Mechanisms of Litchi Response to Postharvest Energy Deficiency via Energy and Sugar Metabolisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Sample Handling
2.2. Determination of Fruit Browning, Respiratory Rate and Membrane Integrity
2.3. Determination of Energy State in Pericarp
2.4. Quantitative Analysis of Energy Metabolism-Associated Gene Expression
2.5. Quantification of Sugar Content via Gas Chromatography-Mass Spectrometry
2.6. Statistical Analysis
3. Results
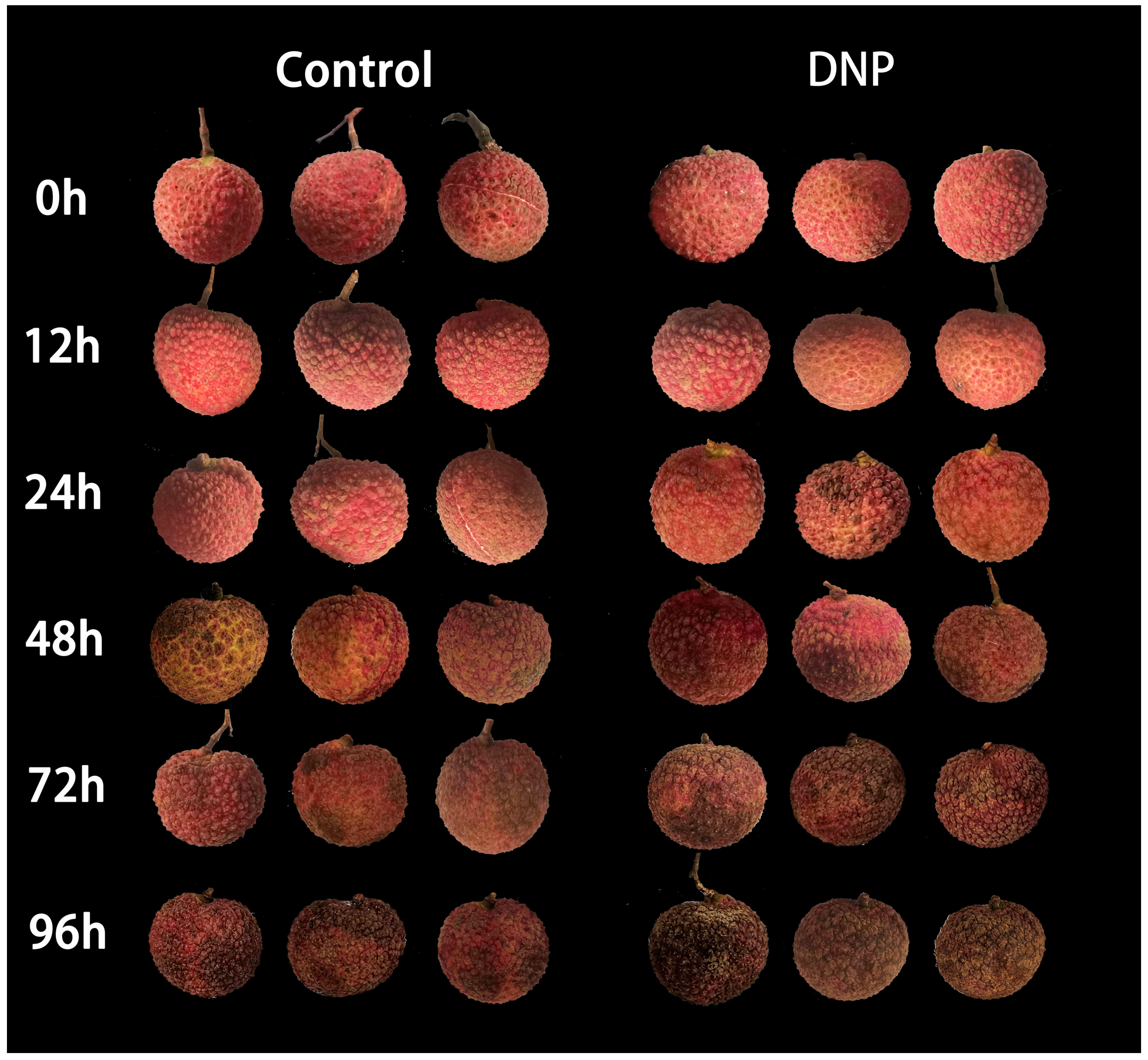
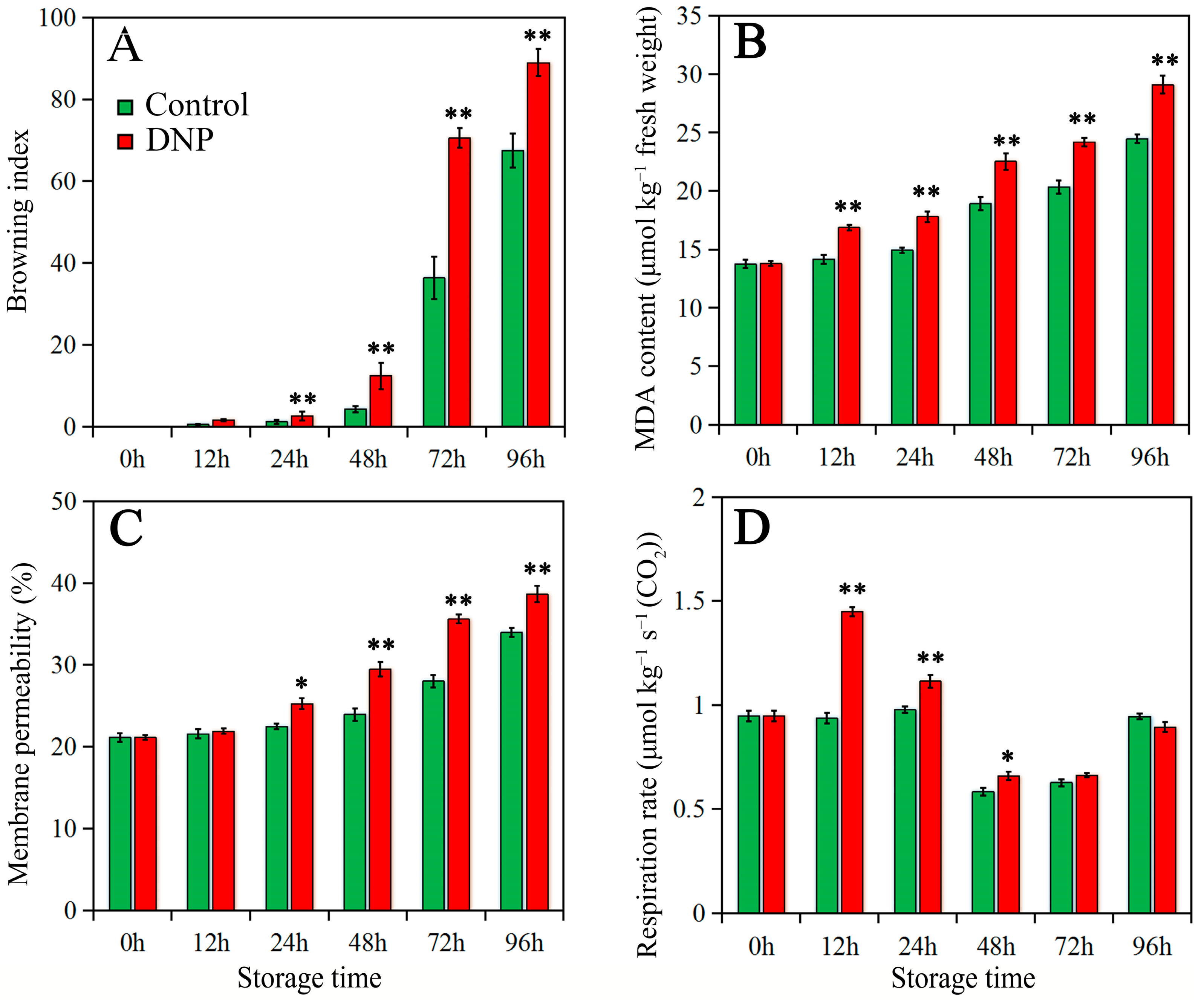
3.1. Effects of DNP Treatment on BI, MDA Content, Membrane Permeability, and Respiratory Rate in Litchi Fruit
3.2. Effects of DNP Treatment on Energy State in Litchi Fruit
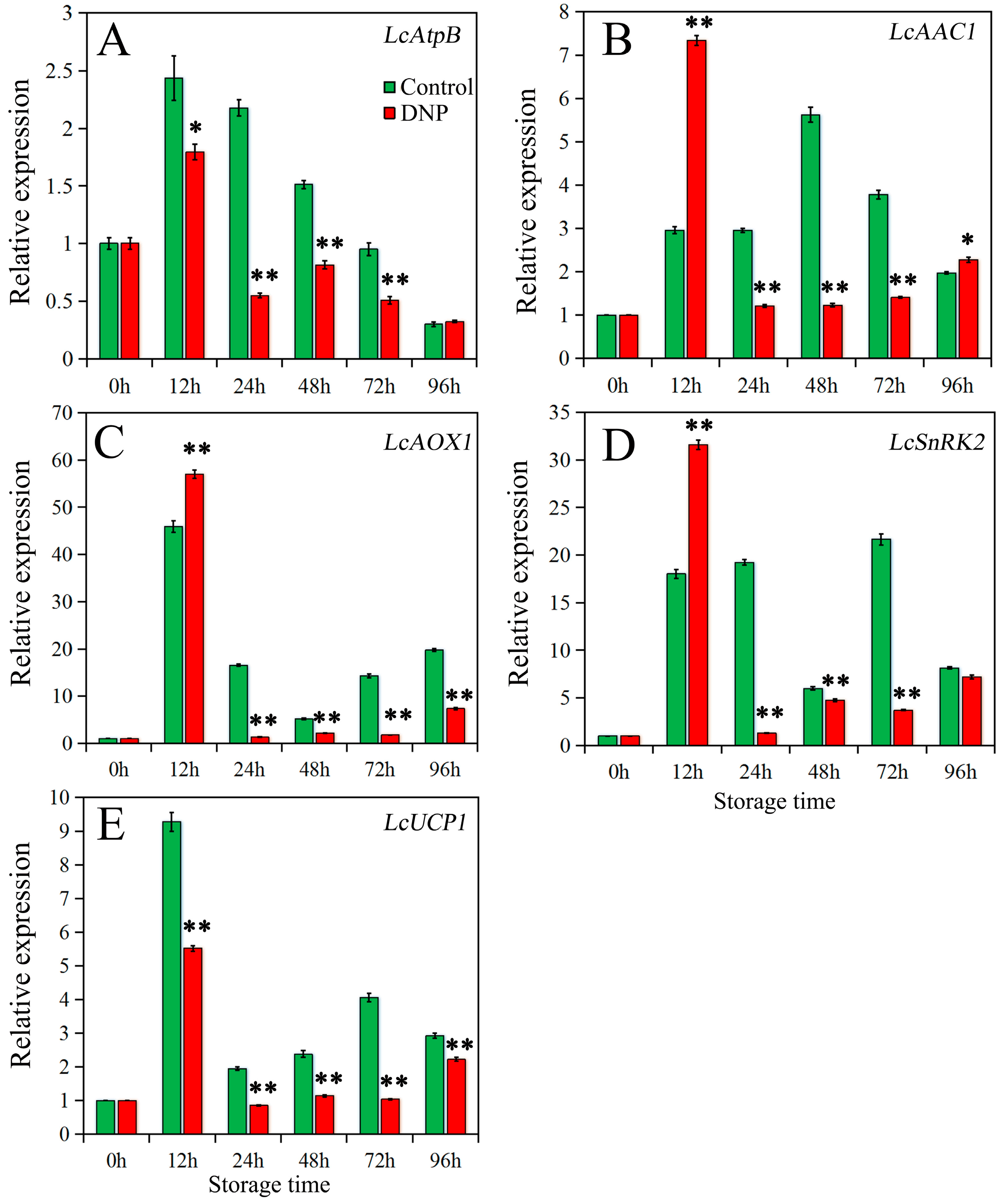
3.3. Effects of DNP Treatment on Expression of Energy Metabolism-Related Genes during Litchi Storage
3.4. Effects of DNP Treatment on Sugar Content Variation in Litchi Fruit
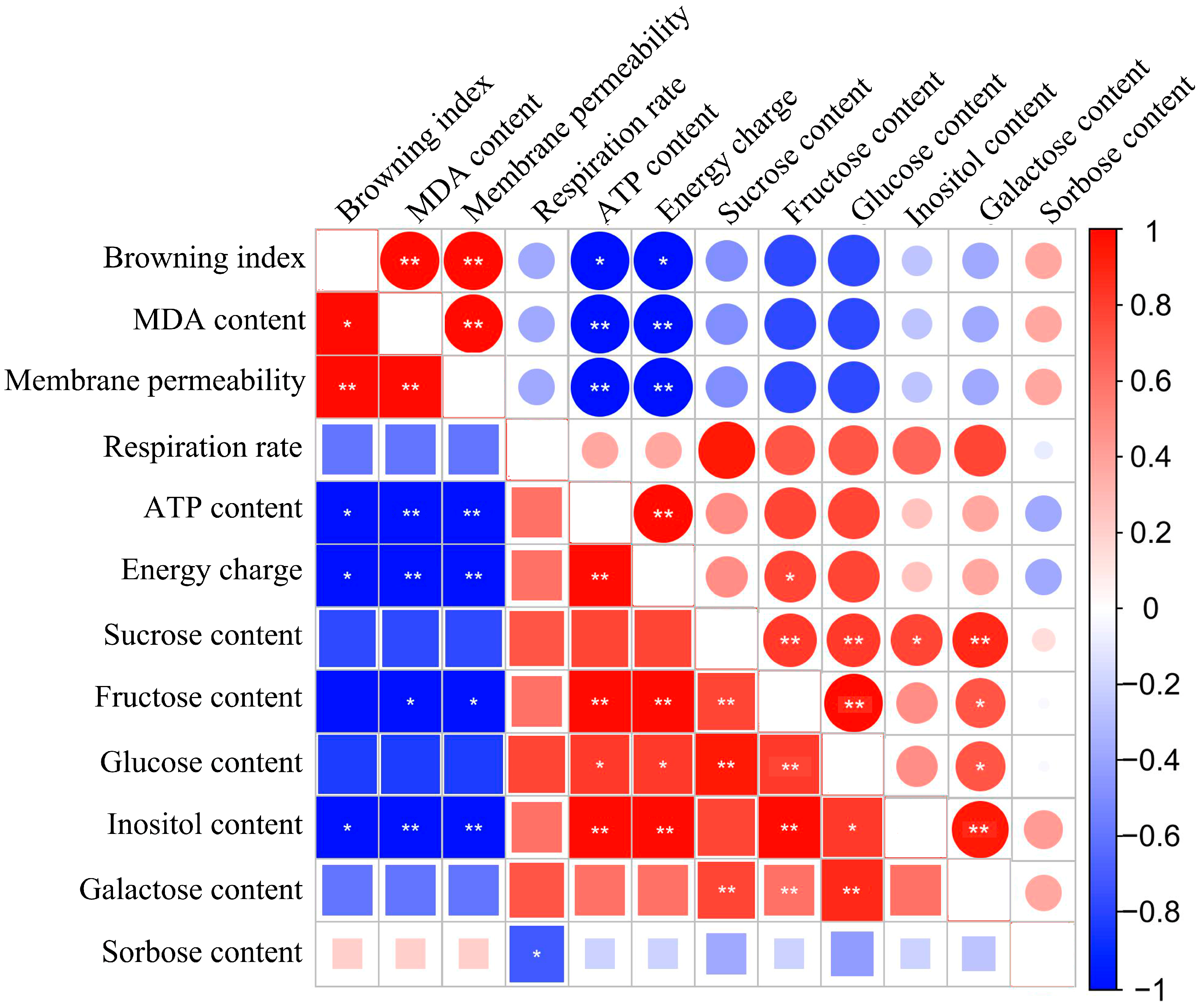
3.5. Correlation Analysis
4. Discussion
4.1. Effect of DNP Treatment for Energy State Is Related to Postharvest Litchi Quality
4.2. Expression of Energy Metabolism-Related Genes under Conditions of Energy Deficiency
4.3. Changes in Sugar Content under Conditions of Energy Deficiency
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, L.; Wang, K.; Wang, K.; Zhu, J.; Hu, Z. Nutrient components, health benefits, and safety of litchi (Litchi chinensis Sonn.): A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2139–2163. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Liu, B.; Fu, L.; Tang, H.; Li, Y.; Pang, X.; Zhang, Z. Water Supply via Pedicel Reduces Postharvest Pericarp Browning of Litchi (Litchi chinensis) Fruit. Foods 2024, 13, 814. [Google Scholar] [CrossRef]
- Huang, C.C.; Paull, R.E.; Wang, T.T. Litchi postharvest physiology and handling. Crop Sci. 2024, 1–50. [Google Scholar] [CrossRef]
- He, M.; Wu, Y.; Hong, M.; Yun, Z.; Li, T.; Jiang, Y. α-Lipoic acid treatment alleviates postharvest pericarp browning of litchi fruit by regulating antioxidant ability and energy metabolism. Postharvest Biol. Technol. 2021, 180, 111629. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, M.; Yun, Z.; Wang, J.; Feng, G.; Gao, Z.; Shi, X.; Jiang, Y. Effect of tea seed oil treatment on browning of litchi fruit in relation to energy status and metabolism. Postharvest Biol. Technol. 2017, 132, 97–104. [Google Scholar] [CrossRef]
- Tiwari, B.S.; Belenghi, B.; Levine, A. Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiol. 2002, 128, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Cao, J.; Jiang, W. Postharvest vibration-induced apple quality deterioration is associated with the energy dissipation system. Food Chem. 2022, 386, 132767. [Google Scholar] [CrossRef] [PubMed]
- Block, M.D.; Verduyn, C.; Brouwer, D.D.; Cornelissen, M. Poly (ADP-ribose) polymerase in plants affects energy homeostasis, cell death and stress tolerance. Plant J. 2005, 41, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, Z.; Khan, Z.U.; Mao, L.; Ying, T. Effect of nitric oxide on energy metabolism in postharvest banana fruit in response to chilling stress. Postharvest Biol. Technol. 2015, 108, 21–27. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, H.; Zhang, S.; Sun, J.; Lin, Y.; Wang, H.; Lin, M.; Shi, J. Phomopsis longanae Chi-induced disease development and pericarp browning of harvested longan fruit in association with energy metabolism. Front. Microbiol. 2018, 9, 1454. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.-W.; Li, G.-D.; Zhou, X.; Fu, W.-W.; Jiang, Y.-G.; Liu, T.; Ji, S.-J. Exogenous ATP alleviated aroma fading by regulating LOX pathway and fatty acids synthesis in ‘Nanguo’ pears after refrigeration. Sci. Hortic. 2018, 240, 522–529. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, X.; Su, M.; Du, J.; Li, X.; Zhang, M.; Hu, Y.; Huan, C.; Ye, Z. Controlled atmosphere storage alleviates internal browning in flat peach fruit by regulating energy and sugar metabolisms. Plant Physiol. Biochem. 2022, 186, 107–120. [Google Scholar] [CrossRef]
- Li, T.; He, M.; Zeng, J.; Chen, Z.; Hongxia, Q.; Duan, X.; Jiang, Y. Choline chloride alleviates the pericarp browning of harvested litchi fruit by inhibiting energy deficiency mediated programmed cell death. Postharvest Biol. Technol. 2020, 167, 111224. [Google Scholar] [CrossRef]
- Liu, J.; Bao, Y.; Liu, S.; Zhu, L.; Xu, X.; Jiang, G.; Zhang, Z. Physiological and transcriptomic analyses reveal mechanisms of exogenous strigolactones to regulate cold tolerance in litchi fruit. Postharvest Biol. Technol. 2024, 210, 112764. [Google Scholar] [CrossRef]
- Ge, Y.; Wei, M.; Li, C.; Chen, Y.; Duan, B.; Li, X.; Tang, Q.; Li, X. Changes in the sucrose metabolism in apple fruit following postharvest acibenzolar-S-methyl treatment. J. Sci. Food Agric. 2019, 99, 1519–1524. [Google Scholar] [CrossRef]
- Liu, J.; Yue, R.; Si, M.; Wu, M.; Cong, L.; Zhai, R.; Yang, C.; Wang, Z.; Ma, F.; Xu, L. Effects of exogenous application of melatonin on quality and sugar metabolism in ‘Zaosu’ pear fruit. J. Plant Growth Regul. 2019, 38, 1161–1169. [Google Scholar] [CrossRef]
- Yao, S.X.; Cao, Q.; Xie, J.; Deng, L.L.; Zeng, K.F. Alteration of sugar and organic acid metabolism in postharvest granulation of Ponkan fruit revealed by transcriptome profling. Postharvest Biol. Technol. 2018, 139, 2–11. [Google Scholar] [CrossRef]
- Durán-Soria, S.; Pott, D.M.; Osorio, S.; Vallarino, J.G. Sugar signaling during fruit ripening. Front. Plant Sci 2020, 11, 564917. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Wang, J.; Wang, L.; Han, C.; Jin, P.; Zheng, Y. Methyl jasmonate enhances wound-induced phenolic accumulation in pitaya fruit by regulating sugar content and energy status. Postharvest Biol. Technol. 2018, 137, 106–112. [Google Scholar] [CrossRef]
- Wang, J.; Lv, M.; He, H.; Jiang, Y.; Yang, J.; Ji, S. Glycine betaine alleviated peel browning in cold-stored ‘Nanguo’ pears during shelf life by regulating phenylpropanoid and soluble sugar metabolisms. Sci. Hortic. 2020, 262, 109100. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Cao, J.; Jiang, W. Advances in biochemical mechanisms and control technologies to treat chilling injury in postharvest fruits and vegetables. Trends Food Sci. Technol. 2021, 113, 355–365. [Google Scholar] [CrossRef]
- Jiang, X.; Lin, H.; Shi, J.; Neethirajan, S.; Lin, Y.; Chen, Y.; Wang, H.; Lin, Y. Effects of a novel chitosan formulation treatment on quality attributes and storage behavior of harvested litchi fruit. Food Chem. 2018, 252, 134–141. [Google Scholar] [CrossRef]
- Cao, X.; Islam, M.N.; Zhong, S.; Pan, X.; Song, M.; Shang, F.; Nie, H.; Xu, W.; Duan, Z. Drying kinetics, antioxidants, and physicochemical properties of litchi fruits by ultrasound-assisted hot air-drying. J. Food Biochem. 2020, 44, e13073. [Google Scholar] [CrossRef]
- Su, X.; Lin, H.; Fu, B.; Mei, S.; Lin, M.; Chen, H.; Zheng, Z.; Bo, H.; Yang, D.P.; Lin, Y. Egg-yolk-derived carbon dots@ albumin bio-nanocomposite as multifunctional coating and its application in quality maintenance of fresh litchi fruit during storage. Food Chem. 2023, 405, 134813. [Google Scholar] [CrossRef]
- Geisler, J.G. 2,4 Dinitrophenol as medicine. Cells 2019, 8, 280. [Google Scholar] [CrossRef]
- Kılıç, Z.; Dumlupınar, R. The changes of 2,4 dinitrophenol substance applied to corn seeds in AOX and ATP synthase gene expression against chilling stress. Turk. J. Food Agric. Sci. 2020, 2, 23–29. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, M.; Lin, H.; Hung, Y.C.; Lin, Y.; Chen, Y.; Wang, H.; Shi, J. DNP and ATP induced alteration in disease development of Phomopsis longanae Chi-inoculated longan fruit by acting on energy status and reactive oxygen species production-scavenging system. Food Chem 2017, 228, 497–505. [Google Scholar] [CrossRef]
- Zhang, D.; Quantick, P.C. Effects of chitosan coating on enzymatic browning and decay during postharvest storage of litchi (Litchi chinensis Sonn.) fruit. Postharvest Biol. Technol. 1997, 12, 195–202. [Google Scholar] [CrossRef]
- Bai, X.Y.; Yang, Z.M.; Shen, W.J.; Shao, Y.Z.; Zeng, J.K.; Li, W. Polyphenol treatment delays the browning of litchi pericarps and promotes the total antioxidant capacity of litchi fruit. Sci. Hortic. 2022, 291, 110563. [Google Scholar] [CrossRef]
- Liu, H.; Song, L.; You, Y.; Li, Y.; Duan, X.; Jiang, Y.; Joyce, D.C.; Ashraf, M.; Lu, W. Cold storage duration affects litchi fruit quality, membrane permeability, enzyme activities and energy charge during shelf time at ambient temperature. Postharvest Biol. Technol. 2011, 60, 24–30. [Google Scholar] [CrossRef]
- Li, T.; Shi, D.; Wu, Q.; Zhang, Z.; Qu, H.; Jiang, Y. Sodium para-aminosalicylate delays pericarp browning of litchi fruit by inhibiting ROS-mediated senescence during postharvest storage. Food Chem. 2019, 278, 552–559. [Google Scholar] [CrossRef]
- Tang, R.; Zhou, Y.; Chen, Z.; Wang, L.; Lai, Y.; Chang, S.K.; Wang, Y.; Qu, H.; Jiang, Y.; Huang, H. Regulation of browning and senescence of litchi fruit mediated by phenolics and energy status: A postharvest comparison on three different cultivars. Postharvest Biol. Technol. 2020, 168, 111280. [Google Scholar] [CrossRef]
- Wang, H.; Qian, Z.; Ma, S.; Zhou, Y.; Patrick, J.W.; Duan, X.; Jiang, Y.; Qu, H. Energy status of ripening and postharvest senescent fruit of Litchi (Litchi chinensis Sonn.). BMC Plant Biol. 2013, 13, 55. [Google Scholar] [CrossRef]
- Wu, Z.C.; Yang, Z.Y.; Li, J.G.; Chen, H.B.; Huang, X.M.; Wang, H.C. Methyl-inositol, γ-aminobutyric acid and other health benefit compounds in the aril of litchi. Int. J. Food Sci. Nutr. 2016, 67, 762–772. [Google Scholar] [CrossRef]
- Devi, S.; Mishra, P.; Gupta, E.; Prakash, H.G. An Overview on Physico-chemical Characteristics and Postharvest Treatments of Litchi (Litchi chinensis Sonn.). Curr. Nutr. Food Sci. 2021, 17, 985–994. [Google Scholar] [CrossRef]
- Koul, B.; Taak, P. Lychee (Litchi chinensis Sonn.): Pre-and Post-harvest Disease Management. In Lychee Disease Management; Springer: Singapore, 2017; pp. 1–26. [Google Scholar] [CrossRef]
- Gao, H.; Ke, Z.; Kang, H.; Cheng, N.; Yang, Y.; Wang, D.N.; Yang, T.; Cao, W. Melatonin treatment delays postharvest senescence and regulates reactive oxygen species metabolism in peach fruit. Postharvest Biol. Technol. 2016, 118, 103–110. [Google Scholar] [CrossRef]
- Huan, C.; Jiang, L.; An, X.; Yu, M.; Xu, Y.; Ma, R.; Yu, Z. Potential role of reactive oxygen species and antioxidant genes in the regulation of peach fruit development and ripening. Plant Physiol. Biochem. 2016, 104, 294–303. [Google Scholar] [CrossRef]
- Gouda, M.B.; Zhang, C.; Wang, J.; Peng, S.; Chen, Y.; Luo, H.; Yu, L.J. ROS and MAPK cascades in the post-harvest senescence of horticultural products. J. Proteom. Bioinf 2020, 13, 1. [Google Scholar] [CrossRef]
- Moradbeygi, H.; Jamei, R.; Heidari, R.; Darvishzadeh, R. Investigating the enzymatic and non-enzymatic antioxidant defense by applying iron oxide nanoparticles in Dracocephalum moldavica L. plant under salinity stress. Sci. Hortic. 2020, 272, 109537. [Google Scholar] [CrossRef]
- Geigenberger, P.; Riewe, D.; Fernie, A.R. The central regulation of plant physiology by adenylates. Trends Plant Sci. 2010, 15, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ji, S.; Wei, B.; Cheng, S.; Wang, Y.; Hao, J.; Wang, S.; Zhou, Q. Transcriptome analysis of postharvest blueberries (Vaccinium corymbosum ‘Duke’) in response to cold stress. BMC Plant Biol. 2020, 20, 80. [Google Scholar] [CrossRef]
- Yi, C.; Jiang, Y.M.; Shi, J.; Qu, H.X.; Xue, S.; Duan, X.W.; Shi, J.; Prasad, N.K. ATP-regulation of antioxidant properties and phenolics in litchi fruit during browning and pathogen infection process. Food Chem. 2010, 118, 42–47. [Google Scholar] [CrossRef]
- Huang, H.; Wang, L.; Bi, F.; Xiang, X. Combined application of malic acid and lycopene maintains content of phenols, antioxidant activity, and membrane integrity to delay the pericarp browning of litchi fruit during storage. Front. Nutr. 2022, 9, 849385. [Google Scholar] [CrossRef]
- Brandt, K.; Maiwald, S.; Herkenhoff-Hesselmann, B.; Gnirss, K.; Greie, J.C.; Dunn, S.D.; Deckers-Hebestreit, G. Individual interactions of the subunits within the stator of the Escherichia coli ATP synthase. J. Biol. Chem. 2013, 288, 24465–24479. [Google Scholar] [CrossRef]
- Huang, Z.; Guo, L.; Wang, H.; Qu, H.; Ma, S.; Liu, Y.; Huang, H.; Jiang, Y. Energy status of kiwifruit stored under different temperatures or exposed to long-term anaerobic conditions or pure oxygen. Postharvest Biol. Technol. 2014, 98, 56–64. [Google Scholar] [CrossRef]
- Song, K.; Baumgartner, D.; Hagemann, M.; Muro-Pastor, A.M.; Maaß, S.; Becher, D.; Hess, W.R. AtpΘ is an inhibitor of F0F1 ATP synthase to arrest ATP hydrolysis during low-energy conditions in cyanobacteria. Curr. Biol. 2022, 32, 136–148. [Google Scholar] [CrossRef]
- Guerinier, T.; Millan, L.; Crozet, P.; Oury, C.; Rey, F.; Valot, B.; Mathieu, C.; Vidal, J.; Hodges, M.; Thomas, M.; et al. Phosphorylation of p27(KIP1) homologs KRP6 and 7 by SNF1-related protein kinase-1 links plant energy homeostasis and cell proliferation. Plant J. 2013, 75, 515–525. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, X.; Jing, R.; Chang, X.; Xie, H. Characterization of a common wheat (Triticum aestivum L.) TaSnRK2. 7 gene involved in abiotic stress responses. J. Exp. Bot. 2011, 62, 975–988. [Google Scholar] [CrossRef]
- Baena-González, E. Energy signaling in the regulation of gene expression during stress. Mol. Plant 2010, 3, 300–313. [Google Scholar] [CrossRef]
- Oliveira, N.F.; Machuqueiro, M. Novel US-CpHMD protocol to study the protonation-dependent mechanism of the ATP/ADP carrier. J. Chem. Inf. Model. 2022, 62, 2550–2560. [Google Scholar] [CrossRef]
- Pu, X.; Lv, X.; Tan, T.; Fu, F.; Qin, G.; Lin, H. Roles of mitochondrial energy dissipation systems in plant development and acclimation to stress. Ann. Bot. 2015, 116, 583–600. [Google Scholar] [CrossRef]
- Shan, Y.; Zhang, D.; Luo, Z.; Li, T.; Qu, H.; Duan, X.; Jiang, Y. Advances in chilling injury of postharvest fruit and vegetable: Extracellular ATP aspects. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4251–4273. [Google Scholar] [CrossRef]
- Mansoor, S.; Ali Wani, O.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive oxygen species in plants: From source to sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef]
- Oliveira, M.G.; Mazorra, L.M.; Souza, A.F.; Silva, G.M.C.; Correa, S.F.; Santos, W.C.; Saraiva, K.D.C.; Teixeira, A.J., Jr.; Melo, D.F.; Silva, M.G.; et al. Involvement of AOX and UCP pathways in the postharvest ripening of papaya fruits. J. Plant Physiol. 2015, 189, 42–50. [Google Scholar] [CrossRef]
- Liu, T.; Wang, H.; Kuang, J.; Sun, C.; Shi, J.; Duan, X.; Qu, H.; Jiang, Y. Short-term anaerobic, pure oxygen and refrigerated storage conditions affect the energy status and selective gene expression in litchi fruit. LWT 2015, 60, 1254–1261. [Google Scholar] [CrossRef]
- Sluse, F.E.; Jarmuszkiewicz, W. Activity and functional interaction of alternative oxidase and uncoupling protein in mitochondria from tomato fruit. Braz. J. Med. Biol. Res. 2000, 33, 259–268. [Google Scholar] [CrossRef]
- Echevarría-Zomeño, S.; Yángüez, E.; Fernández-Bautista, N.; Castro-Sanz, A.B.; Ferrando, A.; Castellano, M. Regulation of translation initiation under biotic and abiotic stresses. Int. J. Mol. Sci. 2013, 14, 4670–4683. [Google Scholar] [CrossRef]
- Trouvelot, S.; Héloir, M.-C.; Poinssot, B.; Gauthier, A.; Paris, F.; Guillier, C.; Combier, M.; Trdá, L.; Daire, X.; Adrian, M. Carbohydrates in plant immunity and plant protection: Roles and potential application as foliar sprays. Front. Plant Sci. 2014, 5, 592. [Google Scholar] [CrossRef]
- Gupta, A.K.; Kaur, N. Sugar signalling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants. J. Biosci. 2005, 30, 761–776. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Nascimento, V.L.; Medeiros, D.B.; Nunes-Nesi, A.; Ribeiro, D.M.; Zsögön, A.; Araújo, W.L. Modifications in organic acid profiles during fruit development and ripening: Correlation or causation? Front. Plant Sci. 2018, 9, 1689. [Google Scholar] [CrossRef]
- Li, J.Z.; Min, D.D.; Li, Z.L.; Fu, X.D.; Zhao, X.; Wang, J.H.; Zhang, X.H.; Li, F.J.; Li, X.A.; Guo, Y.Y. Regulation of sugar metabolism by methyl jasmonate to improve the postharvest quality of tomato fruit. J. Plant Growth Regul. 2021, 41, 1615–1626. [Google Scholar] [CrossRef]
- Fan, S.; Wang, D.; Xie, H.; Wang, H.; Qin, Y.; Hu, G.; Zhao, J. Sugar transport, metabolism and signaling in fruit development of Litchi chinensis sonn: A review. Int. J. Mol. Sci. 2021, 22, 11231. [Google Scholar] [CrossRef]
- Guo, X.; Luo, T.; Han, D.; Zhu, D.; Li, Z.; Wu, Z.; Wu, Z. Multi-omics analysis revealed room temperature storage affected the quality of litchi by altering carbohydrate metabolism. Sci. Hortic. 2022, 293, 110663. [Google Scholar] [CrossRef]
- Saddhe, A.A.; Manuka, R.; Penna, S. Plant sugars: Homeostasis and transport under abiotic stress in plants. Physiol. Plant 2021, 171, 739–755. [Google Scholar] [CrossRef]
- Lim, M.N.; Lee, S.E.; Chang, W.Y.; Yoon, I.S.; Hwang, Y.S. Comparison of transcriptomic adjustments to availability of sugar, cellular energy, and oxygen in germinating rice embryos. J. Plant Physiol. 2021, 264, 153471. [Google Scholar] [CrossRef]
- Gibson, S.I. Sugar and phytohormone response pathways: Navigating a signalling network. J. Exp. Bot. 2004, 55, 253–264. [Google Scholar] [CrossRef]
- Wang, J.; Li, F.; Sun, W.; Ali, M.; Li, B.; Zhang, X.; Li, X.; Zhang, X. Role of sugar and energy metabolism in apple flesh browning during cold storage. Sci. Hortic. 2024, 326, 112758. [Google Scholar] [CrossRef]
- Yu, F.; Ni, Z.; Shao, X.; Yu, L.; Liu, H.; Xu, F.; Wang, H. Differences in sucrose metabolism in peach fruit stored at chilling stress versus nonchilling stress temperatures. HortScience 2015, 50, 1542–1548. [Google Scholar] [CrossRef]
- Habibi, F.; Serrano, M.; Zacarías, L.; Valero, D.; Guillén, F. Postharvest application of 24-epibrassinolide reduces chilling injury symptoms and enhances bioactive compounds content and antioxidant activity of blood orange fruit. Front. Plant Sci. 2021, 12, 629733. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cao, J.; Jiang, W. Changes in sugar metabolism caused by exogenous oxalic acid related to chilling tolerance of apricot fruit. Postharvest Biol. Technol. 2016, 114, 10–16. [Google Scholar] [CrossRef]
- Alok, A.; Singh, S.; Kumar, P.; Bhati, K.K. Potential of engineering the myo-inositol oxidation pathway to increase stress resilience in plants. Mol. Biol. Rep. 2022, 49, 8025–8035. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Wang, F.; Si, Z.; Huo, J.; Xing, L.; An, Y.; He, S.; Liu, Q. A myo-inositol-1-phosphate synthase gene, IbMIPS 1, enhances salt and drought tolerance and stem nematode resistance in transgenic sweet potato. Plant Biotechnol. J. 2015, 14, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhou, K.; Li, Y.; Chen, X.; Liu, B.; Li, C.; Gong, X.; Ma, F. Exogenous myo-inositol alleviates salinity-induced stress in Malus hupehensis Rehd. Plant Physiol. Biochem. 2018, 133, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, J.; Li, C.; Zhang, Z.; Ma, F.; Li, M. Response of sugar metabolism in apple leaves subjected to short-term drought stress. Plant Physiol. Biochem. 2019, 141, 164–171. [Google Scholar] [CrossRef]
- Živanović, B.; Milić Komić, S.; Tosti, T.; Vidović, M.; Prokić, L.; Veljović Jovanović, S. Leaf soluble sugars and free amino acids as important components of abscisic acid—Mediated drought response in tomato. Plants 2020, 9, 1147. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Gao, Z.; Nizamani, M.M.; Hu, M.; Li, M.; Li, X.; Wang, J. Mechanisms of Litchi Response to Postharvest Energy Deficiency via Energy and Sugar Metabolisms. Foods 2024, 13, 2288. https://doi.org/10.3390/foods13142288
Zhao K, Gao Z, Nizamani MM, Hu M, Li M, Li X, Wang J. Mechanisms of Litchi Response to Postharvest Energy Deficiency via Energy and Sugar Metabolisms. Foods. 2024; 13(14):2288. https://doi.org/10.3390/foods13142288
Chicago/Turabian StyleZhao, Kunkun, Zhaoyin Gao, Mir Muhammad Nizamani, Meijiao Hu, Min Li, Xiaohui Li, and Jiabao Wang. 2024. "Mechanisms of Litchi Response to Postharvest Energy Deficiency via Energy and Sugar Metabolisms" Foods 13, no. 14: 2288. https://doi.org/10.3390/foods13142288





