Protective Effect of Caffeine and Chlorogenic Acids of Coffee in Liver Disease
Abstract
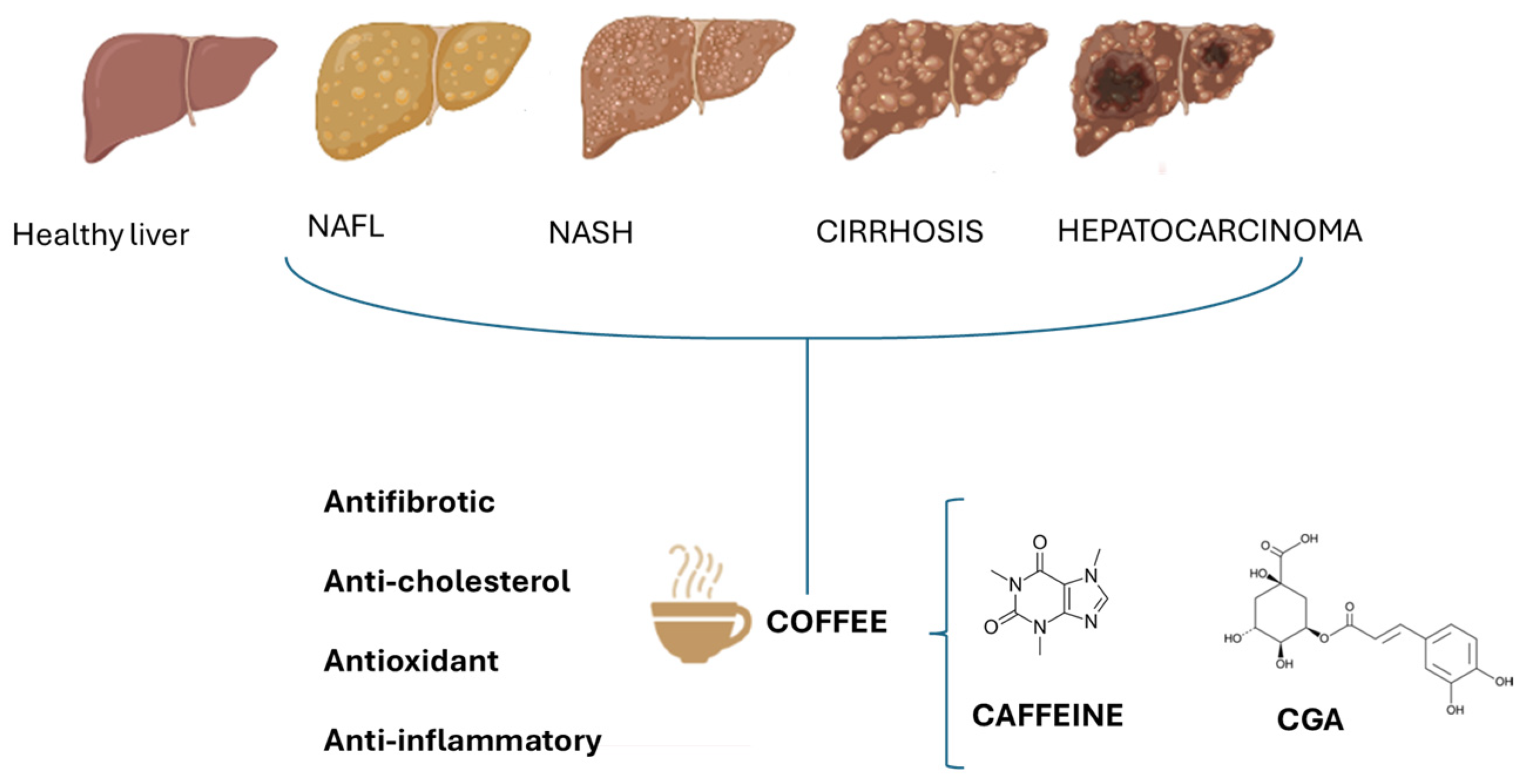
1. Introduction
2. Protective Role of Coffee in Liver Diseases
2.1. HBV/HCV
2.2. Hepatic Steatosis
2.3. Liver Cirrhosis
2.4. Hepatocarcinoma
3. Roles of Bioactive Components of Coffee in Liver Disease
3.1. Caffeine
3.1.1. Pharmacokinetics
3.1.2. Pharmacodynamics
3.2. Chlorogenic Acids
3.2.1. Pharmacokinetics
3.2.2. Pharmacodynamics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bozzola, M.; Charles, S.; Ferretti, T.; Rosser, N.; von der Goltz, P.; Manson, H. The Coffee Guide, 4th ed.; International Trade Centre: Geneva, Switzerland, 2021; ISBN 9789211036831. [Google Scholar]
- Nieber, K. The Impact of Coffee on Health. Planta Med. 2017, 83, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ 2017, 22, j5024. [Google Scholar] [CrossRef] [PubMed]
- Westfall, S.; Pasinetti, G.M. The Gut Microbiota Links Dietary Polyphenols with Management of Psychiatric Mood Disorders. Front. Neurosci. 2019, 13, 1196. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Scapagnini, G. Interactions between dietary polyphenols and aging gut microbiota: A review. Biofactors 2022, 48, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Wang, F.; Zhai, D.; Meng, X.; Liu, J.; Lv, X. Caffeine in liver diseases: Pharmacology and toxicology. Front. Pharmacol. 2022, 13, 1030173. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Bhupathiraju, S.N.; Satija, A.; van Dam, R.M.; Hu, F.B. Long-term coffee consumption and risk of cardiovascular disease: A systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation 2014, 129, 643–659. [Google Scholar] [CrossRef] [PubMed]
- Chieng, D.; Kistler, P.M. Coffee and tea on cardiovascular disease (CVD) prevention. Trends Cardiovasc. Med. 2022, 32, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Hyppönen, E. Long-term coffee consumption, caffeine metabolism genetics, and risk of cardiovascular disease: A prospective analysis of up to 347,077 individuals and 8368 cases. Am. J. Clin. Nutr. 2019, 109, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, I.A.; Mena, P.; Calani, L.; Cid, C.; Del Rio, D.; Lean, M.E.; Crozier, A. Variations in caffeine and chlorogenic acid contents of coffees: What are we drinking? Food Funct. 2014, 5, 1718–1726. [Google Scholar] [CrossRef]
- Caracostea, L.M.; Sîrbu, R.; Buşuricu, F. Determination of Caffeine Content in Arabica and Robusta Green Coffee of Indian Origin. Eur. J. Nat. Sci. Med. 2021, 4, 69–79. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.D.; Behary, J.; Zekry, A. Non-alcoholic fatty liver disease: A review of epidemiology, risk factors, diagnosis and management. Intern. Med. J. 2020, 50, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; Terrault, N.A.; Tacke, F.; Gluud, L.L.; Arrese, M.; Bugianesi, E.; Loomba, R. Global epidemiology of cirrhosis—Aetiology, trends and predictions. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Barré, T.; Fontaine, H.; Ramier, C.; Di Beo, V.; Pol, S.; Carrieri, P.; Marcellin, F.; Cagnot, C.; Dorival, C.; Zucman-Rossi, J.; et al. Elevated coffee consumption is associated with a lower risk of elevated liver fibrosis biomarkers in patients treated for chronic hepatitis B (ANRS CO22 Hepather cohort). Clin. Nutr. 2022, 41, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.W.; Ho, S.C.; Chan, H.L.Y.; Wong, V.; Yeo, W.; Mok, T.S. Moderate coffee consumption reduces the risk of hepatocellular carcinoma in hepatitis B chronic carriers: A case-control study. J. Epidemiol. Community Health 2011, 65, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.D.; Curto, T.M.; Lindsay, K.L.; Wright, E.C.; Sinha, R.; Everhart, J.E.; HALT-C TRIAL Group. Coffee consumption is associated with response to peginterferon and ribavirin therapy in patients with chronic hepatitis C. Gastroenterology 2011, 140, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Taub, R.; Neff, G.W.; Lucas, K.J.; Labriola, D.; Moussa, S.E.; Alkhouri, N.; Bashir, M.R. Resmetirom for nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled phase 3 trial. Nat. Med. 2023, 29, 2919–2928. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves First Treatment for Patients with Liver Scarring Due to Fatty Liver Disease. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-patients-liver-scarring-due-fatty-liver-disease (accessed on 21 June 2024).
- Mantovani, A.; Dalbeni, A. Treatments for NAFLD: State of Art. Int. J. Mol. Sci. 2021, 22, 2350. [Google Scholar] [CrossRef] [PubMed]
- Wadhawan, M.; Anand, A.C. Coffee and Liver Disease. J. Clin. Exp. Hepatol. 2016, 6, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Casiglia, E.; Spolaore, P.; Ginocchio, G.; Ambrosio, G.B. Unexpected effects of coffee consumption on liver enzymes. Eur. J. Epidemiol. 1993, 9, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Lu, F.B.; Hu, Y.B.; Xu, L.M.; Zheng, M.H.; Hu, E.D. A systematic review and a dose-response meta-analysis of coffee dose and nonalcoholic fatty liver disease. Clin. Nutr. 2019, 38, 2552–2557. [Google Scholar] [CrossRef] [PubMed]
- Sewter, R.; Heaney, S.; Patterson, A. Coffee Consumption and the Progression of NAFLD: A Systematic Review. Nutrients 2021, 13, 2381. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 5, 1–51. [Google Scholar] [CrossRef]
- Hayat, U.; Siddiqui, A.A.; Okut, H.; Afroz, S.; Tasleem, S.; Haris, A. The effect of coffee consumption on the non-alcoholic fatty liver disease and liver fibrosis: A meta-analysis of 11 epidemiological studies. Ann. Hepatol. 2021, 20, 100254. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, J.; Ahn, S.B. Different Associations of Coffee Consumption with the Risk of Incident Metabolic Dysfunction-Associated Steatotic Liver Disease and Advanced Liver Fibrosis. Nutrients 2023, 16, 140. [Google Scholar] [CrossRef] [PubMed]
- Niezen, S.; Mehta, M.; Jiang, Z.G.; Tapper, E.B. Coffee Consumption Is Associated with Lower Liver Stiffness: A Nationally Representative Study. Clin. Gastroenterol. Hepatol. 2022, 20, 2032–2040.e6. [Google Scholar] [CrossRef] [PubMed]
- Anty, R.; Marjoux, S.; Iannelli, A.; Patouraux, S.; Schneck, A.S.; Bonnafous, S.; Gire, C.; Amzolini, A.; Ben-Amor, I.; Saint-Paul, M.C.; et al. Regular coffee but not espresso drinking is protective against fibrosis in a cohort mainly composed of morbidly obese European women with NAFLD undergoing bariatric surgery. J. Hepatol. 2012, 57, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Catalano, D.; Martines, G.F.; Tonzuso, A.; Pirri, C.; Trovato, F.M.; Trovato, G.M. Protective role of coffee in non-alcoholic fatty liver disease (NAFLD). Dig. Dis. Sci. 2010, 55, 3200–3206. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Grobe, Y.; Chávez-Tapia, N.; Sánchez-Valle, V.; Gavilanes-Espinar, J.G.; Ponciano-Rodríguez, G.; Uribe, M.; Méndez-Sánchez, N. High coffee intake is associated with lower grade nonalcoholic fatty liver disease: The role of peripheral antioxidant activity. Ann. Hepatol. 2012, 11, 350–355. [Google Scholar] [CrossRef]
- Birerdinc, A.; Stepanova, M.; Pawloski, L.; Younossi, Z.M. Caffeine is protective in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2012, 35, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Wijarnpreecha, K.; Thongprayoon, C.; Ungprasert, P. Coffee consumption and risk of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2017, 29, e8–e12. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Salomone, F.; Webb, M.; Lotan, R.; Yeshua, H.; Halpern, Z.; Santo, E.; Oren, R.; Shibolet, O. Coffee consumption and nonalcoholic fatty liver onset: A prospective study in the general population. Transl. Res. 2015, 165, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, R.; Kobayashi, M.; Matsuda, Y.; Ojika, M.; Shigeoka, S.; Yamamoto, Y.; Tou, Y.; Inoue, T.; Katagiri, T.; Murai, A.; et al. Coffee and caffeine ameliorate hyperglycemia, fatty liver, and inflammatory adipocytokine expression in spontaneously diabetic KK-Ay mice. J. Agric. Food Chem. 2010, 58, 5597–5603. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Mazzone, G.; Lembo, V.; D’Argenio, G.; Rossi, A.; Guido, M.; Savoia, M.; Salomone, F.; Mennella, I.; De Filippis, F.; et al. Coffee prevents fatty liver disease induced by a high-fat diet by modulating pathways of the gut-liver axis. J. Nutr. Sci. 2019, 8, e15. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Pozada, E.E.; Ramos-Tovar, E.; Rodriguez-Callejas, J.D.; Cardoso-Lezama, I.; Galindo-Gómez, S.; Talamás-Lara, D.; Vásquez-Garzón, V.R.; Arellanes-Robledo, J.; Tsutsumi, V.; Villa-Treviño, S.; et al. Caffeine Inhibits NLRP3 Inflammasome Activation by Downregulating TLR4/MAPK/NF-κB Signaling Pathway in an Experimental NASH Model. Int. J. Mol. Sci. 2022, 23, 9954. [Google Scholar] [CrossRef] [PubMed]
- Arauz, J.; Zarco, N.; Segovia, J.; Shibayama, M.; Tsutsumi, V.; Muriel, P. Caffeine prevents experimental liver fibrosis by blocking the expression of TGF-beta. Eur. J. Gastroenterol. Hepatol. 2014, 26, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, M.; Ip, S.; Bhanji, R.A.; Montano-Loza, A.J. Effect of Coffee Consumption on Non-Alcoholic Fatty Liver Disease Incidence, Prevalence and Risk of Significant Liver Fibrosis: Systematic Review with Meta-Analysis of Observational Studies. Nutrients 2021, 13, 3042. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, M.; Angeli, P.; Claria, J.; Moreau, R.; Gines, P.; Jalan, R.; Caraceni, P.; Fernandez, J.; Gerbes, A.L.; O’Brien, A.J.; et al. Albumin in decompensated cirrhosis: New concepts and perspectives. Gut 2020, 69, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Jagdish, R.K.; Roy, A.; Kumar, K.; Premkumar, M.; Sharma, M.; Rao, P.N.; Reddy, D.N.; Kulkarni, A.V. Pathophysiology and management of liver cirrhosis: From portal hypertension to acute-on-chronic liver failure. Front. Med. 2023, 10, 1060073. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef] [PubMed]
- Valenti, L.; Pedica, F.; Colombo, M. Distinctive features of hepatocellular carcinoma in non-alcoholic fatty liver disease. Dig. Liver Dis. 2022, 54, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Defagó, M.D.; Elorriaga, N.; Irazola, V.E.; Rubinstein, A.L. Influence of food patterns on endothelial biomarkers: A systematic review. J. Clin. Hypertens. 2014, 16, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Hori, A.; Kasai, H.; Kawai, K.; Nanri, A.; Sato, M.; Ohta, M.; Mizoue, T. Coffee intake is associated with lower levels of oxidative DNA damage and decreasing body iron storage in healthy women. Nutr. Cancer 2014, 66, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Andrighetto, L.V.; Poziomyck, A.K. Serum leptin levens and hepatocellular carcinoma: Review article. Arq. Bras. Cir. Dig. 2016, 29, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Brimson, J.M.; Prasanth, M.I.; Malar, D.S.; Thitilertdecha, P.; Kabra, A.; Tencomnao, T.; Prasansuklab, A. Plant Polyphenols for Aging Health: Implication from Their Autophagy Modulating Properties in Age-Associated Diseases. Pharmaceuticals 2021, 14, 982. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, M.; Ghidoni, R. Cancer Prevention and Therapy with Polyphenols: Sphingolipid-Mediated Mechanisms. Nutrients 2018, 10, 940. [Google Scholar] [CrossRef] [PubMed]
- Aydın, Z.B.; Erbaş, O. Coffee Intake and Cancer Risk: Exploring the Relationship. J. Exp. Basic Med. Sci. 2024, 5, 194–198. [Google Scholar] [CrossRef]
- Dranoff, J.A. Coffee, adenosine, and the liver. Purinergic Signal. 2024, 20, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, O.J.; Roderick, P.; Buchanan, R.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee, including caffeinated and decaffeinated coffee, and the risk of hepatocellular carcinoma: A systematic review and dose-response meta-analysis. BMJ Open 2017, 7, e013739. [Google Scholar] [CrossRef]
- Aleksandrova, K.; Bamia, C.; Drogan, D.; Lagiou, P.; Trichopoulou, A.; Jenab, M.; Fedirko, V.; Romieu, I.; Bueno-de-Mesquita, H.B.; Pischon, T.; et al. The association of coffee intake with liver cancer risk is mediated by biomarkers of inflammation and hepatocellular injury: Data from the European Prospective Investigation into Cancer and Nutrition. Am. J. Clin. Nutr. 2015, 102, 1498–1508. [Google Scholar] [CrossRef] [PubMed]
- Degertekin, B.; Tozun, N.; Soylemez, A.G.; Gurtay, E.; Bozkurt, U.; Yilmaz, Y.; Yapali, S.; Vardareli, E.; Unal, H.U.; Colakoglu, B.; et al. Regular coffee intake improves liver enzyme levels and liver histology in patients with chronic alcohol consumption, non-alcoholic fatty liver and non-alcoholic steatohepatitis: Report of 259 cases. Hepatol. Forum 2020, 1, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Bravi, F.; Bosetti, C.; Tavani, A.; Gallus, S.; La Vecchia, C. Coffee reduces risk for hepatocellular carcinoma: An updated meta-analysis. Clin. Gastroenterol. Hepatol. 2013, 11, 1413–1421.e1. [Google Scholar] [CrossRef] [PubMed]
- Dranoff, J.A. Coffee Consumption and Prevention of Cirrhosis: In Support of the Caffeine Hypothesis. Gene Expr. 2018, 18, 1–3. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Drinking Coffee, Mate, and Very Hot Beverages; International Agency for Research on Cancer: Lyon, France, 2018. [Google Scholar]
- Blanchard, J.; Sawers, S.J. The absolute bioavailability of caffeine in man. Eur. J. Clin. Pharmacol. 1983, 24, 93–98. [Google Scholar] [CrossRef] [PubMed]
- de Paula, J.; Farah, A. Caffeine Consumption through Coffee: Content in the Beverage, Metabolism, Health Benefits and Risks. Beverages 2019, 5, 37. [Google Scholar] [CrossRef]
- Rasmussen, B.B.; Brix, T.H.; Kyvik, K.O.; Brosen, K. The interindividual differences in the 3-demthylation of caffeine alias CYP1A2 is determined by both genetic and environmental factors. Pharmacogenetics 2002, 12, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, R.P.; Lima, F.D.; Carvalho, N.R.; Bresciani, G.; Royes, L.F. Caffeine effects on systemic metabolism, oxidative-inflammatory pathways, and exercise performance. Nutr. Res. 2020, 80, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Nehlig, A. Interindividual Differences in Caffeine Metabolism and Factors Driving Caffeine Consumption. Pharmacol. Rev. 2018, 70, 384–411. [Google Scholar] [CrossRef] [PubMed]
- Camandola, S.; Plick, N.; Mattson, M.P. Impact of Coffee and Cacao Purine Metabolites on Neuroplasticity and Neurodegenerative Disease. Neurochem. Res. 2019, 44, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Olechno, E.; Puścion-Jakubik, A.; Zujko, M.E.; Socha, K. Influence of various factors on caffeine content in coffee brews. Foods 2021, 10, 1208. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, M.J. Pharmacokinetics and metabolism of natural methylxanthines in animal and man. Handb. Exp. Pharmacol. 2011, 200, 33–91. [Google Scholar] [CrossRef]
- Leviton, A. Biases inherent in studies of coffee consumption in early pregnancy and the risks of subsequent events. Nutrients 2018, 10, 1152. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. EFSA Explains Risk Assessment; European Food Safety Authority: Parma, Italy, 2021. [Google Scholar]
- McPherson, P.S.; Kim, Y.K.; Valdivia, H.; Knudson, C.M.; Takekura, H.; Franzini-Armstrong, C.; Coronado, R.; Campbell, K.P. The brain ryanodine receptor: A caffeine-sensitive calcium release channel. Neuron 1991, 7, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Gliottoni, R.C.; Meyers, J.R.; Arrigrimsson, S.A.; Boglio, S.P.; Motl, R.W. Effect of caffeine on quadriceps muscle pain during acute cycling exercise in low versus high caffeine consumers. Int. J. Sports Nutr. Exerc. Metab. 2009, 19, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.P.; Johnson, D.A.; McVey, D.E. Caffeine and the modulation of brain function. In Caffeine and Behavior: Current Views and Research Trends; Gupta, B.S., Gupta, U., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 17–30. [Google Scholar]
- Nehlig, A.; Daval, J.L.; Debry, G. Caffeine and the central nervous system: Mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res. Rev. 1992, 17, 139–170. [Google Scholar] [CrossRef] [PubMed]
- Vundrala, S.; Reddy, S.; Manikantan, S.; Ramakrishna, S. Pharmacology of caffeine and its effects on the human body. Eur. J. Med. Chem. Rep. 2024, 10, 100138. [Google Scholar]
- Gahr, M. Caffeine, the most frequently consumed psychostimulant: A narrative review article. Fortschr. Neurol. Psychiatr. 2020, 88, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Watson, J. Encyclopedia of Food Sciences and Nutrition; Caballero, B., Finglas, P.M., Toldra, F., Eds.; Academic Press: Cambridge, UK, 2003; p. 745. [Google Scholar]
- Fredholm, B.B. Are methylxanthine effects due to antagonism of endogenous adenosine? Trends Pharmacol. Sci. 1980, 1, 129–132. [Google Scholar] [CrossRef]
- Chan, E.S.; Montesinos, M.C.; Fernandez, P.; Desai, A.; Delano, D.L.; Yee, H.; Reiss, A.B.; Pillinger, M.H.; Chen, J.F.; Schwarzschild, M.A.; et al. Adenosine A(2A) receptors play a role in the pathogenesis of hepatic cirrhosis. Br. J. Pharmacol. 2006, 148, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Gressner, O.A.; Lahme, B.; Rehbein, K.; Siluschek, M.; Weiskirchen, R.; Gressner, A.M. Pharmacological application of caffeine inhibits TGF-beta-stimulated connective tissue growth factor expression in hepatocytes via PPAR and SMAD2/3-dependent pathways. J. Hepatol. 2008, 49, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.G.; Mazen, N.F.; Mohamed, A.M. Caffeine intake decreases oxidative stress and inflammatory biomarkers in experimental liver diseases induced by thioacetamide: Biochemical and histological study. Int. J. Immunopathol. Pharmacol. 2017, 30, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, H.; Lv, X.; Wang, Q.; Zhao, H.; Yang, F.; Yang, Y.; Li, J. Involvement of cAMP-PKA pathway in adenosine A1 and A2A receptor-mediated regulation of acetaldehyde-induced activation of HSCs. Biochimie 2015, 115, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, N.; Spanevello, R.M.; Passamonti, S.; Porciúncula, L.; Bonan, C.D.; Olabiyi, A.A.; Teixeira da Rocha, J.B.; Assmann, C.E.; Morsch, V.M.; Schetinger, M.R.C. Coffee, caffeine, chlorogenic acid, and the purinergic system. Food Chem. Toxicol. 2019, 123, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, F.; Feldman, S.R. A review of phosphodiesterase-inhibition and the potential role for phosphodiesterase 4-inhibitors in clinical dermatology. Dermatol. Online J. 2014, 20, 22608. [Google Scholar] [CrossRef]
- Burg, A.W.; Werner, E. Tissue distribution of caffeine and its metabolites in the mouse. Biochem. Pharmacol. 1972, 21, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Daly, J.W. Alkylxanthines as research tools. J. Auton. Nerv. Syst. 2000, 81, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, W.; von der Leyen, H.; Meyer, W.; Neumann, J.; Scholz, H. Phosphodiesterase inhibition and positive inotropic effects. J. Cardiovasc. Pharmacol. 1989, 14 (Suppl. 3), S11–S14. [Google Scholar] [CrossRef] [PubMed]
- Brackett, L.E.; Shamim, M.T.; Daly, J.W. Activities of caffeine, theophylline, and enprofylline analogs as tracheal relaxants. Biochem. Pharmacol. 1990, 39, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Persson, C.G.; Andersson, K.E.; Kjellin, G. Effects of enprofylline and theophylline may show the role of adenosine. Life Sci. 1986, 38, 1057–1072. [Google Scholar] [CrossRef] [PubMed]
- Alhersh, E.; Abushanab, D.; Al-Shaibi, S.; Al-Badriyeh, D. Caffeine for the Treatment of Apnea in the Neonatal Intensive Care Unit: A Systematic Overview of Meta-Analyses. Paediatr. Drugs 2020, 22, 399–408. [Google Scholar] [CrossRef]
- Sei, Y.; Gallagher, K.L.; Daly, J.W. Multiple effects of caffeine on Ca2+ release and influx in human B lymphocytes. Cell Calcium 2001, 29, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Faudone, G.; Arifi, S.; Merk, D. The Medicinal Chemistry of Caffeine. J. Med. Chem. 2021, 64, 7156–7178. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.E.; Daly, J.W. Stimulation of calcium release by caffeine analogs in pheochromocytoma cells. Biochem. Pharmacol. 1993, 46, 1825–1829. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, P.F.; Byun, J.H.; Platko, K.; Saliba, P.; Sguazzin, M.; MacDonald, M.E.; Paré, G.; Steinberg, G.R.; Janssen, L.J.; Igdoura, S.-A.; et al. Caffeine blocks SREBP2-induced hepatic PCSK9 expression to enhance LDLR-mediated cholesterol clearance. Nat. Commun. 2022, 13, 770. [Google Scholar] [CrossRef] [PubMed]
- de Angelis, L.; Bertolissi, M.; Nardini, G.; Traversa, U.; Vertua, R. Interaction of caffeine with benzodiazepines: Behavioral effects in mice. Arch. Int. Pharmacodyn. Ther. 1982, 255, 89–102. [Google Scholar] [PubMed]
- Mattila, M.E.; Mattila, M.J.; Nuotto, E. Caffeine moderately antagonizes the effects of triazolam and zopiclone on the psychomotor performance of healthy subjects. Pharmacol. Toxicol. 1992, 70, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.N. Drugs which induce anxiety: Caffeine. N. Z. J. Psychol. 1996, 25, 1. [Google Scholar]
- Nehlig, A.; Daval, J.L.; Pereira de Vasconcelos, A.; Boyet, S. Caffeine-diazepam interaction and local cerebral glucose utilization in the conscious rat. Brain Res. 1987, 419, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Weir, R.L.; Hruska, R.E. Interaction between methylxanthines and the benzodiazepine receptor. Arch. Int. Pharmacodyn. Ther. 1983, 265, 42–48. [Google Scholar] [PubMed]
- Lee, C. Antioxidant ability of caffeine and its metabolites based on the study of oxygen radical absorbing capacity and inhibition of LDL peroxidation. Clin. Chim. Acta 2000, 295, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Rittera, M.; Hohenberger, K.; Altera, P.; Herzuma, M.; Tebbe, J.; Maisch, M. Caffeine inhibits cytokine expression in lymphocytes. Cytokine 2005, 30, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Jeszka-Skowron, M.; Sentkowska, A.; Pyrzynska, K.; De Peña, M.P. Chlorogenic acids, caffeine content and antioxidant properties of green coffee extracts: Influence of green coffee bean preparation. Eur. Food Res. Technol. 2016, 242, 1403–1409. [Google Scholar] [CrossRef]
- Farah, A.; de Paula, J. Consumption of Chlorogenic Acids through Coffee and Health Implications. Beverages 2019, 5, 11. [Google Scholar] [CrossRef]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef] [PubMed]
- Rojas-González, A.; Figueroa-Hernández, C.Y.; González-Rios, O.; Suárez-Quiroz, M.L.; González-Amaro, R.M.; Hernández-Estrada, Z.J.; Rayas-Duarte, P. Coffee Chlorogenic Acids Incorporation for Bioactivity Enhancement of Foods: A Review. Molecules 2022, 27, 3400. [Google Scholar] [CrossRef] [PubMed]
- Bagdas, D.; Gul, Z.; Meade, J.A.; Cam, B.; Cinkilic, N.; Gurun, M.S. Pharmacologic Overview of Chlorogenic Acid and its Metabolites in Chronic Pain and Inflammation. Curr. Neuropharmacol. 2020, 18, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Hada, Y.; Uchida, H.A.; Otaka, N.; Onishi, Y.; Okamoto, S.; Nishiwaki, M.; Takemoto, R.; Takeuchi, H.; Wada, J. The Protective Effect of Chlorogenic Acid on Vascular Senescence via the Nrf2/HO-1 Pathway. Int. J. Mol. Sci. 2020, 21, 4527. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feng, Y.; Li, Y.; Hu, Y.; Zhang, Q.; Huang, Y.; Shi, K.; Ran, C.; Hou, J.; Zhou, G.; et al. Chlorogenic Acid Decreases Malignant Characteristics of Hepatocellular Carcinoma Cells by Inhibiting DNMT1 Expression. Front. Pharmacol. 2020, 11, 867. [Google Scholar] [CrossRef]
- Yang, F.; Luo, L.; Zhu, Z.D.; Zhou, X.; Wang, Y.; Xue, J.; Zhang, J.; Cai, X.; Chen, Z.L.; Ma, Q.; et al. Chlorogenic acid inhibits liver fibrosis by blocking the miR-21-regulated TGF-β1/Smad7 signaling pathway in vitro and in vivo. Front. Pharmacol. 2017, 8, 929. [Google Scholar] [CrossRef]
- Shi, H.; Shi, A.; Dong, L.; Lu, X.; Wang, Y.; Zhao, J.; Dai, F.; Guo, X. Chlorogenic acid protects against liver fibrosis in vivo and in vitro through inhibition of oxidative stress. Clin. Nutr. 2016, 35, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Dong, L.; Jiang, J.; Zhao, J.; Zhao, G.; Dang, X.; Lu, X.; Jia, M. Chlorogenic acid reduces liver inflammation and fibrosis through inhibition of toll-like receptor 4 signaling pathway. Toxicology 2013, 303, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, K.; Niu, Z.; Mei, D.; Zhang, B. Protective effect of isochlorogenic acid B on liver fibrosis in non-alcoholic steatohepatitis of mice. Basic Clin. Pharmacol. Toxicol. 2019, 124, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Ismail, T.; Donati-Zeppa, S.; Akhtar, S. Coffee in cancer chemoprevention: An updated review. Expert Opin. Drug Metab. Toxicol. 2021, 17, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Refolo, M.G.; Lippolis, C.; Carella, N.; Cavallini, A.; Messa, C.; D’Alessandro, R. Chlorogenic acid improves the regorafenib effects in human hepatocellular carcinoma cells. Int. J. Mol. Sci. 2018, 19, 1518. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, J.H.; Cho, S.S.; Kim, J.H.; Xu, J.; Seo, K.; Ki, S.H. 5-Caffeoylquinic acid ameliorates oxidative stress-mediated cell death via Nrf2 activation in hepatocytes. Pharm. Biol. 2020, 58, 999–1005. [Google Scholar] [CrossRef]
- Pimpley, V.; Patil, S.; Srinivasan, K.; Desai, N.; Murthy, P.S. The chemistry of chlorogenic acid from green coffee and its role in attenuation of obesity and diabetes. Prep. Biochem. Biotechnol. 2020, 50, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, J.; Ballevre, O.; Luo, H.; Zhang, W. Antihypertensive effects and mechanisms of chlorogenic acids. Hypertens. Res. 2012, 35, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Hwang, S.J.; Park, J.H.; Lee, H.J. Chlorogenic acid inhibits hypoxia-induced angiogenesis via down-regulation of the HIF-1α/AKT pathway. Cell. Oncol. 2015, 38, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, O.J.; Fallowfield, J.A.; Poole, R.; Hayes, P.C.; Parkes, J.; Roderick, P.J. All coffee types decrease the risk of adverse clinical outcomes in chronic liver disease: A UK Biobank study. BMC Public Health 2021, 21, 970. [Google Scholar] [CrossRef] [PubMed]
- Addicott, M.A. Caffeine Use Disorder: A Review of the Evidence and Future Implications. Curr. Addict. Rep. 2014, 1, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Nwafor, E.O.; Lu, P.; Zhang, Y.; Liu, R.; Peng, H.; Xing, B.; Liu, Y.; Li, Z.; Zhang, K.; Zhang, Y.; et al. Chlorogenic acid: Potential source of natural drugs for the therapeutics of fibrosis and cancer. Transl. Oncol. 2022, 15, 101294. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Qiu, Y.; Zhang, Q.F.; Li, D. Chlorogenic acid and caffeine in combination inhibit fat accumulation by regulating hepatic lipid metabolism-related enzymes in mice. Br. J. Nutr. 2014, 112, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Dutra, N.S.; da Silva D’Ávila, C.M.; da Silva, T.C.; de Oliveira Mendes, T.; Livinalli, I.C.; Bertoncelli, A.C.Z.; Saccol, F.K.; Cadoná, F.C. Biological properties of caffeine, (+)-catechin, and theobromine: An in silico study. 3 Biotech 2024, 14, 94. [Google Scholar] [CrossRef] [PubMed]
| Component of Coffee | Mechanism |
|---|---|
| Caffeine | The upregulation of PPARγ and reduction in SMAD2/3 The inhibition of α-smooth muscle actin expression The enhancement of HSC apoptosis and intracellular F-actin and cAMP expression The inhibition of procollagen type 1C expression |
| CGAs | The suppression of inflammation The modification of the extracellular matrix, The increased biosynthesis of TGF-β Fibroblast proliferation and differentiation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Pietrantonio, D.; Pace Palitti, V.; Cichelli, A.; Tacconelli, S. Protective Effect of Caffeine and Chlorogenic Acids of Coffee in Liver Disease. Foods 2024, 13, 2280. https://doi.org/10.3390/foods13142280
Di Pietrantonio D, Pace Palitti V, Cichelli A, Tacconelli S. Protective Effect of Caffeine and Chlorogenic Acids of Coffee in Liver Disease. Foods. 2024; 13(14):2280. https://doi.org/10.3390/foods13142280
Chicago/Turabian StyleDi Pietrantonio, Daniela, Valeria Pace Palitti, Angelo Cichelli, and Stefania Tacconelli. 2024. "Protective Effect of Caffeine and Chlorogenic Acids of Coffee in Liver Disease" Foods 13, no. 14: 2280. https://doi.org/10.3390/foods13142280
APA StyleDi Pietrantonio, D., Pace Palitti, V., Cichelli, A., & Tacconelli, S. (2024). Protective Effect of Caffeine and Chlorogenic Acids of Coffee in Liver Disease. Foods, 13(14), 2280. https://doi.org/10.3390/foods13142280







