Upcycling of Defatted Sesame Seed Meal via Protein Amyloid-Based Nanostructures: Preparation, Characterization, and Functional and Antioxidant Attributes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
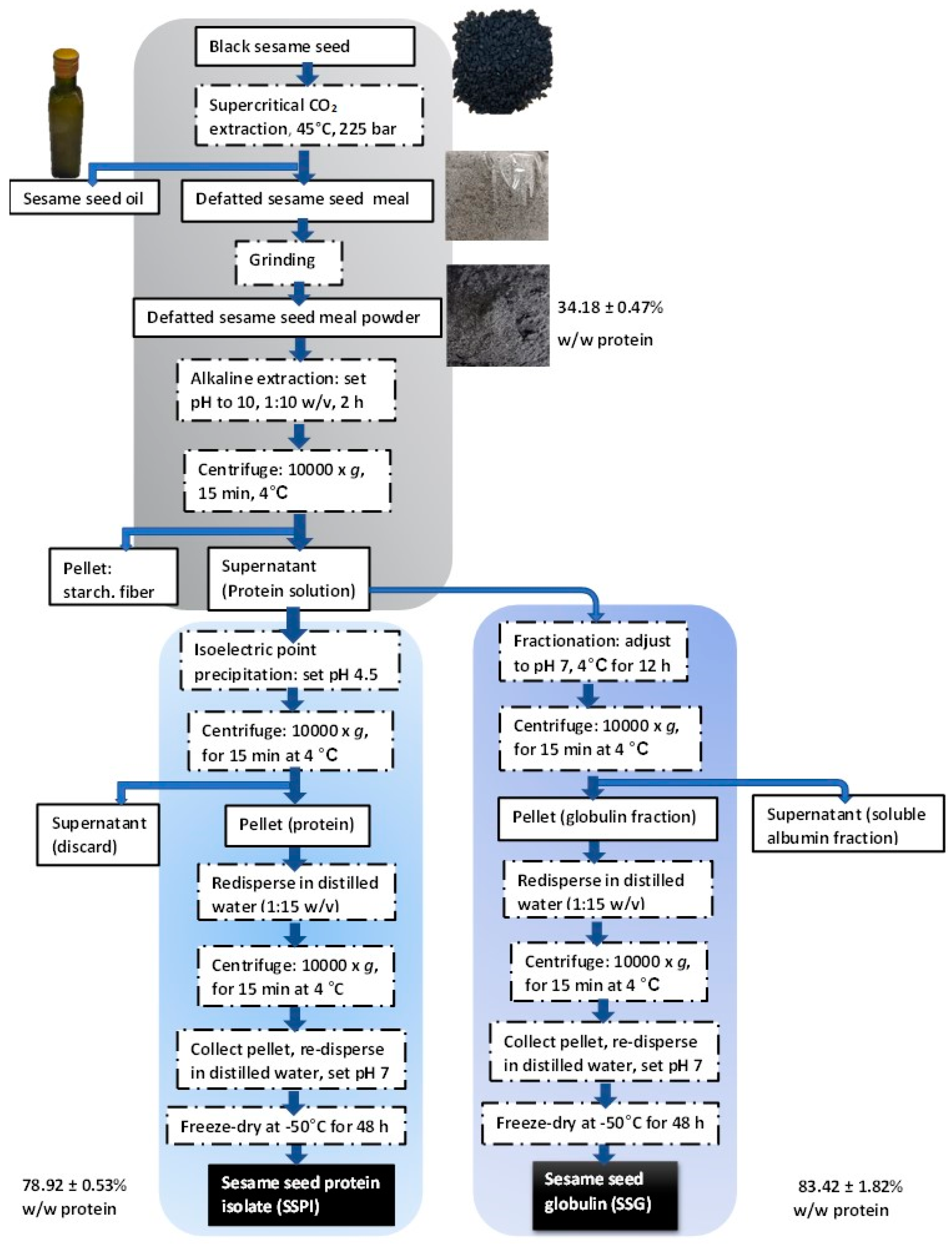
2.2. Preparation of Sesame Seed Protein Isolate (SSPI)
2.3. Preparation of Sesame Seed Globulin (SSG)
2.4. Determination of Protein Yield and Recovery Rate
2.5. Preparation of Sesame Seed Protein Amyloid-Based Nanostructures
2.6. Color Analysis
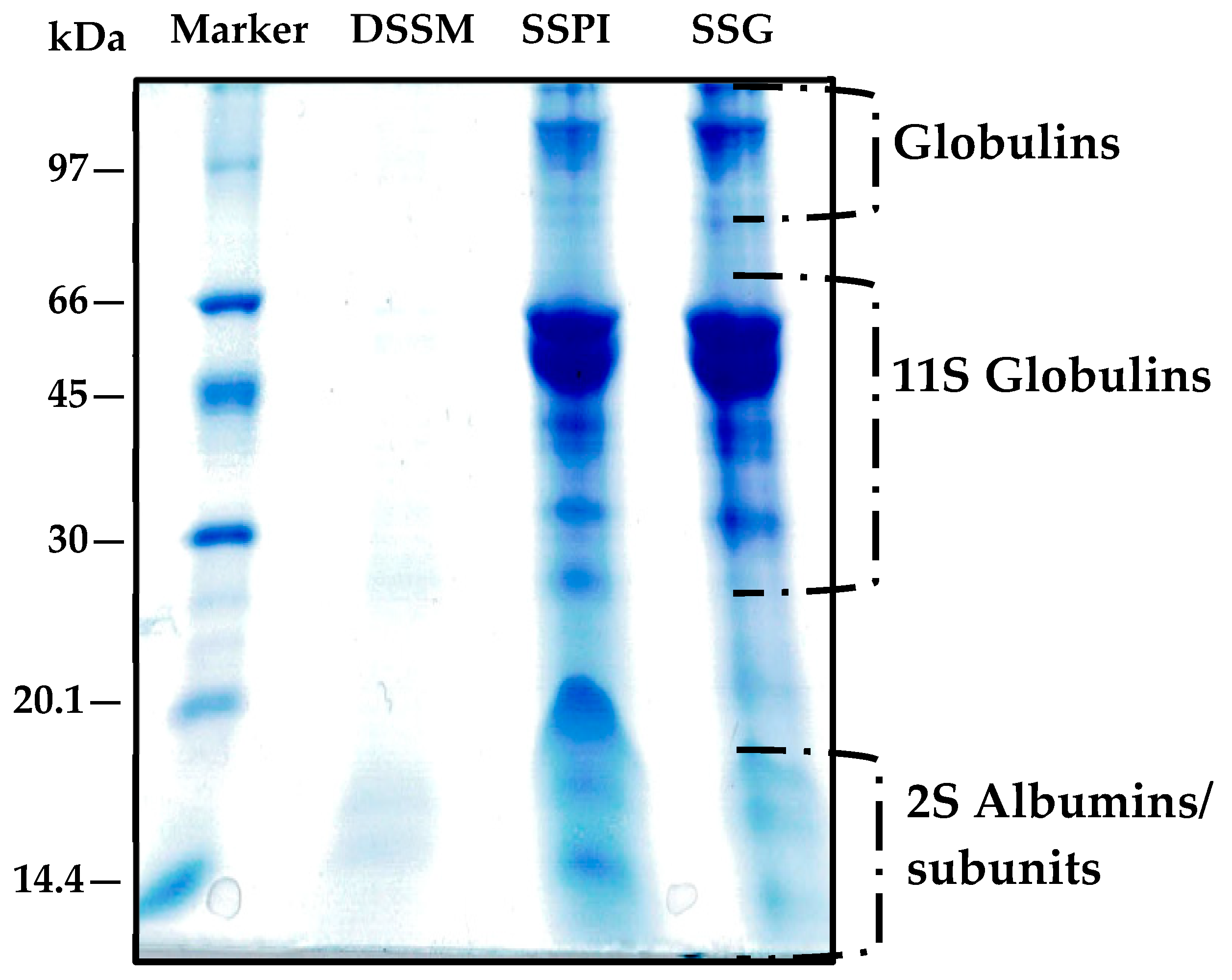
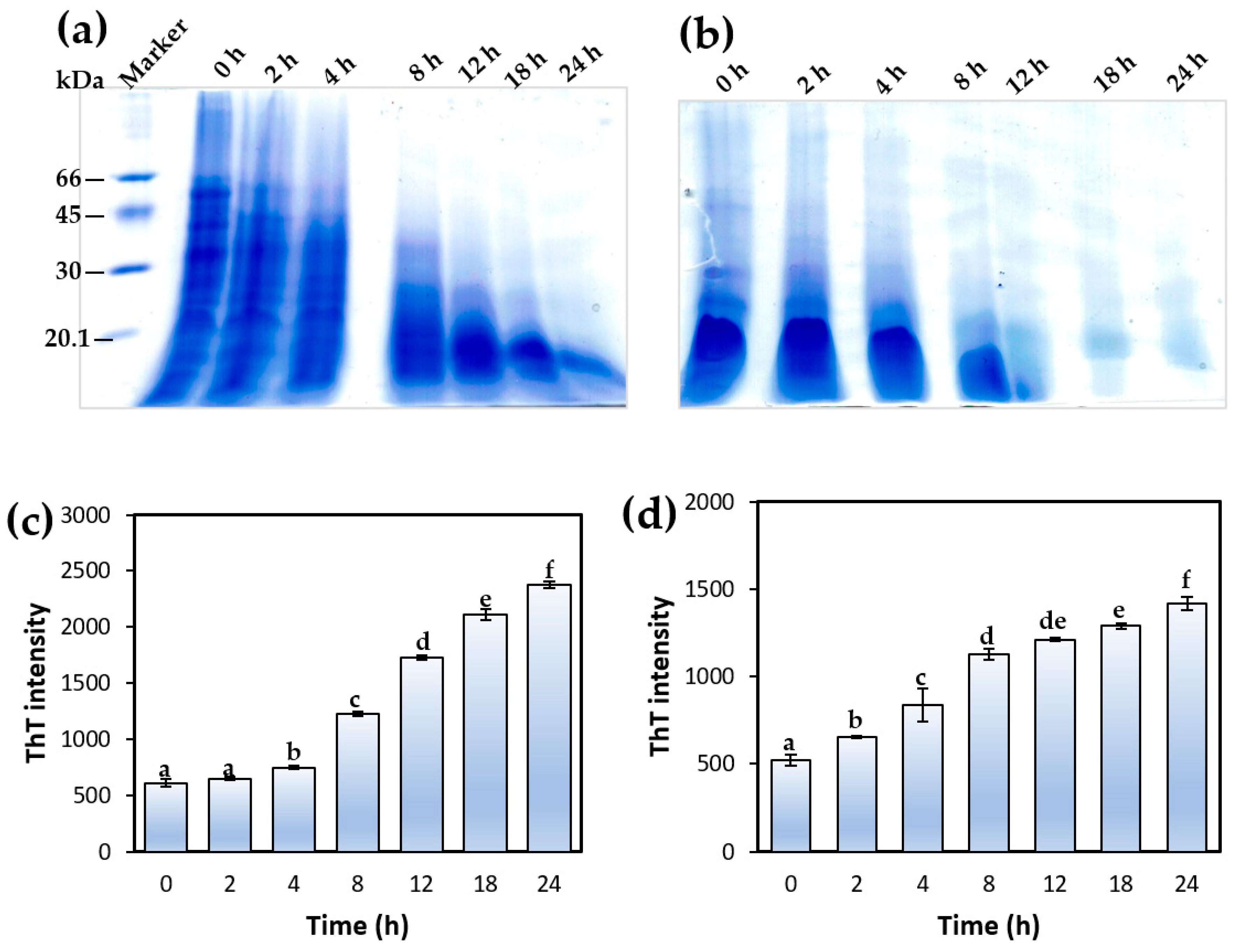
2.7. Tricine SDS-PAGE
2.8. Scanning Electron Microscopy Analysis
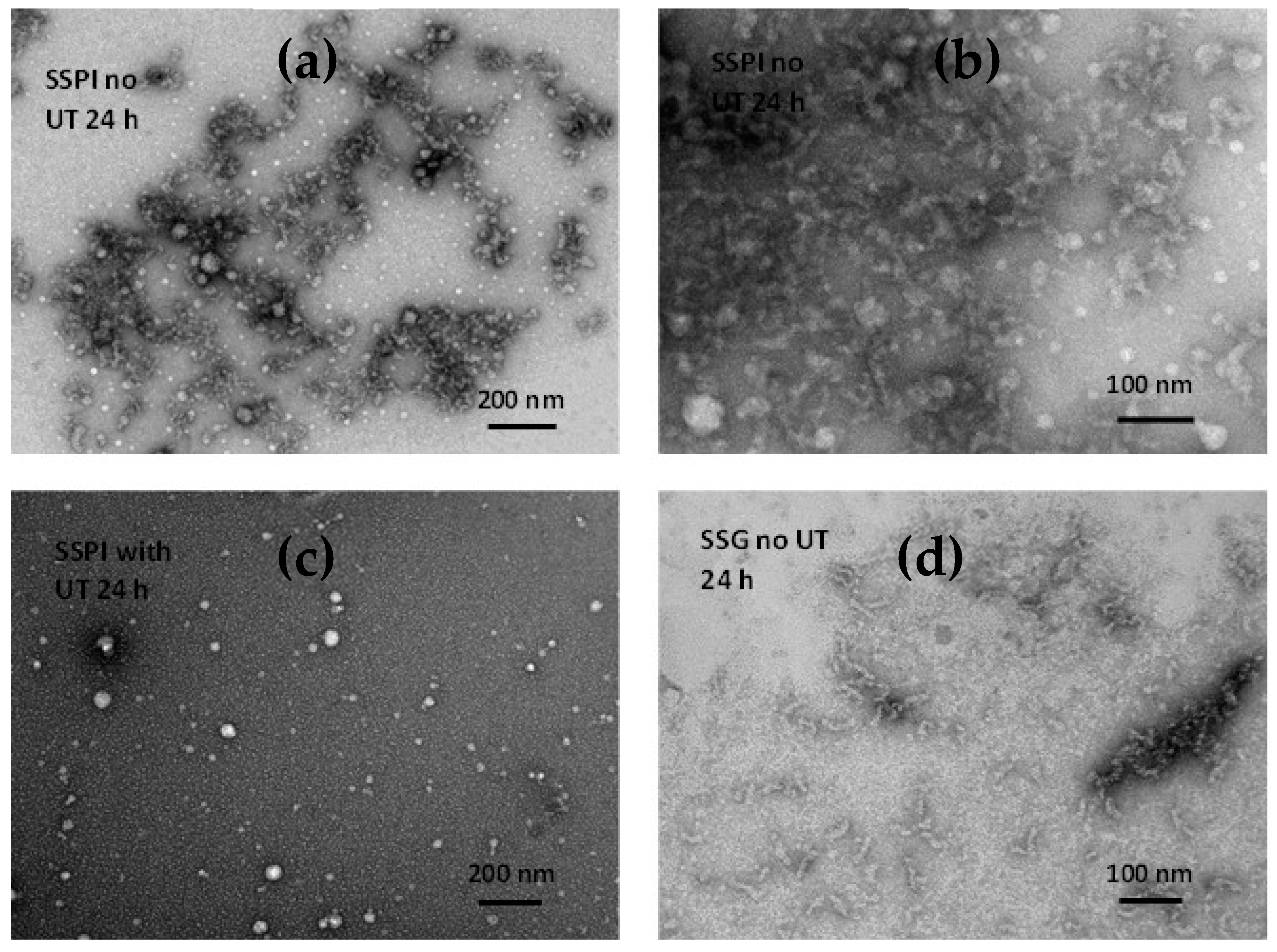
2.9. Transmission Electron Microscopy Analysis
2.10. FT-IR Analysis
2.11. Thioflavin-T Fluorescence Spectroscopy
2.12. Surface Hydrophobicity
2.13. Functional Attributes
2.13.1. Solubility
2.13.2. Fluid Holding Capacity
2.14. Antioxidant Activity
2.15. Biocompatibility
2.15.1. In Vitro Cytotoxicity
2.15.2. Hemolytic Effect
2.16. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of Sesame Proteins from DSSM
3.1.1. Protein Extraction and Recovery
3.1.2. Physical Appearance of Sesame Protein Samples
3.1.3. Microstructure of Sesame Seed Protein Samples
3.1.4. Electrophoretic Mobility of Sesame Protein Samples
3.2. Formation of Sesame Protein Amyloid Nanostructures
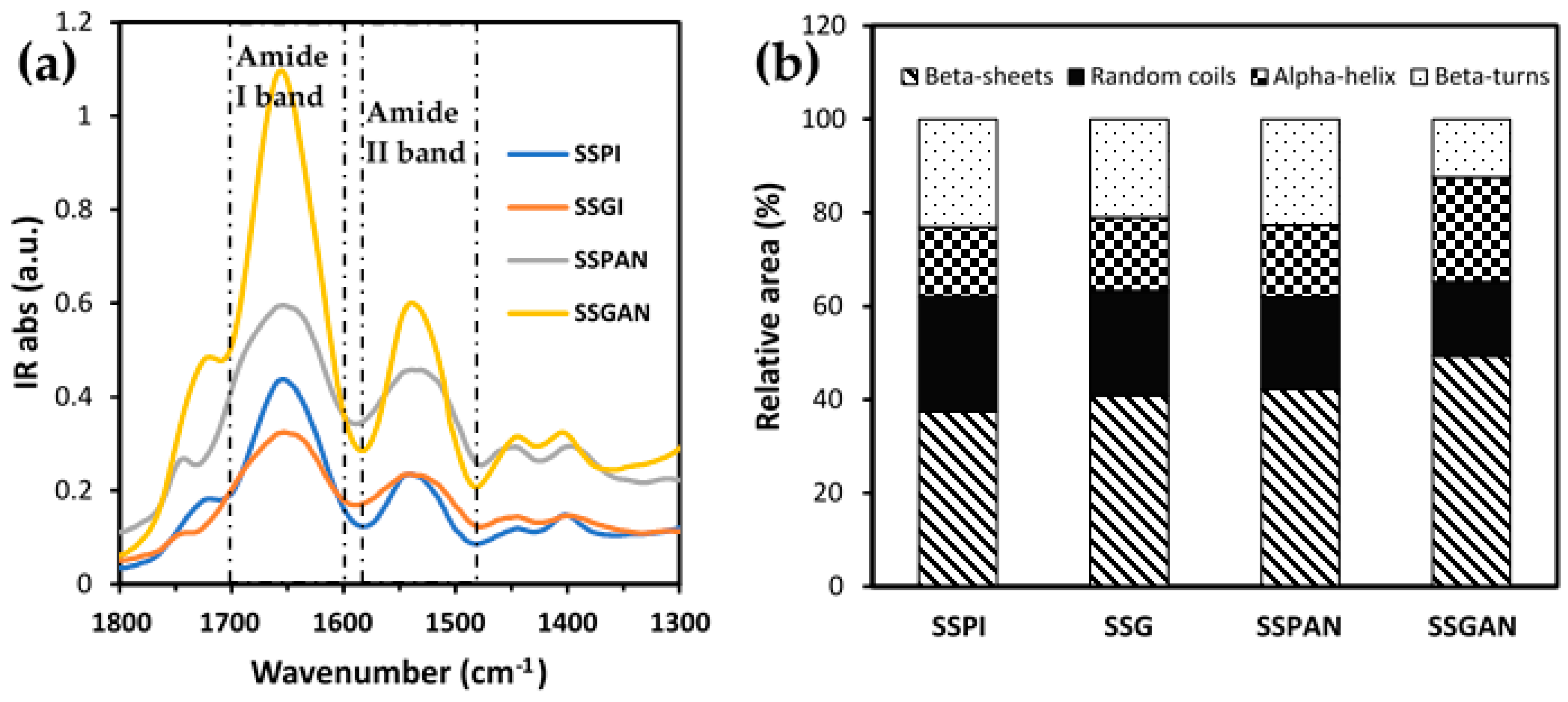
3.3. Secondary Structure Analysis of Sesame Proteins and Amyloid Nanostructures
3.4. Solubility and Surface Hydrophobicity
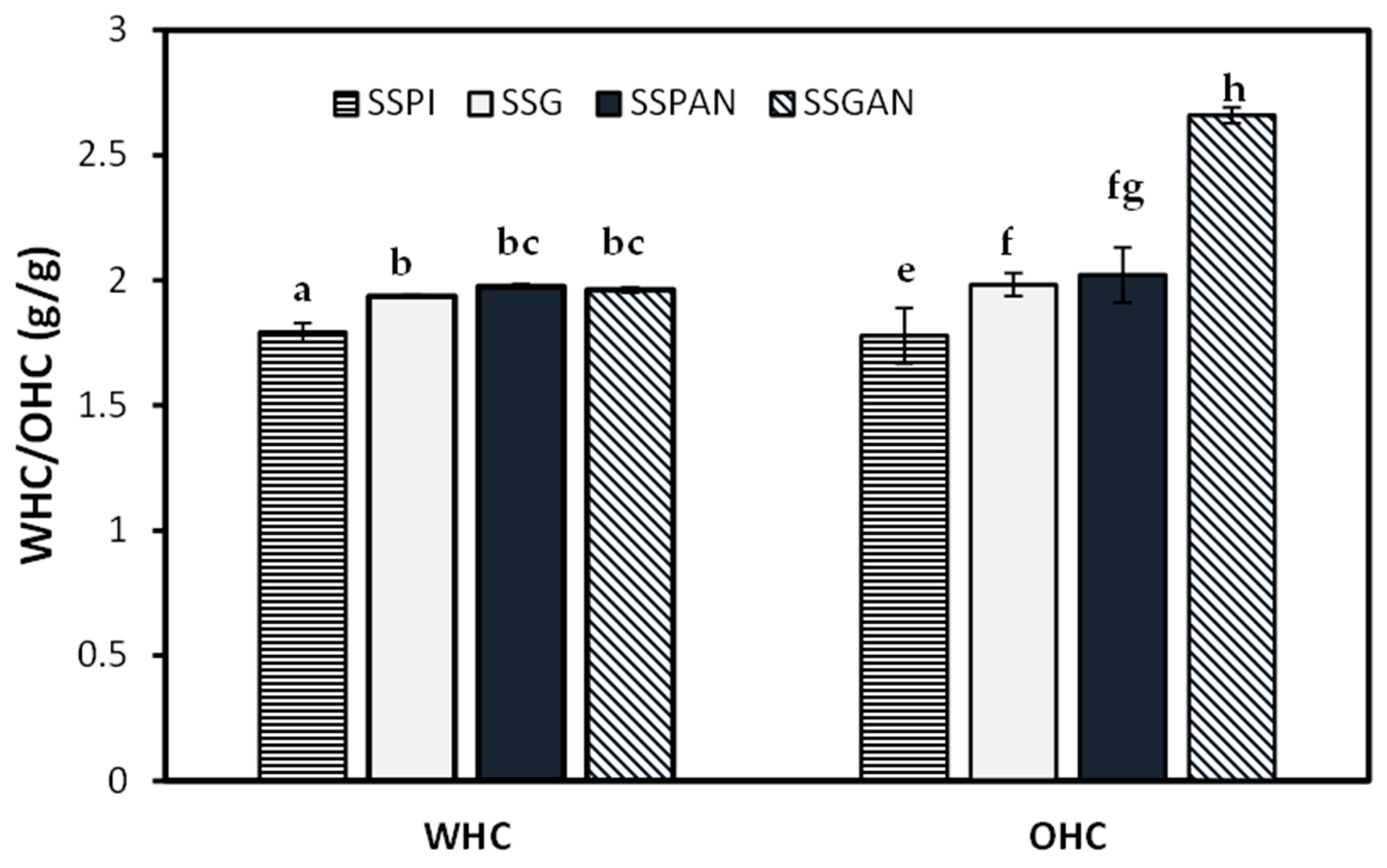
3.5. Water Holding and Oil Holding Capacity of Sesame Protein and Amyloid Structures
3.6. Antioxidant Activity of Sesame Protein Amyloid Nanostructures
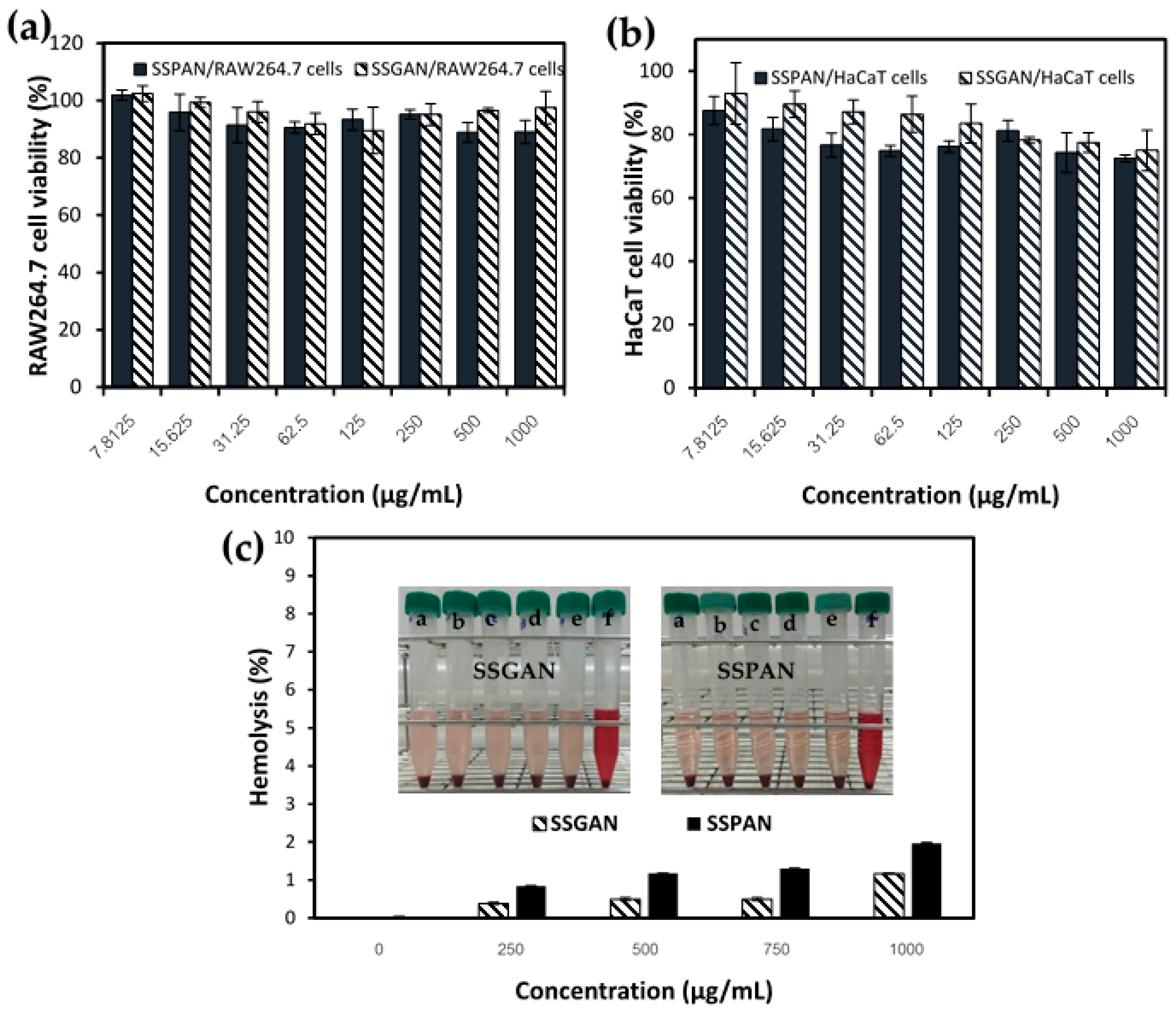
3.7. Biocompatibility of Sesame Protein Nanofibrils
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, D.; Zhou, J.; Soon, W.L.; Kutzli, I.; Molière, A.; Diedrich, S.; Radiom, M.; Handschin, S.; Li, B.; Li, L.; et al. Food Amyloid Fibrils Are Safe Nutrition Ingredients Based on In-Vitro and in-Vivo Assessment. Nat. Commun. 2023, 14, 6806. [Google Scholar] [CrossRef]
- Chen, X.; Yi, J.; Wen, Z.; Fan, Y. Ultrasonic Pretreatment and Epigallocatechin Gallate Incorporation Enhance the Formation, Apparent Viscosity, and Antioxidant Activity of Pea Protein Amyloid-like Fibrils. Food Hydrocoll. 2024, 149, 109630. [Google Scholar] [CrossRef]
- Dong, Y.; Lan, T.; Wang, L.; Wang, X.; Xu, Z.; Jiang, L.; Zhang, Y.; Sui, X. Development of Composite Electrospun Films Utilizing Soy Protein Amyloid Fibrils and Pullulan for Food Packaging Applications. Food Chem. X 2023, 20, 100995. [Google Scholar] [CrossRef]
- Li, T.; Zhou, J.; Peydayesh, M.; Yao, Y.; Bagnani, M.; Kutzli, I.; Chen, Z.; Wang, L.; Mezzenga, R. Plant Protein Amyloid Fibrils for Multifunctional Sustainable Materials. Adv. Sustain. Syst. 2023, 7, 2200414. [Google Scholar] [CrossRef]
- Wei, Z.; Dai, S.; Huang, J.; Hu, X.; Ge, C.; Zhang, X.; Yang, K.; Shao, P.; Sun, P.; Xiang, N. Soy Protein Amyloid Fibril Scaffold for Cultivated Meat Application. ACS Appl. Mater. Interfaces 2023, 15, 15108–15119. [Google Scholar] [CrossRef]
- Liu, S.; Sun, N.; Ren, K.; Tan, X.; Li, L.; Wang, Z.; Dai, S.; Tong, X.; Wang, H.; Jiang, L. Utilization of Self-Assembled Soy Protein Nanoparticles as Carriers for Natural Pigments: Examining Non-Interaction Mechanisms and Stability. Food Hydrocoll. 2024, 148, 109491. [Google Scholar] [CrossRef]
- Wang, Y.-R.; Yang, Q.; Jiang, Y.-X.; Chen, H.-Q. Enhanced Solubility, Thermal Stability and Antioxidant Activity of Resveratrol by Complexation with Ovalbumin Amyloid-like Fibrils: Effect of pH. Food Hydrocoll. 2024, 148, 109463. [Google Scholar] [CrossRef]
- Li, T.; Zhou, J.; Wu, Q.; Zhang, X.; Chen, Z.; Wang, L. Modifying Functional Properties of Food Amyloid-Based Nanostructures from Rice Glutelin. Food Chem. 2023, 398, 133798. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Lu, W.; Sun, C.; Zhu, X.; Fang, Y. Evolution of Physicochemical and Antioxidant Properties of Whey Protein Isolate during Fibrillization Process. Food Chem. 2021, 357, 129751. [Google Scholar] [CrossRef]
- Xu, J.; Tang, M.; Wang, D.; Xie, Q.; Xu, X. Exploring the Self-Assembly Journey of Oat Globulin Fibrils: From Structural Evolution to Modified Functionality. Food Hydrocoll. 2024, 149, 109587. [Google Scholar] [CrossRef]
- Mykolenko, S.; Soon, W.L.; Mezzenga, R. Production and Characterization of Amaranth Amyloid Fibrils from Food Protein Waste. Food Hydrocoll. 2024, 149, 109604. [Google Scholar] [CrossRef]
- Kutzli, I.; Zhou, J.; Li, T.; Baier, S.K.; Mezzenga, R. Formation and Characterization of Plant-Based Amyloid Fibrils from Hemp Seed Protein. Food Hydrocoll. 2023, 137, 108307. [Google Scholar] [CrossRef]
- Ramachandran, S.; Singh, S.K.; Larroche, C.; Soccol, C.R.; Pandey, A. Oil Cakes and Their Biotechnological Applications—A Review. Bioresour. Technol. 2007, 98, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Sá, A.G.A.; Pacheco, M.T.B.; Moreno, Y.M.F.; Carciofi, B.A.M. Cold-Pressed Sesame Seed Meal as a Protein Source: Effect of Processing on the Protein Digestibility, Amino Acid Profile, and Functional Properties. J. Food Compos. Anal. 2022, 111, 104634. [Google Scholar] [CrossRef]
- Sarkis, J.R.; Boussetta, N.; Blouet, C.; Tessaro, I.C.; Marczak, L.D.F.; Vorobiev, E. Effect of Pulsed Electric Fields and High Voltage Electrical Discharges on Polyphenol and Protein Extraction from Sesame Cake. Innov. Food Sci. Emerg. Technol. 2015, 29, 170–177. [Google Scholar] [CrossRef]
- Koysuren, B.; Oztop, M.H.; Mazi, B.G. Sesame Seed as an Alternative Plant Protein Source: A Comprehensive Physicochemical Characterisation Study for Alkaline, Salt and Enzyme-Assisted Extracted Samples. Int. J. Food Sci. Technol. 2021, 56, 5471–5484. [Google Scholar] [CrossRef]
- Saini, C.S.; Sharma, H.K.; Sharma, L. Thermal, Structural and Rheological Characterization of Protein Isolate from Sesame Meal. Food Meas. 2018, 12, 426–432. [Google Scholar] [CrossRef]
- Hu, S.; Gao, H.; Ouyang, L.; Li, X.; Zhu, S.; Wu, Y.; Yuan, L.; Zhou, J. Mechanistic Insights into the Improving Effects of Germination on Physicochemical Properties and Antioxidant Activity of Protein Isolate Derived from Black and White Sesame. Food Chem. 2023, 429, 136833. [Google Scholar] [CrossRef]
- Biswas, A.; Dhar, P.; Ghosh, S. Antihyperlipidemic Effect of Sesame (Sesamum indicum L.) Protein Isolate in Rats Fed a Normal and High Cholesterol Diet. J. Food Sci. 2010, 75, H274–H279. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Huang, J.; Xiong, S.; Zhang, L.; Xu, X.; Xu, Y.; Peng, F.; Huang, T.; Xiao, M.; Xiong, T. Effects of Enzyme Treatment on the Antihypertensive Activity and Protein Structure of Black Sesame Seed (Sesamum indicum L.) after Fermentation Pretreatment. Food Chem. 2023, 428, 136781. [Google Scholar] [CrossRef]
- Shu, Z.; Liu, L.; Geng, P.; Liu, J.; Shen, W.; Tu, M. Sesame Cake Hydrolysates Improved Spatial Learning and Memory of Mice. Food Biosci. 2019, 31, 100440. [Google Scholar] [CrossRef]
- Buranachokpaisan, K.; Chalermchat, Y.; Muangrat, R. Economic Evaluation of the Production of Oil Extracted from Pressed Sesame Seed Cake Using Supercritical CO2 in Thailand. J. Appl. Res. Med. Aromat. Plants 2022, 31, 100410. [Google Scholar] [CrossRef]
- Goyeneche, R.; Agüero, M.V.; Roura, S.; Di Scala, K. Application of Citric Acid and Mild Heat Shock to Minimally Processed Sliced Radish: Color Evaluation. Postharvest Biol. Technol. 2014, 93, 106–113. [Google Scholar] [CrossRef]
- Eze, F.N.; Ingkaninan, K.; Prapunpoj, P. Transthyretin Anti-Amyloidogenic and Fibril Disrupting Activities of Bacopa monnieri (L.) Wettst (Brahmi) Extract. Biomolecules 2019, 9, 845. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Liu, S.; Zhao, C.; Wu, C. Developing Protocols of Tricine-SDS-PAGE for Separation of Polypeptides in the Mass Range 1-30 kDa with Minigel Electrophoresis System. Int. J. Electrochem. Sci. 2016, 11, 640–649. [Google Scholar] [CrossRef]
- Bühler, J.M.; Dekkers, B.L.; Bruins, M.E.; van der Goot, A.J. Modifying Faba Bean Protein Concentrate Using Dry Heat to Increase Water Holding Capacity. Foods 2020, 9, 1077. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhu, S.; Luo, J.; Ouyang, L.; Feng, J.; Zhou, J. Effect of Extrusion on Physicochemical Properties and Antioxidant Potential of Protein Isolate Derived from Baijiu Vinasse. Food Chem. 2022, 384, 132527. [Google Scholar] [CrossRef] [PubMed]
- Ernst, O.; Zor, T. Linearization of the Bradford Protein Assay. J. Vis. Exp. 2010, 12, 1918. [Google Scholar] [CrossRef]
- Hadnađev, M.; Dapčević-Hadnađev, T.; Lazaridou, A.; Moschakis, T.; Michaelidou, A.-M.; Popović, S.; Biliaderis, C.G. Hempseed Meal Protein Isolates Prepared by Different Isolation Techniques. Part I. Physicochemical Properties. Food Hydrocoll. 2018, 79, 526–533. [Google Scholar] [CrossRef]
- Hu, J.; Qi, Q.; Zhu, Y.; Wen, C.; Olatunji, O.J.; Jayeoye, T.J.; Eze, F.N. Unveiling the Anticancer, Antimicrobial, Antioxidative Properties, and UPLC-ESI-QTOF-MS/ GC–MS Metabolite Profile of the Lipophilic Extract of Siam Weed (Chromolaena odorata). Arab. J. Chem. 2023, 16, 104834. [Google Scholar] [CrossRef]
- Singh, S.; Chidrawar, V.R.; Hermawan, D.; Nwabor, O.F.; Olatunde, O.O.; Jayeoye, T.J.; Samee, W.; Ontong, J.C.; Chittasupho, C. Solvent-Assisted Dechlorophyllization of Psidium guajava Leaf Extract: Effects on the Polyphenol Content, Cytocompatibility, Antibacterial, Anti-Inflammatory, and Anticancer Activities. S. Afr. J. Bot. 2023, 158, 166–179. [Google Scholar] [CrossRef]
- Eze, F.N.; Nwabor, O.F. Valorization of Pichia Spent Medium via One-Pot Synthesis of Biocompatible Silver Nanoparticles with Potent Antioxidant, Antimicrobial, Tyrosinase Inhibitory and Reusable Catalytic Activities. Mater. Sci. Eng. C 2020, 115, 111104. [Google Scholar] [CrossRef]
- Yang, K.; Xu, T.-R.; Fu, Y.-H.; Cai, M.; Xia, Q.-L.; Guan, R.-F.; Zou, X.-G.; Sun, P.-L. Effects of Ultrasonic Pre-Treatment on Physicochemical Properties of Proteins Extracted from Cold-Pressed Sesame Cake. Food Res. Int. 2021, 139, 109907. [Google Scholar] [CrossRef]
- Gundogan, R.; Can Karaca, A. Physicochemical and Functional Properties of Proteins Isolated from Local Beans of Turkey. LWT 2020, 130, 109609. [Google Scholar] [CrossRef]
- Tanger, C.; Engel, J.; Kulozik, U. Influence of Extraction Conditions on the Conformational Alteration of Pea Protein Extracted from Pea Flour. Food Hydrocoll. 2020, 107, 105949. [Google Scholar] [CrossRef]
- Segla Koffi Dossou, S.; Xu, F.; You, J.; Zhou, R.; Li, D.; Wang, L. Widely Targeted Metabolome Profiling of Different Colored Sesame (Sesamum indicum L.) Seeds Provides New Insight into Their Antioxidant Activities. Food Res. Int. 2022, 151, 110850. [Google Scholar] [CrossRef]
- Dossou, S.S.K.; Luo, Z.; Wang, Z.; Zhou, W.; Zhou, R.; Zhang, Y.; Li, D.; Liu, A.; Dossa, K.; You, J.; et al. The Dark Pigment in the Sesame (Sesamum indicum L.) Seed Coat: Isolation, Characterization, and Its Potential Precursors. Front. Nutr. 2022, 9, 858673. [Google Scholar] [CrossRef]
- Panzella, L.; Eidenberger, T.; Napolitano, A.; d’Ischia, M. Black Sesame Pigment: DPPH Assay-Guided Purification, Antioxidant/Antinitrosating Properties, and Identification of a Degradative Structural Marker. J. Agric. Food Chem. 2012, 60, 8895–8901. [Google Scholar] [CrossRef]
- Misra, S.; Pandey, P.; Panigrahi, C.; Mishra, H.N. A Comparative Approach on the Spray and Freeze Drying of Probiotic and Gamma-Aminobutyric Acid as a Single Entity: Characterization and Evaluation of Stability in Simulated Gastrointestinal Conditions. Food Chem. Adv. 2023, 3, 100385. [Google Scholar] [CrossRef]
- Sahni, P.; Sharma, S.; Surasani, V.K.R. Influence of Processing and pH on Amino Acid Profile, Morphology, Electrophoretic Pattern, Bioactive Potential and Functional Characteristics of Alfalfa Protein Isolates. Food Chem. 2020, 333, 127503. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, J.; Zhang, C.; Kong, X.; Hua, Y. Sesame Water-Soluble Proteins Fraction Contains Endopeptidases and Exopeptidases with High Activity: A Natural Source for Plant Proteases. Food Chem. 2021, 353, 129519. [Google Scholar] [CrossRef]
- Orruño, E.; Morgan, M.R.A. Purification and Characterisation of the 7S Globulin Storage Protein from Sesame (Sesamum indicum L.). Food Chem. 2007, 100, 926–934. [Google Scholar] [CrossRef]
- Nouska, C.; Deligeorgaki, M.; Kyrkou, C.; Michaelidou, A.-M.; Moschakis, T.; Biliaderis, C.G.; Lazaridou, A. Structural and Physicochemical Properties of Sesame Cake Protein Isolates Obtained by Different Extraction Methods. Food Hydrocoll. 2024, 151, 109757. [Google Scholar] [CrossRef]
- Zhou, J.; Li, T.; Peydayesh, M.; Usuelli, M.; Lutz-Bueno, V.; Teng, J.; Wang, L.; Mezzenga, R. Oat Plant Amyloids for Sustainable Functional Materials. Adv. Sci. 2022, 9, 2104445. [Google Scholar] [CrossRef]
- Afify, A.E.-M.M.R.; Rashed, M.M.; Mahmoud, E.A.; El-Beltagi, H.S. Effect of Gamma Radiation on Protein Profile, Protein Fraction and Solubility’s of Three Oil Seeds: Soybean, Peanut and Sesame. Not. Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 90–98. [Google Scholar] [CrossRef]
- Idowu, A.O.; Alashi, A.M.; Nwachukwu, I.D.; Fagbemi, T.N.; Aluko, R.E. Functional Properties of Sesame (Sesamum indicum L.) Seed Protein Fractions. Food Prod. Process. Nutr. 2021, 3, 4. [Google Scholar] [CrossRef]
- Tai, S.S.K.; Lee, T.T.T.; Tsai, C.C.Y.; Yiu, T.-J.; Tzen, J.T.C. Expression Pattern and Deposition of Three Storage Proteins, 11S Globulin, 2S Albumin and 7S Globulin in Maturing Sesame Seeds§. Plant Physiol. Biochem. 2001, 39, 981–992. [Google Scholar] [CrossRef]
- Yang, J.; Kornet, R.; Diedericks, C.F.; Yang, Q.; Berton-Carabin, C.C.; Nikiforidis, C.V.; Venema, P.; van der Linden, E.; Sagis, L.M.C. Rethinking Plant Protein Extraction: Albumin—From Side Stream to an Excellent Foaming Ingredient. Food Struct. 2022, 31, 100254. [Google Scholar] [CrossRef]
- Hu, A.; Li, L. Effect Mechanism of Ultrasound Pretreatment on Fibrillation Kinetics, Physicochemical Properties and Structure Characteristics of Soy Protein Isolate Nanofibrils. Ultrason. Sonochem. 2021, 78, 105741. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, X.; Gao, Y.; Zhang, Y.; Jiang, L.; Sui, X. Structural Insights into Acidic Heating-Induced Amyloid Fibrils Derived from Soy Protein as a Function of Protein Concentration. Food Hydrocoll. 2023, 145, 109085. [Google Scholar] [CrossRef]
- Yu, Z.; Li, N.; Liu, Y.; Zhang, B.; Zhang, M.; Wang, X.; Wang, X. Formation, Structure and Functional Characteristics of Amyloid Fibrils Formed Based on Soy Protein Isolates. Int. J. Biol. Macromol. 2024, 254, 127956. [Google Scholar] [CrossRef] [PubMed]
- Gade Malmos, K.; Blancas-Mejia, L.M.; Weber, B.; Buchner, J.; Ramirez-Alvarado, M.; Naiki, H.; Otzen, D. ThT 101: A Primer on the Use of Thioflavin T to Investigate Amyloid Formation. Amyloid 2017, 24, 1–16. [Google Scholar] [CrossRef]
- Liu, C.; Wu, D.; Wang, P.; McClements, D.J.; Cui, S.; Liu, H.; Leng, F.; Sun, Q.; Dai, L. Study on the Formation Mechanism of Pea Protein Nanofibrils and the Changes of Structural Properties of Fibril under Different pH and Temperature. Food Hydrocoll. 2024, 150, 109735. [Google Scholar] [CrossRef]
- Liu, J.; Tang, C.-H. Heat-Induced Fibril Assembly of Vicilin at pH 2.0: Reaction Kinetics, Influence of Ionic Strength and Protein Concentration, and Molecular Mechanism. Food Res. Int. 2013, 51, 621–632. [Google Scholar] [CrossRef]
- Martínez-Velasco, A.; Lobato-Calleros, C.; Hernández-Rodríguez, B.E.; Román-Guerrero, A.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. High Intensity Ultrasound Treatment of Faba Bean (Vicia faba L.) Protein: Effect on Surface Properties, Foaming Ability and Structural Changes. Ultrason. Sonochem. 2018, 44, 97–105. [Google Scholar] [CrossRef]
- Herneke, A.; Lendel, C.; Johansson, D.; Newson, W.; Hedenqvist, M.; Karkehabadi, S.; Jonsson, D.; Langton, M. Protein Nanofibrils for Sustainable Food–Characterization and Comparison of Fibrils from a Broad Range of Plant Protein Isolates. ACS Food Sci. Technol. 2021, 1, 854–864. [Google Scholar] [CrossRef]
- Song, Y.; Li, T.; Zhang, X.; Wang, L. Investigating the Effects of Ion Strength on Amyloid Fibril Formation of Rice Proteins. Food Biosci. 2023, 51, 102068. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.-R.; Du, Y.-N.; Chen, H.-Q. Comparison of the Assembly Behavior and Structural Characteristics of Arachin and Conarachin Amyloid-like Fibrils. Food Hydrocoll. 2023, 138, 108479. [Google Scholar] [CrossRef]
- Farrokhi, F.; Badii, F.; Ehsani, M.R.; Hashemi, M. Functional and Thermal Properties of Nanofibrillated Whey Protein Isolate as Functions of Denaturation Temperature and Solution pH. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 124002. [Google Scholar] [CrossRef]
- Mohammadian, M.; Madadlou, A. Characterization of Fibrillated Antioxidant Whey Protein Hydrolysate and Comparison with Fibrillated Protein Solution. Food Hydrocoll. 2016, 52, 221–230. [Google Scholar] [CrossRef]
- Wei, Z.; Huang, Q. Impact of Covalent or Non-Covalent Bound Epigallocatechin-3-Gallate (EGCG) on Assembly, Physicochemical Characteristics and Digestion of Ovotransferrin Fibrils. Food Hydrocoll. 2020, 98, 105314. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, J.; Zheng, J. Molecular Understanding of a Potential Functional Link between Antimicrobial and Amyloid Peptides. Soft Matter 2014, 10, 7425–7451. [Google Scholar] [CrossRef] [PubMed]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant Activity of Proteins and Peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Mezzenga, R. Food Protein Amyloid Fibrils: Origin, Structure, Formation, Characterization, Applications and Health Implications. Adv. Colloid Interface Sci. 2019, 269, 334–356. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Kalita, S.; Kalita, S.; Sukumar, P.; Mandal, B. Disaggregation of Amylin Aggregate by Novel Conformationally Restricted Aminobenzoic Acid Containing α/β and α/γ Hybrid Peptidomimetics. Sci. Rep. 2017, 7, 40095. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.W.; Liu, K.-N.; How, S.-C.; Chen, W.-A.; Lai, C.-M.; Liu, H.-S.; Hu, C.-J.; Wang, S.S.-S. Carnosine’s Effect on Amyloid Fibril Formation and Induced Cytotoxicity of Lysozyme. PLoS ONE 2013, 8, e81982. [Google Scholar] [CrossRef] [PubMed]
- Lassé, M.; Ulluwishewa, D.; Healy, J.; Thompson, D.; Miller, A.; Roy, N.; Chitcholtan, K.; Gerrard, J.A. Evaluation of Protease Resistance and Toxicity of Amyloid-like Food Fibrils from Whey, Soy, Kidney Bean, and Egg White. Food Chem. 2016, 192, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose–Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef]
- Amin, K.; Dannenfelser, R.-M. In Vitro Hemolysis: Guidance for the Pharmaceutical Scientist. J. Pharm. Sci. 2006, 95, 1173–1176. [Google Scholar] [CrossRef]




| Sample | Protein Content (%) | Yield (%) | Recovery Rate (%) |
|---|---|---|---|
| DSSM | 34.18 ± 0.47 a | - | - |
| SSPI | 78.92 ± 0.53 b | 21.53 ± 1.22 c | 49.71 ± 0.98 b |
| SSG | 83.42 ± 1.81 c | 16.33 ± 0.91 d | 39.86 ± 0.76 a |
| Sample | L* | a* | b* | ΔE |
|---|---|---|---|---|
| DSSM | 44.92 ± 0.31 a | 0.40 ± 0.05 c,d | 5.02 ± 0.08 a | - |
| SSPI | 33.39 ± 0.07 b | 0.11 ± 0.04 c,d,e | 4.40 ± 0.40 b | 11.66 ± 0.37 a |
| SSG | 33.24 ± 0.29 b | −0.38 ± 0.01 c,e | 3.37 ± 0.05 c | 11.83 ± 0.32 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eze, F.N.; Muangrat, R.; Singh, S.; Jirarattanarangsri, W.; Siriwoharn, T.; Chalermchat, Y. Upcycling of Defatted Sesame Seed Meal via Protein Amyloid-Based Nanostructures: Preparation, Characterization, and Functional and Antioxidant Attributes. Foods 2024, 13, 2281. https://doi.org/10.3390/foods13142281
Eze FN, Muangrat R, Singh S, Jirarattanarangsri W, Siriwoharn T, Chalermchat Y. Upcycling of Defatted Sesame Seed Meal via Protein Amyloid-Based Nanostructures: Preparation, Characterization, and Functional and Antioxidant Attributes. Foods. 2024; 13(14):2281. https://doi.org/10.3390/foods13142281
Chicago/Turabian StyleEze, Fredrick Nwude, Rattana Muangrat, Sudarshan Singh, Wachira Jirarattanarangsri, Thanyaporn Siriwoharn, and Yongyut Chalermchat. 2024. "Upcycling of Defatted Sesame Seed Meal via Protein Amyloid-Based Nanostructures: Preparation, Characterization, and Functional and Antioxidant Attributes" Foods 13, no. 14: 2281. https://doi.org/10.3390/foods13142281
APA StyleEze, F. N., Muangrat, R., Singh, S., Jirarattanarangsri, W., Siriwoharn, T., & Chalermchat, Y. (2024). Upcycling of Defatted Sesame Seed Meal via Protein Amyloid-Based Nanostructures: Preparation, Characterization, and Functional and Antioxidant Attributes. Foods, 13(14), 2281. https://doi.org/10.3390/foods13142281







