Natural Pigments Recovery from Food By-Products: Health Benefits towards the Food Industry
Abstract
1. Introduction
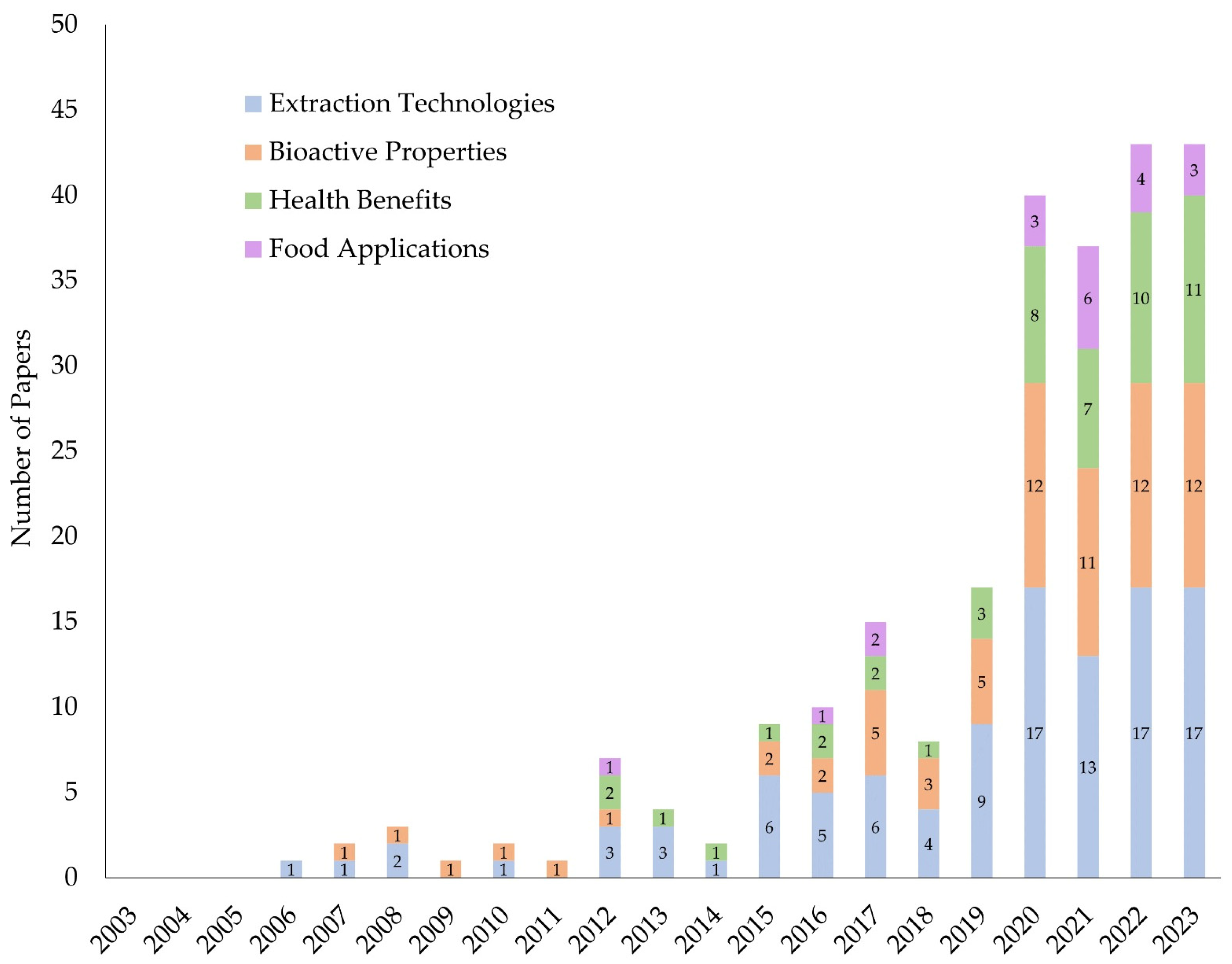
2. Studies Published in the Last Two Decades—An Overview
2.1. Research Methodology
2.2. Results
3. Natural Pigments
3.1. Anthocyanins
3.2. Phycobiliproteins
3.3. Chlorophylls
3.4. Carotenoids
3.5. Betalains
4. Extraction Methodologies for Natural Pigments Recovery
4.1. Ultrasound-Assisted Extraction
4.2. Microwave-Assisted Extraction
4.3. Supercritical Fluid Extraction
4.4. Subcritical Water Extraction
4.5. Pressurized Fluid Extraction
4.6. Pulsed Electric Fields
4.7. Enzyme-Assisted Extraction (EAE)
| By-Product | Pigment | Extraction Method | Solvents/ Co-Solvents | Other Conditions | Yield | References |
|---|---|---|---|---|---|---|
| Cantaloupes peels | Carotenoids | Ultrasound-assisted extraction | Optimal solvent: hexane/ethanol (1:1, v/v). | 3.3% (w/v) sample to solvent ratio; 21.0 ± 2.0 °C. | Yield obtained when working under optimal conditions: 107.74 µg βCE/g DW of by-product. | [71] |
| Tomato pomace | Carotenoids | Ultrasound-assisted extraction | Hexane/ethanol (1:4, v/v) | 3% (w/v) sample to solvent ratio; 25 °C; 10 min. | 8.43 ± 0.26% (w/w) | [82] |
| Tomato peels | Carotenoids | Solvent extraction | Ethanol/acetone/hexane (3:1:1, v/v/v) | 13.3% (w/v) sample to solvent ratio; 40 °C; 23.5 min. | 128 mg βCE/100 g DW of by-product. | [72] |
| Opuntia spp. peels | Betacyanins | Solvent extraction | Ethanol/water (80:20 v/v) | 4% (w/v) sample to solvent ratio; 25 °C; 150 rpm agitation; 1 h. | Extraction from Opuntia ficus-indica vargialla: 1.25 ± 0.01 mg/g DW of extract; extraction from Opuntia ficus-indica var. sanguigna: 3.97 ± 0.03 mg/g DW of extract; extraction from Opuntia engelmannii: 19.4 ± 0.4 mg/g DW of extract. | [74] |
| Raspberries pomace | Anthocyanins | Enzymatic extraction | 100% distilled water (v/v) | Enzymes tested: polygalacturonase, pectinmethylesterase, or polygalacturonase + cellulase; enzyme dosage of 10 mL/100 kg of by-product; 45 °C; 1 h. | Extraction with polygalacturonase: 0.25 ± 0.018 mg/g FW of by-product; extraction with pectinmethylesterase: 0.24 ± 0.017 mg/g FW of by-product; extraction with polygalacturonase + cellulase: 0.32 ± 0.022 mg/g FW of by-product. | [100] |
| Non-compliant tomatoes | Lycopene | Ultrasound- and Microwave-assisted extraction | 100% ethyl acetate (v/v) | Optimal conditions: 10.6:1 (w/v) sample to solvent ratio; microwave power, 98 W; ultrasound power, 50 W; 6.2 min. | Yields obtained when working under optimal conditions: 97.4% (v/v). | [83,87] |
| Carrot and spinach | β-carotene | Microwave-assisted and in vacuo extraction | Acetone/ethanol (1:2 v/v) | 8.3% (w/v) sample to solvent ratio; 25 °C; 20 min; degree of vacuum at 0.04 Mpa. | 9.86 mg/100 g FW of carrot; 0.6 mg/100 g FW of spinach. | [85,87] |
| Hylocereus polyrhizus peels | Betaines | CO2 supercritical fluid extraction | 50% ethanol (v/v) | 1:10 (w/v) sample to ethanol ratio (pre-extraction); 40 °C; 30 Mpa. | Four fractions were obtained (F1, F2, F3, F4): F1: 30.67 mg/100 g DW of by-product; F2: 15.84 mg/100 g DW of by-product, F3: 12.80 mg/100 g DW of by-product; F4: 29.92 mg/100 g DW of by-product. | [81,101] |
| Arthrospira platensis biomass | Chlorophyll; carotenoids and phycobiliproteins | CO2 supercritical fluid extraction | Optimal solvents: distilled water; phycobiliproteins: phosphate buffer | Optimal conditions: 350 bar; 50 °C (for chlorophylls and carotenoids extraction)/40 °C (for phycobiliproteins extraction); static extraction for 60 min and dynamic extraction for 240 min; flow rate of 3 L/min. | Chlorophyll: 133.73 mg/mg DW of biomass; carotenoids: 77.95 µg/mg DW of biomass; phycobiliprotein: 73.40 mg/g DW of biomass. | [89,90] |
| Stevia rebaudiana Bertoni leaves | Chlorophyll A; chlorophyll B and carotenoids | Subcritical water extraction | 100% distilled water (v/v) | Optimal extraction conditions: 60 min of sonication (40 kHz); constant pressure of 10.34 Mpa; mixed with diatomaceous earth (1:3 w/v); 60% (v/w) solvent flushing volume; 160 °C; 10 min. | Yields (optimal conditions): chlorophyll A: 1.58 ± 0.41 mg/g DW of by-product; chlorophyll B: 1.90 ± 0.05 mg/g DW of by-product; carotenoids: 3.62 ± 0.08 mg/100 g DW of by-product. | [80,90] |
| Arthropira platensis biomass | Phycobiliproteins | Pressurized fluid extraction | 0.1 mol/L sodium phosphate buffer, pH 7 | Optimal conditions: 10% (w/v) sample to solvent ratio; 100 bar; 360 min. | Yield (optimal conditions): phycocyanin: 4.4 g/L of extract; allophycocyanin: 1.6 g/L of extract. | [79] |
| Cranberry pomace | Anthocyanins | Pressurized fluid extraction | Optimal solvent: 100% ethanol (v/v) | Optimal conditions: 10% (w/v) sample to solvent ratio; 25 g of 3 mm glass beads; flow rate of 5 mL/min; 50 bar; 60–120 °C; 10 min. | Yield (optimal conditions): 7.78 mg of cyanidin-3-glucoside equivalent. | [78] |
| Rhodotorula glutinis biomass | Carotenoids | Pulsed electric field-assisted | 96% ethanol (v/v) | Flow rate of 4 L/h; residence time of 1.09 s; 15 kV/cm; 150 µs; 10 °C–40 °C; 1 h of incubation, using a ratio of 1:1 (v/v) suspension to 96% ethanol (v/v). | Yield (optimal conditions): 240 μg/g DW of biomass | [77] |
| Beetroot | Betanin and vulgaxanthin | Pulsed electric field-assisted | Phosphate buffer, pH 6.5 | 1% (v/v) sample to solvent ratio; 4.38 kV/cm; pulse number of 20; 4.10 kJ/kg; 20 min of solvent incubation. | Yield (optimal conditions): 4.4 mg betanin/100 g DW of by-product; around 3.2 mg vulgaxanthin/100 g DW of by-product. | [76] |
| Sunflower waste | Carotenoids | Enzyme-assisted extraction | Hexane, limonene; sunflower oil, turpentine; menthol; M/Hac; M/HLaur; M/HLac (d,l-menthol:d,l-lactic acid) | Multi-enzyme complex viscozyme; optimal conditions: solvent/water: 0.6; sunflower/liquid: 0.015; enzyme: 0.5%. | Yield (optimal conditions): 1449 mg/100 g biomass. | [102] |
| Tomato waste | Carotenoids/ lycopene | Enzyme-assisted extraction | Hexane, acetone, and ethyl lactate | Incubation temperature: pectinase (45 °C) and cellulase (55 °C); optimal conditions: enzyme-treated samples with ethyl lactate (solvent–solid = 10:1 mL:g). | Pectinase increased the extraction of total carotenoids/lycopene, compared to cellulase. Yield (optimal conditions): total carotenoid (127 mg/kg DW) and lycopene (89.4 mg/kg DW). | [98] |
| Unsold red beets | Betalains: betacyanins and betaxanthins | Enzyme-assisted extraction | Acetate buffer | Tailored enzymatic mix: Cellulase (37%), xylanase (35%), and pectinase (28%); optimal conditions: 25 U/g total dose of enzymatic mix, temperature 25 °C, and processing time 240 min. | Yield (optimal conditions): (betaxanthins = 11.37 ± 0.45 and Betacyanins = 14.67 ± 0.49 (mg/L)/U). | [103] |
| Spinach pulp | Chlorophyll | Enzyme-assisted extraction | Ethanol | Pectinex Ultra SP-L; optimal conditions: 8% enzyme concentration, 45 °C, and 30 min. | Yield (optimal conditions): 50.747 mg of total chlorophyll content/100 g spinach pulp. | [104] |
| Spinach leaves | Chlorophyll | Enzyme-assisted extraction | McIlvaine buffer (pH 5; 0.1 M) | Optimal conditions (highest amount of recovered chlorophyll, and best quality of green (T: 25 °C, Zn: 150 ppm e B/S: 17.5, t: <2 h, and enzyme mix dose between 12 and 45 U/g). | Yield (optimal conditions/design of experiment (DOE)): 1299 µg/g. | [99] |
5. Bioactivities of Pigments Recovered from Food By-Products
6. Application of Pigments in the Food Industry and Potential Health Benefits
6.1. Anthocyanins
6.2. Phycobiliproteins
6.3. Chlorophylls
6.4. Carotenoids
6.5. Betalains
7. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A Meta-Analysis of Projected Global Food Demand and Population at Risk of Hunger for the Period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.A.; Gómez-García, R.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M.M. Management of Fruit Industrial By-Products—A Case Study on Circular Economy Approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef] [PubMed]
- Novais, C.; Molina, A.K.; Abreu, R.M.V.; Santo-Buelga, C.; Ferreira, I.C.F.R.; Pereira, C.; Barros, L. Natural Food Colorants and Preservatives: A Review, a Demand, and a Challenge. J. Agric. Food Chem. 2022, 70, 2789–2805. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Sarkar, T.; Das, A.; Chakraborty, R. Natural Colorants from Plant Pigments and Their Encapsulation: An Emerging Window for the Food Industry. LWT 2022, 153, 112527. [Google Scholar] [CrossRef]
- Pasdaran, A.; Zare, M.; Hamedi, A.; Hamedi, A. A Review of the Chemistry and Biological Activities of Natural Colorants, Dyes, and Pigments: Challenges, and Opportunities for Food, Cosmetics, and Pharmaceutical Application. Chem. Biodivers. 2023, 20, e202300561. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.; Nieto, G.; Martínez-Zamora, L.; Ros, G.; Kamiloglu, S.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M.; Fernández-López, J.; Viuda-Martos, M.; et al. Novel Approaches for the Recovery of Natural Pigments with Potential Health Effects. J. Agric. Food Chem. 2022, 70, 6864–6883. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green Extraction of Natural Products. Origins, Current Status, and Future Challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Valorization of Fruits and Vegetable Wastes and By-Products to Produce Natural Pigments. Crit. Rev. Biotechnol. 2021, 41, 535–563. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Pandey, V.K.; Dash, K.K.; Zanwar, S.; Singh, R. Natural Bio-Colorant and Pigments: Sources and Applications in Food Processing. J. Agric. Food Res. 2023, 12, 100628. [Google Scholar] [CrossRef]
- Di Salvo, E.; Lo Vecchio, G.; De Pasquale, R.; De Maria, L.; Tardugno, R.; Vadalà, R.; Cicero, N. Natural Pigments Production and Their Application in Food, Health and Other Industries. Nutrients 2023, 15, 1923. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef] [PubMed]
- Speer, H.; D’Cunha, N.M.; Alexopoulos, N.I.; McKune, A.J.; Naumovski, N. Anthocyanins and Human Health—A Focus on Oxidative Stress, Inflammation and Disease. Antioxidants 2020, 9, 366. [Google Scholar] [CrossRef]
- Wallace, T.C. Anthocyanins in Cardiovascular Disease. Adv. Nutr. 2011, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rocha, H.R.; Coelho, M.C.; Gomes, A.M.; Pintado, M.E. Carotenoids Diet: Digestion, Gut Microbiota Modulation, and Inflammatory Diseases. Nutrients 2023, 15, 2265. [Google Scholar] [CrossRef] [PubMed]
- Bolhassani, A.; Milani, A.; Basirnejad, M.; Shahbazi, S. Carotenoids: Biochemistry, Pharmacology and Treatment Correspondence Associate Professor LINKED ARTICLES. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Darvin, M.E.; Sterry, W.; Lademann, J.; Vergou, T. The Role of Carotenoids in Human Skin. Molecules 2011, 16, 10491–10506. [Google Scholar] [CrossRef]
- Nabi, F.; Arain, M.A.; Rajput, N.; Alagawany, M.; Soomro, J.; Umer, M.; Soomro, F.; Wang, Z.; Ye, R.; Liu, J. Health Benefits of Carotenoids and Potential Application in Poultry Industry: A Review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1809–1818. [Google Scholar] [CrossRef]
- Martins, T.; Barros, A.N.; Rosa, E.; Antunes, L. Enhancing Health Benefits through Chlorophylls and Chlorophyll-Rich Agro-Food: A Comprehensive Review. Molecules 2023, 28, 5344. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Wu, S.; Li, X.; Ge, B.; Zhou, C.; Yan, X.; Ruan, R.; Cheng, P. The Structure, Functions and Potential Medicinal Effects of Chlorophylls Derived from Microalgae. Mar. Drugs 2024, 22, 65. [Google Scholar] [CrossRef]
- Gengatharan, A.; Dykes, G.A.; Choo, W.S. Betalains: Natural Plant Pigments with Potential Application in Functional Foods. LWT-Food Sci. Technol. 2015, 64, 645–649. [Google Scholar] [CrossRef]
- Moreno-Ley, C.M.; Osorio-Revilla, G.; Hernández-Martínez, D.M.; Ramos-Monroy, O.A.; Gallardo-Velázquez, T. Anti-Inflammatory Activity of Betalains: A Comprehensive Review. Hum. Nutr. Metab. 2021, 25, 200126. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Y.; Hu, Z.; Wu, S.; Jin, C. Beetroot as a Functional Food with Huge Health Benefits: Antioxidant, Antitumor, Physical Function, and Chronic Metabolomics Activity. Food Sci. Nutr. 2021, 9, 6406–6420. [Google Scholar] [CrossRef]
- Aslam, A.; Fazal, T.; uz Zaman, Q.; Shan, A.; Rehman, F.; Iqbal, J.; Rashid, N.; Rehman, M.S.U. Biorefinery of Microalgae for Nonfuel Products. In Microalgae Cultivation for Biofuels Production; Elsevier: Amsterdam, The Netherlands, 2020; pp. 197–209. [Google Scholar]
- Li, W.; Su, H.-N.; Pu, Y.; Chen, J.; Liu, L.-N.; Liu, Q.; Qin, S. Phycobiliproteins: Molecular Structure, Production, Applications, and Prospects. Biotechnol. Adv. 2019, 37, 340–353. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Qin, S.; Li, W. Phycocyanin: Anti-Inflammatory Effect and Mechanism. Biomed. Pharmacother. 2022, 153, 113362. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, Y.; Yin, Q.; Liu, G.; Liu, H.; Huang, Y.; Li, B. Phycocyanin: A Potential Drug for Cancer Treatment. J. Cancer 2017, 8, 3416–3429. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Giusti, M.M. Anthocyanins. Adv. Nutr. 2015, 6, 620–622. [Google Scholar] [CrossRef]
- Bendokas, V.; Skemiene, K.; Trumbeckaite, S.; Stanys, V.; Passamonti, S.; Borutaite, V.; Liobikas, J. Anthocyanins: From Plant Pigments to Health Benefits at Mitochondrial Level. Crit. Rev. Food Sci. Nutr. 2020, 60, 3352–3365. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health Benefits of Anthocyanins and Molecular Mechanisms: Update from Recent Decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef]
- Arango-Varela, S.S.; Luzardo-Ocampo, I.; Maldonado-Celis, M.E.; Campos-Vega, R. Andean Berry (Vaccinium Meridionale Swartz) Juice in Combination with Aspirin Modulated Anti-Inflammatory Markers on LPS-Stimulated RAW 264.7 Macrophages. Food Res. Int. 2020, 137, 109541. [Google Scholar] [CrossRef]
- Luna-Vital, D.; Weiss, M.; Gonzalez de Mejia, E. Anthocyanins from Purple Corn Ameliorated Tumor Necrosis Factor-α-Induced Inflammation and Insulin Resistance in 3T3-L1 Adipocytes via Activation of Insulin Signaling and Enhanced GLUT4 Translocation. Mol. Nutr. Food Res. 2017, 61, 1700362. [Google Scholar] [CrossRef]
- Luzardo-Ocampo, I.; Ramírez-Jiménez, A.K.; Yañez, J.; Mojica, L.; Luna-Vital, D.A. Technological Applications of Natural Colorants in Food Systems: A Review. Foods 2021, 10, 634. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Rodríguez-Mena, A.; Ochoa-Martínez, L.A.; González-Herrera, S.M.; Rutiaga-Quiñones, O.M.; González-Laredo, R.F.; Olmedilla-Alonso, B. Natural Pigments of Plant Origin: Classification, Extraction and Application in Foods. Food Chem. 2023, 398, 133908. [Google Scholar] [CrossRef]
- Coelho, M.C.; Pereira, R.N.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. The Use of Emergent Technologies to Extract Added Value Compounds from Grape By-Products. Trends Food Sci. Technol. 2020, 106, 182–197. [Google Scholar] [CrossRef]
- Chen, H.; Qi, H.; Xiong, P. Phycobiliproteins—A Family of Algae-Derived Biliproteins: Productions, Characterization and Pharmaceutical Potentials. Mar. Drugs 2022, 20, 450. [Google Scholar] [CrossRef]
- Tounsi, L.; Ben Hlima, H.; Hentati, F.; Hentati, O.; Derbel, H.; Michaud, P.; Abdelkafi, S. Microalgae: A Promising Source of Bioactive Phycobiliproteins. Mar. Drugs 2023, 21, 440. [Google Scholar] [CrossRef]
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and Phycoerythrin: Strategies to Improve Production Yield and Chemical Stability. Algal Res. 2019, 42, 101600. [Google Scholar] [CrossRef]
- Zhang, Z.; Cho, S.; Dadmohammadi, Y.; Li, Y.; Abbaspourrad, A. Improvement of the Storage Stability of C-Phycocyanin in Beverages by High-Pressure Processing. Food Hydrocoll. 2021, 110, 106055. [Google Scholar] [CrossRef]
- Dagnino-Leone, J.; Figueroa, C.P.; Castañeda, M.L.; Youlton, A.D.; Vallejos-Almirall, A.; Agurto-Muñoz, A.; Pavón Pérez, J.; Agurto-Muñoz, C. Phycobiliproteins: Structural Aspects, Functional Characteristics, and Biotechnological Perspectives. Comput. Struct. Biotechnol. J. 2022, 20, 1506–1527. [Google Scholar] [CrossRef]
- de Andrade, A.F.; Porto, A.L.F.; Bezerra, R.P. Photosynthetic Microorganisms and Their Bioactive Molecules as New Product to Healing Wounds. Appl. Microbiol. Biotechnol. 2022, 106, 497–504. [Google Scholar] [CrossRef]
- Bannu, S.M.; Lomada, D.; Gulla, S.; Chandrasekhar, T.; Reddanna, P.; Reddy, M.C. Potential Therapeutic Applications of C-Phycocyanin. Curr. Drug Metab. 2020, 20, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Piniella-Matamoros, B.; Marín-Prida, J.; Pentón-Rol, G. Nutraceutical and Therapeutic Potential of Phycocyanobilin for Treating Alzheimer’s Disease. J. Biosci. 2021, 46, 42. [Google Scholar] [CrossRef]
- Pagels, F.; Guedes, A.C.; Amaro, H.M.; Kijjoa, A.; Vasconcelos, V. Phycobiliproteins from Cyanobacteria: Chemistry and Biotechnological Applications. Biotechnol. Adv. 2019, 37, 422–443. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, P.; Shokramraji, Z.; Tavakkoli, S.; Mihaylova, D.; Lante, A. Chlorophylls as Natural Bioactive Compounds Existing in Food By-Products: A Critical Review. Plants 2023, 12, 1533. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.C.; Ferreira, A.M.; Morais, E.S.; Khan, I.; Freire, M.G.; Coutinho, J.A.P. Cloud Point Extraction of Chlorophylls from Spinach Leaves Using Aqueous Solutions of Nonionic Surfactants. ACS Sustain. Chem. Eng. 2018, 6, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and Chlorophylls as Antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef] [PubMed]
- Queiroz Zepka, L.; Jacob-Lopes, E.; Roca, M. Catabolism and Bioactive Properties of Chlorophylls. Curr. Opin. Food Sci. 2019, 26, 94–100. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Carotenoids: How Effective Are They to Prevent Age-Related Diseases? Molecules 2019, 24, 1801. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.C.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Integral Valorisation of Tomato By-Products towards Bioactive Compounds Recovery: Human Health Benefits. Food Chem. 2023, 410, 135319. [Google Scholar] [CrossRef]
- Reboul, E. Mechanisms of Carotenoid Intestinal Absorption: Where Do We Stand? Nutrients 2019, 11, 838. [Google Scholar] [CrossRef]
- Arain, M.A.; Mei, Z.; Hassan, F.U.; Saeed, M.; Alagawany, M.; Shar, A.H.; Rajput, I.R. Lycopene: A Natural Antioxidant for Prevention of Heat-Induced Oxidative Stress in Poultry. Worlds Poult. Sci. J. 2017, 74, 89–100. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef]
- Donhowe, E.G.; Kong, F. Beta-Carotene: Digestion, Microencapsulation, and In Vitro Bioavailability. Food Bioprocess. Technol. 2014, 7, 338–354. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Carotenoids: Potential Allies of Cardiovascular Health? Food Nutr. Res. 2015, 59, 26762. [Google Scholar] [CrossRef] [PubMed]
- Kijlstra, A.; Tian, Y.; Kelly, E.R.; Berendschot, T.T.J.M. Lutein: More than Just a Filter for Blue Light. Prog. Retin. Eye Res. 2012, 31, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-H.; Yu, R.-B.; Liu, R.; Hao, Z.-X.; Han, C.-C.; Zhu, Z.-H.; Ma, L. Association between Lutein and Zeaxanthin Status and the Risk of Cataract: A Meta-Analysis. Nutrients 2014, 6, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.S.; Lima, M.J.R.; Oliveira, J.; Lemos, E.T.D. Teixeira-Lemos Tomato Lycopene: Functional Proprieties and Health. Int. J. Agric. Biosyst. Eng. 2015, 9, 458–468. [Google Scholar] [CrossRef]
- Nagao, A.; Kotake-Nara, E.; Hase, M. Effects of Fats and Oils on the Bioaccessibility of Carotenoids and Vitamin E in Vegetables. Biosci. Biotechnol. Biochem. 2013, 77, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Gloria, N.F.; Soares, N.; Brand, C.; Oliveira, F.L.; Borojevic, R.; Teodoro, A.J. Lycopene and Beta-Carotene Induce Cell-Cycle Arrest and Apoptosis in Human Breast Cancer Cell Lines. Anticancer. Res. 2014, 34, 1377–1386. [Google Scholar] [PubMed]
- Kaulmann, A.; Bohn, T. Carotenoids, Inflammation, and Oxidative Stress-Implications of Cellular Signaling Pathways and Relation to Chronic Disease Prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef]
- Khan, M.I.; Giridhar, P. Plant Betalains: Chemistry and Biochemistry. Phytochemistry 2015, 117, 267–295. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Biological Properties and Applications of Betalains. Molecules 2021, 26, 2520. [Google Scholar] [CrossRef] [PubMed]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Biological Activities of Plant Pigments Betalains. Crit. Rev. Food Sci. Nutr. 2016, 56, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, K.; Saw, N.M.M.T.; Mohdaly, A.A.A.; Gabr, A.M.M.; Kastell, A.; Riedel, H.; Cai, Z.; Knorr, D.; Smetanska, I. Impact of Processing of Red Beet on Betalain Content and Antioxidant Activity. Food Res. Int. 2013, 50, 670–675. [Google Scholar] [CrossRef]
- Madadi, E.; Mazloum-Ravasan, S.; Yu, J.S.; Ha, J.W.; Hamishehkar, H.; Kim, K.H. Therapeutic Application of Betalains: A Review. Plants 2020, 9, 1219. [Google Scholar] [CrossRef] [PubMed]
- Boo, H.-O.; Hwang, S.-J.; Bae, C.-S.; Park, S.-H.; Heo, B.-G.; Gorinstein, S. Extraction and Characterization of Some Natural Plant Pigments. Ind. Crop. Prod. 2012, 40, 129–135. [Google Scholar] [CrossRef]
- Bursal, E.; Gülçin, İ. Polyphenol Contents and in Vitro Antioxidant Activities of Lyophilised Aqueous Extract of Kiwifruit (Actinidia Deliciosa). Food Res. Int. 2011, 44, 1482–1489. [Google Scholar] [CrossRef]
- Park, Y.-S.; Namiesnik, J.; Vearasilp, K.; Leontowicz, H.; Leontowicz, M.; Barasch, D.; Nemirovski, A.; Trakhtenberg, S.; Gorinstein, S. Bioactive Compounds and the Antioxidant Capacity in New Kiwi Fruit Cultivars. Food Chem. 2014, 165, 354–361. [Google Scholar] [CrossRef]
- Altemimi, A.; Choudhary, R.; Watson, D.G.; Lightfoot, D.A. Effects of Ultrasonic Treatments on the Polyphenol and Antioxidant Content of Spinach Extracts. Ultrason. Sonochem. 2015, 24, 247–255. [Google Scholar] [CrossRef]
- Sood, A.; Gupta, M. Extraction Process Optimization for Bioactive Compounds in Pomegranate Peel. Food Biosci. 2015, 12, 100–106. [Google Scholar] [CrossRef]
- Kajdžanoska, M.; Petreska, J.; Stefova, M. Comparison of Different Extraction Solvent Mixtures for Characterization of Phenolic Compounds in Strawberries. J. Agric. Food Chem. 2011, 59, 5272–5278. [Google Scholar] [CrossRef]
- Sutheimer, S.; Caster, J.M.; Smith, S.H. Green Soap: An Extraction and Saponification of Avocado Oil. J. Chem. Educ. 2015, 92, 1763–1765. [Google Scholar] [CrossRef]
- Garcia-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenolic-Compound-Extraction Systems for Fruit and Vegetable Samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef] [PubMed]
- Montibeller, M.J.; de Lima Monteiro, P.; Tupuna-Yerovi, D.S.; Rios, A.D.O.; Manfroi, V. Stability Assessment of Anthocyanins Obtained from Skin Grape Applied in Kefir and Carbonated Water as a Natural Colorant. J. Food Process. Preserv. 2018, 42, e13698. [Google Scholar] [CrossRef]
- Nowacka, M.; Tappi, S.; Wiktor, A.; Rybak, K.; Miszczykowska, A.; Czyzewski, J.; Drozdzal, K.; Witrowa-Rajchert, D.; Tylewicz, U. The Impact of Pulsed Electric Field on the Extraction of Bioactive Compounds from Beetroot. Foods 2019, 8, 244. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Angulo, J.; Álvarez, I.; Raso, J. Pulsed Electric Field-Assisted Extraction of Carotenoids from Fresh Biomass of Rhodotorula Glutinis. Innov. Food Sci. Emerg. Technol. 2018, 47, 421–427. [Google Scholar] [CrossRef]
- Saldaña, M.D.A.; Martinez, E.R.; Sekhon, J.K.; Vo, H. The Effect of Different Pressurized Fluids on the Extraction of Anthocyanins and Total Phenolics from Cranberry Pomace. J. Supercrit. Fluids 2021, 175, 105279. [Google Scholar] [CrossRef]
- Viana Carlos, T.A.; dos Santos Pires Cavalcante, K.M.; de Cássia Evangelista de Oliveira, F.; do Ó Pessoa, C.; Sant’Ana, H.B.; Feitosa, F.X.; Rocha, M.V.P. Pressurized Extraction of Phycobiliproteins from Arthrospira Platensis and Evaluation of Its Effect on Antioxidant and Anticancer Activities of These Biomolecules. J. Appl. Phycol. 2021, 33, 929–938. [Google Scholar] [CrossRef]
- Bursać Kovačević, D.; Barba, F.J.; Granato, D.; Galanakis, C.M.; Herceg, Z.; Dragović-Uzelac, V.; Putnik, P. Pressurized Hot Water Extraction (PHWE) for the Green Recovery of Bioactive Compounds and Steviol Glycosides from Stevia Rebaudiana Bertoni Leaves. Food Chem. 2018, 254, 150–157. [Google Scholar] [CrossRef]
- Yu, Z.-R.; Weng, Y.-M.; Lee, H.-Y.; Wang, B.-J. Partition of Bioactive Components from Red Pitaya Fruit (Hylocereus Polyrhizus) Peels into Different Fractions Using Supercritical Fluid Fractionation Technology. Food Biosci. 2023, 51, 102270. [Google Scholar] [CrossRef]
- Luengo, E.; Condón-Abanto, S.; Condón, S.; Álvarez, I.; Raso, J. Improving the Extraction of Carotenoids from Tomato Waste by Application of Ultrasound under Pressure. Sep. Purif. Technol. 2014, 136, 130–136. [Google Scholar] [CrossRef]
- Lianfu, Z.; Zelong, L. Optimization and Comparison of Ultrasound/Microwave Assisted Extraction (UMAE) and Ultrasonic Assisted Extraction (UAE) of Lycopene from Tomatoes. Ultrason. Sonochem. 2008, 15, 731–737. [Google Scholar] [CrossRef]
- Coelho, M.; Silva, S.; Costa, E.; Pereira, R.N.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M. Anthocyanin Recovery from Grape By-Products by Combining Ohmic Heating with Food-Grade Solvents: Phenolic Composition, Antioxidant, and Antimicrobial Properties. Molecules 2021, 26, 3838. [Google Scholar] [CrossRef]
- Bagade, S.B.; Patil, M. Recent Advances in Microwave Assisted Extraction of Bioactive Compounds from Complex Herbal Samples: A Review. Crit. Rev. Anal. Chem. 2021, 51, 138–149. [Google Scholar] [CrossRef]
- Isabel Landim Neves, M.; Keven Silva, E.; Meireles, M.A.A. Trends and Challenges in the Industrialization of Natural Colorants. Food Public Health 2019, 9, 33–44. [Google Scholar] [CrossRef]
- Grodstein, F. A randomized trial of beta carotene supplementation and cognitive function in men: The Physicians’ Health Study II. Arch. Intern. Med. 2007, 167, 2184. [Google Scholar] [CrossRef]
- Srivastava, A.; Kalwani, M.; Chakdar, H.; Pabbi, S.; Shukla, P. Biosynthesis and Biotechnological Interventions for Commercial Production of Microalgal Pigments: A Review. Bioresour. Technol. 2022, 352, 127071. [Google Scholar] [CrossRef]
- Pan-utai, W.; Iamtham, S.; Boonbumrung, S.; Mookdasanit, J. Improvement in the Sequential Extraction of Phycobiliproteins from Arthrospira Platensis Using Green Technologies. Life 2022, 12, 1896. [Google Scholar] [CrossRef]
- Coelho, M.C.; Ribeiro, T.B.; Oliveira, C.; Batista, P.; Castro, P.; Monforte, A.R.; Rodrigues, A.S.; Teixeira, J.; Pintado, M. In Vitro Gastrointestinal Digestion Impact on the Bioaccessibility and Antioxidant Capacity of Bioactive Compounds from Tomato Flours Obtained after Conventional and Ohmic Heating Extraction. Foods 2021, 10, 554. [Google Scholar] [CrossRef]
- Gbashi, S.; Adebo, O.A.; Piater, L.; Madala, N.E.; Njobeh, P.B. Subcritical Water Extraction of Biological Materials. Sep. Purif. Rev. 2017, 46, 21–34. [Google Scholar] [CrossRef]
- Vieira, F.A.; Guilherme, R.J.R.; Neves, M.C.; Abreu, H.; Rodrigues, E.R.O.; Maraschin, M.; Coutinho, J.A.P.; Ventura, S.P.M. Single-Step Extraction of Carotenoids from Brown Macroalgae Using Non-Ionic Surfactants. Sep Purif Technol 2017, 172, 268–276. [Google Scholar] [CrossRef]
- Alvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Mendiola, J.A.; Ibañez, E. Pressurized Liquid Extraction. In Liquid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2020; pp. 375–398. [Google Scholar]
- Li, W.; Pu, Y.; Tang, Z.; Zhao, F.; Xie, M.; Qin, S. Energy Transfer Dynamics in B-Phycoerythrin from the Red Alga Porphyridium Purpureum. Chin. J. Phys. 2020, 66, 24–35. [Google Scholar] [CrossRef]
- Buchmann, L.; Mathys, A. Perspective on Pulsed Electric Field Treatment in the Bio-Based Industry. Front. Bioeng. Biotechnol. 2019, 7, 265. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, N.; Gupta, N.; Bhat, A.; Singh, J.; Bandral, J.; Sood, M.; Bashir, M.; Thakur, N. Extraction Techniques and Utilization of Natural Pigments. Chem. Sci. Rev. Lett. 2022, 11, 239–245. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-Assisted Extraction of Bioactives from Plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Strati, I.F.; Gogou, E.; Oreopoulou, V. Enzyme and High Pressure Assisted Extraction of Carotenoids from Tomato Waste. Food Bioprod. Process. 2015, 94, 668–674. [Google Scholar] [CrossRef]
- Mazzocchi, C.; Benucci, I.; Lombardelli, C.; Esti, M. Enzyme-Assisted Extraction for the Recovery of Food-Grade Chlorophyll-Based Green Colorant. Foods 2023, 12, 3440. [Google Scholar] [CrossRef]
- Lechner, J.F.; Stoner, G.D. Red Beetroot and Betalains as Cancer Chemopreventative Agents. Molecules 2019, 24, 1602. [Google Scholar] [CrossRef]
- Coelho, M.; Pereira, R.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Extraction of Tomato By-Products’ Bioactive Compounds Using Ohmic Technology. Food Bioprod. Process. 2019, 117, 329–339. [Google Scholar] [CrossRef]
- Ricarte, G.N.; Coelho, M.A.Z.; Marrucho, I.M.; Ribeiro, B.D. Enzyme-Assisted Extraction of Carotenoids and Phenolic Compounds from Sunflower Wastes Using Green Solvents. 3 Biotech 2020, 10, 405. [Google Scholar] [CrossRef]
- Lombardelli, C.; Benucci, I.; Mazzocchi, C.; Esti, M. A Novel Process for the Recovery of Betalains from Unsold Red Beets by Low-Temperature Enzyme-Assisted Extraction. Foods 2021, 10, 236. [Google Scholar] [CrossRef]
- Özkan, G.; Ersus Bilek, S. Enzyme-Assisted Extraction of Stabilized Chlorophyll from Spinach. Food Chem. 2015, 176, 152–157. [Google Scholar] [CrossRef]
- Lu, W.; Shi, Y.; Wang, R.; Su, D.; Tang, M.; Liu, Y.; Li, Z. Antioxidant Activity and Healthy Benefits of Natural Pigments in Fruits: A Review. Int. J. Mol. Sci. 2021, 22, 4945. [Google Scholar] [CrossRef]
- Abou Elmaaty, T.; Sayed-Ahmed, K.; Elsisi, H.; Magdi, M. Optimization of Extraction of Natural Antimicrobial Pigments Using Supercritical Fluids: A Review. Processes 2022, 10, 2111. [Google Scholar] [CrossRef]
- Kim, H.; Koo, K.A.; Park, W.S.; Kang, D.; Kim, H.S.; Lee, B.Y.; Goo, Y.; Kim, J.; Lee, M.K.; Woo, D.K.; et al. Anti-obesity Activity of Anthocyanin and Carotenoid Extracts from Color-fleshed Sweet Potatoes. J. Food Biochem. 2020, 44, e13438. [Google Scholar] [CrossRef] [PubMed]
- Szymanowska, U.; Baraniak, B. Antioxidant and Potentially Anti-Inflammatory Activity of Anthocyanin Fractions from Pomace Obtained from Enzymatically Treated Raspberries. Antioxidants 2019, 8, 299. [Google Scholar] [CrossRef]
- Ould Amara-Leffad, L.; Ramdane, H.; Nekhoul, K.; Ouznadji, A.; Koceir, E.A. Spirulina Effect on Modulation of Toxins Provided by Food, Impact on Hepatic and Renal Functions. Arch. Physiol. Biochem. 2019, 125, 184–194. [Google Scholar] [CrossRef]
- Rodriguez, E.B.; Vidallon, M.L.P.; Mendoza, D.J.R.; Reyes, C.T. Health-Promoting Bioactivities of Betalains from Red Dragon Fruit (Hylocereus Polyrhizus (Weber) Britton and Rose) Peels as Affected by Carbohydrate Encapsulation. J. Sci. Food Agric. 2016, 96, 4679–4689. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca Machado, A.P.; Alves Rezende, C.; Alexandre Rodrigues, R.; Fernández Barbero, G.; de Tarso Vieira e Rosa, P.; Martínez, J. Encapsulation of Anthocyanin-Rich Extract from Blackberry Residues by Spray-Drying, Freeze-Drying and Supercritical Antisolvent. Powder Technol. 2018, 340, 553–562. [Google Scholar] [CrossRef]
- Sarabandi, K.; Jafari, S.M.; Mahoonak, A.S.; Mohammadi, A. Application of Gum Arabic and Maltodextrin for Encapsulation of Eggplant Peel Extract as a Natural Antioxidant and Color Source. Int. J. Biol. Macromol. 2019, 140, 59–68. [Google Scholar] [CrossRef]
- Bao, X.; Huang, Y.; Chen, F. C-Phycocyanin Alleviates Bladder Inflammation and Dysfunction in Cyclophosphamide-Induced Cystitis in a Mouse Model by Inhibiting COX-2 and EP4. Evid.-Based Complement. Altern. Med. 2019, 2019, 8424872. [Google Scholar] [CrossRef] [PubMed]
- Chaubey, M.G.; Patel, S.N.; Rastogi, R.P.; Srivastava, P.L.; Singh, A.K.; Madamwar, D.; Singh, N.K. Therapeutic Potential of Cyanobacterial Pigment Protein Phycoerythrin: In Silico and in Vitro Study of BACE1 Interaction and in Vivo Aβ Reduction. Int. J. Biol. Macromol. 2019, 134, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Zeyada, N.N.; Zeitoum, M.A.M.; Barbary, O.M. Utilization of Some Vegetables and Fruits Waste as Natural Antioxidants. Alex. J. Food Sci. Technol. 2008, 5, 1–11. [Google Scholar] [CrossRef]
- Miller, F.A.; Fundo, J.F.; Garcia, E.; Santos, J.R.; Silva, C.L.M.; Brandão, T.R.S. Physicochemical and Bioactive Characterisation of Edible and Waste Parts of “Piel de Sapo” Melon. Horticulturae 2020, 6, 60. [Google Scholar] [CrossRef]
- Sowmya, R.; Sachindra, N.M. Evaluation of Antioxidant Activity of Carotenoid Extract from Shrimp Processing Byproducts by in Vitro Assays and in Membrane Model System. Food Chem. 2012, 134, 308–314. [Google Scholar] [CrossRef]
- Benmeziane, A.; Boulekbache-Makhlouf, L.; Mapelli-Brahm, P.; Khaled Khodja, N.; Remini, H.; Madani, K.; Meléndez-Martínez, A.J. Extraction of Carotenoids from Cantaloupe Waste and Determination of Its Mineral Composition. Food Res. Int. 2018, 111, 391–398. [Google Scholar] [CrossRef]
- Rizk, E.M.; El-Kady, A.T.; El-Bialy, A.R. Charactrization of Carotenoids (Lyco-Red) Extracted from Tomato Peels and Its Uses as Natural Colorants and Antioxidants of Ice Cream. Ann. Agric. Sci. 2014, 59, 53–61. [Google Scholar] [CrossRef]
- Rezende, Y.R.R.S.; Nogueira, J.P.; Narain, N. Microencapsulation of Extracts of Bioactive Compounds Obtained from Acerola (Malpighia Emarginata DC) Pulp and Residue by Spray and Freeze Drying: Chemical, Morphological and Chemometric Characterization. Food Chem. 2018, 254, 281–291. [Google Scholar] [CrossRef]
- Melgar, B.; Dias, M.I.; Ciric, A.; Sokovic, M.; Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Barros, L.; Ferreira, I. By-Product Recovery of Opuntia Spp. Peels: Betalainic and Phenolic Profiles and Bioactive Properties. Ind. Crop. Prod. 2017, 107, 353–359. [Google Scholar] [CrossRef]
- Saad, F.; Al-Shaikh, T.M.; Zouidi, F.; Taher, M.A.; Saidi, S.A.; Hamden, K. Betalain-Enriched Beetroots Exhibit Antiulcer and Anti-Inflammatory Potentials. J. Food Process. Preserv. 2023, 2023, 9522830. [Google Scholar] [CrossRef]
- Abdelkader Saidi, S.; Al-Shaikh, T.M.; Hamden, K. Evaluation of Gastroprotective Effect of Betalain-Rich Ethanol Extract from Opuntia Stricta Var. Dillenii Employing an In Vivo Rat Model. J. Food Qual. 2023, 2023, 2215454. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Update on Natural Food Pigments—A Mini-Review on Carotenoids, Anthocyanins, and Betalains. Food Res. Int. 2019, 124, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Amchova, P.; Kotolova, H.; Ruda-Kucerova, J. Health Safety Issues of Synthetic Food Colorants. Regul. Toxicol. Pharmacol. 2015, 73, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Benucci, I.; Lombardelli, C.; Mazzocchi, C.; Esti, M. Natural Colorants from Vegetable Food Waste: Recovery, Regulatory Aspects, and Stability—A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2715–2737. [Google Scholar] [CrossRef] [PubMed]
- Lala, G.; Malik, M.; Zhao, C.; He, J.; Kwon, Y.; Giusti, M.M.; Magnuson, B.A. Anthocyanin-Rich Extracts Inhibit Multiple Biomarkers of Colon Cancer in Rats. Nutr. Cancer 2006, 54, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Vojdani, C. Immune Reactivity to Food Coloring. Altern. Ther. Health Med. 2015, 21, 48–85. [Google Scholar]
- Kwon, Y.H.; Banskota, S.; Wang, H.; Rossi, L.; Grondin, J.A.; Syed, S.A.; Yousefi, Y.; Schertzer, J.D.; Morrison, K.M.; Wade, M.G.; et al. Chronic Exposure to Synthetic Food Colorant Allura Red AC Promotes Susceptibility to Experimental Colitis via Intestinal Serotonin in Mice. Nat. Commun. 2022, 13, 7617. [Google Scholar] [CrossRef]
- Claus, S.P.; Guillou, H.; Ellero-Simatos, S. The Gut Microbiota: A Major Player in the Toxicity of Environmental Pollutants? NPJ Biofilms Microbiomes 2016, 2, 16003. [Google Scholar] [CrossRef] [PubMed]
- Chen, H. Toxicological Significance of Azo Dye Metabolism by Human Intestinal Microbiota. Front. Biosci. 2012, E4, 400. [Google Scholar] [CrossRef]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N. Natural Colorants: Pigment Stability and Extraction Yield Enhancement via Utilization of Appropriate Pretreatment and Extraction Methods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3243–3259. [Google Scholar] [CrossRef]
- Nabi, B.G.; Mukhtar, K.; Ahmed, W.; Manzoor, M.F.; Ranjha, M.M.A.N.; Kieliszek, M.; Bhat, Z.F.; Aadil, R.M. Natural Pigments: Anthocyanins, Carotenoids, Chlorophylls, and Betalains as Colorants in Food Products. Food Biosci. 2023, 52, 102403. [Google Scholar] [CrossRef]
- Mazza, G. Anthocyanins in Fruits, Vegetables, and Grains; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781351069700. [Google Scholar]
- Abdel-Moemin, A.R. Effect of Roselle Calyces Extract on the Chemical and Sensory Properties of Functional Cupcakes. Food Sci. Hum. Wellness 2016, 5, 230–237. [Google Scholar] [CrossRef]
- da Silva, L.P.; Pereira, E.; Prieto, M.A.; Simal-Gandara, J.; Pires, T.C.S.P.; Alves, M.J.; Calhelha, R.; Barros, L.; Ferreira, I.C.F.R. Rubus Ulmifolius Schott as a Novel Source of Food Colorant: Extraction Optimization of Coloring Pigments and Incorporation in a Bakery Product. Molecules 2019, 24, 2181. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, B.R.; Pinela, J.; Barros, L.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Anthocyanin-Rich Extract of Jabuticaba Epicarp as a Natural Colorant: Optimization of Heat- and Ultrasound-Assisted Extractions and Application in a Bakery Product. Food Chem. 2020, 316, 126364. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.M.; Attia, M.H.; Rashed, E.N. Enhancing the Stability of Strawberry Anthocyanins Complexed to β-Cyclodextrin and Starch toward Heat, Oxidation, and Irradiation. ACS Omega 2024, 9, 5319–5329. [Google Scholar] [CrossRef] [PubMed]
- Luna-Vital, D.; Cortez, R.; Ongkowijoyo, P.; Gonzalez de Mejia, E. Protection of Color and Chemical Degradation of Anthocyanin from Purple Corn (Zea mays L.) by Zinc Ions and Alginate through Chemical Interaction in a Beverage Model. Food Res. Int. 2018, 105, 169–177. [Google Scholar] [CrossRef]
- Aguilera, Y.; Mojica, L.; Rebollo-Hernanz, M.; Berhow, M.; de Mejía, E.G.; Martín-Cabrejas, M.A. Black Bean Coats: New Source of Anthocyanins Stabilized by β-Cyclodextrin Copigmentation in a Sport Beverage. Food Chem. 2016, 212, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Tensiska, T.; Marta, H.; Cahyana, Y.; Amirah, N.S. Application of Encapsulated Anthocyanin Pigments from Purple Sweet Potato (Ipomoea batatas L.) in Jelly Drink. KnE Life Sci. 2017, 2, 482. [Google Scholar] [CrossRef]
- Benchikh, Y.; Aissaoui, A.; Allouch, R.; Mohellebi, N. Optimising Anthocyanin Extraction from Strawberry Fruits Using Response Surface Methodology and Application in Yoghurt as Natural Colorants and Antioxidants. J. Food Sci. Technol. 2021, 58, 1987–1995. [Google Scholar] [CrossRef]
- Rugină, D.; Sconţa, Z.; Leopold, L.; Pintea, A.; Bunea, A.; Socaciu, C. Antioxidant Activities of Chokeberry Extracts and the Cytotoxic Action of Their Anthocyanin Fraction on HeLa Human Cervical Tumor Cells. J. Med. Food 2012, 15, 700–706. [Google Scholar] [CrossRef]
- Barrios, J.; Cordero, C.P.; Aristizabal, F.; Heredia, F.J.; Morales, A.L.; Osorio, C. Chemical Analysis and Screening as Anticancer Agent of Anthocyanin-Rich Extract from Uva Caimarona (Pourouma Cecropiifolia Mart.) Fruit. J. Agric. Food Chem. 2010, 58, 2100–2110. [Google Scholar] [CrossRef] [PubMed]
- Singletary, K.W.; Jung, K.-J.; Giusti, M. Anthocyanin-Rich Grape Extract Blocks Breast Cell DNA Damage. J. Med. Food 2007, 10, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.; Bin, Y.; Xiaoping, Y.; Long, Y.; Chunye, C.; Mantian, M.; Wenhua, L. Anticancer Activities of an Anthocyanin-Rich Extract From Black Rice Against Breast Cancer Cells In Vitro and In Vivo. Nutr. Cancer 2010, 62, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.S.; Kumar, M.S.; Das, S.M. Evaluation of Antiproliferative Activity of Red Sorghum Bran Anthocyanin on a Human Breast Cancer Cell Line (MCF-7). Int. J. Breast Cancer 2011, 2011, 891481. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Gupta, A.; Munagala, R.; Jeyabalan, J.; Kausar, H.; Sharma, R.J.; Singh, I.P.; Gupta, R.C. Antioxidant and Antiproliferative Activities of Anthocyanin/Ellagitannin-Enriched Extracts From Syzygium Cumini L. (Jamun, the Indian Blackberry). Nutr. Cancer 2012, 64, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Háznagy-Radnai, E.; Mbimba, T.; Sipos, P.; Morazzoni, P.; Darvesh, A.S.; Bhatia, D.; Hohmann, J. Anthocyanin-Rich Black Currant Extract Suppresses the Growth of Human Hepatocellular Carcinoma Cells. Nat. Prod. Commun. 2010, 5, 1934578X1000501. [Google Scholar] [CrossRef]
- Bishayee, A.; Mbimba, T.; Thoppil, R.J.; Háznagy-Radnai, E.; Sipos, P.; Darvesh, A.S.; Folkesson, H.G.; Hohmann, J. Anthocyanin-Rich Black Currant (Ribes nigrum L.) Extract Affords Chemoprevention against Diethylnitrosamine-Induced Hepatocellular Carcinogenesis in Rats. J. Nutr. Biochem. 2011, 22, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Longo, L.; Platini, F.; Scardino, A.; Alabiso, O.; Vasapollo, G.; Tessitore, L. Autophagy Inhibition Enhances Anthocyanin-Induced Apoptosis in Hepatocellular Carcinoma. Mol. Cancer Ther. 2008, 7, 2476–2485. [Google Scholar] [CrossRef] [PubMed]
- Reddivari, L.; Vanamala, J.; Chintharlapalli, S.; Safe, S.H.; Miller, J.C. Anthocyanin Fraction from Potato Extracts Is Cytotoxic to Prostate Cancer Cells through Activation of Caspase-Dependent and Caspase-Independent Pathways. Carcinogenesis 2007, 28, 2227–2235. [Google Scholar] [CrossRef]
- Lim, S.; Xu, J.; Kim, J.; Chen, T.-Y.; Su, X.; Standard, J.; Carey, E.; Griffin, J.; Herndon, B.; Katz, B.; et al. Role of Anthocyanin-Enriched Purple-Fleshed Sweet Potato P40 in Colorectal Cancer Prevention. Mol. Nutr. Food Res. 2013, 57, 1908–1917. [Google Scholar] [CrossRef]
- Srivastava, A.; Akoh, C.C.; Fischer, J.; Krewer, G. Effect of Anthocyanin Fractions from Selected Cultivars of Georgia-Grown Blueberries on Apoptosis and Phase II Enzymes. J. Agric. Food Chem. 2007, 55, 3180–3185. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Bomser, J.A.; Schwartz, S.J.; He, J.; Magnuson, B.A.; Giusti, M.M. Structure−Function Relationships of Anthocyanins from Various Anthocyanin-Rich Extracts on the Inhibition of Colon Cancer Cell Growth. J. Agric. Food Chem. 2008, 56, 9391–9398. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Giusti, M.M.; Malik, M.; Moyer, M.P.; Magnuson, B.A. Effects of Commercial Anthocyanin-Rich Extracts on Colonic Cancer and Nontumorigenic Colonic Cell Growth. J. Agric. Food Chem. 2004, 52, 6122–6128. [Google Scholar] [CrossRef] [PubMed]
- Filipiak, K.; Hidalgo, M.; Silvan, J.M.; Fabre, B.; Carbajo, R.J.; Pineda-Lucena, A.; Ramos, A.; de Pascual-Teresa, B.; de Pascual-Teresa, S. Dietary Gallic Acid and Anthocyanin Cytotoxicity on Human Fibrosarcoma HT1080 Cells. A Study on the Mode of Action. Food Funct. 2014, 5, 381–389. [Google Scholar] [CrossRef]
- Bunea, A.; Rugină, D.; Sconţa, Z.; Pop, R.M.; Pintea, A.; Socaciu, C.; Tăbăran, F.; Grootaert, C.; Struijs, K.; VanCamp, J. Anthocyanin Determination in Blueberry Extracts from Various Cultivars and Their Antiproliferative and Apoptotic Properties in B16-F10 Metastatic Murine Melanoma Cells. Phytochemistry 2013, 95, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-C.; Huang, H.-P.; Chang, Y.-C.; Wang, C.-J. An Anthocyanin-Rich Extract from Hibiscus Sabdariffa Linnaeus Inhibits N. -Nitrosomethylurea-Induced Leukemia in Rats. J. Agric. Food Chem. 2014, 62, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Eggersdorfer, M.; Wyss, A. Carotenoids in Human Nutrition and Health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Spence, C. What Is so Unappealing about Blue Food and Drink? Int. J. Gastron. Food Sci. 2018, 14, 1–8. [Google Scholar] [CrossRef]
- Nwoba, E.G.; Ogbonna, C.N.; Ishika, T.; Vadiveloo, A. Microalgal Pigments: A Source of Natural Food Colors. In Microalgae Biotechnology for Food, Health and High Value Products; Springer: Singapore, 2020; pp. 81–123. [Google Scholar]
- Yu, P.; Wu, Y.; Wang, G.; Jia, T.; Zhang, Y. Purification and Bioactivities of Phycocyanin. Crit. Rev. Food Sci. Nutr. 2017, 57, 3840–3849. [Google Scholar] [CrossRef]
- Ferraro, G.; Imbimbo, P.; Marseglia, A.; Illiano, A.; Fontanarosa, C.; Amoresano, A.; Olivieri, G.; Pollio, A.; Monti, D.M.; Merlino, A. A Thermophilic C-Phycocyanin with Unprecedented Biophysical and Biochemical Properties. Int. J. Biol. Macromol. 2020, 150, 38–51. [Google Scholar] [CrossRef]
- Garcia-Pliego, E.; Franco-Colin, M.; Rojas-Franco, P.; Blas-Valdivia, V.; Serrano-Contreras, J.I.; Pentón-Rol, G.; Cano-Europa, E. Phycocyanobilin Is the Molecule Responsible for the Nephroprotective Action of Phycocyanin in Acute Kidney Injury Caused by Mercury. Food Funct. 2021, 12, 2985–2994. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-L.; Wang, G.-H.; Xiang, W.-Z.; Li, T.; He, H. Stability and Antioxidant Activity of Food-Grade Phycocyanin Isolated from Spirulina Platensis. Int. J. Food Prop. 2016, 19, 2349–2362. [Google Scholar] [CrossRef]
- LIU, Y.; PERERA, C.; SURESH, V. Comparison of Three Chosen Vegetables with Others from South East Asia for Their Lutein and Zeaxanthin Content. Food Chem. 2007, 101, 1533–1539. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae Biomass as an Alternative Ingredient in Cookies: Sensory, Physical and Chemical Properties, Antioxidant Activity and in Vitro Digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Sanna, D.; Fadda, A. Waste from Food and Agro-Food Industries as Pigment Sources: Recovery Techniques, Stability and Food Applications. Nutraceuticals 2022, 2, 365–383. [Google Scholar] [CrossRef]
- Liu, M.-H.; Li, Y.-F.; Chen, B.-H. Preparation of Chlorophyll Nanoemulsion from Pomelo Leaves and Its Inhibition Effect on Melanoma Cells A375. Plants 2021, 10, 1664. [Google Scholar] [CrossRef] [PubMed]
- da Silva Ferreira, V.; Sant’Anna, C. Impact of Culture Conditions on the Chlorophyll Content of Microalgae for Biotechnological Applications. World J. Microbiol. Biotechnol. 2017, 33, 20. [Google Scholar] [CrossRef] [PubMed]
- Lanfer-Marquez, U.M.; Barros, R.M.C.; Sinnecker, P. Antioxidant Activity of Chlorophylls and Their Derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Chao, P.-Y.; Hu, S.-P.; Yang, C.-M. The Antioxidant and Free Radical Scavenging Activities of Chlorophylls and Pheophytins. Food Nutr. Sci. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- de Vogel, J.; Jonker-Termont, D.S.M.L.; Katan, M.B.; van der Meer, R. Natural Chlorophyll but Not Chlorophyllin Prevents Heme-Induced Cytotoxic and Hyperproliferative Effects in Rat Colon. J. Nutr. 2005, 135, 1995–2000. [Google Scholar] [CrossRef]
- Cheng, H.-H.; Wang, H.-K.; Ito, J.; Bastow, K.F.; Tachibana, Y.; Nakanishi, Y.; Xu, Z.; Luo, T.-Y.; Lee, K.-H. Cytotoxic Pheophorbide-Related Compounds from Clerodendrum alamitosum and C. cyrtophyllum. J. Nat. Prod. 2001, 64, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Lourenço-Lopes, C.; Carreira-Casais, A.; Fraga-Corral, M.; Garcia-Oliveira, P.; Soria, A.; Jarboui, A.; Barral, M.; Otero, P.; Simal-Gandara, J.; Prieto, M.A. Carotenoids as Natural Colorful Additives for the Food Industry. In Natural Food Additives; IntechOpen: London, UK, 2022. [Google Scholar]
- de Mejia, E.G.; Zhang, Q.; Penta, K.; Eroglu, A.; Lila, M.A. The Colors of Health: Chemistry, Bioactivity, and Market Demand for Colorful Foods and Natural Food Sources of Colorants. Annu. Rev. Food Sci. Technol. 2020, 11, 145–182. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Mapelli-Brahm, P. The Undercover Colorless Carotenoids Phytoene and Phytofluene: Importance in Agro-Food and Health in the Green Deal Era and Possibilities for Innovation. Trends Food Sci. Technol. 2021, 116, 255–263. [Google Scholar] [CrossRef]
- Krahl, T.; Fuhrmann, H.; Dräger, S. Ice Cream. In Handbook on Natural Pigments in Food and Beverages; Elsevier: Amsterdam, The Netherlands, 2024; pp. 283–293. [Google Scholar]
- Porto Dalla Costa, A.; Cruz Silveira Thys, R.; De Oliveira Rios, A.; Hickmann Flôres, S. Carrot Flour from Minimally Processed Residue as Substitute of Β-Carotene Commercial in Dry Pasta Prepared with Common Wheat (Triticum aestivum). J. Food Qual. 2016, 39, 590–598. [Google Scholar] [CrossRef]
- Cerreti, M.; Liburdi, K.; Del Franco, F.; Esti, M. Heat and Light Stability of Natural Yellow Colourants in Model Beverage Systems. Food Addit. Contam. Part A 2020, 37, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Lourenço-Lopes, C.; Garcia-Oliveira, P.; Carpena, M.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Scientific Approaches on Extraction, Purification and Stability for the Commercialization of Fucoxanthin Recovered from Brown Algae. Foods 2020, 9, 1113. [Google Scholar] [CrossRef]
- Van Wayenbergh, E.; Struyf, N.; Rezaei, M.N.; Sagalowicz, L.; Bel-Rhlid, R.; Moccand, C.; Courtin, C.M. Cereal Bran Protects Vitamin A from Degradation during Simmering and Storage. Food Chem. 2020, 331, 127292. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Q.; Davis, C.R.; Schmaelzle, S.T.; Rocheford, T.; Cook, M.E.; Tanumihardjo, S.A. β-Cryptoxanthin Biofortified Maize (Zea mays) Increases β-Cryptoxanthin Concentration and Enhances the Color of Chicken Egg Yolk. Poult. Sci. 2012, 91, 432–438. [Google Scholar] [CrossRef]
- Breithaupt, D.E. Modern Application of Xanthophylls in Animal Feeding—A Review. Trends Food Sci. Technol. 2007, 18, 501–506. [Google Scholar] [CrossRef]
- Hassaan, M.S.; Mohammady, E.Y.; Soaudy, M.R.; Sabae, S.A.; Mahmoud, A.M.A.; El-Haroun, E.R. Comparative Study on the Effect of Dietary β-Carotene and Phycocyanin Extracted from Spirulina Platensis on Immune-Oxidative Stress Biomarkers, Genes Expression and Intestinal Enzymes, Serum Biochemical in Nile Tilapia, Oreochromis Niloticus. Fish Shellfish Immunol. 2021, 108, 63–72. [Google Scholar] [CrossRef]
- Stoll, L.; Rech, R.; Flôres, S.H.; Nachtigall, S.M.B.; de Oliveira Rios, A. Poly(Acid Lactic) Films with Carotenoids Extracts: Release Study and Effect on Sunflower Oil Preservation. Food Chem. 2019, 281, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Grune, T.; Lietz, G.; Palou, A.; Ross, A.C.; Stahl, W.; Tang, G.; Thurnham, D.; Yin, S.A.; Biesalski, H.K. β-Carotene Is an Important Vitamin A Source for Humans. J. Nutr. 2010, 140, 2268S–2285S. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef]
- Barker, F.M.; Snodderly, D.M.; Johnson, E.J.; Schalch, W.; Koepcke, W.; Gerss, J.; Neuringer, M. Nutritional Manipulation of Primate Retinas, V: Effects of Lutein, Zeaxanthin, and n–3 Fatty Acids on Retinal Sensitivity to Blue-Light–Induced Damage. Investig. Opthalmol. Vis. Sci. 2011, 52, 3934. [Google Scholar] [CrossRef] [PubMed]
- Koklesova, L.; Liskova, A.; Samec, M.; Buhrmann, C.; Samuel, S.M.; Varghese, E.; Ashrafizadeh, M.; Najafi, M.; Shakibaei, M.; Büsselberg, D.; et al. Carotenoids in Cancer Apoptosis—The Road from Bench to Bedside and Back. Cancers 2020, 12, 2425. [Google Scholar] [CrossRef] [PubMed]
- Black, H.S.; Boehm, F.; Edge, R.; Truscott, T.G. The Benefits and Risks of Certain Dietary Carotenoids That Exhibit Both Anti- and Pro-Oxidative Mechanisms—A Comprehensive Review. Antioxidants 2020, 9, 264. [Google Scholar] [CrossRef] [PubMed]
- Zu, K.; Mucci, L.; Rosner, B.A.; Clinton, S.K.; Loda, M.; Stampfer, M.J.; Giovannucci, E. Dietary Lycopene, Angiogenesis, and Prostate Cancer: A Prospective Study in the Prostate-Specific Antigen Era. JNCI J. Natl. Cancer Inst. 2014, 106, djt430. [Google Scholar] [CrossRef] [PubMed]
- Rowles, J.L.; Ranard, K.M.; Smith, J.W.; An, R.; Erdman, J.W. Increased Dietary and Circulating Lycopene Are Associated with Reduced Prostate Cancer Risk: A Systematic Review and Meta-Analysis. Prostate Cancer Prostatic Dis. 2017, 20, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, A.; Stahl, W.; Sies, H.; Lirussi, F.; Donner, A.; Häussinger, D. Plasma Levels of Vitamin e and Carotenoids Are Decreased in Patients with Nonalcoholic Steatohepatitis (Nash). Eur. J. Med. Res. 2011, 16, 76. [Google Scholar] [CrossRef]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer Chemoprevention by Carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef]
- Yoshida, H.; Yanai, H.; Ito, K.; Tomono, Y.; Koikeda, T.; Tsukahara, H.; Tada, N. Administration of Natural Astaxanthin Increases Serum HDL-Cholesterol and Adiponectin in Subjects with Mild Hyperlipidemia. Atherosclerosis 2010, 209, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Landrier, J.-F.; Marcotorchino, J.; Tourniaire, F. Lipophilic Micronutrients and Adipose Tissue Biology. Nutrients 2012, 4, 1622–1649. [Google Scholar] [CrossRef] [PubMed]
- Tourniaire, F.; Gouranton, E.; von Lintig, J.; Keijer, J.; Luisa Bonet, M.; Amengual, J.; Lietz, G.; Landrier, J.-F. β-Carotene Conversion Products and Their Effects on Adipose Tissue. Genes Nutr. 2009, 4, 179–187. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Johnson, E.J. Carotenoid Actions and Their Relation to Health and Disease. Mol. Asp. Med. 2005, 26, 459–516. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E. Adiposopathy. J. Am. Coll. Cardiol. 2011, 57, 2461–2473. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, R.; Kuchan, M.J.; Sen, S.; Johnson, E.J. Lutein and Preterm Infants With Decreased Concentrations of Brain Carotenoids. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Estévez-Santiago, R.; Olmedilla-Alonso, B.; Fernández-Jalao, I. Bioaccessibility of Provitamin A Carotenoids from Fruits: Application of a Standardised Static in Vitro Digestion Method. Food Funct. 2016, 7, 1354–1366. [Google Scholar] [CrossRef] [PubMed]
- Zin, M.M.; Anucha, C.B.; Bánvölgyi, S. Recovery of Phytochemicals via Electromagnetic Irradiation (Microwave-Assisted-Extraction): Betalain and Phenolic Compounds in Perspective. Foods 2020, 9, 918. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Santiago, V.; Cavia, M.M.; Alonso-Torre, S.R.; Carrillo, C. Relationship between Color and Betalain Content in Different Thermally Treated Beetroot Products. J. Food Sci. Technol. 2020, 57, 3305–3313. [Google Scholar] [CrossRef]
- Castro-Enríquez, D.D.; Montaño-Leyva, B.; Del Toro-Sánchez, C.L.; Juaréz-Onofre, J.E.; Carvajal-Millan, E.; Burruel-Ibarra, S.E.; Tapia-Hernández, J.A.; Barreras-Urbina, C.G.; Rodríguez-Félix, F. Stabilization of Betalains by Encapsulation—A Review. J. Food Sci. Technol. 2020, 57, 1587–1600. [Google Scholar] [CrossRef]
- Attia, G.Y.; Moussa, M.M.; Sheashea, E.E.D.R. Characterization of red pigments extracted from red beet (Beta vulgaris, L.) And its potential uses as antioxidant and natural food colorants. Egypt. J. Agric. Res. 2013, 91, 1095–1110. [Google Scholar] [CrossRef]
- Kumar, S.S.; Arya, M.; Chauhan, A.S.; Giridhar, P. Basella Rubra Fruit Juice Betalains as a Colorant in Food Model Systems and Shelf-life Studies to Determine Their Realistic Usability. J. Food Process. Preserv. 2020, 44, e14595. [Google Scholar] [CrossRef]
- Coria-Cayupán, Y.; Nazareno, M.A. Cactus betalains can be used as antioxidant colorants protecting food constituents from oxidative damage. Acta Hortic. 2015, 319–325. [Google Scholar] [CrossRef]
- da Silva, D.V.T.; dos Santos Baião, D.; de Oliveira Silva, F.; Alves, G.; Perrone, D.; Del Aguila, E.M.; Paschoalin, V.M.F. Betanin, a Natural Food Additive: Stability, Bioavailability, Antioxidant and Preservative Ability Assessments. Molecules 2019, 24, 458. [Google Scholar] [CrossRef]
- Kanatt, S.R. Development of Active/Intelligent Food Packaging Film Containing Amaranthus Leaf Extract for Shelf Life Extension of Chicken/Fish during Chilled Storage. Food Packag. Shelf Life 2020, 24, 100506. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Zhang, X.; Liu, J. Development of Active and Intelligent Packaging by Incorporating Betalains from Red Pitaya (Hylocereus Polyrhizus) Peel into Starch/Polyvinyl Alcohol Films. Food Hydrocoll. 2020, 100, 105410. [Google Scholar] [CrossRef]
- Yao, X.; Hu, H.; Qin, Y.; Liu, J. Development of Antioxidant, Antimicrobial and Ammonia-Sensitive Films Based on Quaternary Ammonium Chitosan, Polyvinyl Alcohol and Betalains-Rich Cactus Pears (Opuntia Ficus-Indica) Extract. Food Hydrocoll. 2020, 106, 105896. [Google Scholar] [CrossRef]
- Hu, H.; Yao, X.; Qin, Y.; Yong, H.; Liu, J. Development of Multifunctional Food Packaging by Incorporating Betalains from Vegetable Amaranth (Amaranthus tricolor L.) into Quaternary Ammonium Chitosan/Fish Gelatin Blend Films. Int. J. Biol. Macromol. 2020, 159, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, E.; Kulawik, P.; Guzik, P.; Duda, I. The Verification of Intelligent Properties of Furcellaran Films with Plant Extracts on the Stored Fresh Atlantic Mackerel during Storage at 2 °C. Food Hydrocoll. 2019, 97, 105211. [Google Scholar] [CrossRef]
- Carrillo, C.; Rey, R.; Hendrickx, M.; del Mar Cavia, M.; Alonso-Torre, S. Antioxidant Capacity of Beetroot: Traditional vs Novel Approaches. Plant Foods Hum. Nutr. 2017, 72, 266–273. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Wagner, A.E.; Schini-Kerth, V.B.; Rimbach, G. Betanin-A Food Colorant with Biological Activity. Mol. Nutr. Food Res. 2015, 59, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Shi, J.; Xie, S.-Y.; Zhang, T.-Y.; Soladoye, O.P.; Aluko, R.E. Red Beetroot Betalains: Perspectives on Extraction, Processing, and Potential Health Benefits. J. Agric. Food Chem. 2020, 68, 11595–11611. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, P.; Abedimanesh, S.; Mesbah-Namin, S.A.; Ostadrahimi, A. Betalains, the Nature-Inspired Pigments, in Health and Diseases. Crit. Rev. Food Sci. Nutr. 2019, 59, 2949–2978. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, P.; Mesbah-Namin, S.A.; Ostadrahimi, A.; Separham, A.; Asghari Jafarabadi, M. Betalain- and Betacyanin-Rich Supplements’ Impacts on the PBMC SIRT1 and LOX1 Genes Expression and Sirtuin-1 Protein Levels in Coronary Artery Disease Patients: A Pilot Crossover Clinical Trial. J. Funct. Foods 2019, 60, 103401. [Google Scholar] [CrossRef]
- Rahimi, P.; Mesbah-Namin, S.A.; Ostadrahimi, A.; Abedimanesh, S.; Separham, A.; Asghary Jafarabadi, M. Effects of Betalains on Atherogenic Risk Factors in Patients with Atherosclerotic Cardiovascular Disease. Food Funct. 2019, 10, 8286–8297. [Google Scholar] [CrossRef]


| By-Products | Pigments | Bioactivities | Results | References | |
|---|---|---|---|---|---|
| Blackberry residues | Anthocyanins | Cyanidin-3-O-glucosidase, cyanidin-3-O-rutinoside, cyanidin-3-O-malonyl-glucoside, cyanidin-3-O-dioxalyl-glucoside | Antioxidant activity | DPPH assay: (98.05–113.60 µmoL TE/g dp); FRAP: (154.93–188.48 µmoL TE/g dp). | [111] |
| Grape by-products | Delphinidin-3-O-glucoside, cyanidin-3-O-glucoside, petunidine-3-O-glucoside, peonide-3-O-glucoside, malvidin-3-O-glucoside | Antioxidant activity | ORAC assay: 2.02–2.34 g/100 g ascorbic acid equivalent. | [84] | |
| Antimicrobial activity | Antimicrobial potential against Yersinia enterocolitica, Pseudomonas aeruginosa, Salmonella enteritidis, Staphylococcus aureus, and Bacillus cereus. | ||||
| Eggplant peel | Total anthocyanins | Antioxidant activity | Higher reducing power (39 ± 2.5 mg QE/100 g DE) and scavenging activity (IC50 = 2.88 ± 0.02 mg/mL). | [112] | |
| Raspberry pomace | Cyanidin-3-O-sophoroside, cyanidin-3-O-glucoside, cyanidin-3-O-rutinoside | Antioxidant activity | The highest ability to neutralize DPPH radicals was IC50 = 8.15 mg FW/mL. | [108] | |
| Anti-inflammatory activity | Strong COX-2 inhibitory properties. The highest inhibitory activity was IC50 = 0.87 mg FW/mL. | ||||
| Spirulina sp. | Phycobiliproteins | Phycocyanin | Antioxidant activity | Dietary supplementation reduces the oxidative stress in liver and kidney induced by a diet enriched with lipid peroxides in Wistar strain rats. | [109] |
| Spirulina platensis | C-phycocyanin | Anti-inflammatory activity | Reduces micturition frequency and bladder inflammation in mice with cyclophosphamide-induced cystitis by inhibiting COX-2 and prostaglandin E receptor 4. | [113] | |
| Porphyra haitanensis | Phycoerythrin | Anti-neurodegenerative activity | Inhibits the precursor protein of BACE1 and, therefore, reduces accumulation of amyloid-β precursor protein. | [114] | |
| Cucumber and watermelon peels | Chlorophylls | Chlorophyll | Antioxidant activity | Antioxidant activity when compared to the control sample without extract addition. | [115] |
| Melon juice, pulp, peel, and seeds | Chlorophyll a and b | Antioxidant activity | Chlorophylls were only detected in peels. | [116] | |
| Shrimp process by-products | Carotenoids | Astaxanthin, astaxanthin esters, and other carotenoids | Antioxidant activity | DPPH-scavenging activity of crude extract and its fractions were in the range of 3.08–3.74 mg TBHQ equivalent/mg of sample. | [117] |
| Cantaloupe waste | Lutein, β-carotene, violaxanthin | Antioxidant activity | The concentration needed to reduce IC50 was 7.33 μg/mL. This concentration is lower than that of β-carotene and Trolox standards (350 and 102.34 μg/mL, respectively). | [118] | |
| Tomato peels | Lycopene, phytoene, phytofluene, β-carotene, cis-lycopene and lutein | Antioxidant activity | Induction period of sunflower oil was increased by increasing the concentration of carotenoids. | [119] | |
| Acerola pulp and residue | Total carotenoids | Antioxidant activity | Pulp had greater antioxidant activity. | [120] | |
| Red dragon fruit peels | Betalains | Total betalains | Antioxidant activity | ABTS assay: encapsulated betalains possessed higher antioxidant capacities (195.39–201.76 μmol Trolox/g microparticles). | [110] |
| Anti-inflammatory activity | Duck embryo chorioallantoic membrane (CAM) vascular irritation assay showed that the anti-inflammatory activity of encapsulated betalains was five- to six-fold higher than that of non-encapsulated betalains. | ||||
| Antigenic activity | Glutathione S-transferase (GST)-inducing activity of betalains was likewise improved four- to five-fold. | ||||
| Opuntia spp. peels | Betaxanthins (Indicaxanthin isomer I and II), Betacyanins (Betanidin-5-O-β-sophoroside, betanidin-5-O-β-glucoside, isobetanin, gomphrenin, betanidin) | Antioxidant activity | DPPH scavenging activity: 1.96–4.6 mg/mL extract. | [121] | |
| Antifungal activity | A higher activity performance of the extract was attained on Trichoderma viride and Penicillum ochrochloron strains. | ||||
| Antimicrobial activity | The hydroethanolic extract exhibited effect on 6 of the 8 strains tested, being Micrococcus flavus and Escherichia coli the only resistant strains. | ||||
| Beetroots | Betanidin 5-glucoside, isobetanidin 5-glucoside, 2,17-bidecarboxy-neobetanin, 2-O-glucosyl-betanin, 17-decarboxy-betanidin, neobetanin | Antiulcer activity and anti-inflammatory activity | Betalain administration at doses 200, 400, and 800 mg/kg decreases the ulcer areas (UA) and index (UI); increases the curative index (CI) by 78.1, 78.4, and 78%, respectively; ameliorates the pathological damage induced by ethanol; prevented the decrease in gastric mucus content (116%) and reduced the stress oxidant; decrease in gastric mucosa thiobarbituric acid reactive species (TBARS) (28%) and mucus juice pepsin (56%). | [122] | |
| Opuntia stricta pulp and peel (var. Dillenii) | Betalain-rich extract (BRE) | Gastroprotective activity | BRE supplementation (800 mg/kg) from pulp and peel to rats with ethanol-induced gastric ulcer significantly reduced: volume of gastric secretion (VGS) decreased by 35% and 34%, respectively; UI by 41% and 68%, respectively; and the curative radio (CR) by 41% and 68%, respectively, as compared to untreated ethanol-induced gastric ulcer. | [123] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magalhães, D.; Gonçalves, R.; Rodrigues, C.V.; Rocha, H.R.; Pintado, M.; Coelho, M.C. Natural Pigments Recovery from Food By-Products: Health Benefits towards the Food Industry. Foods 2024, 13, 2276. https://doi.org/10.3390/foods13142276
Magalhães D, Gonçalves R, Rodrigues CV, Rocha HR, Pintado M, Coelho MC. Natural Pigments Recovery from Food By-Products: Health Benefits towards the Food Industry. Foods. 2024; 13(14):2276. https://doi.org/10.3390/foods13142276
Chicago/Turabian StyleMagalhães, Daniela, Ricardo Gonçalves, Cristina V. Rodrigues, Helena R. Rocha, Manuela Pintado, and Marta C. Coelho. 2024. "Natural Pigments Recovery from Food By-Products: Health Benefits towards the Food Industry" Foods 13, no. 14: 2276. https://doi.org/10.3390/foods13142276
APA StyleMagalhães, D., Gonçalves, R., Rodrigues, C. V., Rocha, H. R., Pintado, M., & Coelho, M. C. (2024). Natural Pigments Recovery from Food By-Products: Health Benefits towards the Food Industry. Foods, 13(14), 2276. https://doi.org/10.3390/foods13142276








