Developing Effective Radio Frequency Vacuum Drying Processes for Moutan Cortex: Effect on Moisture Migration, Drying Kinetics, Physicochemical Quality, and Microstructure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
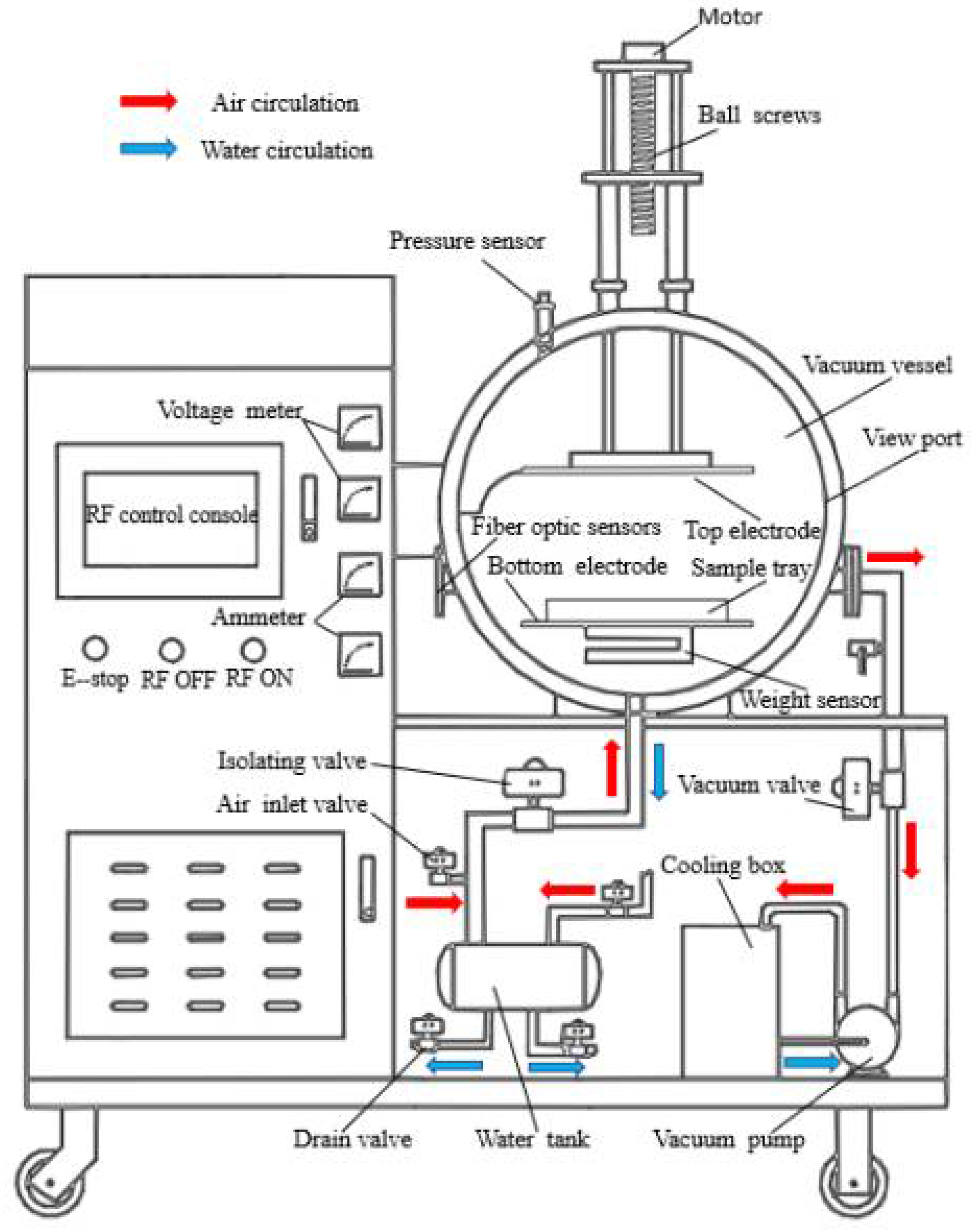
2.2. Experimental Methods
2.3. Moisture Content and Drying Rate
2.4. Color Measurement
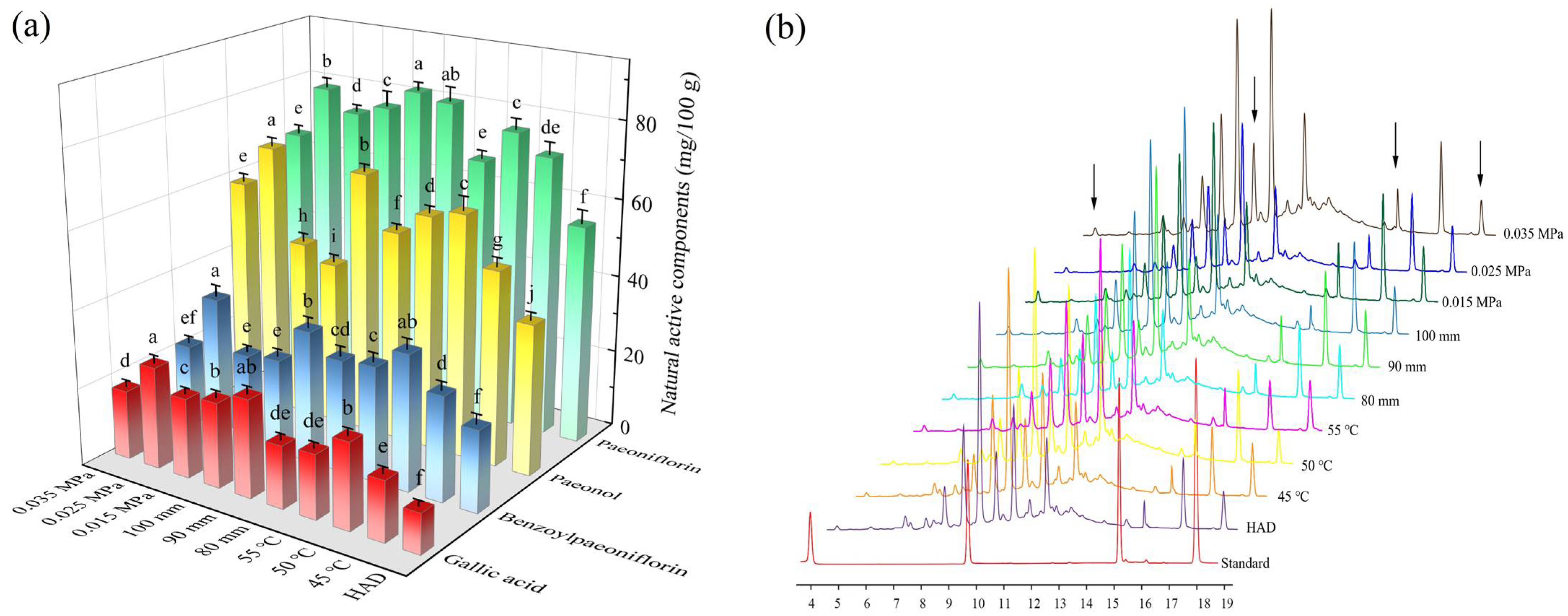
2.5. Determination of Natural Active Substances
2.6. Measurement of Basic Nutrient Compound
2.6.1. Preparation of Polysaccharides, Total Phenolics, Total Flavonoids, and Antioxidant Extracts
2.6.2. Determination of Polysaccharide Content
2.6.3. Determination of Total Phenolic Content
2.6.4. Determination of Total Flavonoid Content
2.6.5. Determination of Antioxidant Activity
2.7. Microstructure
2.8. Statistical Analysis
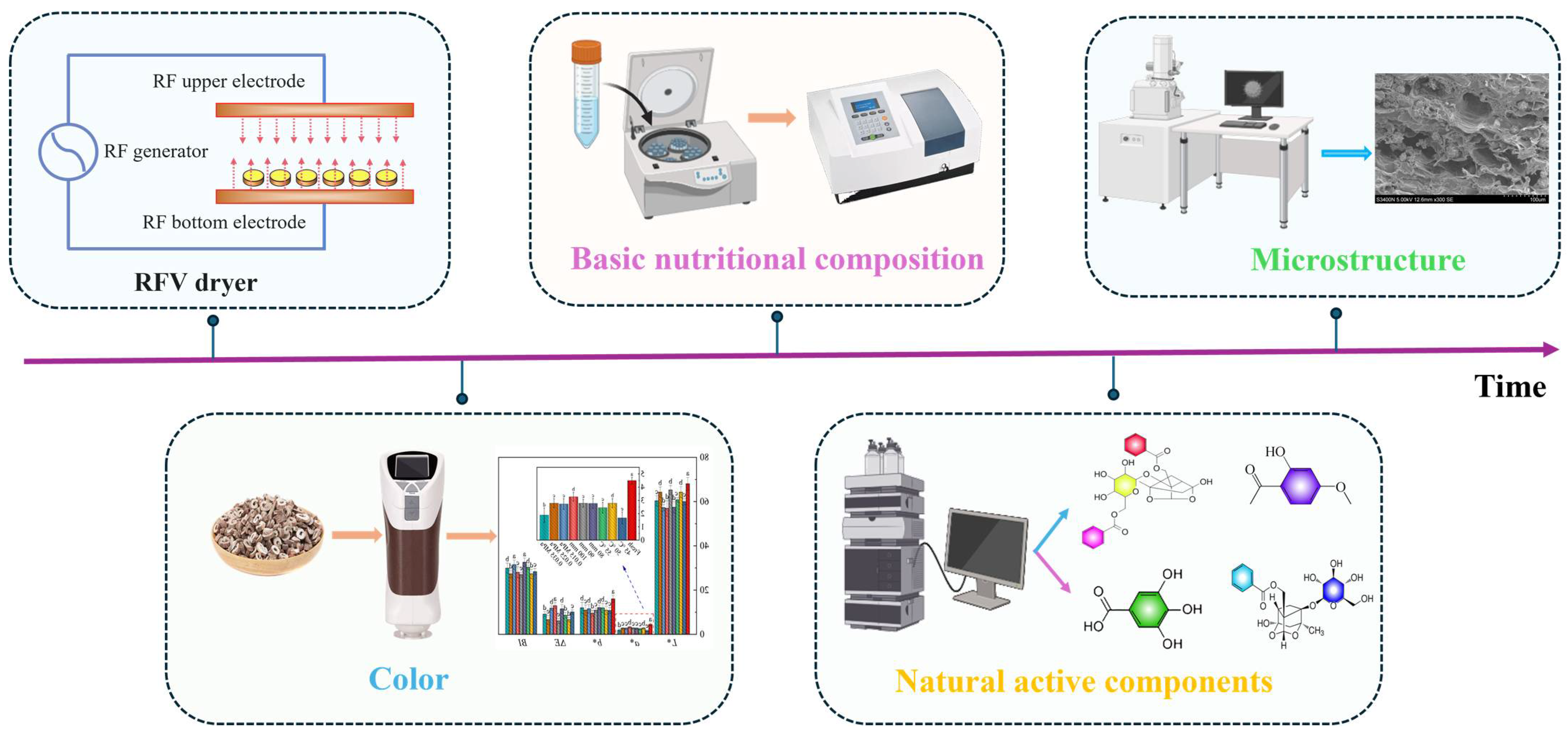
3. Results and Discussion
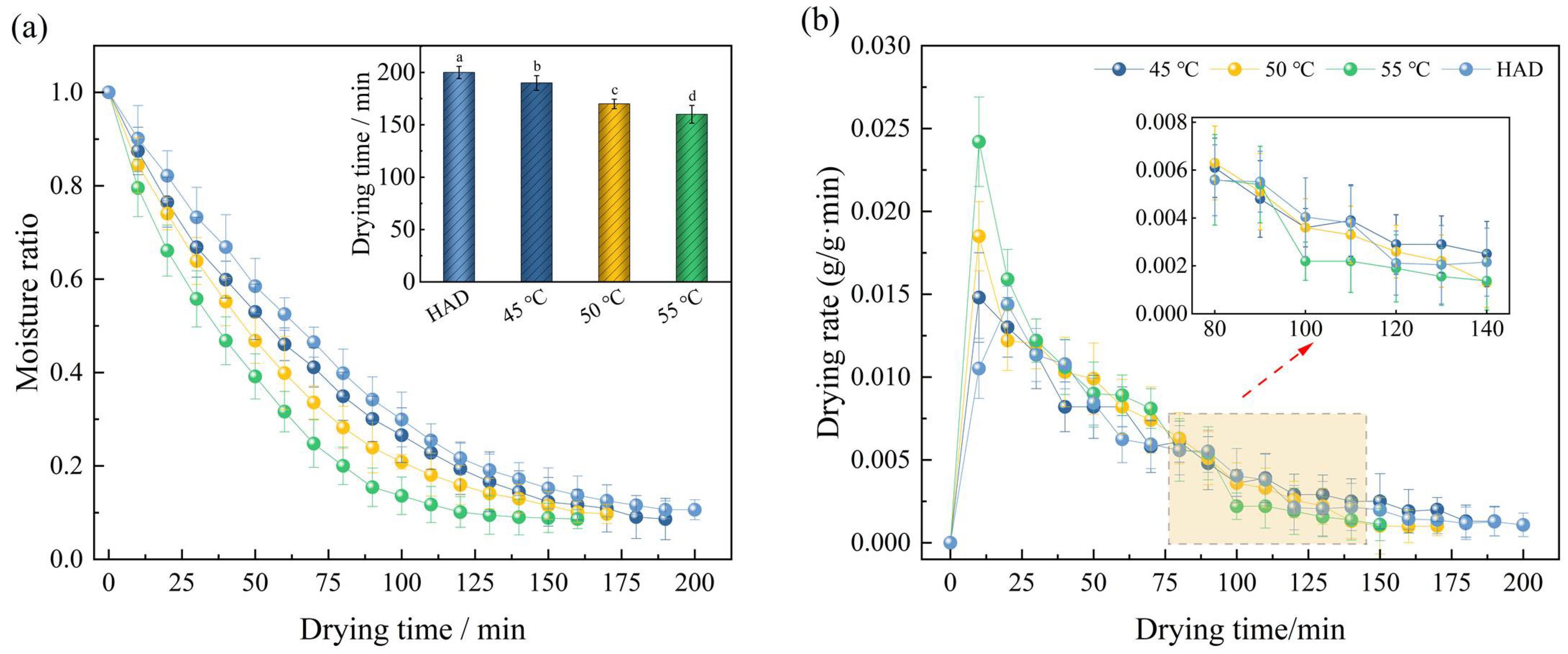
3.1. Drying Characteristics
3.1.1. Effect of Drying Temperatures on the Drying Characteristics
3.1.2. Effect of Plate Spacing on Drying Characteristics
3.1.3. Effect of Vacuum Degree on Drying Characteristics
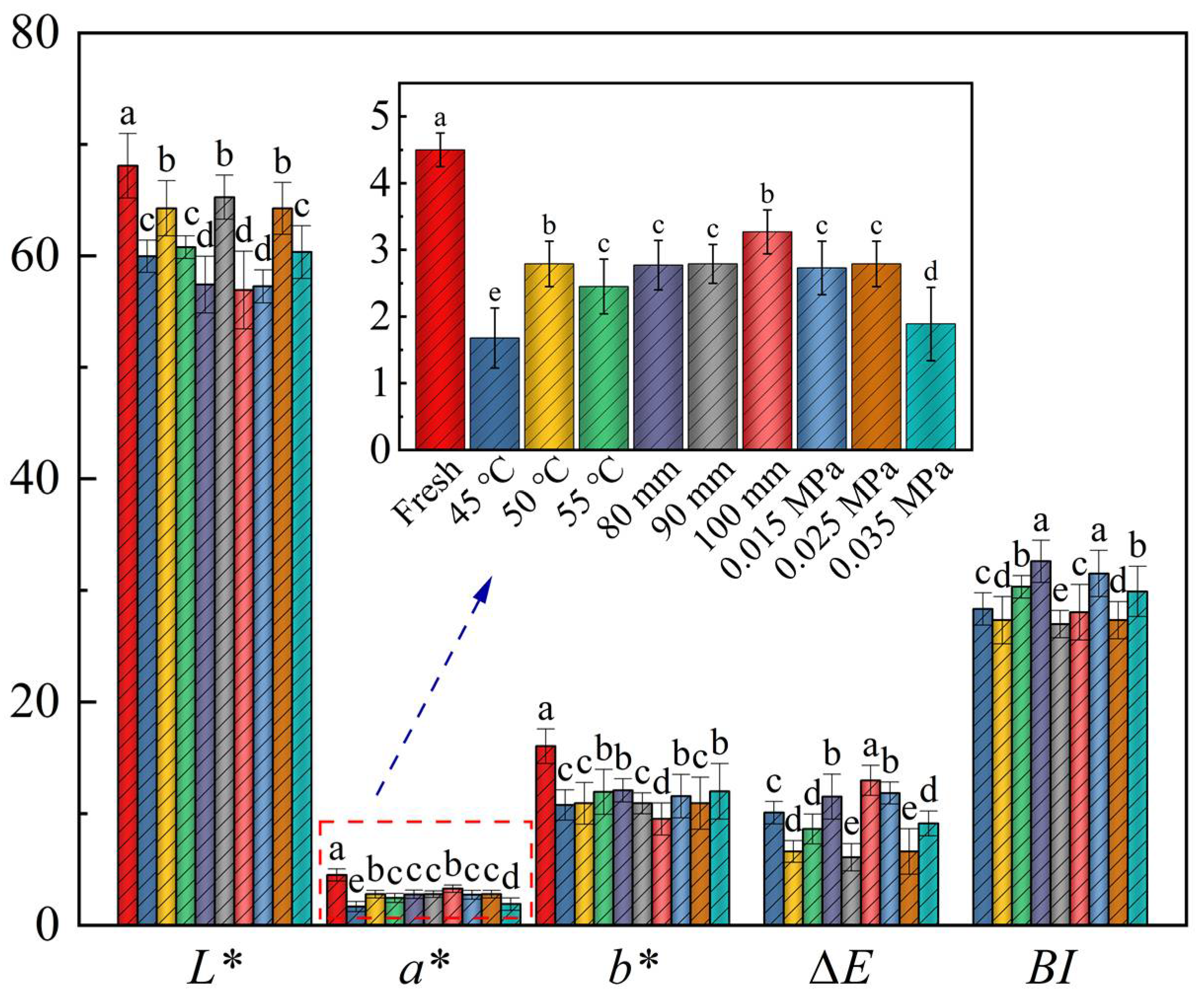
3.2. Color
3.3. Natural Active Components Content
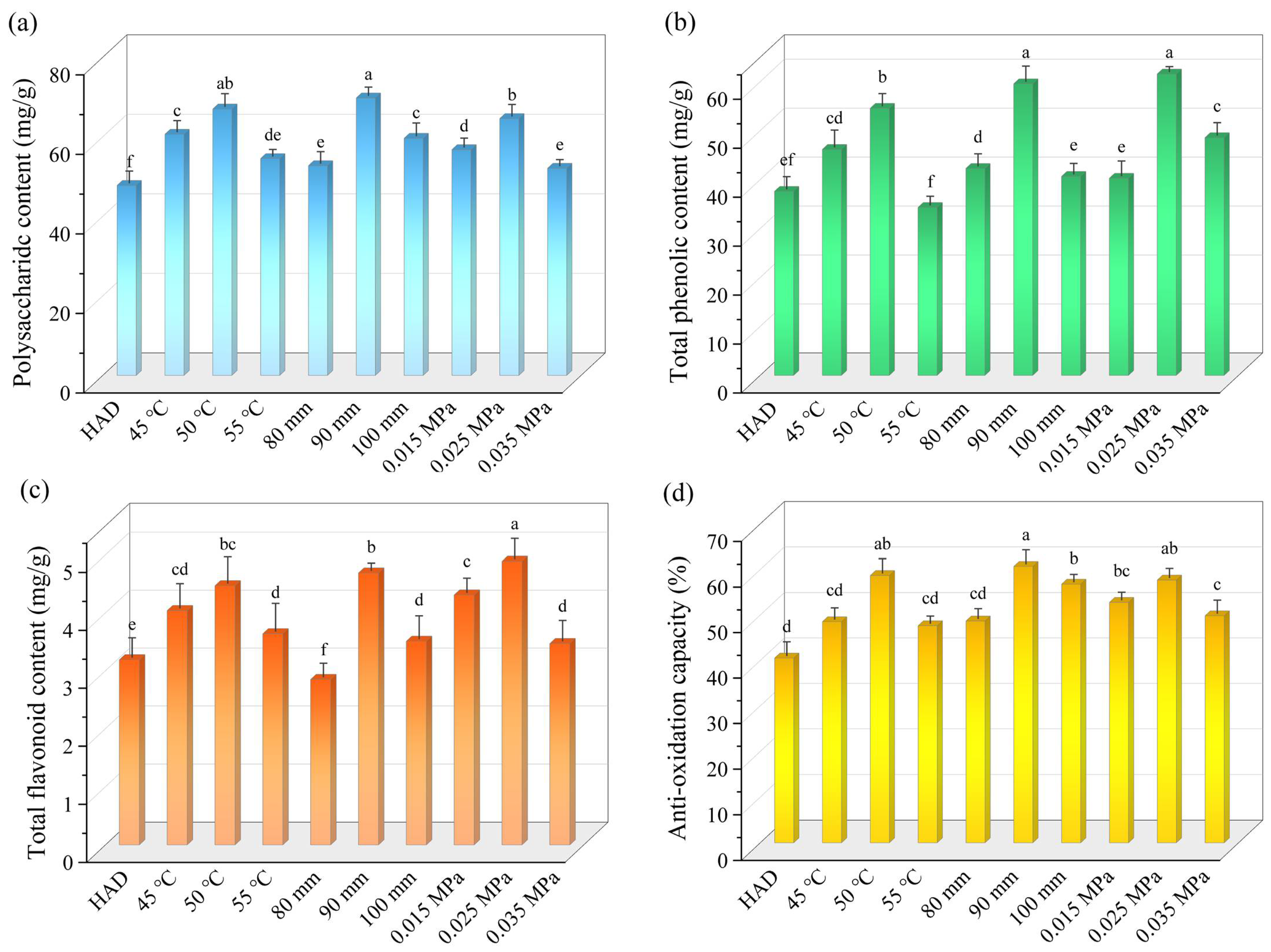
3.4. Polysaccharides Content
3.5. Total Phenolic Content
3.6. Total Flavonoid Content
3.7. DPPH Free-Radical Scavenging Capacity
3.8. Microstructure
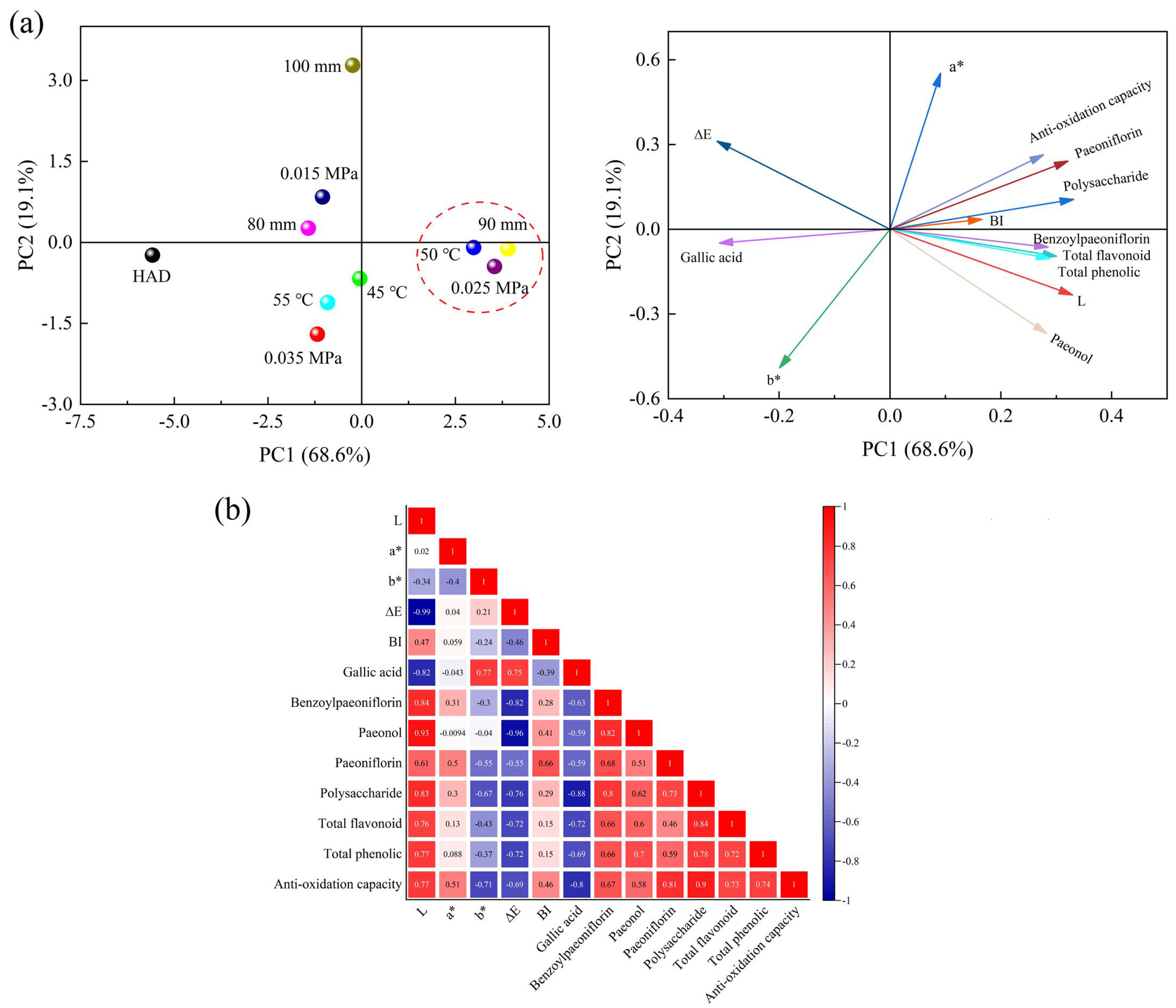
3.9. PCA Analysis and Correlation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, C.; Chen, Q.; Li, J.; Lin, X.; Xue, X.; Cai, X.; Chen, Y.; Sun, Y.; Wang, S.; Zhang, Y.; et al. Identification of potential anti-inflammatory components in Moutan Cortex by bio-affinity ultrafiltration coupled with ultra-performance liquid chromatography mass spectrometry. Front. Pharmacol. 2024, 15, 1358640. [Google Scholar] [CrossRef]
- Meng, L.; Chen, Y.; Zheng, Z.; Wang, L.; Xu, Y.; Li, X.; Xiao, Z.; Tang, Z.; Wang, Z. Ultrasound-Assisted Extraction of Paeonol from Moutan Cortex: Purification and Component Identification of Extract. Molecules 2024, 29, 622. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, L.; Zhu, M.; Yang, B.; Yang, Y.; Jia, X.; Feng, L. Moutan Cortex polysaccharide ameliorates diabetic kidney disease via modulating gut microbiota dynamically in rats. Int. J. Biol. Macromol. 2022, 206, 849–860. [Google Scholar] [CrossRef]
- Li, C.; Li, M.; Yang, H.; Li, P.; Gao, W. Rapid characterization of chemical markers for discrimination of Moutan Cortex and its processed products by direct injection-based mass spectrometry profiling and metabolomic method. Phytomedicine 2018, 45, 76–83. [Google Scholar] [CrossRef]
- Hu, D.; Yang, G.; Liu, X.; Qin, Y.; Zhang, F.; Sun, Z.; Wang, X. Comparison of different drying technologies for coffee pulp tea: Changes in color, taste, bioactive and aroma component. LWT-Food Sci. Technol. 2024, 200, 116193. [Google Scholar] [CrossRef]
- Wu, B.; Guo, X.; Guo, Y.; Ma, H.; Zhou, C. Enhancing jackfruit infrared drying by combining ultrasound treatments: Effect on drying characteristics, quality properties and microstructure. Food Chem. 2021, 358, 129845. [Google Scholar] [CrossRef]
- Zang, Z.; Wan, F.; Ma, G.; Xu, Y.; Wang, T.; Wu, B.; Huang, X. Enhancing peach slices radio frequency vacuum drying by combining ultrasound and ultra-high pressure as pretreatments: Effect on drying characteristics, physicochemical quality, texture and sensory evaluation. Ultrason. Sonochem. 2024, 103, 106786. [Google Scholar] [CrossRef]
- An, K.; Fu, M.; Zhang, H.; Tang, D.; Xu, Y.; Xiao, G. Effect of ethyl oleate pretreatment on blueberry (Vaccinium corymbosum L.): Drying kinetics, antioxidant activity, and structure of wax layer. J. Food Sci. Technol. 2019, 56, 783–791. [Google Scholar] [CrossRef]
- Jiang, C.; Wan, F.; Zang, Z.; Zhang, Q.; Ma, G.; Huang, X. Effect of an Ultrasound Pre-Treatment on the Characteristics and Quality of Far-Infrared Vacuum Drying with Cistanche Slices. Foods 2022, 11, 866. [Google Scholar] [CrossRef]
- Tao, Y.; Han, M.; Gao, Y.; Han, Y.; Show, P.L.; Liu, C.; Ye, Y.; Xie, G. Applications of water blanching, surface contacting ultrasound-assisted air drying, and their combination for dehydration of white cabbage: Drying mechanism, bioactive profile, color and rehydration property. Ultrason. Sonochem. 2019, 53, 192–201. [Google Scholar] [CrossRef]
- Zang, Z.; Zhang, Q.; Huang, X.; Jiang, C.; He, C.; Wan, F. Effect of Ultrasonic Combined with Vacuum Far-infrared on the Drying Characteristics and Physicochemical Quality of Angelica sinensis. Food Bioprocess Technol. 2023, 16, 2455–2470. [Google Scholar] [CrossRef]
- Shi, X.; Yang, Y.; Li, Z.; Wang, X.; Liu, Y. Moisture transfer and microstructure change of banana slices during contact ultrasound strengthened far-infrared radiation drying. Innov. Food Sci. Emerg. Technol. 2020, 66, 102537. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, C.; Zhang, H.; Yang, N.; Wang, C.; Jike, X.; Zhang, T.; Lei, H. Comparison of different drying technologies for kiwifruit pomace: Changes in physical characteristics, nutritional properties and antioxidant capacities. Food Chem. 2024, 451, 139497. [Google Scholar] [CrossRef] [PubMed]
- Yamchi, A.A.; Sharifian, F.; Khalife, E.; Kaveh, M. Drying kinetic, thermodynamic and quality analyses of infrared drying of truffle slices. J. Food Sci. 2024, 89, 3666–3686. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, S. Recent developments in radio frequency drying of food and agricultural products: A review. Dry. Technol. 2019, 37, 271–286. [Google Scholar] [CrossRef]
- Zhou, X.; Li, R.; Lyng, J.G.; Wang, S.; Wang, S. Dielectric properties of kiwifruit associated with a combined radio frequency vacuum and osmotic drying. J. Food Eng. 2018, 239, 72–82. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, L.; Wang, S. Computer simulation of radio frequency selective heating of insects in soybeans. Int. J. Heat Mass Transf. 2015, 90, 406–417. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, B.; Marra, F.; Wang, S. Computational modelling of the iMPact of polystyrene containers on radio frequency heating uniformity improvement for dried soybeans. Innov. Food Sci. Emerg. Technol. 2016, 33, 365–380. [Google Scholar] [CrossRef]
- Mahmood, N.; Liu, Y.; Munir, Z.; Zhang, Y.; Niazi, B.M.K. Effects of hot air assisted radio frequency drying on heating uniformity, drying characteristics and quality of paddy. LWT-Food Sci. Technol. 2022, 158, 113131. [Google Scholar] [CrossRef]
- Zang, Z.; Huang, X.; He, C.; Zhang, Q.; Jiang, C.; Wan, F. Improving Drying Characteristics and Physicochemical Quality of Angelica sinensis by Novel Tray Rotation Microwave Vacuum Drying. Foods 2023, 12, 1202. [Google Scholar] [CrossRef]
- Xu, Y.; Zang, Z.; Zhang, Q.; Wang, T.; Shang, J.; Huang, X.; Wan, F. Characteristics and Quality Analysis of Radio Frequency-Hot Air Combined Segmented Drying of Wolfberry (Lycium barbarum). Foods 2022, 11, 1645. [Google Scholar] [CrossRef] [PubMed]
- Shewale, S.R.; Rajoriya, D.; Bhavya, M.L.; Hebbar, H.U. Application of radiofrequency heating and low humidity air for sequential drying of apple slices: Process intensification and quality improvement. LWT-Food Sci. Technol. 2019, 135, 109904. [Google Scholar] [CrossRef]
- Li, M.; Tian, Y.; Jiang, L.; Xu, J.; Li, R.; Wang, S. Developing effective radio frequency drying processes for tiger nuts: Dynamic analysis of moisture state, dielectric properties and quality. J. Food Eng. 2024, 375, 112058. [Google Scholar] [CrossRef]
- Mao, Y.; Wang, S. Simultaneous hot-air assisted radio frequency drying and disinfestation for in-shell walnuts using a two-stage strategy. LWT-Food Sci. Technol. 2021, 151, 112134. [Google Scholar] [CrossRef]
- Wang, C.; Kou, X.; Zhou, X.; Li, R.; Wang, S. Effects of layer arrangement on heating uniformity and product quality after hot air assisted radio frequency drying of carrot. Innov. Food Sci. Emerg. Technol. 2021, 69, 102667. [Google Scholar] [CrossRef]
- Shang, J.; Ma, G.; Wan, F.; Zang, Z.; Xu, Y.; Zhang, Q.; Wang, T.; Huang, X. Drying Characteristics of Moutan Cortex by Rotary Wheel Microwave Vacuum Drying and Its Influence on Quality. Agriculture 2024, 14, 563. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Q.; Pan, W.; Zhang, Y.; Li, J.; Xue, X.; Lei, X.; Wang, S.; Meng, J. Moutan cortex exerts blood-activating and anti-inflammatory effects by regulating coagulation-inflammation cascades pathway in cells, rats and zebrafish. J. Ethnopharmacol. 2023, 320, 117398. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Ye, L.; Zhang, Y.; Wu, M.; Cao, H.; Ma, Z. Study on drying characteristics and kinetics of Moutan Cortex decoction pieces based on LF-NMR/MRI and TA-HD plus. J. Jinan Univ. 2023, 44, 203–210. [Google Scholar]
- Huang, X.; Li, W.; Wang, Y.; Wan, F. Drying characteristics and quality of Stevia rebaudiana leaves by far-infrared radiation. LWT-Food Sci. Technol. 2021, 140, 110638. [Google Scholar] [CrossRef]
- Shang, J.; Zhang, Q.; Wang, T.; Xu, Y.; Zang, Z.; Wang, F.; Yue, Y.; Huang, X. Effect of Ultrasonic Pretreatment on the Far-Infrared Drying Process and Quality Characteristics of Licorice. Foods 2023, 12, 2414. [Google Scholar] [CrossRef]
- Zhang, Q.; Wan, F.; Zang, Z.; Jiang, C.; Xu, Y.; Huang, X. Effect of ultrasonic far-infrared synergistic drying on the characteristics and qualities of wolfberry (Lycium barbarum L.). Ultrason. Sonochem. 2022, 89, 106134. [Google Scholar] [CrossRef] [PubMed]
- National Pharmacopoeia Commission. Chinese Pharmacopoeia, 11th ed.; China Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Ni, J.; Zielinska, M.; Wang, J.; Fang, X.; Sutar, P.P.; Li, S.; Li, X.; Wang, H.; Xiao, H. Post-harvest ripening affects drying behavior, antioxidant capacity and flavor release of peach via alteration of cell wall polysaccharides content and nanostructures, water distribution and status. Food Res. Int. 2023, 170, 113037. [Google Scholar] [CrossRef] [PubMed]
- Yamchi, A.A.; Yeganeh, R.; Kouchakzadeh, A. Effect of ultrasonic pretreatment on drying kinetics and physio-mechanical characteristics of peach slices. J. Food Process Eng. 2022, 45, e14053. [Google Scholar] [CrossRef]
- Chao, E.; Tian, J.; Fan, L.; Zhang, T. Drying methods influence the physicochemical and functional properties of seed-used pumpkin. Food Chem. 2021, 369, 130937. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Zang, Z.; Wan, F.; Zhang, Q.; Shang, J.; Xu, Y.; Jiang, C.; Wang, T.; Huang, X. Effect of Ultrasonic Pretreatment on Radio Frequency Vacuum Drying Characteristics and Quality of Codonopsis pilosula Slices. Agriculture. 2022, 13, 72. [Google Scholar] [CrossRef]
- Feng, Y.; Tan, C.P.; Zhou, C.; Yagoub, A.E.G.A.; Xu, B.; Sun, Y.; Ma, H.; Xu, X.; Yu, X. Effect of freeze-thaw cycles pretreatment on the vacuum freeze-drying process and physicochemical properties of the dried garlic slices. Food Chem. 2020, 324, 126883. [Google Scholar] [CrossRef] [PubMed]
- Lay, M.; Anuar, S.A.; Sadeh, M.; Nurestri, A. Phytochemical constituents, nutritional values, phenolics, flavonols, flavonoids, antioxidant and cytotoxicity studies on phaleria macrocarpa (Scheff.) Boerl fruits. BMC Complement. Altern. Med. 2014, 14, 152. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xiao, D.; Lagnika, C.; Song, J.; Li, D.; Liu, C.; Jiang, N.; Zhang, M.; Duan, X. A comparative study of drying methods on physical characteristics, nutritional properties and antioxidant capacity of broccoli. Dry. Technol. 2020, 38, 1378–1388. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Vidyarthi, S.K.; Wang, Q.; Gao, L.; Li, B.; Wei, Q.; Liu, Y.; Xiao, H. Effects of different drying methods on drying kinetics, physicochemical properties, microstructure, and energy consumption of potato (Solanum tuberosum L.) cubes. Dry. Technol. 2020, 39, 418–431. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Cheng, J.; Wang, J.; Ding, Z.; Yuan, X.; Zhou, S.; Liu, X. Effects of Moisture, Temperature, and Salt Content on the Dielectric Properties of Pecan Kernels during Microwave and Radio Frequency Drying Processes. Foods 2019, 8, 385. [Google Scholar] [CrossRef]
- Jiang, C.; Wan, F.; Zang, Z.; Zhang, Q.; Xu, Y.; Huang, X. Influence of far-infrared vacuum drying on drying kinetics and quality characteristics of Cistanche slices. J. Food Process. Preserv. 2022, 46, e17144. [Google Scholar] [CrossRef]
- Huang, Z.; Zhu, H.; Yan, R.; Wang, S. Simulation and prediction of radio frequency heating in dry soybeans. Biosyst. Eng. 2015, 129, 34–47. [Google Scholar] [CrossRef]
- Wang, W.; Tang, J.; Zhao, Y. Investigation of hot-air assisted continuous radio frequency drying for improving drying efficiency and reducing shell cracks of inshell hazelnuts: The relationship between cracking level and nut quality. Food Bioprod. Process. 2021, 125, 46–56. [Google Scholar] [CrossRef]
- Ji, Z.; Zhao, D.; Yin, J.; Ding, S.; Liu, X.; Hao, J. Quality analysis and pectin characteristics of winter jujube processed by microwave coupled with pulsed vacuum drying (MPVD). LWT-Food Sci. Technol. 2024, 201, 116236. [Google Scholar] [CrossRef]
- Dinani, S.T.; Hamdami, N.; Shahedi, M.; Havet, M. Quality assessment of mushroom slices dried by hot air combined with an electrohydrodynamic (EHD) drying system. Food Bioprod. Process. 2015, 94, 572–580. [Google Scholar] [CrossRef]
- Zang, Z.; Huang, X.; Zhang, Q.; Jiang, C.; Wang, T.; Shang, J.; He, C.; Wan, F. Evaluation of the effect of ultrasonic pretreatment on vacuum far-infrared drying characteristics and quality of Angelica sinensis based on entropy weight-coefficient of variation method. J. Food Sci. 2023, 88, 1905–1923. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Xiong, C.; Sutar, P.P.; Mujumdar, A.S.; Pei, Y.; Yang, X.; Ji, X.; Zhang, Q.; Xiao, H. An emerging pretreatment technology for reducing postharvest loss of vegetables-a case study of red pepper (Capsicum annuum L.) drying. Dry. Technol. 2022, 40, 1620–1628. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, X.; Xiao, H.; Shan, C.; Li, Y.; Yang, T. Quality and Process Optimization of Infrared Combined Hot Air Drying of Yam Slices Based on BP Neural Network and Gray Wolf Algorithm. Foods 2024, 13, 434. [Google Scholar] [CrossRef]
- Zhao, Y.; Bi, J.; Yi, J.; Njoroge, D.; Peng, J.; Hou, C. Comparison of dynamic water distribution and micro-structure formation of shiitake mushrooms during hot air and far infrared radiation drying by low-field nuclear magnetic resonance and scanning electron microscopy. J. Sci. Food Agric. 2018, 99, 2826–2834. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, R.; Zhang, B.; Pei, S.; Liu, Q.; Ramaswamy, H.S.; Wang, S. Radio Frequency-Vacuum Drying of Kiwifruits: Kinetics, Uniformity, and Product Quality. Food Bioprocess Technol. 2018, 11, 2094–2109. [Google Scholar] [CrossRef]
- Yao, L.; Fan, L.; Duan, Z. Effect of different pretreatments followed by hot-air and far-infrared drying on the bioactive compounds, physicochemical property and microstructure of mango slices. Food Chem. 2020, 305, 125477. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, R.; Xia, Y.; Shao, X.; Wang, Y.; Zhang, W. Image recognition technology provides insights into relationships between anthocyanin degradation and color variation during jet drying of black carrot. Food Chem. 2024, 450, 139460. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, O.; Malvandi, A.; Vargas, L.; Feng, H. Drying characteristics and quality attributes of apple slices dried by a non-thermal ultrasonic contact drying method. Ultrason. Sonochem. 2021, 73, 105510. [Google Scholar] [CrossRef] [PubMed]
- Garrido, I.; Hernández, M.S.; Llerena, J.L.; Espinosa, F. Effect of Water Supplementation on Oxidant/Antioxidant Activities and Total Phenol Content in Growing Olives of the Morisca and Manzanilla Varieties. Antioxidants 2022, 11, 729. [Google Scholar] [CrossRef] [PubMed]
- Salehi, F.; Inanloodoghouz, M. Effects of gum-based coatings combined with ultrasonic pretreatment before drying on quality of sour cherries. Ultrason. Sonochem. 2023, 100, 106633. [Google Scholar] [CrossRef] [PubMed]
- Konstantinos, P.; Penta, P.; John, B.G.; Costas, E.S.; Michael, C.B.; Christopher, J.S.; Quan, V.V. Effect of vacuum-drying, hot air-drying and freeze-drying on polyphenols and antioxidant capacity of lemon (Citrus limon) pomace aqueous extracts. Int. J. Food Sci. Technol. 2017, 52, 880–887. [Google Scholar] [CrossRef]
- Peinado, J.; Lerma, N.L.; Moreno, J.A.; Peinado, R.A. Antioxidant activity of different phenolics fractions isolated in must from Pedro Ximenez grapes at different stages of the off-vine drying process. Food Chem. 2008, 114, 1050–1055. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zang, Z.; Wan, F.; Jia, H.; Ma, G.; Xu, Y.; Zhao, Q.; Wu, B.; Lu, H.; Huang, X. Developing Effective Radio Frequency Vacuum Drying Processes for Moutan Cortex: Effect on Moisture Migration, Drying Kinetics, Physicochemical Quality, and Microstructure. Foods 2024, 13, 2294. https://doi.org/10.3390/foods13142294
Zang Z, Wan F, Jia H, Ma G, Xu Y, Zhao Q, Wu B, Lu H, Huang X. Developing Effective Radio Frequency Vacuum Drying Processes for Moutan Cortex: Effect on Moisture Migration, Drying Kinetics, Physicochemical Quality, and Microstructure. Foods. 2024; 13(14):2294. https://doi.org/10.3390/foods13142294
Chicago/Turabian StyleZang, Zepeng, Fangxin Wan, Haiwen Jia, Guojun Ma, Yanrui Xu, Qiaozhu Zhao, Bowen Wu, Hongyang Lu, and Xiaopeng Huang. 2024. "Developing Effective Radio Frequency Vacuum Drying Processes for Moutan Cortex: Effect on Moisture Migration, Drying Kinetics, Physicochemical Quality, and Microstructure" Foods 13, no. 14: 2294. https://doi.org/10.3390/foods13142294




