Mangosteen Pericarp Processing Technology to Create Economic Value and Reduce Biowaste
Abstract
1. Introduction
2. Materials and Methods
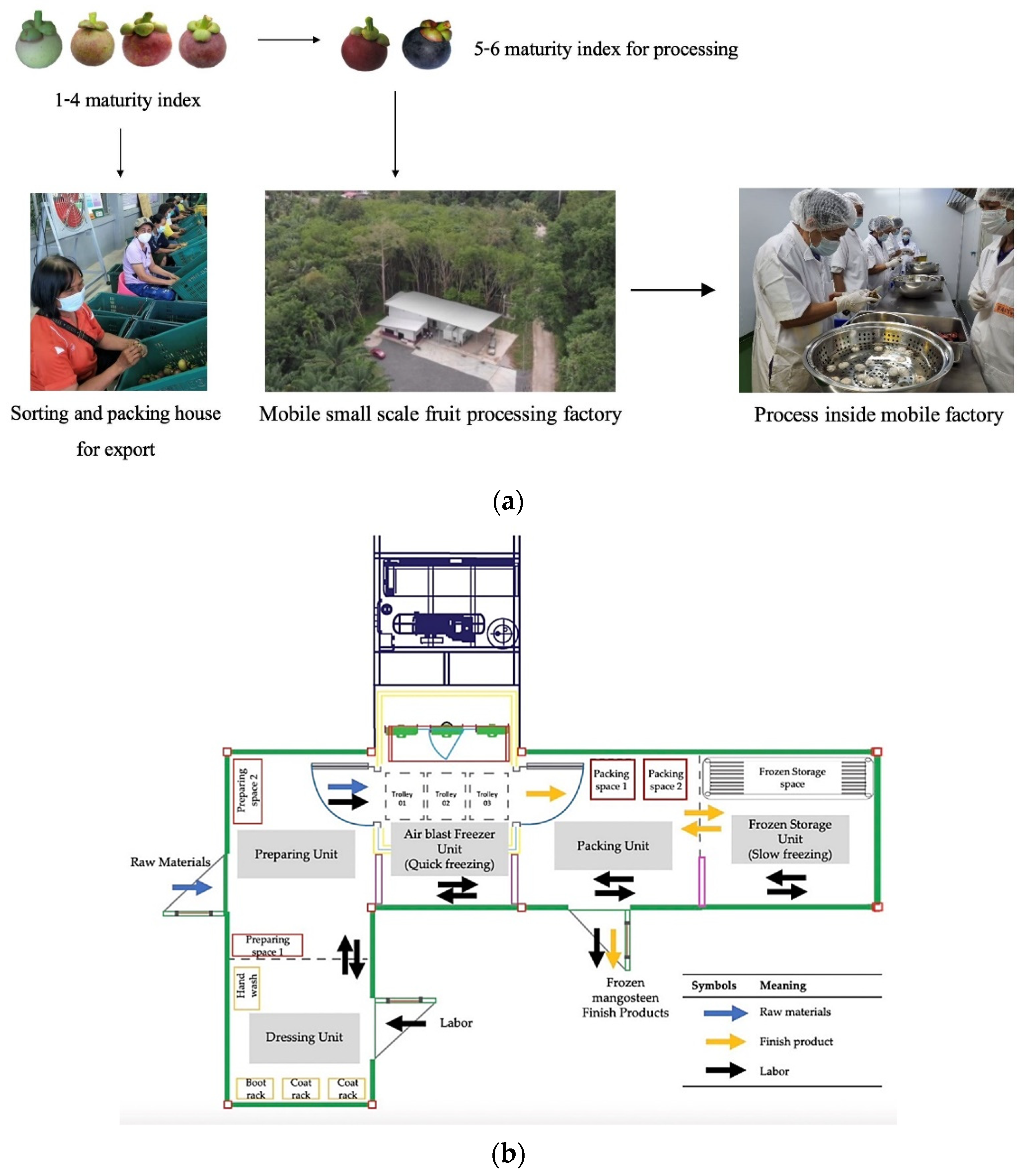
2.1. Raw Materials and Sample Preparation
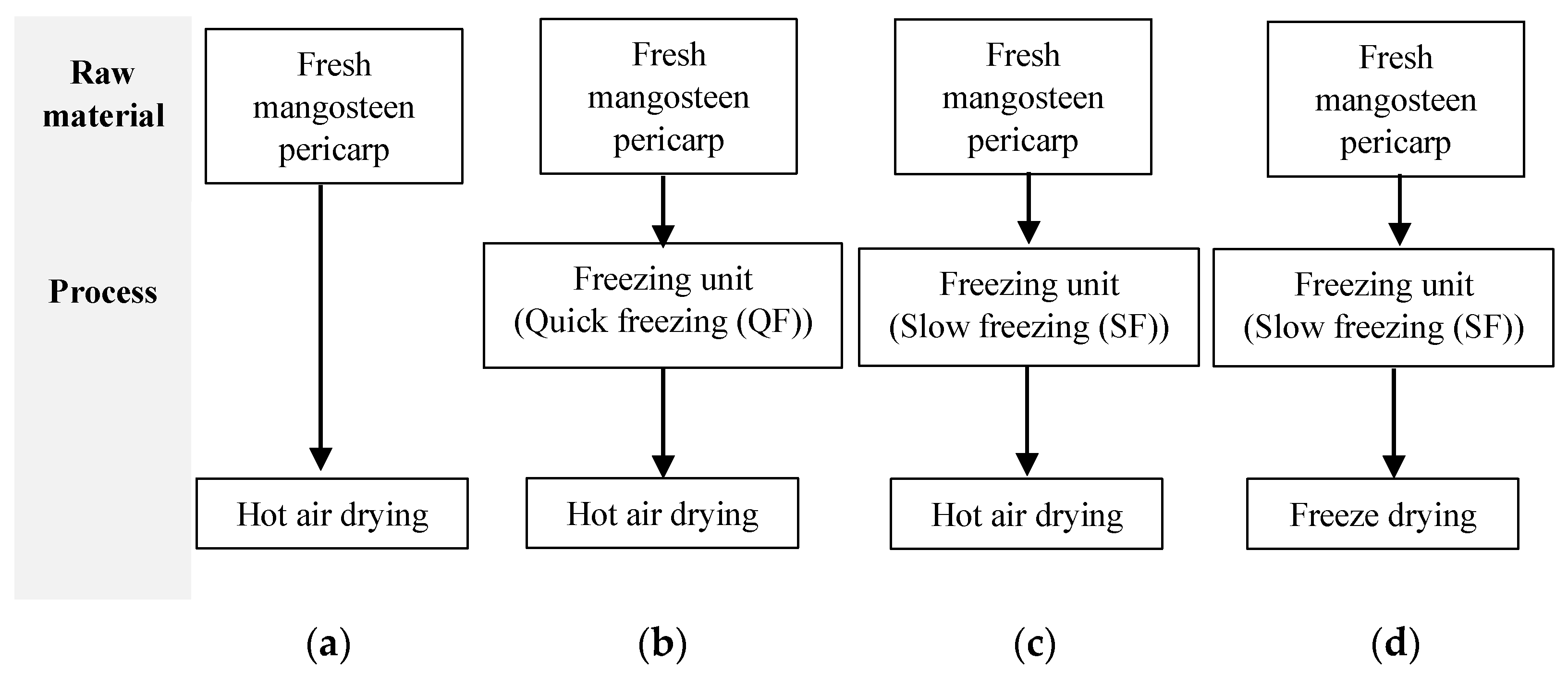
2.2. Freezing and Drying Process Description
2.3. Analytical Methods
2.3.1. Drying Rate
2.3.2. Moisture Content
2.3.3. Water Activity
2.3.4. Color
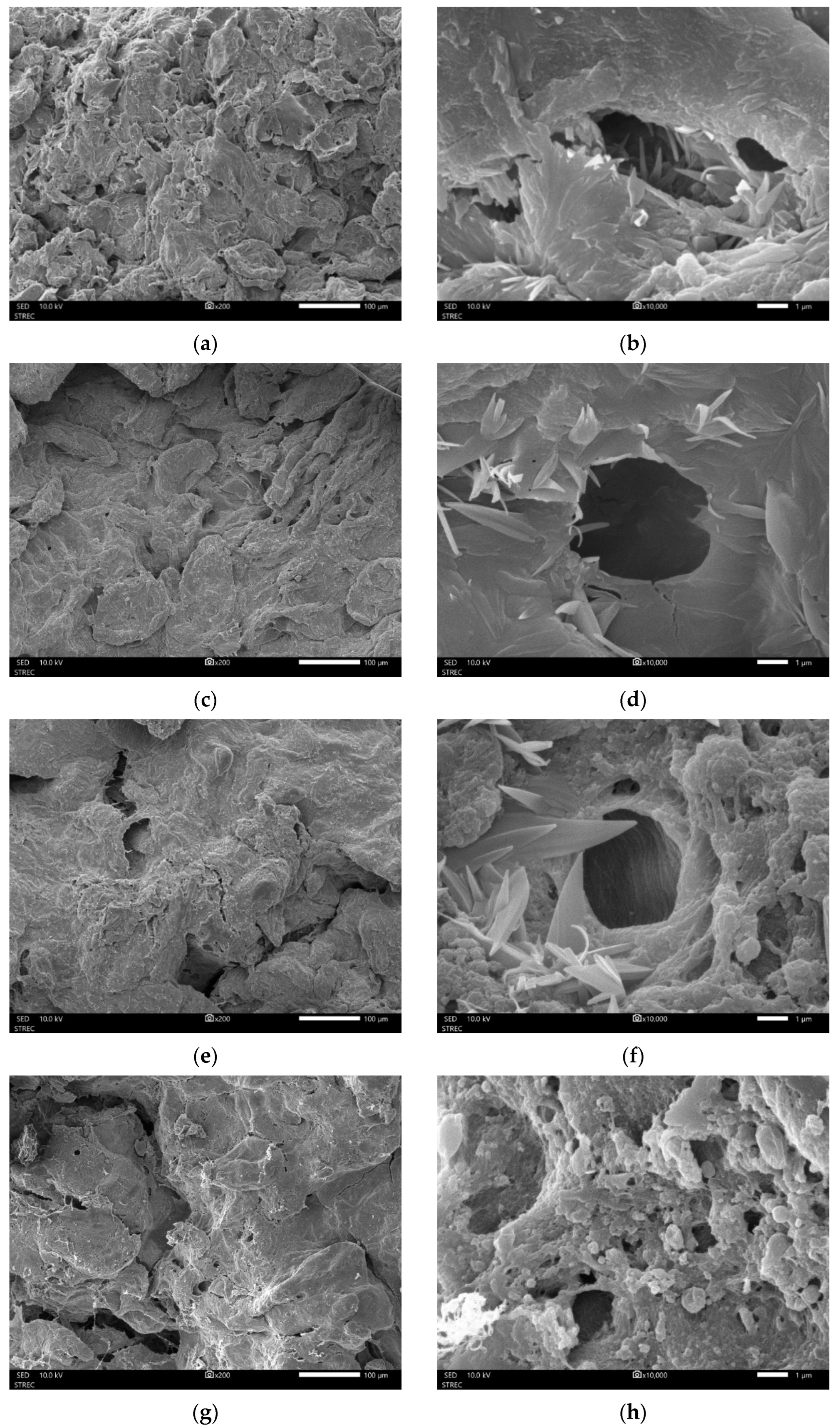
2.3.5. Microstructural Analyses
2.3.6. Pore Property Analyses
2.4. Quantification of Bioactive Compounds and Antioxidant Activities
2.4.1. Xanthone Extraction and α-Mangostin Assay
2.4.2. Determination of Total Phenolic Compounds (TPC)
2.4.3. DPPH Radical Scavenging Activity
2.4.4. ABTS Radical Scavenging Activity
2.4.5. Ferric Reducing Antioxidant Potential (FRAP) Assay
2.5. Statistical Analyses
3. Results and Discussion
3.1. Effects of Different Freezing and Hot Air Drying Processes on Qualities of Ground Mangosteen Pericarps
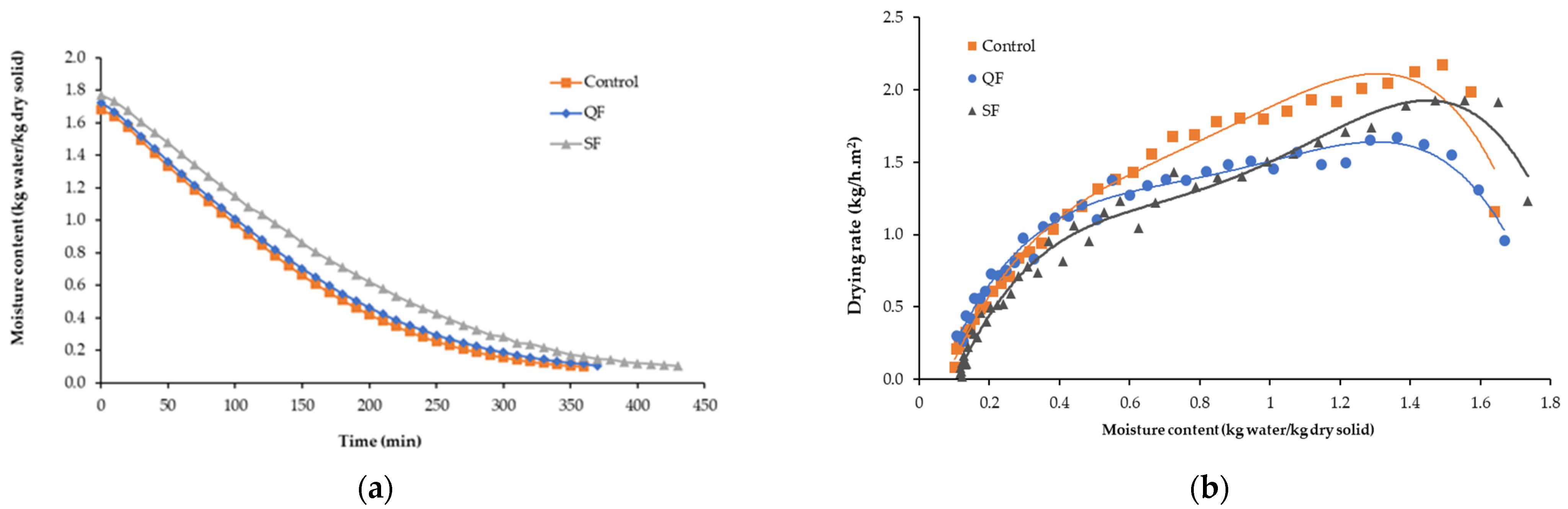
3.1.1. Drying Kinetics
3.1.2. Physicochemical Properties
3.1.3. Bioactive Compounds
3.2. Effects of Slow Freezing Unit and Different Drying Processes on Qualities of Ground Mangosteen Pericarps
3.2.1. Physicochemical Properties
3.2.2. Bioactive Compounds
3.3. Potential Economic Advantage of Recovered α-Mangostin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis (3-ethylbenzthiazoline-6- sulphonic acid)) radical cation decolorization assay |
| BET | Brunauer-Emmett-Teller surface area analysis |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity |
| FRAP | Ferric reducing antioxidant potential assay |
| GAE | Gallic acid |
| HAD | Hot air drying |
| FD | Freeze-drying |
| PPO | Polyphenol oxidase |
| POD | Peroxidase |
| QF | Quick freezing |
| QF + HAD | Quick freezing and hot air drying process |
| SF | Slow freezing |
| SF + HAD | Slow freezing and hot air drying process |
| SF + FD | Slow freezing and freeze drying process |
| SEM | Scanning electron microscope |
| TPC | Total phenolic content |
References
- Yao, S.L.; Solihin, I.M.; Chompoorat, P.; Ying, L.L.; Phing, L.P. Quality Assessment of Mangosteen in Different Maturity Stages by Handheld Near Infrared Spectroscopy. Malays. J. Anal. Sci. 2021, 25, 751–765. [Google Scholar]
- Cantos, E.; Espín, J.C.; Tomás-Barberán, F.A. Postharvest induction modeling method using UV irradiation pulses for obtaining resveratrol-enriched table grapes: A new functional fruit. J. Agric. Food Chem. 2001, 49, 5052–5058. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, M.; Elfving, S.W.; and Jackson, M. The factory-in-a-box concept and its maintenance application. In Proceedings of the 19th International Conference on Condition Monitoring and Diagnostic Engineering Management, Luleå, Sweden, 12–15 June 2006. [Google Scholar]
- Hedelind, M.; Jackson, M.; Funk, P.; Stahre, J.; Söderberg, R.; Carlson, J.; Björkman, M.; Winroth, M. Factory-in-a-Box–Solutions for availability and mobility of flexible production capacity. In Proceedings of the 1st Swedish Production Symposium, Göteborg, Sverige, 28–30 August 2007. [Google Scholar]
- Moorapun, C.; Bunyarittikit, S.; Pornchaloempong, P.; Rakmae, S. Mobile food processing plant design project for fruit process. Food Focus Thai. 2020, 25, 26–35. [Google Scholar]
- Zadernowski, R.; Czaplicki, S.; Naczk, M. Phenolic acid profiles of mangosteen fruits (Garcinia mangostana). Food Chem. 2009, 112, 685–689. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, B.; Huang, M.; Cai, B. On the preparation and characterization of activated carbon from mangosteen shell. J. Taiwan Inst. Chem. Eng. 2011, 42, 837–842. [Google Scholar] [CrossRef]
- Cheok, Y.C.; Adzahan, M.N.; Rahman, A.R.; Abedin, H.Z.N.; Hussain, N.; Sulaiman, R.; Chong, H.G. Current trends of tropical fruit waste utilization. Crit. Rev. Food Sci. Nutr. 2018, 58, 335–361. [Google Scholar] [CrossRef] [PubMed]
- Hemshekhar, M.; Sunitha, K.; Santhosh, M.S.; Devaraja, S.; Kemparaju, K.; Vishwanath, B.; Niranjana, S.; Girish, K. An overview on genus garcinia: Phytochemical and therapeutical aspects. Phytochem 2011, 10, 325–351. [Google Scholar] [CrossRef]
- Zarena, A.; Sankar, K.U. Phenolic acids, flavonoid profile and antioxidant activity in mangosteen (Garcinia mangostana L.) pericarp. J. Food Biochem. 2012, 36, 627–633. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, Z.E.H.; Baghdadi, A.; Tayebi-Meigooni, A. Alpha-Mangostin-Rich Extracts from Mangosteen Pericarp: Optimization of Green Extraction Protocol and Evaluation of Biological Activity. Molecules 2018, 23, 1852. [Google Scholar] [CrossRef]
- Suksamrarn, S.; Suwannapoch, N.; Phakhodee, W. Anti-mycobacterial activity of prenylated xanthones from the fruits of Garcinia mangostana. Chem. Pharm. Bull. 2003, 51, 857–859. [Google Scholar] [CrossRef]
- Pinto, M.; Sousa, M.; Nascimento, M.S. Xanthone derivatives: New insights in biological activities. Curr. Med. Chem. 2005, 12, 2517–2538. [Google Scholar] [CrossRef] [PubMed]
- Pedraza-Chaverrí, J.; Reyes-Fermín, L.M.; Nolasco-Amaya, E.G.; Orozco-Ibarra, M.; Medina-Campos, O.N.; González-Cuahutencos, O.; Rivero-Cruz, I.; Mata, R. ROS scavenging capacity and neuroprotective effect of α-mangostin against 3-nitropropionic acid in cerebellar granule neurons. Exp. Toxicol. Pathol. 2009, 61, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Geetha, R.; Roy, A.; Lakshmi, T. Evaluation of antibacterial activity of fruit rind extract of Garcinia mangostana linn on enteric pathogens—An in vitro study. Asian J. Pharm. Clin. Res. 2011, 4, 115–118. [Google Scholar]
- Gutierrez-Orozco, F.; Failla, M.L. Biological activities and bioavailability of mangosteen xanthones: A critical review of the current evidence. Nutrients 2013, 5, 3163–3183. [Google Scholar] [CrossRef]
- Liu, S.H.; Lee, L.T.; Hu, N.Y.; Huange, K.K.; Shih, Y.C.; Munekazu, I.; Li, J.M.; Chou, T.Y.; Wang, W.H.; Chen, T.S. Effects of α-mangostin on the expression of anti-inflammatory genes in U937 cells. Chin. Med. 2012, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.Y.; Hashim, N.M.; Mariod, A.A.; Mohan, S.; Abdulla, M.A.; Abdelwahab, S.I.; Arbab, I.A. α-Mangostin from Garcinia mangostana linn: An updated review of its pharmacological properties. Arab. J. Chem. 2016, 9, 317–329. [Google Scholar] [CrossRef]
- Marquez, C.A.; Mechalis, A.D. Comparison of drying kinetics of small fruits with or without particle shrinkage considerations. Food Bioprocess Technol. 2011, 4, 1212–1218. [Google Scholar] [CrossRef]
- Galoburda, R.; Kuka, M.; Cakste, I.; Klava, D. The effect of blanching temperature on the quality of microwave-vacuum dried mushroom Cantharellus cibarius. Agron. Res. 2015, 13, 929–938. [Google Scholar]
- Xiao, H.W.; Pan, Z.; Deng, L.Z.; El-Mashad, H.M.; Yang, X.H.; Mujumdar, A.S.; Gao, Z.J.; Zhang, Q. Recent developments and trends in thermal blanching. Inf. Process. Agric. 2017, 4, 101–127. [Google Scholar]
- Orikasa, T.; Ono, N.; Watanabe, T.; Ando, Y.; Shiina, T.; Koide, S. Impact of blanching pretreatment on the drying rate and energy consumption during far-infrared drying of Paprika (Capsicum annuum L.). Food Qual. Saf. 2018, 2, 97–103. [Google Scholar] [CrossRef]
- Banasik, A.; Kanellopoulos, A.; Claassen, G.D.H.; Bloemhof-Ruwaard, J.M.; van der Vorst, G.A.J. Assessing alternative production options for eco-efficient food supply chains using multi-objective optimization. Ann. Oper. Res. 2017, 250, 341–362. [Google Scholar] [CrossRef]
- van der Sman, R.G.M. Impact of Processing Factors on Quality of Frozen Vegetables and Fruits. Food Eng. Rev. 2020, 12, 399–420. [Google Scholar] [CrossRef]
- Raja, K.S.; Taip, F.S.; Azmi, M.M.Z.; Shishir, M.R.I. Effect of pre-treatment and different drying methods on the physicochemical properties of Carica papaya L. leaf powder. J. Saudi Soc. Agric. Sci. 2019, 18, 150–156. [Google Scholar] [CrossRef]
- Fellows, P. Food Processing Technology—Principles and Practice. In Food Processing Technology; Woodhead Publishing Limited: Washington, DC, USA, 2000. [Google Scholar]
- Nowak, D.; Syta, M. Identification of the impact of grinding degree, pretreatment and drying method on content of betalaine dyes in dried beet material. Inżynieria Rol 2009, 13, 131–137. [Google Scholar]
- De Ancos, B.; Sanchez-Moreno, C.; De Pascual-Teresa, S.; Cano, M. Handbook of Fruit and Fruits Processing, 2nd ed.; Wiley-Blackwell: Oxford, UK, 2012. [Google Scholar]
- Yoswathana, N. Accelerated extraction of Xanthone from Mangosteen pericarp using ultrasonic technique. Afr. J. Pharm. Pharmacol. 2012, 7, 302–309. [Google Scholar] [CrossRef]
- Satong-aun, W.; Assawarachan, R.; Noomhorm, A. The Influence of Drying Temperature and Extraction Methods on α-Mangostin in Mangosteen Pericarp. J. Food Sci. Eng. 2011, 1, 85–92. [Google Scholar]
- Sukatta, U.; Takenaka, M.; Ono, H.; Okadome, H.; Sotome, I.; Nanayama, K.; Thanapase, W.; Isobe, S. Distribution of Major Xanthones in the Pericarp, Aril, and Yellow Gum of Mangosteen (Garcinia Mangostana Linn.) Fruit and Their Contribution to Antioxidative Activity. Biosci. Biotechnol. Biochem. 2013, 77, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Pongsuttiyakorn, T.; Pitikhate Sooraksa, P.; Pornchalermpong, P. Simple Effective and Robust Weight Sensor for Measuring Moisture Content in Food Drying Process. Sens. Mater. 2019, 31, 2393–2404. [Google Scholar] [CrossRef]
- Saohin, W.; Boonchoong, P.; Iamlikitkuakoon, S.; Jamnoiprom, I.; Mungdee, W. Effects of drying temperature and residual moisture content of Fa-Tha-Li (Andrographis paniculata (Burm.f.) Nees) crude powder for capsule preparation. Thai J. Pharm. Sci. 2007, 31, 28–35. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Zhang, Y.; Jiao, S.; Lian, Z.; Deng, Y.; Zhao, Y. Effect of single- and two-cycle high hydrostatic pressure treatments on water properties, physicochemical and microbial qualities of minimally processed squids (Todarodes pacificus). J. Food Sci. 2015, 80, 1012–1020. [Google Scholar] [CrossRef]
- Xiao, W.H.; Gao, J.Z. The Application of Scanning Electron Microscope (SEM) to Study the Microstructure Changes in the Field of Agricultural Products Drying. Scanning Electron Microscopy; Kazmiruk, V., Ed.; InTech: London, UK, 2012; pp. 213–226. [Google Scholar]
- Condon, J.B. Surface Area and Porosity Determinations by Phyisorption: Measurement and Theory; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Singleton, V.L.; Rossi, J. Colorimetry of Total Phenolic with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as measurement of antioxidant power the FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Watanabe, T.; Ando, Y.; Orikasa, T.; Shiina, T.; Kohyama, K. Effect of short time heating on the mechanical fracture and electrical impedance properties of spinach (Spinacia oleracea L.). J. Food Eng. 2017, 194, 9–14. [Google Scholar] [CrossRef]
- Watanabe, T.; Orikasa, T.; Sasaki, K.; Koide, S.; Shiina, T.; Tagawa, A. Influence of blanching on water transpiration rate and quality changes during far-infrared drying of cut cabbage. JSAM J. 2014, 76, 387–394. [Google Scholar]
- Pathare, P.B.; Sharma, G.P. Effective moisture diffusivity of onion slices undergoing infrared convective drying. Biosyst. Eng. 2006, 93, 285–291. [Google Scholar] [CrossRef]
- Mphahlele, R.R.; Pankaj, B.; Pathare, B.P.; Opar, L.U. Drying kinetics of pomegranate fruit peel (cv. Wonderful). Sci. Afr. 2019, 5, e00145. [Google Scholar] [CrossRef]
- TACFS 9007-2005; National Bureau of Agricultural Commodity and Food Standards; Part 4: Fruit and Seeds. Thai Agricultural Standard: Bangkok, Thailand, 2020.
- Mermelstein, H.N. Measuring Moisture Content & Water Activity. Food Technol. Mag. 2009, 63. [Google Scholar]
- Ahmed, J. Drying of vegetables: Principles and dryerdesign. In Handbook of Vegetables and Vegetable Processing; Sinha, N.K., Hui, Y.H., Ozgul Evranuz, E., Siddiq, M., Ahmed, J., Eds.; Wiley-Blackwell Publishing: Hoboken, NJ, USA, 2011; pp. 279–298. [Google Scholar]
- Neelapong, W.; Phonyotin, B.; Sittikijyothin, W. Extraction of active compounds from Thai herbs: Powder and extract. KMUTNB 2019, 29, 157–166. [Google Scholar]
- Rhim, J.W.; Koh, S.; Kim, J.M. Effect of freezing temperature on rehydration and water vapor adsorption characteristics of freeze-dried rice porridge. J. Food Eng. 2011, 104, 484–491. [Google Scholar] [CrossRef]
- Van Buggenhout, S.; Sila, D.N.; Duvetter, T.; van Loey, A.; Hendrickx, M. Pectins in processed fruits and vegetables: Part III—Texture engineering. Compr. Rev. Food Sci. Food Saf. 2009, 8, 105–117. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.K.; Pourgouri, S.; Taoukis, P.S. Kinetic study of the effect of the osmotic dehydration pretreatment to the shelf life of frozen cucumber. Innov. Food Sci. Emerg. Technol. 2008, 9, 542–549. [Google Scholar] [CrossRef]
- Holzwarth, M.; Korhummel, S.; Carle, R.; Kammerer, D.R. Evaluation of the effects of different freezing and thawing methods on color, polyphenol and ascorbic acid retention in strawberries (Fragaria xananassa Duch.). Int. Food Res. J. 2012, 48, 241–248. [Google Scholar] [CrossRef]
- Rahman, S.M. Mass-Volume-Area–Related Properties of Foods. In Engineering Properties of Foods, 3rd ed.; Rao, M.A., Rizvi, S.H.S., Datta, K.A., Eds.; Taylor & Francis Group: New York, NY, USA, 2005; pp. 1–35. [Google Scholar]
- Gabrijela Horvat, G.; Pantic, M.; Knez, Z.; Novak, Z. A Brief Evaluation of Pore Structure Determination for Bio aerogels. Gels 2022, 8, 438. [Google Scholar] [CrossRef] [PubMed]
- Levin, P.; Meunier, V.; Kessler, U.; Heinrich, S. Influence of Freezing Parameters on the Formation of Internal Porous Structure and Its Impact on Freeze-Drying Kinetics. Processes 2021, 9, 1273. [Google Scholar] [CrossRef]
- Tamer, C.; Isci, A.; Kutlu, N.; Sakiyan, O.; Sahin, S.; Sumnu, G. Effect of Drying on Porous Characteristics of Orange Peel. Int. J. Food Eng. 2016, 12, 921–928. [Google Scholar] [CrossRef]
- Nasrulla, A.; Saad, B.; Bhat, H.A.; Khan, S.A.; Danish, M.; Mohamed Hasnain Isa, H.M.; Naeem, A. Mangosteen peel waste as a sustainable precursor for high surface area mesoporous activated carbon: Characterization and application for methylene blue removal. J. Clean. Prod. 2019, 211, 1190–1200. [Google Scholar] [CrossRef]
- Hüseyinbaş, M.; Korkmaz, B.; Yücelen, S.; Güvenç, A. Recycling of pomegranate peel and mandarin peel with ultrasound assisted solvent extraction. Acad. Perspect. Procedia 2020, 3, 161–169. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, D.; Chang, Y.; Xiao, Y. Effect of Freeze-Thaw Pretreatment on Extraction Yield and Antioxidant Bioactivity of Corn Carotenoids (Lutein and Zeaxanthin). J. Food Qual. 2018, 2018, 9843503. [Google Scholar] [CrossRef]
- Vallespir, F.; Rodríguez, Ó.; Eim, S.V.; Rosselló, C.; Simal, S. Effects of freezing treatments before convective drying on quality parameters: Vegetables with different microstructures. J. Food Eng. 2019, 249, 15–24. [Google Scholar] [CrossRef]
- Figiel, A. Drying kinetics and quality of beetroots dehydrated by combination of convective and vacuum-microwave methods. J. Food Eng. 2010, 98, 461–470. [Google Scholar] [CrossRef]
- de Ancos, B.; González, E.M.; Cano, M.P. Ellagic Acid, Vitamin C, and Total Phenolic Contents and Radical Scavenging Capacity Affected by Freezing and Frozen Storage in Raspberry Fruit. J. Agric. Food Chem. 2000, 48, 4565–4570. [Google Scholar] [CrossRef] [PubMed]
- Ketsa, S.; Paull, R.E. Mangosteen (Garcinia mangostana L.). In Postharvest Biology and Technology of Tropical and Subtropical Fruit; Elsevier Ltd.: London, UK, 2011; Volume 4, pp. 1–30. [Google Scholar]
- Versari, A.; Parpinello, G.P.; Tornielli, G.B.; Ferrarini, R.; Giulivo, C. Stilbene compounds and stilbene synthase expression during ripening, wilting, and UV treatment in grape cv. Corvina. J. Agric. Food Chem. 2001, 49, 5531–5536. [Google Scholar] [CrossRef] [PubMed]
- Çoklar, H.; Akbulut, M. Effect of Sun Oven and Freeze-Drying on Anthocyanins, Phenolic Compounds and Antioxidant Activity of Black Grape (Ekşikara) (Vitis vinifera L.). S. Afr. J. Enol. Vitic. 2017, 38, 264–267. [Google Scholar] [CrossRef]
- Haminiuk, C.W.; Maciel, G.M.; Plata-Oviedo, M.S.; Peralta, R.M. Phenolic compounds in fruits—An overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Coklar, H.; Akbulut, M. Effect of different solvents on extraction of phenolic compounds and antioxidant activity of hawthorn (Crataegus orientalis) fruits. Derim 2016, 33, 237–248. [Google Scholar]
- Yuvanatemiya, V.; Srean, P.; Klangbud, W.K.; Venkatachalam, K.; Wongsa, J.; Parametthanuwat, T.; Charoenphun, N. A Review of the Influence of Various Extraction Techniques and the Biological Effects of the Xanthones from Mangosteen (Garcinia mangostana L.) Pericarps. Molecules 2022, 27, 8775. [Google Scholar] [CrossRef]
- Santos, P.H.S.; Silva, M.A. Retention of vitamin C in drying processes of fruits and vegetables—A review. Dry. Technol. 2008, 26, 1421–1437. [Google Scholar] [CrossRef]
- Orrego-Alzate, C.E.; Pamplona-López, F.D.; Perez, V.H. Low pressure water diffusivity measurements of freeze-dried tomato tree (Cyphomandra betacea (Cav) Send) Juice. Int. Rev. Chem. Eng. 2009, 1, 453–459. [Google Scholar]
- Peres, V.; Nagem, T.J.; Oliveira, F.F. Tetra oxygenated naturally occurring xanthones. Phytochemistry 2000, 55, 683–710. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Su, B.N.; Keller, W.J.; Mehta, R.G.; Kinghorn, A.D. Antioxidant xanthones from the pericarp of Garcinia mangostana (mangosteen). J. Agric. Food Chem. 2006, 54, 2077–2082. [Google Scholar] [CrossRef] [PubMed]
- Okonogi, S.; Duangrat, C.; Anuchpreeda, S.; Tachakittirungrod, S.; Chowwanapoonpohn, S. Comparison of antioxidant capacities and cytotoxicities of certain fruit peels. Food Chem. 2007, 103, 839–846. [Google Scholar] [CrossRef]
- Tachakittirungrod, S.; Okonogi, S.; Chowwanapoonpohn, S. Study on antioxidant activity of certain plants in Thailand: Mechanism of antioxidant action of guava leaf extract. Food Chem. 2007, 103, 381–388. [Google Scholar] [CrossRef]
- Suvarnakuta, P.; Chaweerungrat, C.; Devahastin, S. Effects of drying methods on assay and antioxidant activity of xanthones in mangosteen rind. Food Chem. 2011, 25, 240–247. [Google Scholar] [CrossRef]
| Methods | Model | ||
|---|---|---|---|
| Control | 0.8971 | 0.9837 | |
| QF + HAD | 0.8890 | 0.9454 | |
| SF + HAD | 0.8371 | 0.9145 |
| Process | ||||
|---|---|---|---|---|
| Control | QF + HAD | SF + HAD | SF + Freeze-Drying | |
| Physical properties | ||||
| Moisture content (%) | 9.89 ± 0.21 d | 6.16 ± 0.18 c | 4.60 ± 0.11 b | 2.42 ± 0.16 a |
| Water activity (aw) | 0.51 ± 0.01 d | 0.43 ± 0.00 c | 0.34 ± 0.00 b | 0.13 ± 0.00 a |
| Color | ||||
| L* | 43.22 ± 0.06 b | 40.44 ± 0.12 a | 43.29 ± 0.13 b | 44.72 ± 0.12 c |
| a* | 19.55 ± 0.02 d | 17.73 ± 0.08 b | 17.35 ± 0.14 a | 18.99 ± 0.06 c |
| b* | 26.23 ± 0.19 b | 24.77 ± 0.12 a | 25.80 ± 0.22 b | 18.20 ± 0.19 a |
| Bioactive compounds | ||||
| α-mangostin (mg/g DW of mangosteen pericarp) | 66.94 ± 0.80 a | 84.16 ± 0.46 b | 78.88 ± 0.74 b | 82.30 ± 0.27 b |
| Total phenolic compounds (mg GAE/g DW of mangosteen pericarp) | 792.34 ± 33.23 a | 750.45 ± 40.06 a | 783.24 ± 49.58 a | 1065.57 ± 30.17 a |
| Antioxidant activity | ||||
| DPPH | 32.90 ± 1.03 ab | 32.05 ± 3.21 a | 28.20 ± 0.54 a | 40.68 ± 1.41 b |
| ABTS | 25.47 ± 1.13 a | 27.18 ± 0.52 a | 26.86 ± 0.22 a | 41.20 ± 1.17 b |
| FRAP | 0.32 ± 0.07 a | 0.27 ± 0.05 a | 0.28 ± 0.01 a | 0.38 ± 0.07 a |
| Pore Properties | Control (HAD) | QF + HAD | SF + HAD | SF + Freeze-Drying |
|---|---|---|---|---|
| Specific surface area (m2/g) Single point surface area a | 40.16 ± 0.26 d | 80.75 ± 0.15 c | 29.61 ± 0.09 b | 20.93 ± 0.04 a |
| BET surface area b | 58.28 ± 0.18 c | 131.50 ± 0.20 d | 45.28 ± 0.28 b | 36.26 ± 0.11 a |
| Total pore volume (cm3/g) c | 0.070 ± 0.005 b | 0.137 ± 0.003 c | 0.054 ± 0.002 a | 0.066 ± 0.003 b |
| Pore size (average pore diameter, nm) d | 4.772 ± 0.04 b | 4.174 ± 0.07 a | 4.850 ± 0.02 c | 7.274 ± 0.01 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soontornwat, A.; Pongsuttiyakorn, T.; Rakmae, S.; Sritham, E.; Sirisomboon, P.; Pun, U.K.; Krusong, W.; Pornchaloempong, P. Mangosteen Pericarp Processing Technology to Create Economic Value and Reduce Biowaste. Foods 2024, 13, 2286. https://doi.org/10.3390/foods13142286
Soontornwat A, Pongsuttiyakorn T, Rakmae S, Sritham E, Sirisomboon P, Pun UK, Krusong W, Pornchaloempong P. Mangosteen Pericarp Processing Technology to Create Economic Value and Reduce Biowaste. Foods. 2024; 13(14):2286. https://doi.org/10.3390/foods13142286
Chicago/Turabian StyleSoontornwat, Alisa, Thadchapong Pongsuttiyakorn, Samak Rakmae, Eakasit Sritham, Panmanas Sirisomboon, Umed Kumar Pun, Warawut Krusong, and Pimpen Pornchaloempong. 2024. "Mangosteen Pericarp Processing Technology to Create Economic Value and Reduce Biowaste" Foods 13, no. 14: 2286. https://doi.org/10.3390/foods13142286
APA StyleSoontornwat, A., Pongsuttiyakorn, T., Rakmae, S., Sritham, E., Sirisomboon, P., Pun, U. K., Krusong, W., & Pornchaloempong, P. (2024). Mangosteen Pericarp Processing Technology to Create Economic Value and Reduce Biowaste. Foods, 13(14), 2286. https://doi.org/10.3390/foods13142286






