 216, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Schnell, O.; Weng, J.; Sheu, W.H.-H.; Watada, H.; Kalra, S.; Soegondo, S.; Yamamoto, N.; Rathod, R.; Zhang, C.; Grzeszczak, W. Acarbose Reduces Body Weight Irrespective of Glycemic Control in Patients with Diabetes: Results of a Worldwide, Non-Interventional, Observational Study Data Pool. J. Diabetes Complicat. 2016, 30, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yin, Y.; Zhao, W.; Liu, J.; Chen, F. Anti-Diabetic Activity Peptides from Albumin against α-Glucosidase and α-Amylase. Food Chem. 2012, 135, 2078–2085. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, Á.G.; de la Rosa, L.A.; Vargas-Requena, C.L.; Bustos-Jaimes, I.; Alvarez-Parrilla, A.E. Polyphenolic Compounds and Digestive Enzymes: In Vitro Non-Covalent Interactions. Molecules 2017, 22, 669. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ali, M.C.; Ruma, R.A.; Mahmud, S.; Paul, G.K.; Saleh, M.A.; Alshahrani, M.M.; Obaidullah, A.J.; Biswas, S.K.; Rahman, M.M.; et al. Molecular Docking and Dynamics Simulation of Natural Compounds from Betel Leaves (Piper betle L.) for Investigating the Potential Inhibition of Alpha-Amylase and Alpha-Glucosidase of Type 2 Diabetes. Molecules. 2022, 27, 4526. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Hayat, S.; Rahim, F.; Uddin, N.; Wadood, A.; Nawaz, M.; Gollapalli, M.; Rehman, A.U.; Khan, K.M.; Farooq, R.K. Exploring Thiazole-Based Schiff Base Analogs as Potent α-Glucosidase and α-Amylase Inhibitor: Their Synthesis and in-Silico Study. J. Mol. Struct. 2023, 1287, 135672. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, C.; Guven, E.C.; Paoli, P.; Simal-Gandara, J.; Ramkumar, K.M.; Wang, S.; Buleu, F.; Pah, A.; Turi, V.; et al. Dietary Polyphenols as Antidiabetic Agents: Advances and Opportunities. Food Front. 2020, 1, 18–44. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Miao, M. Inhibition of α-Amylase by Polyphenolic Compounds: Substrate Digestion, Binding Interactions and Nutritional Intervention. Trends Food Sci. Technol. 2020, 104, 190–207. [Google Scholar] [CrossRef]
- Rasouli, H.; Hosseini-Ghazvini, S.M.; Adibi, H.; Khodarahmi, R. Differential α-Amylase/α-Glucosidase Inhibitory Activities of Plant-Derived Phenolic Compounds: A Virtual Screening Perspective for the Treatment of Obesity and Diabetes. Food Funct. 2017, 8, 1942–1954. [Google Scholar] [CrossRef] [PubMed]
- Safitri, A.; Sari, D.R.T.; Fatchiyah, F.; Roosdiana, A. Modeling of Aqueous Root Extract Compounds of Ruellia tuberosa L. for Alpha-Glucosidase Inhibition Through in Silico Study. Makara J. Sci. 2021, 25, 3–31. [Google Scholar] [CrossRef]
- Hua, F.; Zhou, P.; Wu, H.-Y.; Chu, G.-X.; Xie, Z.-W.; Bao, G.-H. Inhibition of α-Glucosidase and α-Amylase by Flavonoid Glycosides from Lu’an GuaPian Tea: Molecular Docking and Interaction Mechanism. Food Funct. 2018, 9, 4173–4183. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Huang, L.; Sun, C.; Zhao, D. Exploring the Structure–Activity Relationship and Interaction Mechanism of Flavonoids and α-Glucosidase Based on Experimental Analysis and Molecular Docking Studies. Food Funct. 2020, 11, 3332–3350. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Liu, X.; Jiang, Z.; Geng, S.; Ma, H.; Liu, B. Interaction Mechanism of Flavonoids and α-Glucosidase: Experimental and Molecular Modelling Studies. Foods 2019, 8, 355. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Mango Bar | Pineapple Bar | Control Bar |
|---|---|---|---|
| Moisture | 10.23 ± 0.03 a | 11.54 ± 0.01 b | 13.15 ± 0.01 c |
| Ashes | 2.08 ± 0.03 a | 1.93 ± 0.00 b | 1.24 ± 0.00 c |
| Protein | 2.41 ± 0.06 a | 2.73 ± 0.04 b | 1.63 ± 0.04 c |
| Lipids | 1.09 ± 0.00 a | 0.19 ± 0.00 b | 4.64 ± 0.04 c |
| Carbohydrates | 15.06 ± 0.2 a | 14.88 ± 0.2 a | 17.01 ± 0.1 b |
| Total dietary fiber | 9.55 ± 0.2 a | 7.37 ± 0.3 b | 1.96 ± 0.1 c |
| Total phenolics (mg GAE/g) | 46.47 ± 0.1 a | 14.09 ± 0.0 b | 5.01 ± 0.2 c |
| Concentration | Control Bar | Mango Bar | Pineapple Bar | Acarbose | Gallic Acid |
|---|---|---|---|---|---|
| α-amylase | |||||
| 25% | 4.25 ± 0.1 a | 16.29 ± 0.23 b | 15.37 ± 0.05 b | 15.94 ± 0.01 b | 14.27 ± 0.01 b |
| 50% | 5.53 ± 0.11 a | 33.68 ± 0.15 b | 31.79 ± 0.31 c | 29.77 ± 0.24 c | 27.27 ± 0.07 d |
| 75% | 8.08 ± 0.34 a | 49.62 ± 0.1 b | 45.93 ± 0.2 c | 42.24 ± 0.01 d | 38.38 ± 0.17 e |
| 100% | 9.83 ± 0.5 a | 61.44 ± 0.35 b | 59.37 ± 0.1 b | 54.23 ± 0.6 c | 52.39 ± 0.44 c |
| α-glucosidase | |||||
| 25% | 6.84 ± 0.1 a | 25.85 ± 0.37 b | 27.54 ± 0.8 c | 24.21 ± 0.04 d | 20.57 ± 0.2 e |
| 50% | 7.42 ± 0.4 a | 37.46 ± 0.12 b | 45.31 ± 0.14 c | 39.84 ± 0.27 d | 34.24 ± 0.31 e |
| 75% | 10.15 ± 0.2 a | 48.66 ± 0.51 b | 50.82 ± 0.6 c | 47.04 ± 0.87 b | 45.51 ± 0.16 d |
| 100% | 12.46 ± 0.08 a | 64.97 ± 0.26 b | 64.57 ± 0.34 b | 62.98 ± 0.61 c | 57.02 ± 0.07 d |
| Bind | α-Amylase | α-Glucosidase |
|---|---|---|
| (Kcal/mol) | ||
| Ferulic acid | −1692.8904 | −2760.3513 |
| Buteine | −1690.6622 | −2752.7178 |
| Catechin | −1664.7638 | −2727.2085 |
| Kaempferol | - | −2733.0417 |
| Naringenin | −1692.5985 | −2757.674 |
| Quercetin | −1662.3651 | −2729.2283 |
| Acarbose | −1211.8814 | −1745.7192 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Beltrán, Y.E.; Wall-Medrano, A.; Valencia Estrada, M.A.; Sánchez-Burgos, J.A.; Blancas-Benítez, F.J.; Tovar, J.; Sáyago-Ayerdi, S.G. In Vivo Glycemic Response of Fruit-Based Mango (Mangifera indica) and Pineapple (Ananas comosus) Bars in In Vitro and In Silico Enzyme Inhibitory Effects Studies. Foods 2024, 13, 2258. https://doi.org/10.3390/foods13142258
Pérez-Beltrán YE, Wall-Medrano A, Valencia Estrada MA, Sánchez-Burgos JA, Blancas-Benítez FJ, Tovar J, Sáyago-Ayerdi SG. In Vivo Glycemic Response of Fruit-Based Mango (Mangifera indica) and Pineapple (Ananas comosus) Bars in In Vitro and In Silico Enzyme Inhibitory Effects Studies. Foods. 2024; 13(14):2258. https://doi.org/10.3390/foods13142258
Chicago/Turabian StylePérez-Beltrán, Yolanda E., Abraham Wall-Medrano, Monserrat A. Valencia Estrada, Jorge A. Sánchez-Burgos, Francisco Javier Blancas-Benítez, Juscelino Tovar, and Sonia G. Sáyago-Ayerdi. 2024. "In Vivo Glycemic Response of Fruit-Based Mango (Mangifera indica) and Pineapple (Ananas comosus) Bars in In Vitro and In Silico Enzyme Inhibitory Effects Studies" Foods 13, no. 14: 2258. https://doi.org/10.3390/foods13142258
APA StylePérez-Beltrán, Y. E., Wall-Medrano, A., Valencia Estrada, M. A., Sánchez-Burgos, J. A., Blancas-Benítez, F. J., Tovar, J., & Sáyago-Ayerdi, S. G. (2024). In Vivo Glycemic Response of Fruit-Based Mango (Mangifera indica) and Pineapple (Ananas comosus) Bars in In Vitro and In Silico Enzyme Inhibitory Effects Studies. Foods, 13(14), 2258. https://doi.org/10.3390/foods13142258










