Light Flux Density and Photoperiod Affect Growth and Secondary Metabolism in Fully Expanded Basil Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Germplasm and Growing Conditions
2.3. Biometric Determinations
2.4. Chlorophyll Concentration Index (CCI) Determination
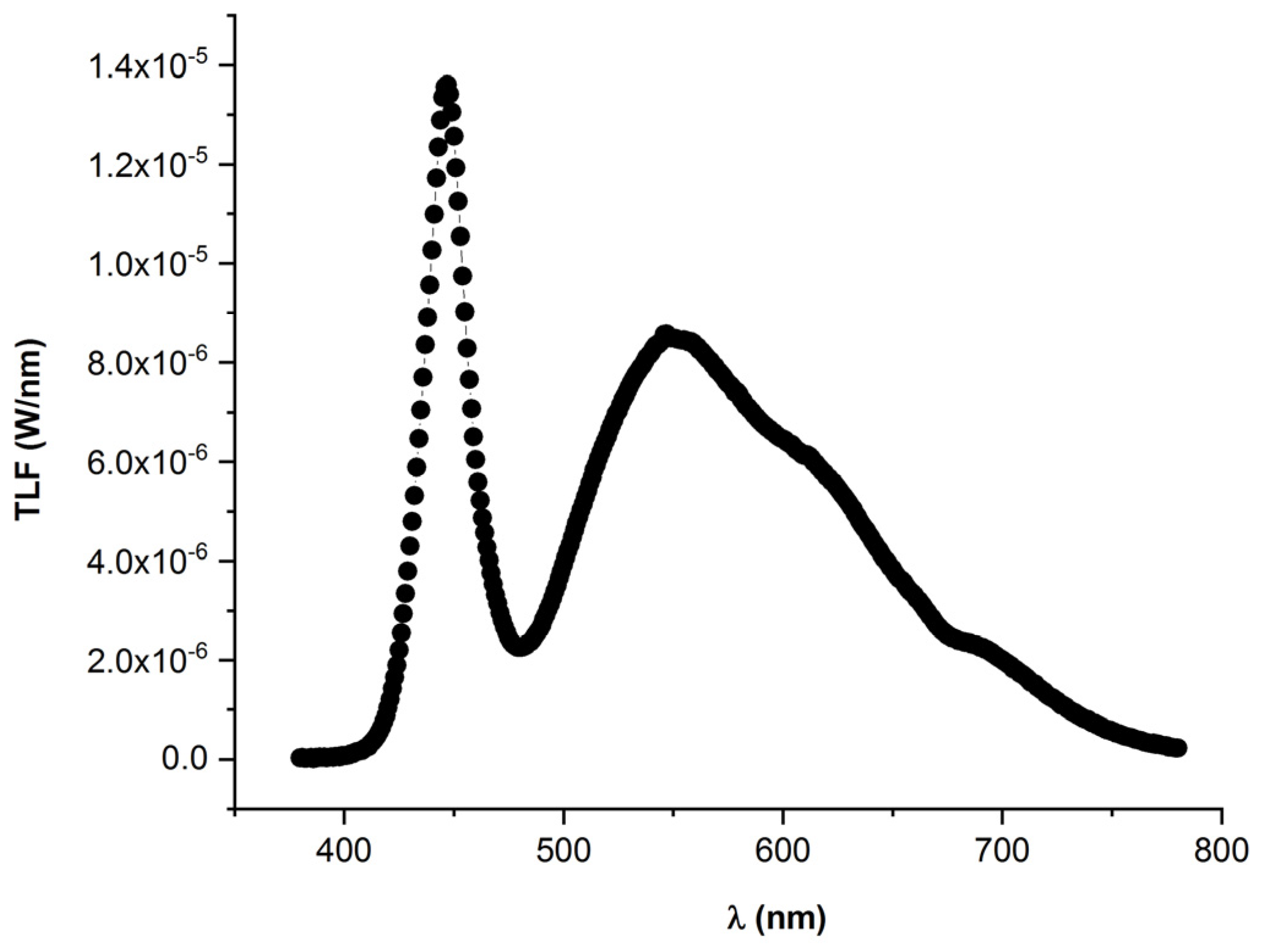
2.5. Liquid Chromatography–Electrospray Ionization–Linear Ion Trap Quadrupole–Orbitrap–Mass Spectrometry (LC-ESI-LTQ-Orbitrap-MS) Analysis
2.6. Sample Preparation and Headspace Solid Phase Microextraction (SPME) Procedure
2.7. Gas Chromatography—Quadrupole Mass Spectrometry (GC-qMS) Analysis
2.8. Multivariate Data Analysis
3. Results
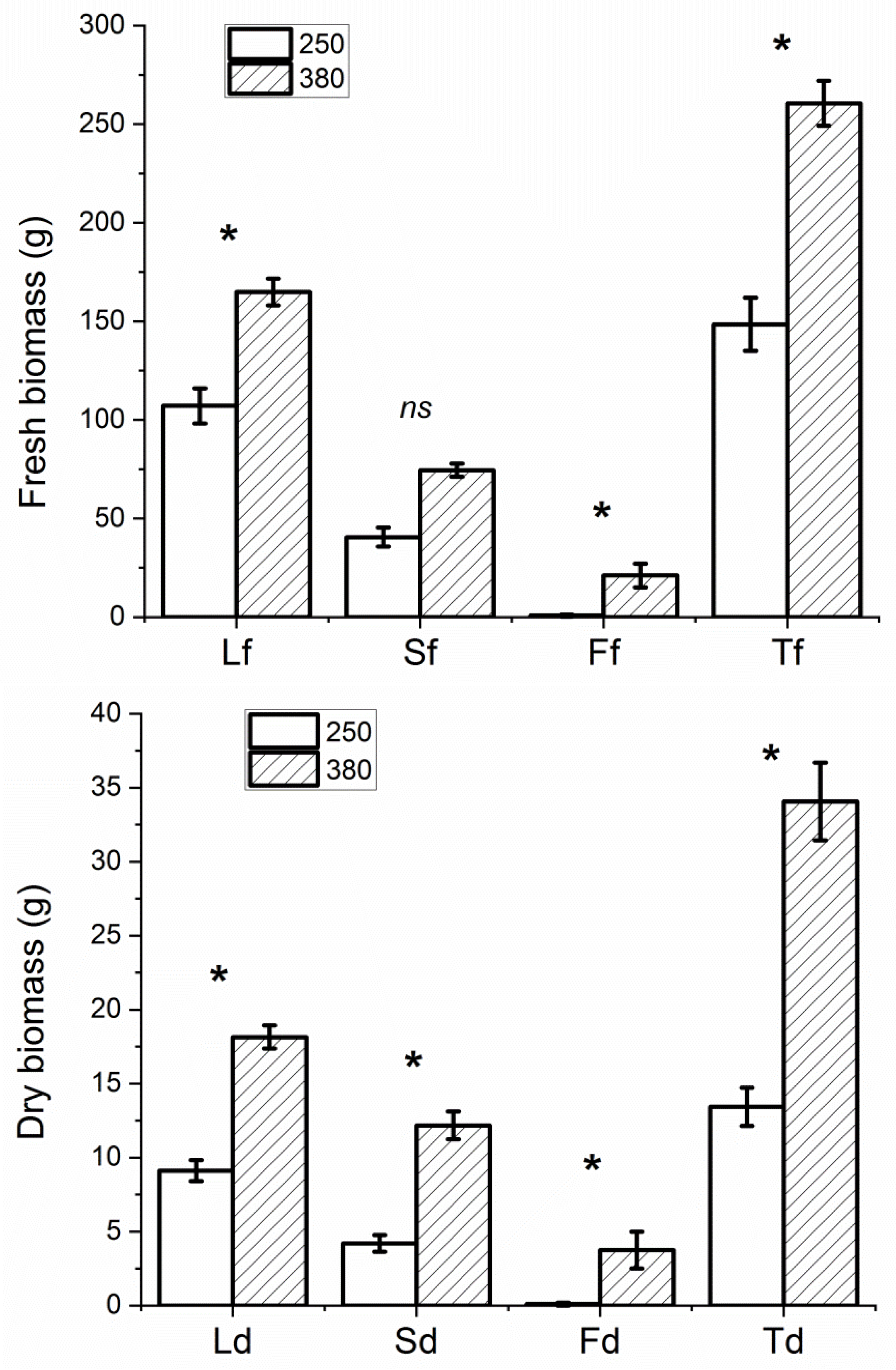
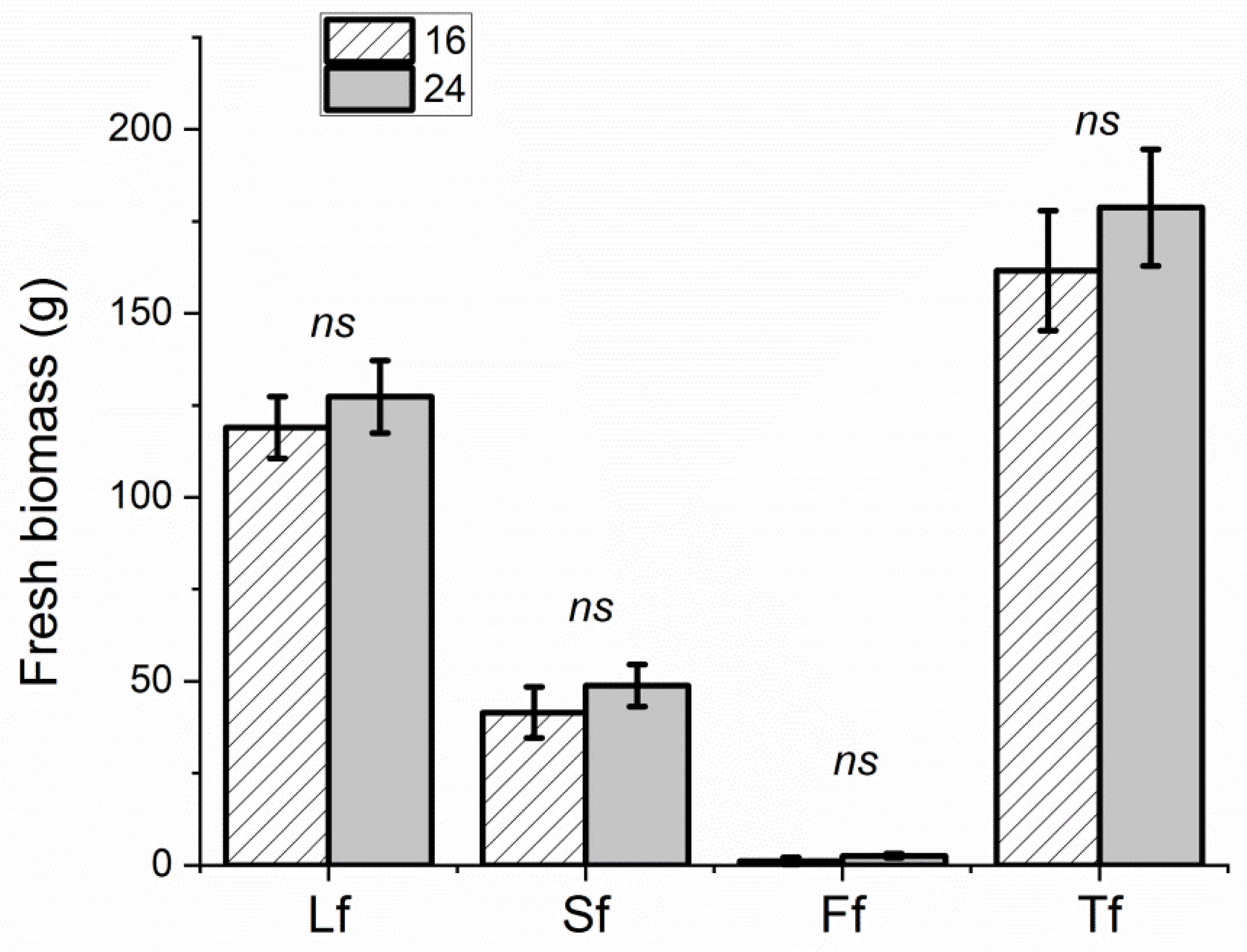
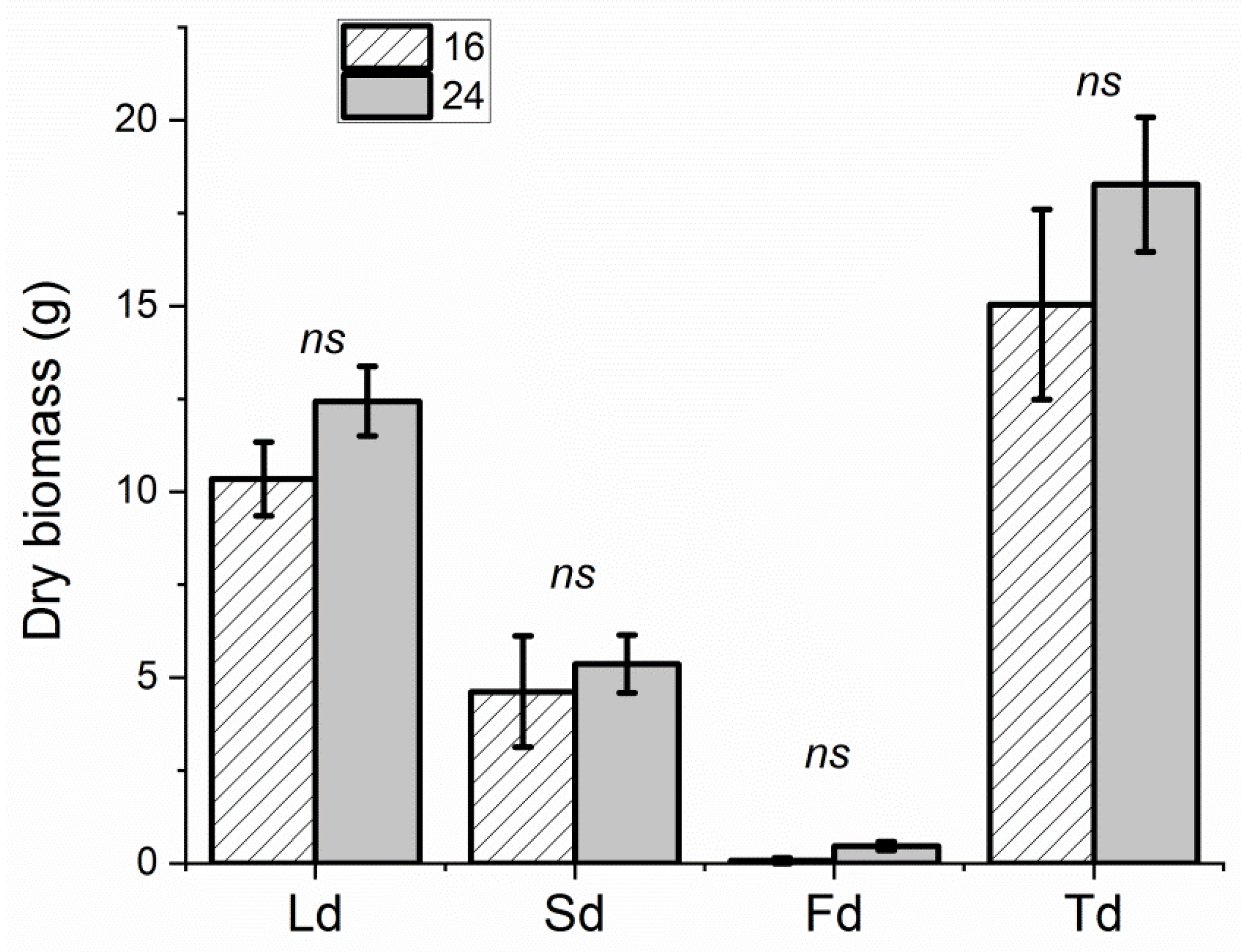
3.1. Biometric Determinations
3.2. CCI Determination
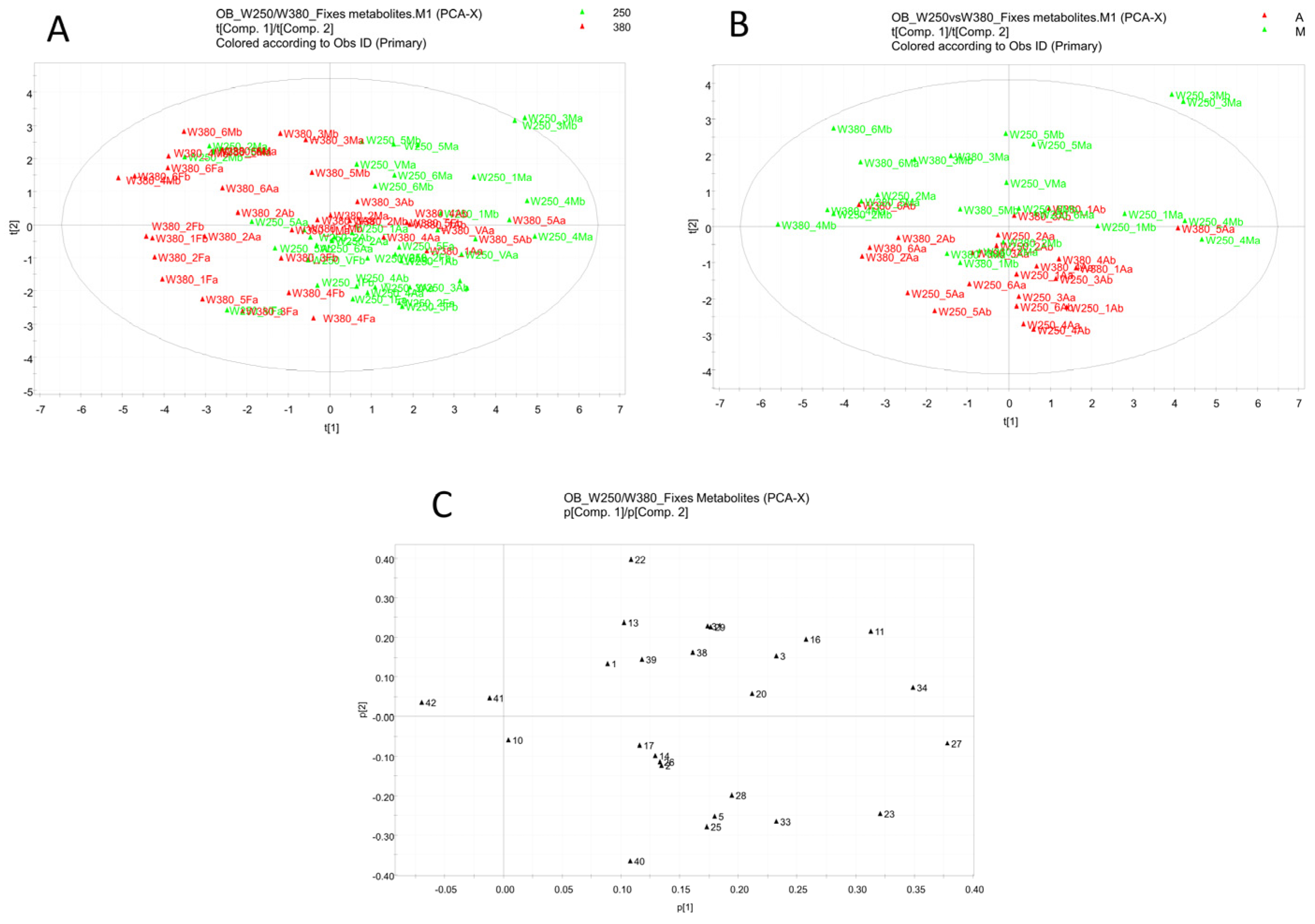
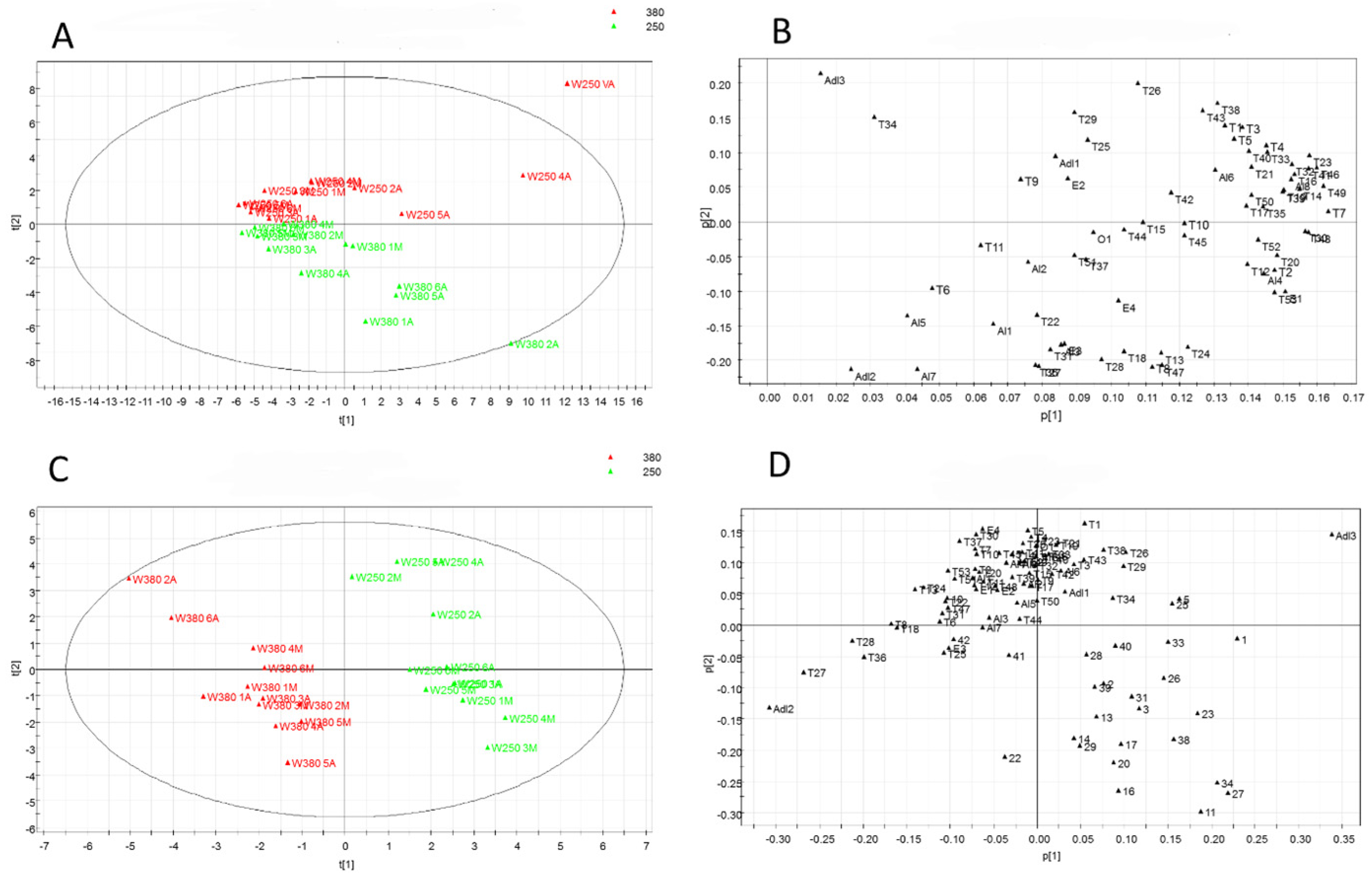
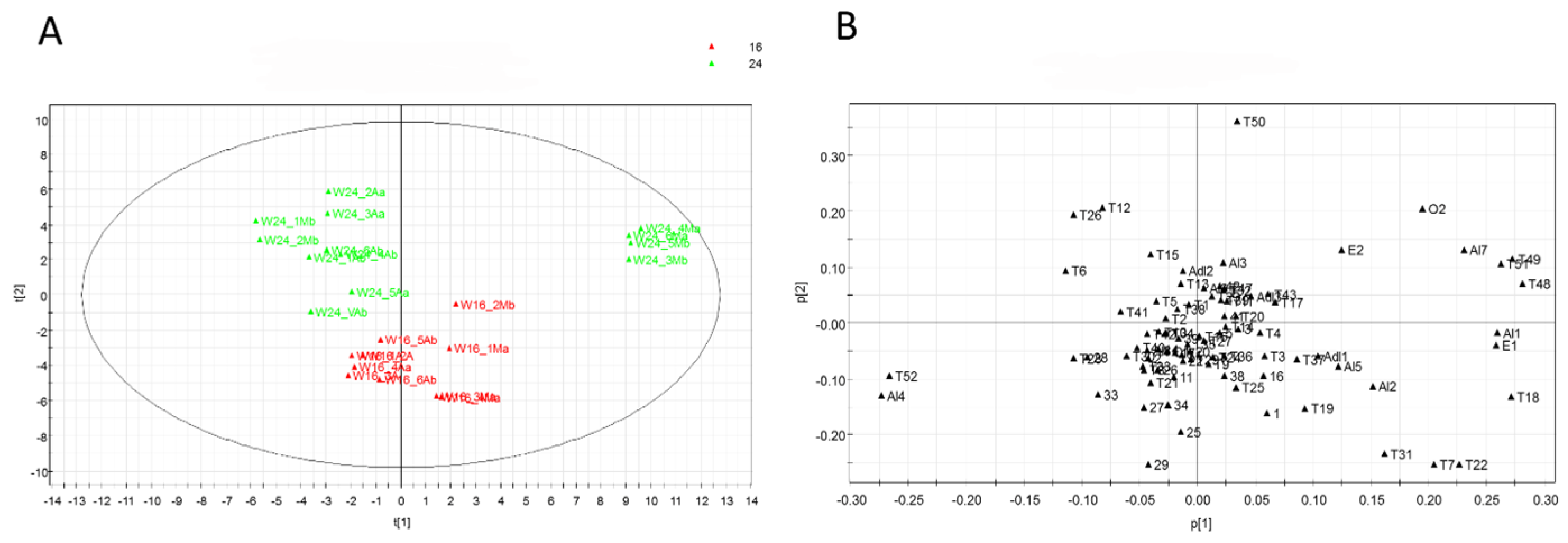
3.3. LC-ESI-LTQ-Orbitrap-MS Analysis
3.4. GC-qMS Analysis
3.5. Multivariate Data Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benke, K.; Tomkins, B. Future food-production systems: Vertical farming and controlled-environment agriculture. Sustain. Sci. Pract. Policy 2017, 13, 13–26. [Google Scholar] [CrossRef]
- Skar, S.L.G.; Pineda-Martos, R.; Timpe, A.; Pölling, B.; Bohn, K.; Külvik, M.; Delgado, C.; Pedrash, C.M.G.; Paçoi, T.A.; Ćujić, M.; et al. Urban agriculture as a keystone contribution towards securing sustainable and healthy development for cities in the future. Blue-Green Syst. 2020, 2, 1–27. [Google Scholar] [CrossRef]
- Viršilė, A.; Olle, M.; Duchovskis, P. LED Lighting in Horticulture. In Light Emitting Diodes for Agriculture; Dutta Gupta, S., Ed.; Springer: Singapore, 2017; pp. 113–147. [Google Scholar] [CrossRef]
- Darko, E.; Heydarizadeh, P.; Schoefs, B.; Sabzalian, M.R. Photosynthesis under artificial light: The shift in primary and secondary metabolism. Philos. Trans. R. Soc. B 2018, 369, 20130243. [Google Scholar] [CrossRef] [PubMed]
- Gawande, V.; Raut, D.; Rai, S.; Beese, S.; Singh, B.V.; Agnihotri, N. Artificial Light Spectra and Its Impact on Plant Physiological Processes and Secondary Metabolism. Int. J. Plant Soil Sci. 2023, 35, 2060–2070. [Google Scholar] [CrossRef]
- Landi, M.; Zivcak, M.; Sytarb, O.; Brestic, M.; Allakhverdiev, S.I. Plasticity of photosynthetic processes and the accumulation of secondary metabolites in plants in response to monochromatic light environments: A review. Biochim. Biophys. Acta (BBA)-Bioenerg. 2020, 1861, 148131. [Google Scholar] [CrossRef] [PubMed]
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C. Spectral effects of artificial light on plant physiology and secondary metabolism: A review. HortScience 2015, 50, 1128–1135. [Google Scholar] [CrossRef]
- Amaki, W.; Yamazaki, N.; Ichimura, M.; Watanabe, H. Effects of light quality on the growth and essential oil content in sweet basil. Acta Hortic. 2011, 907, 91–94. [Google Scholar] [CrossRef]
- Bantis, F.; Ouzounis, T.; Radoglou, K. Artificial LED lighting enhances growth characteristics and total phenolic content of Ocimum basilicum, but variably affects transplant success. Sci. Hortic. 2016, 198, 277–283. [Google Scholar] [CrossRef]
- Carvalho, S.D.; Schwieterman, M.L.; Abrahan, C.E.; Colquhoun, T.A.; Folta, K.M. Light Quality Dependent Changes in Morphology, Antioxidant Capacity, and Volatile Production in Sweet Basil (Ocimum basilicum). Front. Plant Sci. 2016, 7, 1328. [Google Scholar] [CrossRef]
- Chutimanukul, P.; Wanichananan, P.; Janta, S.; Toojinda, T.; Darwell, C.T.; Mosaleeyanon, K. The influence of different light spectra on physiological responses, antioxidant capacity and chemical compositions in two holy basil cultivars. Sci. Rep. 2022, 12, 588. [Google Scholar] [CrossRef]
- d’Aquino, L.; Cozzolino, R.; Nardone, G.; Borelli, G.; Gambale, E.; Sighicelli, M.; Menegoni, P.; Modarelli, G.C.; Rimauro, J.; Chianese, E.; et al. Effects of White and Blue-Red Light on Growth and Metabolism of Basil Grown under Microcosm Conditions. Plants 2023, 12, 1450. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Responses of sweet basil to different daily light integrals in photosynthesis, morphology, yield, and nutritional quality. HortScience 2018, 53, 496–503. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M. Photosynthesis, morphology, yield, and phytochemical accumulation in basil plants influenced by substituting green light for partial red and/or blue light. HortScience 2019, 54, 1769–1776. [Google Scholar] [CrossRef]
- Hammock, H.A.; Kopsell, D.A.; Sams, C.E. Narrowband Blue and Red LED Supplements Impact Key Flavor Volatiles in Hydroponically Grown Basil Across Growing Seasons. Front. Plant Sci. 2021, 12, 623314. [Google Scholar] [CrossRef] [PubMed]
- Hammock, H.A.; Sams, C.E. Variation in supplemental lighting quality influences key aroma volatiles in hydroponically grown ‘Italian Large Leaf’ basil. Front. Plant Sci. 2023, 14, 1184664. [Google Scholar] [CrossRef] [PubMed]
- Kivimäenpä, M.; Mofikoya, A.; El-Raheem, A.M.A.; Riikonen, J.; Julkunen-Tiitto, R.; Holopainen, J.K. Alteration in Light Spectra Causes Opposite Responses in Volatile Phenylpropanoids and Terpenoids Compared with Phenolic Acids in Sweet Basil (Ocimum basilicum) Leaves. J. Agric. Food Chem. 2022, 70, 12287–12296. [Google Scholar] [CrossRef] [PubMed]
- Lauria, G.; Piccolo, E.L.; Davini, A.; Castiglione, M.R.; Pieracci, Y.; Flamini, G.; Martens, S.; Angeli, A.; Ceccanti, C.; Guidi, L.; et al. Modulation of VOC fingerprint and alteration of physiological responses after supplemental LED light in green-and red-leafed sweet basil (Ocimum basilicum L.). Sci. Hortic. 2023, 315, 111970. [Google Scholar] [CrossRef]
- Litvin, A.G.; Currey, C.J.; Wilson, L.A. Effects of Supplemental Light Source on Basil, Dill, and Parsley Growth, Morphology, Aroma, and Flavor. J. Am. Soc. Hortic. Sci. 2020, 145, 18–29. [Google Scholar] [CrossRef]
- Lobiuc, A.; Vasilache, V.; Pintilie, O.; Stoleru, T.; Burducea, M.; Oroian, M.; Zamfirache, M. Blue and Red LED Illumination Improves Growth and Bioactive Compounds Contents in Acyanic and Cyanic Ocimum basilicum L. Microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef]
- Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Unraveling the Role of Red:Blue LED Lights on Resource Use Efficiency and Nutritional Properties of Indoor Grown Sweet Basil. Front. Plant Sci. 2019, 10, 305. [Google Scholar] [CrossRef]
- Pennisi, G.; Pistillo, A.; Orsini, F.; Cellini, A.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Crepaldi, A.; Gianquinto, G.; Marcelis, L.F.M. Optimal light intensity for sustainable water and energy use in indoor cultivation of lettuce and basil under red and blue LEDs. Sci. Hortic. 2020, 272, 109508. [Google Scholar] [CrossRef]
- Walters, K.J.; Lopez, R.G.; Behe, B.K. Leveraging Controlled-Environment Agriculture to Increase Key Basil Terpenoid and Phenylpropanoid Concentrations: The Effects of Radiation Intensity and CO2 Concentration on Consumer Preference. Front. Plant Sci. 2021, 11, 598519. [Google Scholar] [CrossRef] [PubMed]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Chemical components and pharmacological benefits of Basil (Ocimum basilicum): A review. Int. J. Food Prop. 2020, 23, 1961–1970. [Google Scholar] [CrossRef]
- d’Aquino, L.; Lanza, B.; Gambale, E.; Sighicelli, M.; Menegoni, P.; Modarelli, G.C.; Rimauro, J.; Chianese, E.; Nenna, G.; Fasolino, T.; et al. Growth and metabolism of basil grown in a new-concept microcosm under different lighting conditions. Sci. Hortic. 2022, 299, 111035. [Google Scholar] [CrossRef]
- Cozzolino, R.; Pace, B.; Cefola, M.; Martignetti, A.; Stocchero, M.; Fratianni, F.; Nazzaro, F.; De Giulio, B. Assessment of volatile profile as potential marker of chilling injury of basil leaves during postharvest storage. Food Chem. 2016, 2013, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Kovats, V.E. Gas-chromatographische charakterisierung organischer verbindungen. Teil 1: Retentionsindices aliphatischer halogenide, alkohole, aldehyde und ketone. Helv. Chim. Acta 1958, 41, 1915–1932. [Google Scholar] [CrossRef]
- Sarais, G.; D’Urso, G.; Lai, C.; Pirisi, F.M.; Pizza, C.; Montoro, P. Targeted and untargeted mass spectrometric approaches in discrimination between Myrtus communis cultivars from Sardinia region. J. Mass. Spectrom. 2016, 51, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Birse, N.; Quinn, B.; Li, Y.; Jia, W.; McCarron, P.; Rosas da Silva, G.; Vanhaecke, L.; Van Ruth, S.; Elliott, C.T. Data fusion and multivariate analysis for food authenticity analysis. Nat. Commun. 2023, 14, 3309. [Google Scholar] [CrossRef] [PubMed]
- Larsen, D.H.; Woltering, E.J.; Nicole, C.C.S.; Marcelis, L.F.M. Response of Basil Growth and Morphology to Light Intensity and Spectrum in a Vertical Farm. Front. Plant Sci. 2020, 11, 597906. [Google Scholar] [CrossRef]
- Schenkels, L.; Saeys, W.; Lauwers, A.; Maurice, P. Green light induces shade avoidance to alter plant morphology and increases biomass production in Ocimum basilicum L. Sci. Hortic. 2020, 261, 109002. [Google Scholar] [CrossRef]
- Brosset, A.; Blande, J.D. Volatile-mediated plant–plant interactions: Volatile organic compounds as modulators of receiver plant defence, growth, and reproduction. J. Exp. Bot. 2022, 73, 511–528. [Google Scholar] [CrossRef]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Khare, S.; Singh, N.B.; Singh, A.; Hussain, I.; Niharika, K.; Yadav, V.; Bano, C.; Yadav, R.K.; Amist, N. Plant secondary metabolites synthesis and their regulations under biotic and abiotic constraints. J. Plant Biol. 2020, 63, 203–216. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, M.; Wu, M.; Li, X.; Liu, H.; Niu, N.; Li, S.; Chen, L. Volatile organic compounds (VOCs) from plants: From release to detection. TrAC Trends Anal. Chem. 2023, 158, 116872. [Google Scholar] [CrossRef]
- Ninkovic, V.; Markovic, D.; Rensing, M. Plant volatiles as cues and signals in plant communication. Plant Cell Environ. 2021, 44, 1030–1043. [Google Scholar] [CrossRef] [PubMed]
- Twaij, B.M.; Hasan, M.N. Bioactive Secondary Metabolites from Plant Sources: Types, Synthesis, and Their Therapeutic Uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Ballestrieri, D.; Strani, L.; Cocchi, M.; Durante, C. Characterization of Basil Volatile Fraction and Study of Its Agronomic Variation by ASCA. Molecules 2021, 26, 3842. [Google Scholar] [CrossRef]
- Lee, S.-J.; Katumi, U.; Takayuki, S.; Kwang-Geun, L. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem. 2005, 91, 131. [Google Scholar] [CrossRef]
- Murarikova, A.; Tazky, A.; Neugebaureova, J.; Plankova, A.; Jampilek, J.; Mucaji, P.; Mikus, P. Characterization of Essential Oil Composition in Different Basil Species and Pot Cultures by a GC-MS Method. Molecules 2017, 22, 1221. [Google Scholar] [CrossRef]
- Hoch, C.C.; Petry, J.; Griesbaum, L.; Weiser, T.; Werner, K.; Ploch, M.; Verschoor, A.; Multhoff, G.; Bashiri Dezfouli, A.; Wollenberg, B. 1,8-cineole (eucalyptol): A versatile phytochemical with therapeutic applications across multiple diseases. Biomed. Pharmacother. 2023, 167, 115467. [Google Scholar] [CrossRef] [PubMed]
- Racea, R.; Macasoi, I.; Dinu, S.; Pinzaru, I.; Marcovici, I.; Dehelean, C.; Rusu, L.; Chioran, D.; Rivis, M.; Buzatu, R. Eugenol: In Vitro and In Ovo Assessment to Explore Cytotoxic Effects on Osteosarcoma and Oropharyngeal Cancer Cells. Plants 2023, 12, 3549. [Google Scholar] [CrossRef] [PubMed]
- Tholl, D. Biosynthesis and Biological Functions of Terpenoids in Plants. In Biotechnology of Isoprenoids. Advances in Biochemical Engineering/Biotechnology; Schrader, J., Bohlmann, J., Eds.; Springer: Cham, Switzerland, 2015; Volume 148, pp. 63–106. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Kivimäenpää, M.; Julkunen-Tiitto, R. New light for phytochemicals. Trends Biotechnol. 2018, 36, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Palmer-Young, E.C.; Veit, D.; Gershenzon, J.; Schuman, M.C. The Sesquiterpenes(E)-ß-Farnesene and (E)-α-Bergamotene Quench Ozone but Fail to Protect the Wild Tobacco Nicotiana attenuata from Ozone, UVB, and Drought Stresses. PLoS ONE 2015, 10, e0127296. [Google Scholar] [CrossRef]


| N° | RT | [M-H]− | Formula | Δ ppm | Metabolite Identification | M250 | M380 | ||
|---|---|---|---|---|---|---|---|---|---|
| A | M | A | M | ||||||
| 1 | 5.8 | 263.0049 | C8H7O10 | 4.7 | unknown | ||||
| 2 | 5.8 | 355.1245 | C13H23O11 | 1.9 | methyl-glucopyranosyl-glucopyranoside | x | |||
| 3 | 6.95 | 191.0198 | C6H7O7 | 2.6 | citric acid | x | x | ||
| 4 | 7.96 | 593.1497 | C27H29O15 | 0.5 | vicenin II | x | x | ||
| 5 | 8 | 417.1040 | C17H21O12 | 1 | aloin | x | x | x | |
| 6 | 8.22 | 197.0454 | C9H9O5 | 3.1 | syringic acid | x | x | x | x |
| 7 | 10.6 | 353.0872 | C16H17O9 | 1.4 | chlorogenic acid | x | x | x | x |
| 8 | 10.82 | 193.0505 | C10H9O4 | 5 | ferulic acid | x | x | x | x |
| 9 | 11.08 | 181.0504 | C9H9O4 | 4.5 | homovanillic acid | x | x | ||
| 10 | 12.59 | 609.1468 | C27H29O16 | 2.9 | kaempferol gentiobioside | x | x | x | |
| 11 | 12.85 | 447.0982 | C21H19O11 | 2.8 | luteolin glucoside | x | x | x | |
| 12 | 13.55 | 463.0890 | C21H19O12 | 4.4 | quercetin glucoside | x | x | ||
| 13 | 13.6 | 311.1136 | C15H19O7 | 3.2 | glucopyranosyloxy hydroxy allylbenzene | x | |||
| 14 | 13.6 | 493.1137 | C26H21O10 | 1.5 | salvianolic acid A | x | x | x | |
| 15 | 13.81 | 300.9987 | C14H5O8 | −0.5 | ellagic acid | x | x | ||
| 16 | 13.98 | 665.3887 | C36H57O11 | 1.4 | triterpene | x | x | ||
| 17 | 14.15 | 473.0729 | C22H17O12 | 2.8 | chicoric acid | x | x | ||
| 18 | 14.15 | 475.0869 | C22H19O12 | −0.3 | luteolin glucuronide methyl ester | x | x | ||
| 19 | 14.71 | 339.0723 | C15H15O9 | 2.7 | esculin hydrate | x | |||
| 20 | 14.84 | 431.0982 | C21H19O10 | 2.2 | genistin | x | x | x | |
| 21 | 14.89 | 717.1475 | C36H29O16 | 1.7 | salvianolic acid B | x | x | ||
| 22 | 15.41 | 359.0775 | C18H15O8 | 1.8 | rosmarinic acid | x | x | x | x |
| 23 | 15.41 | 445.0776 | C22H17O11 | 3.9 | apigenin glucuronide | x | x | ||
| 24 | 15.88 | 203.0827 | C11H11O2N2 | 4.5 | L-tryptophan | x | x | x | |
| 25 | 16.14 | 651.2310 | C31H39O15 | −1.1 | epiredisroside A | x | x | x | |
| 26 | 16.36 | 577.1571 | C27H29O14 | 1.3 | rhoifolin | x | x | x | |
| 27 | 16.75 | 459.0923 | C22H19O11 | 1.2 | apigenin glucuronide methyl ester | x | x | ||
| 28 | 16.82 | 327.0873 | C18H15O6 | 3 | salvigenin | x | x | x | |
| 29 | 17.48 | 285.0396 | C15H9O6 | 1.2 | luteolin | x | x | x | |
| 30 | 17.52 | 289.0690 | C15H13O6 | −5.9 | catechin | x | x | x | |
| 31 | 18.19 | 501.1038 | C24H21O12 | 0.5 | diferuloyl tartaric acid | x | |||
| 32 | 19.35 | 269.0457 | C15H9O5 | 0.8 | apigenin | x | x | ||
| 33 | 19.78 | 329.0665 | C17H13O7 | 1.3 | aurantio-obtusin | x | |||
| 34 | 21.99 | 373.0922 | C19H17O8 | 1.4 | rosmarinic acid methyl ester | x | x | ||
| 35 | 22.6 | 271.0618 | C15H11O5 | 1.4 | naringenin | x | x | x | |
| 36 | 22.6 | 283.0596 | C16H11O5 | −1.5 | wogonin | x | x | x | |
| 37 | 22.68 | 343.0223 | C18H15O8 | 4.6 | eupatilin | x | x | x | |
| 38 | 23.11 | 313.0717 | C17H13O6 | 2.6 | pectolinarigenin | x | x | x | |
| 39 | 25.23 | 487.3427 | C30H47O5 | 1.3 | asiatic acid | x | x | ||
| 40 | 27.82 | 291.1997 | C15H31O3S | 2.8 | pentadecanesulfonic acid | x | x | x | |
| 41 | 29.22 | 325.1448 | C20H21O4 | 0.2 | dehydrodiisoeugenol | x | x | x | |
| 42 | 30.95 | 471.3459 | C30H47O4 | −2.2 | hederagenin | x | x | x | |
| 43 | 38.83 | 455.3515 | C30H47O3 | −0.15 | ursolic acid | x | |||
| N° | RT | [M-H]− | Formula | Δ ppm | Metabolite Identification | M16 | M24 | ||
|---|---|---|---|---|---|---|---|---|---|
| A | M | A | M | ||||||
| 1 | 5.5 | 263.0049 | C8H7O10 | - | unknown | ||||
| 2 | 5.7 | 355.1245 | C13H23O11 | 1.9 | methyl-glucopyranosyl-glucopyranoside | x | x | ||
| 3 | 6.75 | 191.0198 | C6H7O7 | 2.6 | citric acid | x | x | x | x |
| 4 | 7.98 | 593.1497 | C27H29O15 | 0.5 | vicenin II | x | x | ||
| 5 | 8.2 | 417.1040 | C17H21O12 | 1 | aloin | x | x | x | x |
| 6 | 8.32 | 197.0454 | C9H9O5 | 3.1 | syringic acid | x | x | x | x |
| 7 | 10.7 | 353.0872 | C16H17O9 | 1.4 | chlorogenic acid | x | x | x | x |
| 8 | 10.92 | 193.0505 | C10H9O4 | 5 | ferulic acid | x | x | x | x |
| 9 | 11.18 | 181.0504 | C9H9O4 | 4.5 | homovanillic acid | x | x | ||
| 10 | 12.69 | 609.1468 | C27H29O16 | 2.9 | kaempferol gentiobioside | x | x | x | x |
| 11 | 12.95 | 447.0982 | C21H19O11 | 2.8 | luteolin glucoside | x | x | x | x |
| 12 | 13.75 | 463.0890 | C21H19O12 | 4.4 | quercetin glucoside | x | x | ||
| 13 | 13.71 | 311.1136 | C15H19O7 | 3.2 | glucopyranosyloxy hydroxy allylbenzene | ||||
| 14 | 13.75 | 493.1137 | C26H21O10 | 1.5 | salvianolic acid A | x | x | x | |
| 15 | 13.89 | 300.9987 | C14H5O8 | −0.5 | ellagic acid | x | x | x | |
| 16 | 14.01 | 665.3887 | C36H57O11 | 1.4 | triterpene | x | x | ||
| 17 | 14.22 | 473.0729 | C22H17O12 | 2.8 | chicoric acid | x | x | ||
| 18 | 14.22 | 475.0869 | C22H19O12 | −0.3 | luteolin glucuronide methyl ester | x | x | ||
| 19 | 14.87 | 339.0723 | C15H15O9 | 2.7 | esculin hydrate | x | x | ||
| 20 | 14.98 | 431.0982 | C21H19O10 | 2.2 | genistin | x | x | x | |
| 21 | 14.99 | 717.1475 | C36H29O16 | 1.7 | salvianolic acid B | x | x | x | x |
| 22 | 15.66 | 359.0775 | C18H15O8 | 1.8 | rosmarinic acid | x | x | x | x |
| 23 | 15.71 | 445.0776 | C22H17O11 | 3.9 | apigenin glucuronide | ||||
| 24 | 15.98 | 203.0827 | C11H11O2N2 | 4.5 | L-tryptophan | x | x | x | x |
| 25 | 16.45 | 651.2310 | C31H39O15 | −1.1 | epiredisroside A | x | x | x | x |
| 26 | 16.56 | 577.1571 | C27H29O14 | 1.3 | rhoifolin | x | x | x | x |
| 27 | 16.79 | 459.0923 | C22H19O11 | 1.2 | apigenin glucuronide methyl ester | x | x | ||
| 28 | 16.88 | 327.0873 | C18H15O6 | 3 | salvigenin | x | x | x | x |
| 29 | 17.55 | 285.0396 | C15H9O6 | 1.2 | luteolin | x | x | x | x |
| 30 | 17.77 | 289.0690 | C15H13O6 | −5.9 | catechin | x | x | x | |
| 31 | 18.10 | 501.1038 | C24H21O12 | 0.5 | diferuloyl tartaric acid | x | |||
| 32 | 19.38 | 269.0457 | C15H9O5 | 0.8 | apigenin | x | x | ||
| 33 | 19.78 | 329.0665 | C17H13O7 | 1.3 | aurantio-obtusin | x | |||
| 34 | 21.98 | 373.0922 | C19H17O8 | 1.4 | rosmarinic acid methyl ester | x | x | ||
| 35 | 22.61 | 271.0618 | C15H11O5 | 1.4 | naringenin | x | x | x | |
| 36 | 22.65 | 283.0596 | C16H11O5 | −1.5 | wogonin | x | x | x | |
| 37 | 22.78 | 343.0223 | C18H15O8 | 4.6 | eupatilin | x | x | ||
| 38 | 23.13 | 313.0717 | C17H13O6 | 2.6 | pectolinarigenin | x | x | ||
| 39 | 25.33 | 487.3427 | C30H47O5 | 1.3 | asiatic acid | x | x | ||
| 40 | 27.99 | 291.1997 | C15H31O3S | 2.8 | pentadecanesulfonic acid | ||||
| 41 | 29.32 | 325.1448 | C20H21O4 | 0.2 | dehydrodiisoeugenol | x | x | ||
| 42 | 32.11 | 471.3459 | C30H47O4 | −2.2 | hederagenin | ||||
| 43 | 38.98 | 455.3515 | C30H47O3 | −0.15 | ursolic acid | x | x | ||
| Metabolite | Code | a RIt/RIsp | b ID | Metabolite | Code | a RIt/RIsp | b ID |
|---|---|---|---|---|---|---|---|
| Terpenes | |||||||
| α-Pinene | T1 | 1026/1021 | RI/MS/S | α-Selinene | T39 | 1700/1688 | RI/MS |
| α-Thujene | T2 | 1030/1025 | RI/MS/S | Bicyclogermacrene | T40 | 1740/1742 | RI/MS |
| Camphene | T3 | 1076/1080 | RI/MS/S | α-Amorphene | T41 | 1681/1681 | RI/MS |
| β-Pinene | T4 | 1106/1118 | RI/MS/S | γ-Cadinene | T42 | 1765/1766 | RI/MS |
| Sabinene | T5 | 1129/1130 | RI/MS/S | β-Sesquiphellandrene | T43 | 1775/1776 | RI/MS |
| α-Phellandrene | T6 | 1167/1177 | RI/MS/S | α-Curcumene | T44 | 1784/1784 | RI/MS |
| β-Myrcene | T7 | 1168/1156 | RI/MS/S | Cadina-1,4-diene | T45 | 1786/1789 | RI/MS |
| α-Terpinene | T8 | 1180/1186 | RI/MS/S | α-Cadinene | T46 | 1808/1815 | RI/MS |
| Limonene | T9 | 1198/1206 | RI/MS/S | Nerol | T47 | 1815/1812 | RI/MS/S |
| β-Phellandrene | T10 | 1202/1202 | RI/MS/S | cis-Calamenene | T48 | 1835/1837 | RI/MS |
| 1,8-Cineole | T11 | 1221/1228 | RI/MS/S | α-Calacorene | T49 | 1926/1926 | RI/MS |
| cis-α-Ocimene | T12 | 1251/1245 | RI/MS/S | β-Ionone | T50 | 1963/1964 | RI/MS/S |
| γ-Terpinene | T13 | 1255/1251 | RI/MS/S | Methyl Eugenol | T51 | 2014/2014 | RI/MS/S |
| trans-β-Ocimene | T14 | 1258/1250 | RI/MS/S | trans-Nerolidol | T52 | 2054/2054 | RI/MS/S |
| p-Cymene | T15 | 1276/1267 | RI/MS/S | Eugenol | T53 | 2141/2141 | RI/MS/S |
| α-Terpinolene | T16 | 1288/1287 | RI/MS/S | Aldehydes | |||
| trans-Allocimene | T117 | 1382/1392 | RI/MS/S | Hexanal | Ald1 | 1090/1088 | RI/MS/S |
| 1,3,8 p-Menthatriene | T18 | 1391/1391 | RI/MS | cis-3-Hexenal | Ald2 | 1118/1118 | RI/MS/S |
| α-Cubebene | T19 | 1466/1463 | RI/MS | trans-2-Hexenal | Ald3 | 1026/1020 | RI/MS/S |
| trans-Sabinene hydrate | T20 | 1474/1474 | RI/MS | Alcohols | |||
| α-Copaene | T21 | 1499/1497 | RI/MS | 2-Penten-1-ol | Al1 | 1316/1316 | RI/MS/S |
| Camphor | T22 | 1521/1518 | RI/MS/S | Hexanol | Al2 | 1363/1360 | RI/MS/S |
| β-Cubebene | T23 | 1545/1541 | RI/MS | trans-3-Hexen-1-ol | Al3 | 1392/1390 | RI/MS/S |
| Linalool | T24 | 1566/1560 | RI/MS/S | 3-Octanol | Al4 | 1404/1396 | RI/MS/S |
| Bornyl acetate | T25 | 1588/1584 | RI/MS/S | trans-2-Hexen-1-ol | Al5 | 1413/1411 | RI/MS/S |
| α-Bergamotene | T26 | 1598/1590 | RI/MS | 1-Octen-3-ol | Al6 | 1464/1465 | RI/MS/S |
| α-Farnesene | T27 | 1698/1698 | RI/MS/S | 2-Ethyl-1-hexanol | Al7 | 1499/1496 | RI/MS/S |
| α-Guaiene | T28 | 1603/1600 | RI/MS | Octanol | Al8 | 1570/1572 | RI/MS/S |
| β-Elemene | T29 | 1600/1593 | RI/MS/S | Esters | |||
| Aromadendrene | T30 | 1649/1650 | RI/MS/S | cis-3-Hexenyl acetate | E1 | 1321/1321 | RI/MS/S |
| 4-Terpineol | T31 | 1614/1616 | RI/MS/S | 1-Octen-3-yl-acetate | E2 | 1389/1379 | RI/MS |
| α-Humulene | T32 | 1674/1672 | RI/MS/S | cis-3-Hexenyl isovalerate | E3 | 1440/1440 | RI/MS |
| Epi-bicyclosesquiphellandrene | T33 | 1653/1633 | RI/MS | Methyl benzoate | E4 | 1628/1628 | RI/MS/S |
| trans-β-Farnesene | T34 | 1677/1673 | RI/MS/S | Ketones | |||
| Ledene | T35 | 1702/1707 | RI/MS/S | 3-Octanone | O1 | 1260/1254 | RI/MS/S |
| L-Borneol | T36 | 1709/1707 | RI/MS/S | 1-Octen-3-one | O2 | 1290/1290 | RI/MS/S |
| α-Terpineol | T37 | 1705/1707 | RI/MS/S | ||||
| Germacrene D | T38 | 1716/1716 | RI/MS/S |
| Metabolite | Code | a RIt/RIsp | b ID | Metabolite | Code | a RIt/RIsp | b ID |
|---|---|---|---|---|---|---|---|
| Terpenes | |||||||
| α-Pinene | T1 | 1026/1021 | RI/MS/S | α-Selinene | T38 | 1700/1688 | RI/MS |
| α-Thujene | T2 | 1030/1025 | RI/MS/S | Bicyclogermacrene | T39 | 1740/1742 | RI/MS |
| Camphene | T3 | 1076/1080 | RI/MS/S | α-Amorphene | T40 | 1681/1681 | RI/MS |
| β-Pinene | T4 | 1106/1118 | RI/MS/S | γ-Cadinene | T41 | 1765/1766 | RI/MS |
| Sabinene | T5 | 1129/1130 | RI/MS/S | β-Sesquiphellandrene | T42 | 1775/1776 | RI/MS |
| δ-3-Carene | T6 | 1149/1151 | RI/MS/S | α-Curcumene | T43 | 1784/1784 | RI/MS |
| α-Phellandrene | T7 | 1167/1177 | RI/MS/S | Cadina-1,4-diene | T44 | 1786/1789 | RI/MS |
| β-Myrcene | T8 | 1168/1156 | RI/MS/S | α-Cadinene | T45 | 1808/1815 | RI/MS |
| α-Terpinene | T9 | 1180/1186 | RI/MS/S | Nerol | T46 | 1815/1812 | RI/MS/S |
| D-Limonene | T10 | 1198/1206 | RI/MS/S | cis-Calamenene | T47 | 1835/1837 | RI/MS |
| β-Phellandrene | T11 | 1202/1202 | RI/MS/S | α-Calacorene | T48 | 1926/1926 | RI/MS |
| 1,8-Cineole | T12 | 1221/1228 | RI/MS/S | β-Ionone | T49 | 1963/1964 | RI/MS/S |
| cis-β-Ocimene | T13 | 1251/1245 | RI/MS/S | Methyl Eugenol | T50 | 2014/2014 | RI/MS/S |
| γ-Terpinene | T14 | 1255/1251 | RI/MS/S | trans-Nerolidol | T51 | 2054/2054 | RI/MS/S |
| trans-β-Ocimene | T15 | 1258/1250 | RI/MS/S | Eugenol | T52 | 2141/2141 | RI/MS/S |
| p-Cymene | T16 | 1276/1267 | RI/MS | Aldehydes | |||
| α-Terpinolene | T17 | 1288/1287 | RI/MS/S | Hexanal | Ald1 | 1090/1088 | RI/MS/S |
| trans-Allocimene | T18 | 1382/1392 | RI/MS/S | cis-3-Hexenal | Ald2 | 1118/1118 | RI/MS/S |
| 1,3,8 p-Menthatriene | T19 | 1391/1391 | RI/MS | trans-2-Hexenal | Ald3 | 1026/1020 | RI/MS/S |
| α-Cubebene | T20 | 1466/1463 | RI/MS | Alcohols | |||
| trans-Sabinene hydrate | T21 | 1474/1474 | RI/MS | ||||
| trans-Linalool oxide | T22 | 1484/1486 | RI/MS | 2-Penten-1-ol | Al1 | 1316/1316 | RI/MS/S |
| α-Copaene | T23 | 1499/1497 | RI/MS | Hexanol | Al2 | 1363/1360 | RI/MS/S |
| Camphor | T27 | 1521/1518 | RI/MS/S | trans-3-Hexen-1-ol | Al3 | 1392/1390 | RI/MS/S |
| β-Cubebene | T25 | 1545/1541 | RI/MS | 3-Octanol | Al4 | 1404/1396 | RI/MS/S |
| Linalool | T26 | 1566/1560 | RI/MS/S | trans-2-Hexen-1-ol | Al5 | 1413/1411 | RI/MS/S |
| Bornyl acetate | T27 | 1588/1584 | RI/MS/S | 1-Octen-3-ol | Al6 | 1464/1465 | RI/MS/S |
| α-Bergamotene | T28 | 1598/1590 | RI/MS | 2-Ethyl-1-hexanol | Al7 | 1499/1496 | RI/MS/S |
| β-Elemene | T29 | 1600/1593 | RI/MS/S | Octanol | Al8 | 1570/1572 | RI/MS/S |
| Aromadendrene | T30 | 1649/1650 | RI/MS/S | Esters | |||
| 4-Terpineol | T31 | 1614/1616 | RI/MS/S | cis-3-Hexenyl acetate | E1 | 1321/1321 | RI/MS/S |
| α-Humulene | T32 | 1674/1672 | RI/MS/S | 1-Octen-3-yl-acetate | E2 | 1389/1379 | RI/MS |
| Epi-bicyclosesquiphellandrene | T33 | 1653/1633 | RI/MS | Ketones | |||
| trans-β-Farnesene | T34 | 1677/1673 | RI/MS/S | 3-Octanone | O1 | 1260/1254 | RI/MS/S |
| Ledene | T35 | 1702/1707 | RI/MS/S | 1-Octen-3-one | O2 | 1290/1290 | RI/MS/S |
| L-Borneol | T36 | 1709/1707 | RI/MS/S | ||||
| α-Terpineol | T37 | 1705/1707 | RI/MS/S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
d’Aquino, L.; Cozzolino, R.; Malorni, L.; Bodhuin, T.; Gambale, E.; Sighicelli, M.; Della Mura, B.; Matarazzo, C.; Piacente, S.; Montoro, P. Light Flux Density and Photoperiod Affect Growth and Secondary Metabolism in Fully Expanded Basil Plants. Foods 2024, 13, 2273. https://doi.org/10.3390/foods13142273
d’Aquino L, Cozzolino R, Malorni L, Bodhuin T, Gambale E, Sighicelli M, Della Mura B, Matarazzo C, Piacente S, Montoro P. Light Flux Density and Photoperiod Affect Growth and Secondary Metabolism in Fully Expanded Basil Plants. Foods. 2024; 13(14):2273. https://doi.org/10.3390/foods13142273
Chicago/Turabian Styled’Aquino, Luigi, Rosaria Cozzolino, Livia Malorni, Thierry Bodhuin, Emilia Gambale, Maria Sighicelli, Brigida Della Mura, Cristina Matarazzo, Sonia Piacente, and Paola Montoro. 2024. "Light Flux Density and Photoperiod Affect Growth and Secondary Metabolism in Fully Expanded Basil Plants" Foods 13, no. 14: 2273. https://doi.org/10.3390/foods13142273







