Effects of Substituting Tenebrio molitor and Elodea nuttallii as Feed on Growth, Flesh Quality and Intestinal Microbiota of Red Swamp Crayfish (Procambarus clarkii)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Experimental Animals
2.3. Experimental Design and Management
2.4. Sample Collection
2.5. Growth Performance Indicators
- IBW: Initial body weight (g).
- FBW: Final body weight (g).
- SR: Survival rate (%) = 100 × final number of crayfish/initial number of crayfish.
- WGR: Weight gain rate (%) = 100 × (FBW − IBW)/IBW.
- SGR: Specific growth rate (%/d) = 100 × (ln FBW − ln IBW)/84 d.
- HSI: Hepatosomatic index (%) = 100 × hepatopancreas weight/FBW.
- MY: Meat yield (%) = 100 × muscle weight/FBW.
- CF: Condition factor (g/cm3) = FBW/(carapace’s length)3.
2.6. Sample Pretreatment and Determination Methods
2.6.1. Digestive Enzyme Activities
2.6.2. Muscle Routine Biochemical Composition
2.6.3. Muscle Amino Acid Content and Composition
2.6.4. Muscle Fatty Acid Content and Composition
2.6.5. Muscle RNA Extraction and Quantitative Polymerase Chain Reaction (qPCR)
2.6.6. Intestinal DNA Extraction and Microbiota Composition Analysis
2.7. Statistical Analysis
3. Results
3.1. Growth Performance
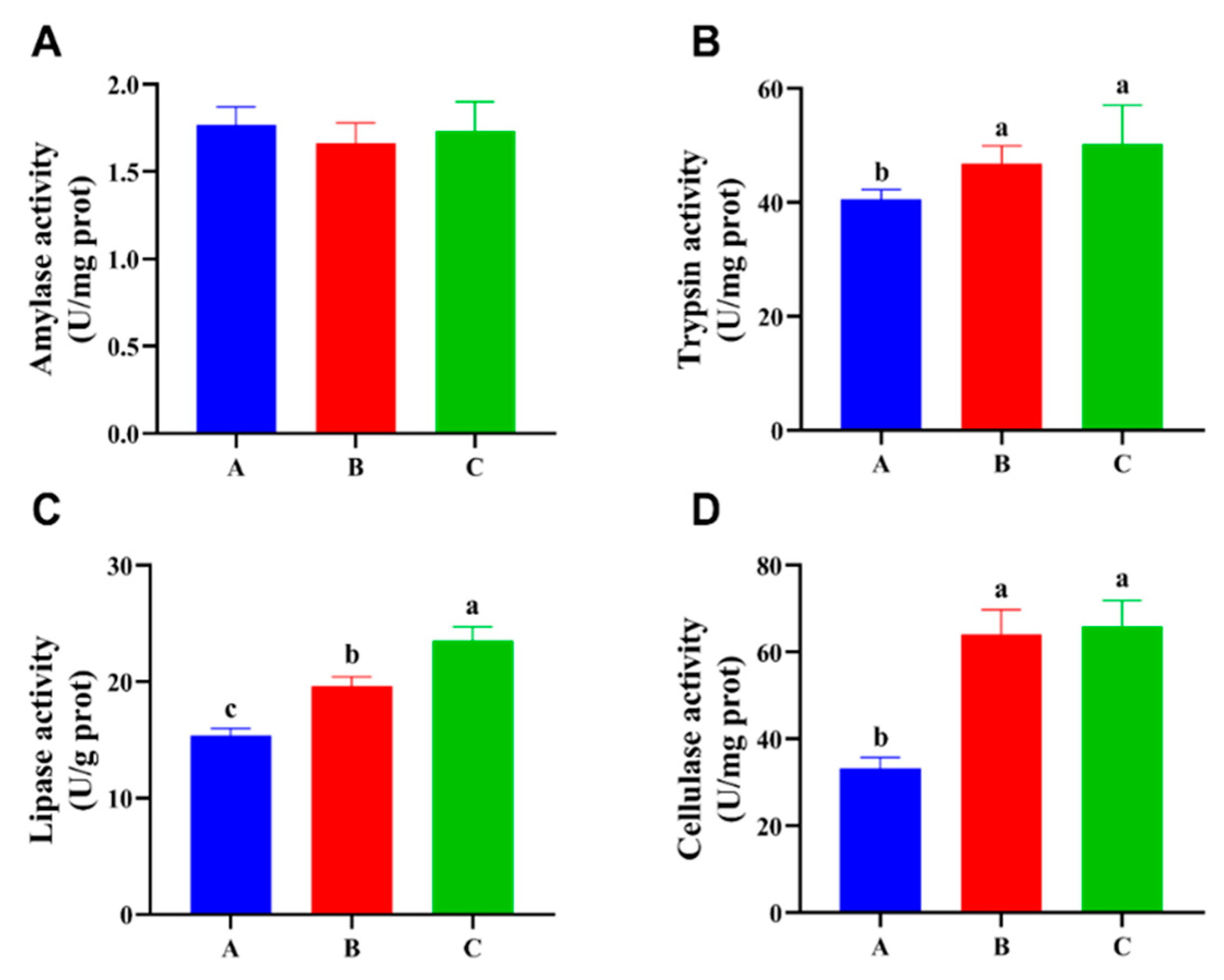
3.2. Digestive Enzyme Activities
3.3. Muscle Proximate Composition
3.4. Muscle Amino Acid Composition
3.5. Muscle Fatty Acid Composition
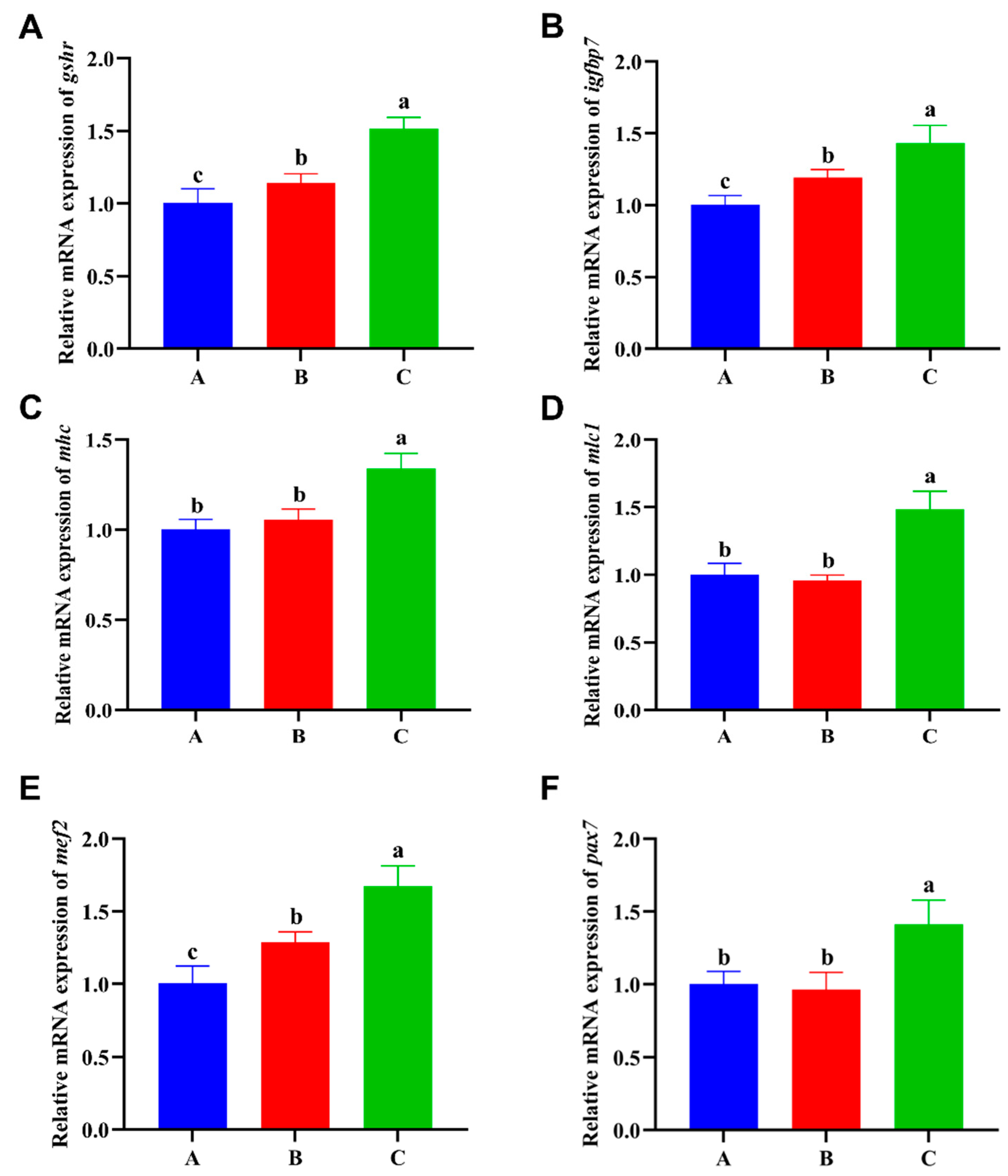
3.6. Muscle Growth and Development-Related Genes Expression
3.7. Intestinal Microbiota Diversity and Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuo, Y.-C.; Ho, T.H.; Bharadwaj, A.; Tran, H.T.Q.; Chu, Y.T.; Wang, S.-H.; Chen, T.Y.; Nan, F.-H.; Lee, P.T. Feasibility assessment of replacing fishmeal with Clostridium autoethanogenum protein in commercial whiteleg shrimp diets: Impacts on growth, muscle characteristics, and health. Anim. Feed Sci. Technol. 2024, 309, 115916. [Google Scholar] [CrossRef]
- Bjorndal, T.; Dey, M.; Tusvik, A. Economic analysis of the contributions of aquaculture to future food security. Aquaculture 2024, 578, 740071. [Google Scholar] [CrossRef]
- Bondad-Reantaso, M.G.; Subasinghe, R.P.; Josupeit, H.; Cai, J.; Zhou, X. The role of crustacean fisheries and aquaculture in global food security: Past, present and future. J. Invertebr. Pathol. 2012, 110, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.E.; McNevin, A.A.; Davis, R.P. The contribution of fisheries and aquaculture to the global protein supply. Food Secur. 2022, 14, 805–827. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, H.; Zhang, C.; Bian, Y.; Yao, W.; Xu, Z.; Wang, Y.; Li, X.; Leng, X. Effects of replacing fishmeal with cottonseed protein concentrate on growth performance, flesh quality and gossypol deposition of largemouth bass (Micropterus salmoides). Aquaculture 2022, 548, 737751. [Google Scholar] [CrossRef]
- Cottrell, R.S.; Blanchard, J.L.; Halpern, B.S.; Metian, M.; Froehlich, H.E. Global adoption of novel aquaculture feeds could substantially reduce forage fish demand by 2030. Nat. Food 2020, 1, 301–308. [Google Scholar] [CrossRef]
- Maulu, S.; Langi, S.; Hasimuna, O.J.; Missinhoun, D.; Munganga, B.P.; Hampuwo, B.M.; Gabriel, N.N.; Elsabagh, M.; Van Doan, H.; Kari, Z.A.; et al. Recent advances in the utilization of insects as an ingredient in aquafeeds: A review. Anim. Nutr. 2022, 11, 334–349. [Google Scholar] [CrossRef] [PubMed]
- Nowak, V.; Persijn, D.; Rittenschober, D.; Charrondiere, U.R. Review of food composition data for edible insects. Food Chem. 2016, 193, 39–46. [Google Scholar] [CrossRef]
- Bai, N.; Li, Q.; Pan, S.; Qi, Z.; Deng, W.; Gu, M. Effects of defatted yellow mealworm (Tenebrio molitor) on growth performance, intestine, and liver health of turbot (Scophthalmus maximus). Anim. Feed Sci. Technol. 2023, 302, 115672. [Google Scholar] [CrossRef]
- Gu, J.; Liang, H.; Ge, X.; Xia, D.; Pan, L.; Mi, H.; Ren, M. A study of the potential effect of yellow mealworm (Tenebrio molitor) substitution for fish meal on growth, immune and antioxidant capacity in juvenile largemouth bass (Micropterus salmoides). Fish Shellfish Immun. 2022, 120, 214–221. [Google Scholar] [CrossRef]
- Li, H.; Hu, Z.; Liu, S.; Sun, J.; Ji, H. Influence of dietary soybean meal replacement with yellow mealworm (Tenebrio molitor) on growth performance, antioxidant capacity, skin color, and flesh quality of mirror carp (Cyprinus carpio var. specularis). Aquaculture 2022, 561, 738686. [Google Scholar] [CrossRef]
- Iaconisi, V.; Marono, S.; Parisi, G.; Gasco, L.; Genovese, L.; Maricchiolo, G.; Bovera, F.; Piccolo, G. Dietary inclusion of Tenebrio molitor larvae meal: Effects on growth performance and final quality treats of blackspot sea bream (Pagellus bogaraveo). Aquaculture 2017, 476, 49–58. [Google Scholar] [CrossRef]
- Belforti, M.; Gai, F.; Lussiana, C.; Renna, M.; Malfatto, V.; Rotolo, L.; De Marco, M.; Dabbou, S.; Schiavone, A.; Zoccarato, I.; et al. Tenebrio molitor meal in rainbow trout (Oncorhynchus mykiss) diets: Effects on animal performance, nutrient digestibility and chemical composition of fillets. Ital. J. Anim. Sci. 2015, 14, 4170. [Google Scholar] [CrossRef]
- Henry, M.A.; Gai, F.; Enes, P.; Perez-Jimenez, A.; Gasco, L. Effect of partial dietary replacement of fishmeal by yellow mealworm (Tenebrio molitor) larvae meal on the innate immune response and intestinal antioxidant enzymes of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immun. 2018, 83, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Gasco, L.; Henry, M.; Piccolo, G.; Marono, S.; Gai, F.; Renna, M.; Lussiana, C.; Antonopoulou, E.; Mola, P.; Chatzifotis, S. Tenebrio molitor meal in diets for European sea bass (Dicentrarchus labrax L.) juveniles: Growth performance, whole body composition and in vivo apparent digestibility. Anim. Feed Sci. Technol. 2016, 220, 34–45. [Google Scholar] [CrossRef]
- Panini, R.L.; Lima Freitas, L.E.; Guimaraes, A.M.; Rios, C.; da Silva, M.F.O.; Vieira, F.N.; Fracalossi, D.M.; Samuels, R.I.; Prudencio, E.S.; Silva, C.P.; et al. Potential use of mealworms as an alternative protein source for Pacific white shrimp: Digestibility and performance. Aquaculture 2017, 473, 115–120. [Google Scholar] [CrossRef]
- Panini, R.L.; Pinto, S.S.; Nobrega, R.O.; Vieira, F.N.; Fracalossi, D.M.; Samuels, R.I.; Prudencio, E.S.; Silva, C.P.; Amboni, R.D.M.C. Effects of dietary replacement of fishmeal by mealworm meal on muscle quality of farmed shrimp Litopenaeus vannamei. Food Res. Int. 2017, 102, 445–450. [Google Scholar] [CrossRef]
- Zheng, Y.; Hou, C.; Chen, J.; Wang, H.; Yuan, H.; Hu, N.; Shi, L.; Zhang, S. Integrating microbiome and transcriptome analyses to understand the effect of replacing fishmeal with Tenebrio molitor meal in Pacific white shrimp (Litopenaeus vannamei) diets. Aquaculture 2023, 575, 739818. [Google Scholar] [CrossRef]
- Feng, P.; He, J.; Lv, M.; Huang, G.; Chen, X.; Yang, Q.; Wang, J.; Wang, D.; Ma, H. Effect of dietary Tenebrio molitor protein on growth performance and immunological parameters in Macrobrachium rosenbergii. Aquaculture 2019, 511, 734247. [Google Scholar] [CrossRef]
- Naseem, S.; Bhat, S.U.; Gani, A.; Bhat, F.A. Perspectives on utilization of macrophytes as feed ingredient for fish in future aquaculture. Rev. Aquac. 2021, 13, 282–300. [Google Scholar] [CrossRef]
- Poveda, J. The use of freshwater macrophytes as a resource in sustainable agriculture. J. Clean. Prod. 2022, 369, 133247. [Google Scholar] [CrossRef]
- Mao, Z.; Gu, X.; Zeng, Q. Food sources and trophic relationships of three decapod crustaceans: Insights from gut contents and stable isotope analyses. Aquac. Res. 2016, 47, 2888–2898. [Google Scholar] [CrossRef]
- Yao, Z.L.; Zhao, Y.; Wang, H.; Chen, H.J.; Ji, X.S. Growth promotion and dietary contribution assessment of three submerged macrophytes to Macrobrachium nipponense. Aquaculture 2019, 504, 70–80. [Google Scholar] [CrossRef]
- Tang, Y.; Qian, C.; Zhao, L.; Wang, C.; Tang, B.; Peng, X.; Cheng, Y.; Guo, X. Comparative metabolomics analysis of Elodea nuttallii and Cladophora sp. in aquaculture systems. Aquaculture 2023, 563, 738950. [Google Scholar] [CrossRef]
- Yang, H.; Li, X.; Rahman, M.M.; Leng, X. Dietary supplementation of leucine improved the flesh quality of largemouth bass, Micropterus salmoides through TOR, FoxO3a and MRFs regulation. Aquaculture 2023, 566, 739237. [Google Scholar] [CrossRef]
- Listrat, A.; Lebret, B.; Louveau, I.; Astruc, T.; Bonnet, M.; Lefaucheur, L.; Picard, B.; Bugeon, J. How Muscle Structure and Composition Influence Meat and Flesh Quality. Thescientificworldjo 2016, 2016, 3182746. [Google Scholar] [CrossRef]
- De Schryver, P.; Vadstein, O. Ecological theory as a foundation to control pathogenic invasion in aquaculture. ISME J. 2014, 8, 2360–2368. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.E.; Jaeger, R.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Townsend, J.R.; West, N.P.; Black, K.; Gleeson, M.; Pyne, D.B.; et al. The athletic gut microbiota. J. Int. Soc. Sports Nutr. 2020, 17, 24. [Google Scholar] [CrossRef]
- Ringo, E.; Zhou, Z.; Vecino, J.L.G.; Wadsworth, S.; Romero, J.; Krogdahl, A.; Olsen, R.E.; Dimitroglou, A.; Foey, A.; Davies, S.; et al. Effect of dietary components on the gut microbiota ofaquatic animals. A never-ending story? Aquac. Nutr. 2016, 22, 219–282. [Google Scholar] [CrossRef]
- Teves, J.F.C.; Ragaza, J.A. The quest for indigenous aquafeed ingredients: A review. Rev. Aquac. 2016, 8, 154–171. [Google Scholar] [CrossRef]
- Hernandez-Perez, A.; Soderhall, I. Intestinal microbiome in crayfish: Its role upon growth and disease presentation. Dev. Comp. Immunol. 2023, 145, 104703. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Xi, Q.; Tang, J.; Liu, T.; Liu, C.; Li, H.; Gu, X.; Shen, M.; Zhang, M.; Fang, J.; et al. Effects of Pelleted and Extruded Feed on Growth Performance, Intestinal Histology and Microbiota of Juvenile Red Swamp Crayfish (Procambarus clarkii). Animals 2022, 12, 2252. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Rahman, M.M.; Li, X.; Sharifuzzaman, S.M.; Leng, X. Dietary leucine requirement of juvenile largemouth bass (Micropterus salmoides) based on growth, nutrient utilization and growth-related gene analyses. Aquaculture 2022, 555, 738207. [Google Scholar] [CrossRef]
- Zhang, Y.; Bing, Y.; Li, Y.; Shaotong, J.; Jianfeng, L.; Lin, L. Comparison of the nutritional qualities of the pond, rice-field and wild crayfish (Procambarus clarkii) meat. Food Chem. Adv. 2023, 2, 100272. [Google Scholar] [CrossRef]
- Basawa, R.; Kabra, S.; Khile, D.A.; Abbu, R.U.F.; Parekkadan, S.J.; Thomas, N.A.; Kim, S.K.; Raval, R. Repurposing chitin-rich seafood waste for warm-water fish farming. Heliyon 2023, 9, e18197. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.A.; Ding, Q.; Yin, L.; Chi, X.; Sun, N.; He, R.; Luo, L.; Ma, H.; Li, Z. Comparison of the nutritional value of mysore thorn borer (Anoplophora chinensis) and mealworm larva (Tenebrio molitor): Amino acid, fatty acid, and element profiles. Food Chem. 2020, 323, 126818. [Google Scholar] [CrossRef] [PubMed]
- Koganti, P.; Yao, J.; Cleveland, B.M. Molecular Mechanisms Regulating Muscle Plasticity in Fish. Animals 2021, 11, 61. [Google Scholar] [CrossRef]
- Song, S.-G.; Chi, S.-Y.; Tan, B.-P.; Liang, G.-L.; Lu, B.-Q.; Dong, X.-H.; Yang, Q.-H.; Liu, H.-Y.; Zhang, S. Effects of fishmeal replacement by Tenebrio molitor meal on growth performance, antioxidant enzyme activities and disease resistance of the juvenile pearl gentian grouper (Epinephelus lanceolatus ♂ x Epinephelus fuscoguttatus ♀). Aquac. Res. 2018, 49, 2210–2217. [Google Scholar] [CrossRef]
- Lovett, D.L.; Felder, D.L. Ontogenetic Change in Digestive Enzyme Activity of Larval and Postlarval White Shrimp Penaeus setiferus (Crustacea, Decapoda, Penaeidae). Biol. Bull. 1990, 178, 144–159. [Google Scholar] [CrossRef]
- Sanchez-Muros, M.J.; De Haro, C.; Sanz, A.; Trenzado, C.E.; Villareces, S.; Barroso, F.G. Nutritional evaluation of Tenebrio molitor meal as fishmeal substitute for tilapia (Oreochromis niloticus) diet. Aquac. Nutr 2016, 22, 943–955. [Google Scholar] [CrossRef]
- Melenchon, F.; Larran, A.M.; de Mercado, E.; Hidalgo, M.C.; Cardenete, G.; Barroso, F.G.; Fabrikov, D.; Lourenco, H.M.; Pessoa, M.R.; Tomas-Almenar, C. Potential use of black soldier fly (Hermetia illucens) and mealworm (Tenebrio molitor) insectmeals in diets for rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 2021, 27, 491–505. [Google Scholar] [CrossRef]
- Mao, Z.; Chen, Y.; Cao, S.; Tang, J.; Qu, F.; Tao, M.; Liu, Z. Effects of the total fish meal replacement by plant meal on growth performance, nutrient utilization and intestinal microbiota of backcross F2 derived from blunt snout bream (Megalobrama amblycephala, ♀) x topmouth culter (Culter alburnus, ♂). Aquac. Rep. 2024, 34, 101889. [Google Scholar] [CrossRef]
- Zhou, Y.; Yuan, X.; Liang, X.-F.; Fang, L.; Li, J.; Guo, X.; Bai, X.; He, S. Enhancement of growth and intestinal flora in grass carp: The effect of exogenous cellulase. Aquaculture 2013, 416, 1–7. [Google Scholar] [CrossRef]
- Ahmed, I.; Jan, K.; Fatma, S.; Dawood, M.A.O. Muscle proximate composition of various food fish species and their nutritional significance: A review. J. Anim. Physiol. Anim. Nutr. 2022, 106, 690–719. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.X.; Wang, P.; Zheng, H.; Smith, R.G. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc. Natl. Acad. Sci. USA 2004, 101, 4679–4684. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Lou, C.; Ren, B.; Pi, J.; Dai, T.; Qin, W.; Zhou, Y. Hepatic transcriptome analysis provides new insights into ghrelin regulation of the liver in Nile tilapia (Oreochromis niloticus). Front. Vet. Sci. 2023, 10, 1192195. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, J.; Cao, X.; Du, J.; Cao, L.; Nie, Z.; Xu, G.; Dong, Z. Integrated Analysis of Transcriptomics and Metabolomics Unveil the Novel Insight of One-Year-Old Precocious Mechanism in the Chinese Mitten Crab, Eriocheir sinensis. Int. J. Mol. Sci. 2023, 24, 11171. [Google Scholar] [CrossRef] [PubMed]
- How, H.K.; Yeoh, A.; Quah, T.C.; Oh, Y.; Rosenfeld, R.G.; Lee, K.O. Insulin-like growth factor binding proteins (IGFBPs) and IGFBP-related protein 1-levels in cerebrospinal fluid of children with acute lymphoblastic leukemia. J. Clin. Endocr. Metab. 1999, 84, 1283–1287. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, R.; Wang, R.; Chen, S. Transcriptomics analysis revealing candidate networks and genes for the body size sexual dimorphism of Chinese tongue sole (Cynoglossus semilaevis). Funct. Integr. Genom. 2018, 18, 327–339. [Google Scholar] [CrossRef]
- Chen, L.; Pan, Y.; Cheng, J.; Zhu, X.; Chu, W.; Meng, Y.Y.; Bin, S.; Zhang, J. Characterization of myosin heavy chain (MYH) genes and their differential expression in white and red muscles of Chinese perch, Siniperca chuatsi. Int. J. Biol. Macromol. 2023, 250, 125907. [Google Scholar] [CrossRef]
- Weeds, A.G.; Lowey, S. Substructure of the myosin molecule. II. The light chains of myosin. J. Mol. Biol. 1971, 61, 701–725. [Google Scholar] [CrossRef] [PubMed]
- Hettige, P.; Tahir, U.; Nishikawa, K.C.; Gage, M.J. Comparative analysis of the transcriptomes of EDL, psoas, and soleus muscles from mice. BMC Genom. 2020, 21, 808. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, J.; Zhong, L.; Chu, W.; Hu, Y. A study on methionine-mediated regulation of muscle fiber growth, development and differentiation in the rice field eel (Monopterus albus). Aquaculture 2022, 547, 737430. [Google Scholar] [CrossRef]
- Li, C.; Liu, J.; Lu, P.; Ma, S.; Zhu, K.; Gao, L.; Li, B.; Chen, K. Identification, expression and function of myosin heavy chain family genes in Tribolium castaneum. Genomics 2019, 111, 719–728. [Google Scholar] [CrossRef]
- Potthoff, M.J.; Olson, E.N. MEF2: A central regulator of diverse developmental programs. Development 2007, 134, 4131–4140. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Tan, X.; Li, M.; Sui, Y.; Du, S.J.; You, F. The duplicated paired box protein 7 (pax7) genes differentially transcribed during Japanese flounder (Paralichthys olivaceus) embryogenesis. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2015, 189, 62–68. [Google Scholar] [CrossRef]
- Minchin, J.E.; Hughes, S.M. Sequential actions of Pax3 and Pax7 drive xanthophore development in zebrafish neural crest. Dev. Biol. 2008, 319, 530. [Google Scholar] [CrossRef]
- Akolkar, D.B.; Asaduzzaman, M.; Kinoshita, S.; Asakawa, S.; Watabe, S. Characterization of Pax3 and Pax7 genes and their expression patterns during different development and growth stages of Japanese pufferfish Takifugu rubripes. Gene 2016, 575, 21–28. [Google Scholar] [CrossRef]
- Jiang, W.-D.; Wen, H.-L.; Liu, Y.; Jiang, J.; Wu, P.; Zhao, J.; Kuang, S.-Y.; Tang, L.; Tang, W.-N.; Zhang, Y.-A.; et al. Enhanced muscle nutrient content and flesh quality, resulting from tryptophan, is associated with anti-oxidative damage referred to the Nrf2 and TOR signalling factors in young grass carp (Ctenopharyngodon idella): Avoid tryptophan deficiency or excess. Food Chem. 2016, 199, 210–219. [Google Scholar] [CrossRef]
- Pyz-Lukasik, R.; Paszkiewicz, W. Species Variations in the Proximate Composition, Amino Acid Profile, and Protein Quality of the Muscle Tissue of Grass Carp, Bighead Carp, Siberian Sturgeon, and Wels Catfish. J. Food Qual. 2018, 2018, 2625401. [Google Scholar] [CrossRef]
- Wong, K.H.; Aziz, S.A.; Mohamed, S. Sensory aroma from Maillard reaction of individual and combinations of amino acids with glucose in acidic conditions. Int. J. Food Sci. Technol. 2008, 43, 1512–1519. [Google Scholar] [CrossRef]
- Costas, B.; Aragao, C.; Mancera, J.M.; Dinis, M.T.; Conceicao, L.E.C. High stocking density induces crowding stress and affects amino acid metabolism in Senegalese sole Solea senegalensis (Kaup 1858) juveniles. Aquac. Res. 2008, 39, 1–9. [Google Scholar] [CrossRef]
- Dong, B.; Wu, L.; Wang, Y.; Han, D.; Liu, H.; Zhu, X.; Yang, Y.; Xie, S.; Liu, Z.; Jin, J. Glutamate improves flesh quality and muscle growth of triploid crucian carp. Aquac. Rep. 2023, 33, 101832. [Google Scholar] [CrossRef]
- Yao, W.; Yang, P.; Zhang, X.; Xu, X.; Zhang, C.; Li, X.; Leng, X. Effects of replacing dietary fish meal with Clostridium autoethanogenum protein on growth and flesh quality of Pacific white shrimp (Litopenaeus vannamei). Aquaculture 2022, 549, 737770. [Google Scholar] [CrossRef]
- Wei, Y.; Shen, H.; Xu, W.; Pan, Y.; Chen, J.; Zhang, W.; Mai, K. Replacement of dietary fishmeal by Antarctic krill meal on growth performance, intestinal morphology, body composition and organoleptic quality of large yellow croaker Larimichthys crocea. Aquaculture 2019, 512, 734281. [Google Scholar] [CrossRef]
- Turchini, G.M.; Moretti, V.M.; Mentasti, T.; Orban, E.; Valfre, F. Effects of dietary lipid source on fillet chemical composition, flavour volatile compounds and sensory characteristics in the freshwater fish tench (Tinca tinca L.). Food Chem. 2007, 102, 1144–1155. [Google Scholar] [CrossRef]
- Harrison, S.; Lemieux, S.; Lamarche, B. Assessing the impact of replacing foods high in saturated fats with foods high in unsaturated fats on dietary fat intake among Canadians. Am. J. Clin. Nutr. 2022, 115, 877–885. [Google Scholar] [CrossRef]
- Sellem, L.; Eichelmann, F.; Jackson, K.G.; Wittenbecher, C.; Schulze, M.B.; Lovegrove, J.A. Replacement of dietary saturated with unsaturated fatty acids is associated with beneficial effects on lipidome metabolites: A secondary analysis of a randomized trial. Am. J. Clin. Nutr. 2023, 117, 1248–1261. [Google Scholar] [CrossRef]
- Harlioglu, M.M.; Koprucu, K.; Harlioglu, A.G.; Yilmaz, O.; Yonar, S.M.; Aydin, S.; Duran, T.C. Effects of dietary n-3 polyunsaturated fatty acids on the nutritional quality of abdomen meat and hepatopancreas in a freshwater crayfish (Astacus leptodactylus). J. Food Compos. Anal. 2015, 41, 144–150. [Google Scholar] [CrossRef]
- Cahu, C.; Salen, P.; de Lorgeril, M. Farmed and wild fish in the prevention of cardiovascular diseases: Assessing possible differences in lipid nutritional values. Nutr. Metab. Cardiovasc. Dis. 2004, 14, 34–41. [Google Scholar] [CrossRef]
- Bae, J.-Y.; Kim, D.-J.; Yoo, K.-Y.; Kim, S.-G.; Lee, J.-Y.; Bai, S.C. Effects of Dietary Arachidonic Acid (20:4n-6) Levels on Growth Performance and Fatty Acid Composition of Juvenile Eel, Anguilla japonica. Asian Austral. J.Anim. 2010, 23, 508–514. [Google Scholar] [CrossRef]
- Luo, Z.; Tan, X.Y.; Li, X.D.; Yin, G.J. Effect of dietary arachidonic acid levels on growth performance, hepatic fatty acid profile, intermediary metabolism and antioxidant responses for juvenile Synechogobius hasta. Aquac. Nutr. 2012, 18, 340–348. [Google Scholar] [CrossRef]
- Rombenso, A.N.; Trushenski, J.T.; Jirsa, D.; Drawbridge, M. Docosahexaenoic acid (DHA) and arachidonic acid (ARA) are essential to meet LC-PUFA requirements of juvenile California Yellowtail (Seriola dorsalis). Aquaculture 2016, 463, 123–134. [Google Scholar] [CrossRef]
- Holt, C.C.; Bass, D.; Stentiford, G.D.; van der Giezen, M. Understanding the role of the shrimp gut microbiome in health and disease. J. Invertebr. Pathol. 2021, 186, 107387. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Kong, Y.; Chang, X.; Feng, J.; Wang, X.; Hou, L.; Zhao, X.; Pei, C.; Kong, X. Effects of two fish-derived probiotics on growth performance, innate immune response, intestinal health, and disease resistance of Procambarus clarkii. Aquaculture 2023, 562, 738765. [Google Scholar] [CrossRef]
- Klase, G.; Lee, S.; Liang, S.; Kim, J.; Zo, Y.-G.; Lee, J. The microbiome and antibiotic resistance in integrated fishfarm water: Implications of environmental public health. Sci. Total Environ. 2019, 649, 1491–1501. [Google Scholar] [CrossRef]
- Ruan, G.; Li, S.; He, N.; Fang, L.; Wang, Q. Short-term adaptability to non-hyperthermal stress: Antioxidant, immune and gut microbial responses in the red swamp crayfish, Procambarus clarkii. Aquaculture 2022, 560, 738497. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. Biomed Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015, 74, 13–22. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, S.; Nie, Q.; He, H.; Tan, H.; Geng, F.; Ji, H.; Hu, J.; Nie, S. Gut firmicutes: Relationship with dietary fiber and role in host homeostasis. Crit. Rev. Food Sci. 2022, 63, 12073–12088. [Google Scholar] [CrossRef]
- Hossain, M.S.; Dai, J.C.; Qiu, D.R. European eel (Anguilla anguilla) GI tract conserves a unique metagenomics profile in the recirculation aquaculture system (RAS). Aquac. Int. 2021, 29, 1529–1544. [Google Scholar] [CrossRef]
- Semova, I.; Carten, J.D.; Stombaugh, J.; Mackey, L.C.; Knight, R.; Farber, S.A.; Rawls, J.F. Microbiota Regulate Intestinal Absorption and Metabolism of Fatty Acids in the Zebrafish. Cell Host Microbe 2012, 12, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Duman, M.; Saticioglu, I.B.; Buyukekiz, A.G.; Balta, F.; Altun, S. Molecular characterization and antimicrobial resistance profile of atypical Citrobacter gillenii and Citrobacter sp isolated from diseased rainbow trout (Oncorhynchus mykiss). J. Glob. Antimicrob. Resist. 2017, 10, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Pazdzior, E. Shewanella putrefaciens—A new opportunistic pathogen of freshwater fish. J. Vet. Res. 2016, 60, 429–434. [Google Scholar] [CrossRef]
- Hou, S.; Li, J.; Huang, J.; Cheng, Y. Effects of dietary phospholipid and cholesterol levels on antioxidant capacity, nonspecial immune response and intestinal microflora of juvenile female crayfish, Procambarus clarkii. Aquac. Rep. 2022, 25, 101245. [Google Scholar] [CrossRef]
- Qian, J.; Zhang, Y.W.; Yi, L.; Shi, M.Y.; Deng, D. Effects of different diets on growth performance, digestive enzyme activities and intestinal bacterial community of juvenile Chinese giant salamander (Andrias davidianus). Aquac. Rep. 2023, 30, 101612. [Google Scholar] [CrossRef]


| Parameters | Numeric Range |
|---|---|
| Temperature (°C) | 20–25 |
| pH | 7.52–8.41 |
| DO (mg/L) | 6.0–7.0 |
| NH4+-N (mg/L) | 0.042–0.053 |
| NO2N (mg/L) | 0.031–0.039 |
| COD (mg/L) | 12.0–14.7 |
| Genes | Primers (5′–3′) | Accession No. |
|---|---|---|
| ghsr | F: 5′ GCCTTCTTGAGGGTGTTC 3′ R: 5′ GTTGGTGCCGTAGTTGAG 3′ | XM_045728526.1 |
| igfbp7 | F: 5′ GGGACAAATGCGACAGGA 3′ R: 5′ AAGAAGTGGGTGACGAGGAA 3′ | XM_045757043.1 |
| mhc | F: 5′ TTCGGTATGGATCTTATGG 3′ R: 5′ GAACACGGGAGACTTGC 3′ | XM_045726227.1 |
| mlc1 | F: 5′ CCATCTTCGCTCAGGTT 3′ R: 5′ GTACATCATAGTGCCATTCTC 3′ | XM_045743365.1 |
| mef2 | F: 5′ TCACCGCCCGTTAGGAT 3′ R: 5′ GCCCAGGATGGTTGGAAGA 3′ | XM_045740701.1 |
| pax7 | F: 5′ ATGACGGTAACAGGAGCG 3′ R: 5′ ATGGCGGAGACTGAGGG 3′ | XM_045750481.1 |
| β-actin | F: 5′ AGGTTGCTGCCCTGGTT 3′ R: 5′ CACGCTTGCTCTGTGCC 3′ | XM_045725824.1 |
| Parameters | A | B | C | p-Value |
|---|---|---|---|---|
| IBW (g) | 9.03 ± 0.19 | 9.08 ± 0.21 | 9.02 ± 0.22 | 0.439 |
| FBW (g) | 37.25 ± 3.47 b | 36.22 ± 2.17 b | 44.85 ± 1.68 a | <0.001 |
| SR (%) | 88.67 ± 0.58 | 90.67 ± 1.53 | 89.67 ± 2.08 | 0.343 |
| WGR (%) | 312.77 ± 39.36 b | 299.01 ± 25.88 b | 397.59 ± 24.30 a | <0.001 |
| SGR (%/d) | 1.68 ± 0.11 b | 1.64 ± 0.08 b | 1.91 ± 0.06 a | <0.001 |
| HSI (%) | 6.88 ± 0.63 b | 5.32 ± 0.87 c | 8.12 ± 1.01 a | <0.001 |
| MY (%) | 10.38 ± 0.57 b | 10.10 ± 0.39 b | 11.11 ± 0.78 a | <0.001 |
| CF (g/cm3) | 0.043 ± 0.002 | 0.044 ± 0.004 | 0.045 ± 0.003 | 0.073 |
| Parameters | A | B | C | p-Value |
|---|---|---|---|---|
| Moisture (%) | 63.57 ± 1.17 b | 73.20 ± 1.64 a | 74.50 ± 2.86 a | <0.001 |
| Crude protein (%) | 16.56 ± 0.96 b | 17.17 ± 0.79 b | 21.31 ± 1.17 a | <0.001 |
| Crude lipid (%) | 2.12 ± 0.14 b | 2.11 ± 0.46 b | 2.71 ± 0.45 a | 0.003 |
| Ash (%) | 2.34 ± 0.15 | 2.33 ± 0.34 | 2.12 ± 0.30 | 0.170 |
| Parameters | A | B | C |
|---|---|---|---|
| Threonine | 0.77 ± 0.03 b | 0.99 ± 0.04 a | 0.96 ± 0.77 a |
| Valine | 0.53 ± 0.04 b | 0.42 ± 0.02 c | 0.63 ± 0.03 a |
| Isoleucine | 0.33 ± 0.02 | 0.33 ± 0.02 | 0.31 ± 0.01 |
| Leucine | 0.80 ± 0.05 a | 0.33 ± 0.03 b | 0.30 ± 0.01 b |
| Phenylalanine * | 0.42 ± 0.02 a | 0.40 ± 0.02 a | 0.26 ± 0.01 b |
| Lysine | 3.30 ± 0.18 a | 3.07 ± 0.18 b | 1.47 ± 0.08 c |
| Methionine | 0.28 ± 0.03 a | 0.27 ± 0.03 a | 0.22 ± 0.01 b |
| ΣEAA | 6.42 ± 0.24 a | 5.81 ± 0.21 b | 4.15 ± 0.09 c |
| Serine | 0.97 ± 0.04 c | 1.30 ± 0.03 b | 1.68 ± 0.11 a |
| Glutamate * | 1.37 ± 0.12 c | 1.61 ± 0.15 b | 2.47 ± 0.10 a |
| Glycine * | 1.86 ± 0.08 b | 2.59 ± 0.12 a | 1.95 ± 0.05 b |
| Alanine * | 1.63 ± 0.05 b | 1.98 ± 0.08 a | 1.92 ± 0.12 a |
| Histidine | 1.78 ± 0.09 c | 2.34 ± 0.07 b | 3.68 ± 0.15 a |
| Arginine | 1.57 ± 0.11 a | 1.37 ± 0.12 b | 1.18 ± 0.07 c |
| Tyrosine * | 0.30 ± 0.04 a,b | 0.34 ± 0.03 a | 0.29 ± 0.04 b |
| Proline | 0.65 ± 0.06 b | 0.56 ± 0.04 b | 3.62 ± 0.24 a |
| ΣNEAA | 10.12 ± 0.13 c | 12.08 ± 0.35 b | 16.79 ± 0.16 a |
| ΣDAA | 5.58 ± 0.16 b | 6.91 ± 0.23 a | 6.89 ± 0.17 a |
| ΣTAA | 16.55 ± 0.25 c | 17.89 ± 0.49 b | 20.94 ± 0.23 a |
| ΣEAA/ΣTAA (%) | 38.81 ± 0.99 a | 32.47 ± 0.72 b | 19.81 ± 0.24 c |
| Parameters | A | B | C |
|---|---|---|---|
| C12:0 | 0.08 ± 0.01 | 0.08 ± 0.00 | 0.09 ± 0.01 |
| C14:0 | 0.35 ± 0.01 c | 0.57 ± 0.05 b | 0.77 ± 0.15 a |
| C15:0 | 0.12 ± 0.02 b | 0.10 ± 0.01 b | 0.17 ± 0.03 a |
| C16:0 | 14.47 ± 0.83 a | 12.97 ± 0.56 b | 15.02 ± 0.51 a |
| C17:0 | 0.87 ± 0.08 a | 0.41 ± 0.04 b | 0.48 ± 0.04 b |
| C18:0 | 13.51 ± 0.32 a | 11.01 ± 0.49 b | 7.71 ± 0.19 c |
| C19:0 | 0.57 ± 0.01 b | 0.62 ± 0.04 a | 0.42 ± 0.01 c |
| C20:0 | 0.29 ± 0.01 | 0.29 ± 0.03 | 0.27 ± 0.02 |
| ΣSFA | 30.25 ± 0.92 a | 26.04 ± 0.65 b | 24.93 ± 0.45 c |
| C16:1n-7 | 7.85 ± 0.41 a | 5.79 ± 0.18 b | 4.68 ± 0.26 c |
| C17:1n-7 | 0.68 ± 0.09 b | 0.83 ± 0.06 a | 0.82 ± 0.07 a |
| C18:1n-9 | 16.65 ± 0.98 b | 15.33 ± 0.38 c | 23.56 ± 0.61 a |
| C20:1n-9 | 1.00 ± 0.04 b | 1.27 ± 0.06 a | 0.96 ± 0.02 b |
| ΣMUFA | 26.18 ± 1.11 b | 23.23 ± 0.50 c | 30.02 ± 0.70 a |
| C18:2n-6 | 11.76 ± 1.07 b | 14.93 ± 0.89 a | 10.81 ± 0.9 b |
| C18:3n-3 | 2.14 ± 0.09 c | 2.94 ± 0.13 b | 3.19 ± 0.21 a |
| C20:2n-6 | 3.31 ± 0.12 c | 4.43 ± 0.07 a | 3.59 ± 0.11 b |
| C20:3n-6 | 3.37 ± 0.09 a | 3.33 ± 0.12 a | 3.02 ± 0.23 b |
| C20:4n-6(ARA) | 8.28 ± 0.31 c | 8.88 ± 0.41 b | 10.57 ± 0.32 a |
| C20:5n-3(EPA) | 7.50 ± 0.29 b | 8.43 ± 0.18 a | 5.25 ± 0.15 c |
| C22:2n-6 | 1.12 ± 0.08 b | 1.34 ± 0.11 a | 0.94 ± 0.05 c |
| C22:4n-6 | 1.02 ± 0.06 b | 1.37 ± 0.08 a | 0.79 ± 0.08 c |
| C22:6n-3(DHA) | 5.07 ± 0.04 b | 5.08 ± 0.11 b | 6.89 ± 0.10 a |
| ΣPUFA | 43.57 ± 1.22 c | 50.73 ± 1.02 a | 45.05 ± 0.84 b |
| Σn-3 PUFA | 14.72 ± 0.30 c | 16.45 ± 0.25 a | 15.33 ± 0.37 b |
| Σn-6 PUFA | 28.86 ± 1.02 b | 34.27 ± 1.03 a | 29.72 ± 0.76 b |
| Σn-3/Σn-6(%) | 51.04 ± 1.48 a | 48.05 ± 1.72 b | 51.62 ± 1.85 a |
| Parameters | A | B | C | p-Value |
|---|---|---|---|---|
| Goods coverage (%) | 99.76 ± 0.06 b | 99.88 ± 0.09 a | 99.90 ± 0.06 a | 0.011 |
| Observed species | 488.25 ± 64.87 b | 635.72 ± 62.41 a | 697.22 ± 74.82 a | <0.001 |
| Shannon | 4.27 ± 0.51 b | 5.71 ± 0.70 a | 6.29 ± 0.64 a | <0.001 |
| Simpson | 0.88 ± 0.02 b | 0.92 ± 0.02 a | 0.93 ± 0.02 a | 0.002 |
| Chao1 | 485.60 ± 64.94 b | 633.53 ± 63.52 a | 694.35 ± 73.76 a | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Lv, W.; Zhao, Y.; Huang, W.; Yuan, Q.; Yang, H.; Wang, A.; Zhou, W.; Li, M. Effects of Substituting Tenebrio molitor and Elodea nuttallii as Feed on Growth, Flesh Quality and Intestinal Microbiota of Red Swamp Crayfish (Procambarus clarkii). Foods 2024, 13, 2292. https://doi.org/10.3390/foods13142292
Li M, Lv W, Zhao Y, Huang W, Yuan Q, Yang H, Wang A, Zhou W, Li M. Effects of Substituting Tenebrio molitor and Elodea nuttallii as Feed on Growth, Flesh Quality and Intestinal Microbiota of Red Swamp Crayfish (Procambarus clarkii). Foods. 2024; 13(14):2292. https://doi.org/10.3390/foods13142292
Chicago/Turabian StyleLi, Muyan, Weiwei Lv, Yifan Zhao, Weiwei Huang, Quan Yuan, Hang Yang, Aimin Wang, Wenzong Zhou, and Mingyou Li. 2024. "Effects of Substituting Tenebrio molitor and Elodea nuttallii as Feed on Growth, Flesh Quality and Intestinal Microbiota of Red Swamp Crayfish (Procambarus clarkii)" Foods 13, no. 14: 2292. https://doi.org/10.3390/foods13142292
APA StyleLi, M., Lv, W., Zhao, Y., Huang, W., Yuan, Q., Yang, H., Wang, A., Zhou, W., & Li, M. (2024). Effects of Substituting Tenebrio molitor and Elodea nuttallii as Feed on Growth, Flesh Quality and Intestinal Microbiota of Red Swamp Crayfish (Procambarus clarkii). Foods, 13(14), 2292. https://doi.org/10.3390/foods13142292








