In Vitro Evaluation of Probiotic Properties and Anti-Pathogenic Effects of Lactobacillus and Bifidobacterium Strains as Potential Probiotics
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Bacterial Strains and Culture Conditions
2.2. Cell-Free Supernatants Preparation
2.3. Tolerance to Simulated Gastrointestinal Conditions
2.4. Auto-Aggregation and Co-Aggregation Assay
2.5. Bacterial Adhesion to Solvents Assay
2.6. Cytotoxicity Assay and Adhesion Determination
2.7. Antimicrobial Activity
2.8. Minimum Inhibitory Concentrations of the Cell-Free Supernatants
2.9. Biofilms Eradication and Inhibition Assay
2.10. Inhibition of Gas Production
2.11. Statistical Analysis
3. Results and Discussion
3.1. Resistance of the Probiotic Strains to Simulated Gastrointestinal Conditions
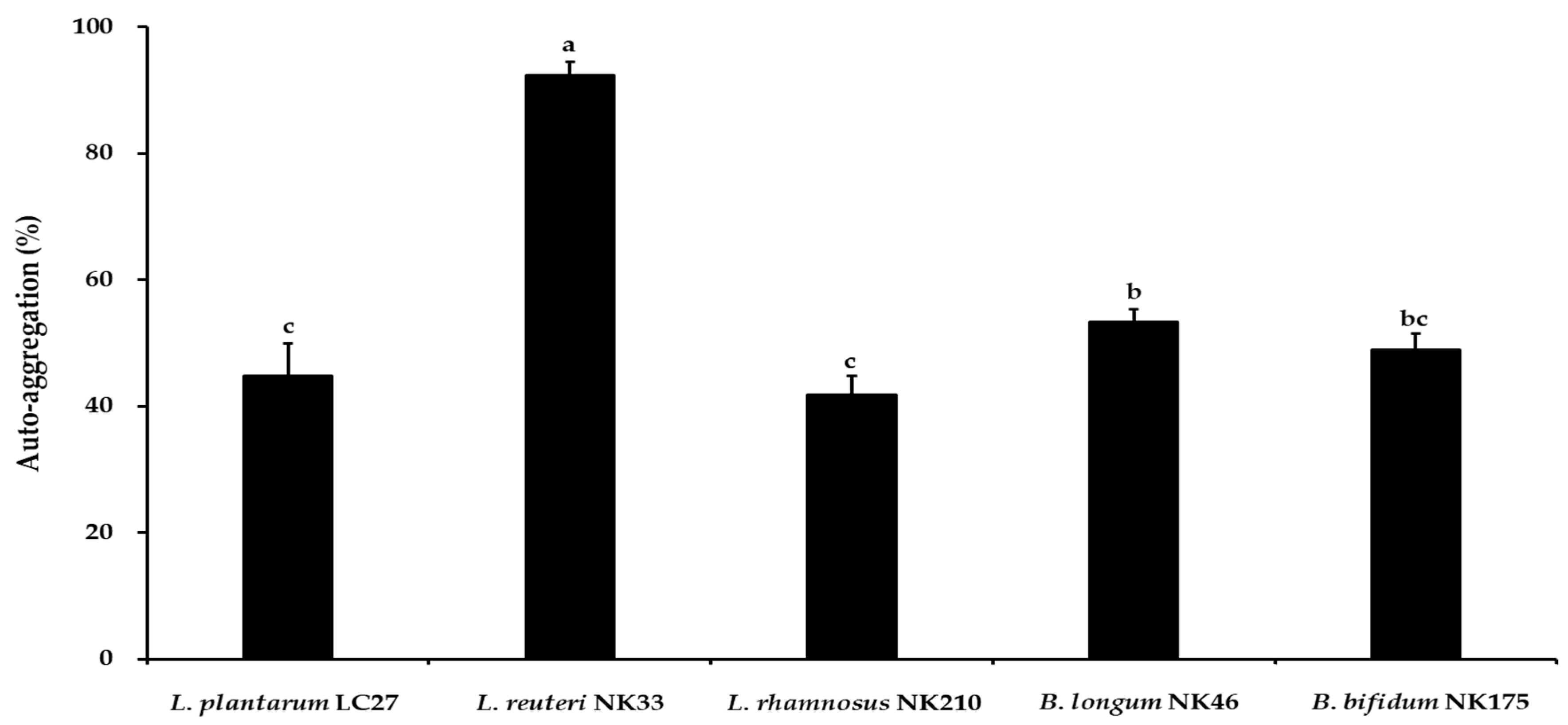
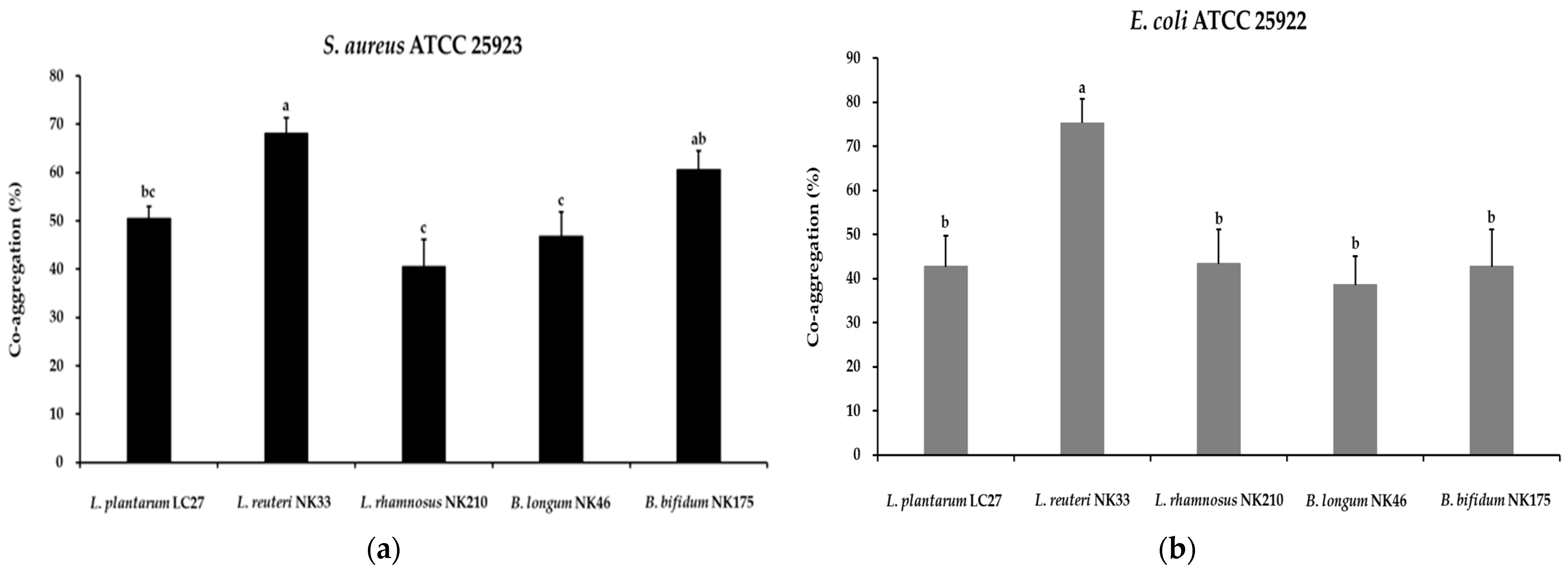
3.2. Auto-Aggregation and Co-Aggregation Activity
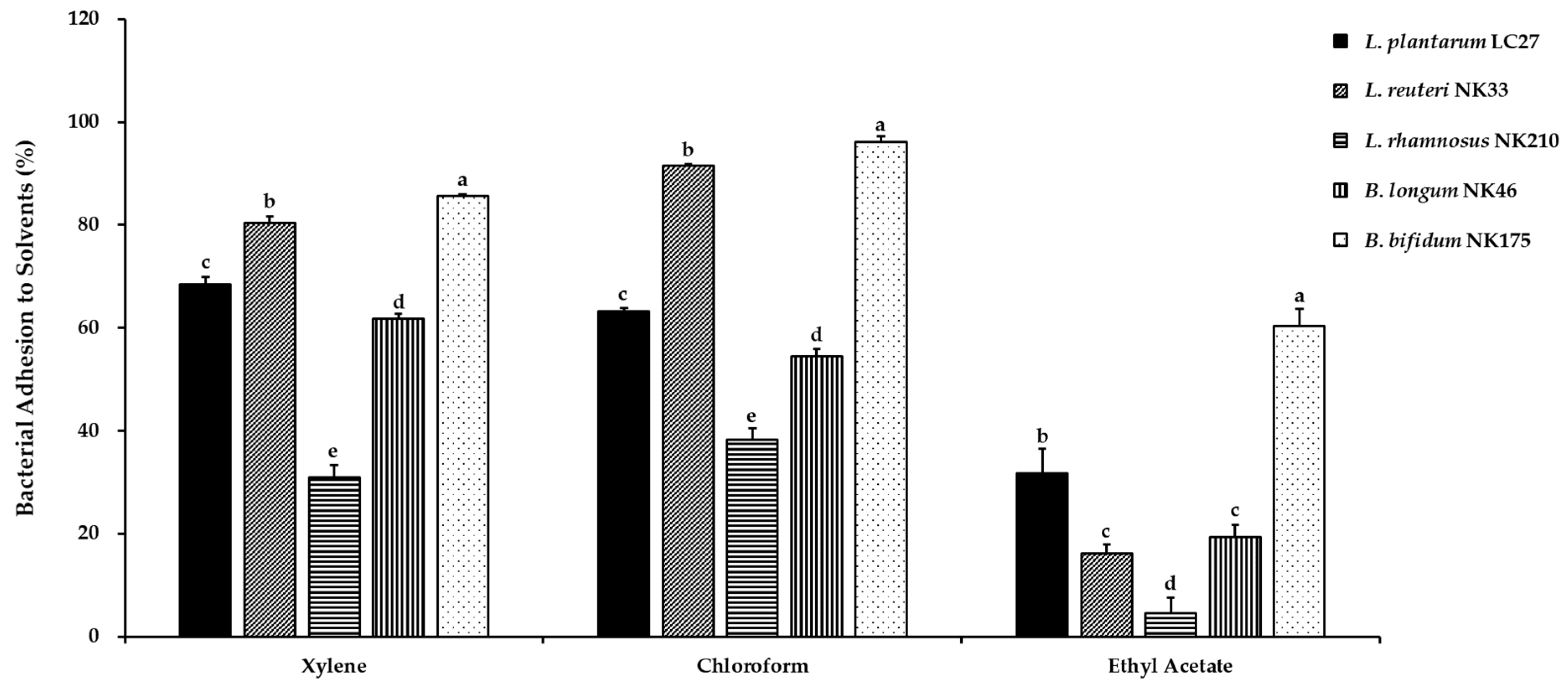
3.3. Cell Surface Hydrophobicity
3.4. Adhesion Ability of the Probiotic Strains
3.5. Antimicrobial Activity of the Probiotic Strains
3.6. Antibiofilm Activity of the Probiotic Strains
3.7. Inhibition of Gas Production by E. coli from the Probiotic Strains
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Martín, R.; Miquel, S.; Ulmer, J.; Langella, P.; Bermudez-Humaran, L. Gut ecosystem: How microbes help us. Benef. Microbes 2014, 5, 219–233. [Google Scholar] [CrossRef]
- Matamoros, S.; Gras-Leguen, C.; Le Vacon, F.; Potel, G.; de La Cochetiere, M.-F. Development of intestinal microbiota in infants and its impact on health. Trends Microbiol. 2013, 21, 167–173. [Google Scholar] [CrossRef]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. ISRN Nutr. 2013, 2013, 481651. [Google Scholar] [CrossRef]
- Rolfe, R.D. The role of probiotic cultures in the control of gastrointestinal health. J. Nutr. 2000, 130, 396S–402S. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Lee, A.; Yoo, H.J.; Kim, M.; Noh, G.M.; Lee, J.H. Supplementation with the probiotic strain Weissella cibaria JW15 enhances natural killer cell activity in nondiabetic subjects. J. Funct. Foods 2018, 48, 153–158. [Google Scholar] [CrossRef]
- Lee, H.J.; Hong, J.K.; Kim, J.-K.; Kim, D.-H.; Jang, S.W.; Han, S.-W.; Yoon, I.-Y. Effects of probiotic NVP-1704 on mental health and sleep in healthy adults: An 8-week randomized, double-blind, placebo-controlled trial. Nutrients 2021, 13, 2660. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-C.; Tu, Y.-T.; Lee, C.-C.; Tsai, S.-C.; Hsu, H.-Y.; Tsai, T.-Y.; Liu, T.-H.; Young, S.-L.; Lin, J.-S.; Huang, C.-C. Lactobacillus plantarum TWK10 improves muscle mass and functional performance in frail older adults: A randomized, double-blind clinical trial. Microorganisms 2021, 9, 1466. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Kim, B.; Ban, J.; Lee, J.; Kim, B.J.; Choi, B.S.; Hwang, S.; Ahn, K.; Kim, J. A randomized trial of L actobacillus plantarum CJLP 133 for the treatment of atopic dermatitis. Pediatr. Allergy Immunol. 2012, 23, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Papizadeh, M.; Rohani, M.; Nahrevanian, H.; Javadi, A.; Pourshafie, M.R. Probiotic characters of Bifidobacterium and Lactobacillus are a result of the ongoing gene acquisition and genome minimization evolutionary trends. Microb. Pathog. 2017, 111, 118–131. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Mehwish, H.M.; Siddiq, M.; Haobin, Z.; Zhu, J.; Yan, L.; Shao, D.; Xu, X.; Shi, J. Identification, characterization, and probiotic potential of Lactobacillus rhamnosus isolated from human milk. LWT 2017, 84, 271–280. [Google Scholar] [CrossRef]
- Tham, C.S.-C.; Peh, K.-K.; Bhat, R.; Liong, M.-T. Probiotic properties of bifidobacteria and lactobacilli isolated from local dairy products. Ann. Microbiol. 2012, 62, 1079–1087. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, P.; Zhang, X. Probiotics regulate gut microbiota: An effective method to improve immunity. Molecules 2021, 26, 6076. [Google Scholar] [CrossRef]
- Kim, S.-K.; Guevarra, R.B.; Kim, Y.-T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.-H. Role of probiotics in human gut microbiome-associated diseases. J. Microbiol. Biotechnol 2019, 29, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Liong, M.-T. Roles of probiotics and prebiotics in colon cancer prevention: Postulated mechanisms and in-vivo evidence. Int. J. Mol. Sci. 2008, 9, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to qualify microorganisms as “probiotic” in foods and dietary supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef] [PubMed]
- Araya, M.; Morelli, L.; Reid, G.; Sanders, M.; Stanton, C.; Pineiro, M.; Ben Embarek, P. Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food; World Health Organization, Food and Agriculture Organization of the United Nations: Geneva, Switzerland, 2002. [Google Scholar]
- Ng, S.Y.; Koon, S.S.; Padam, B.S.; Chye, F.Y. Evaluation of probiotic potential of lactic acid bacteria isolated from traditional Malaysian fermented Bambangan (Mangifera pajang). CYTA J. Food 2015, 13, 563–572. [Google Scholar] [CrossRef]
- Celebi, O.; Taghizadehghalehjoughi, A.; Celebi, D.; Mesnage, R.; Golokhvast, K.S.; Arsene, A.L.; Spandidos, D.A.; Tsatsakis, A. Effect of the combination of Lactobacillus acidophilus (probiotic) with vitamin K3 and vitamin E on Escherichia coli and Staphylococcus aureus: An in vitro pathogen model. Mol. Med. Rep. 2023, 27, 119. [Google Scholar] [CrossRef] [PubMed]
- Kwak, Y.-K.; Vikström, E.; Magnusson, K.-E.; Vécsey-Semjén, B.; Colque-Navarro, P.; Möllby, R. The Staphylococcus aureus alpha-toxin perturbs the barrier function in Caco-2 epithelial cell monolayers by altering junctional integrity. Infect. Immun. 2012, 80, 1670–1680. [Google Scholar] [CrossRef]
- Martinez-Medina, M.; Garcia-Gil, L.J. Escherichia coli in chronic inflammatory bowel diseases: An update on adherent invasive Escherichia coli pathogenicity. World J. Gastrointest. Pathophysiol. 2014, 5, 213. [Google Scholar] [CrossRef]
- Mgomi, F.C.; Yang, Y.-r.; Cheng, G.; Yang, Z.-q. Lactic acid bacteria biofilms and their antimicrobial potential against pathogenic microorganisms. Biofilm 2023, 5, 100118. [Google Scholar] [CrossRef]
- Saidi, N.; Saderi, H.; Owlia, P.; Soleimani, M. Anti-biofilm potential of Lactobacillus casei and Lactobacillus rhamnosus cell-free supernatant extracts against Staphylococcus aureus. Adv. Biomed. Res. 2023, 12, 50. [Google Scholar] [CrossRef]
- Jang, Y.J.; Min, B.; Lim, J.H.; Kim, B.-Y. In Vitro Evaluation of Probiotic Properties of Two Novel Probiotic Mixtures, Consti-Biome and Sensi-Biome. J. Microbiol. Biotechnol 2023, 33, 1149. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-E.; Lee, N.-K.; Paik, H.-D. Antimicrobial and anti-biofilm effects of probiotic Lactobacillus plantarum KU200656 isolated from kimchi. Food Sci. Biotechnol. 2021, 30, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, S.; Kang, C.-H. Screening and probiotic properties of lactic acid bacteria with potential immunostimulatory activity isolated from kimchi. Fermentation 2022, 9, 4. [Google Scholar] [CrossRef]
- Balakrishna, A. In vitro evaluation of adhesion and aggregation abilities of four potential probiotic strains isolated from guppy (Poecilia reticulata). Braz. Arch. Biol. Technol. 2013, 56, 793–800. [Google Scholar] [CrossRef]
- Kumari, M.; Patel, H.K.; Kokkiligadda, A.; Bhushan, B.; Tomar, S. Characterization of probiotic lactobacilli and development of fermented soymilk with improved technological properties. LWT 2022, 154, 112827. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Zhang, L.; Zhao, Y.; Niu, C.; Yang, Z.; Li, S. Potential probiotic characterization of Lactobacillus plantarum strains isolated from Inner Mongolia. J. Microbiol. Biotechnol 2014, 24, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jo, J.; Seo, H.; Han, S.-W.; Kim, D.-H. The Probiotic Properties and Safety of Limosilactobacillus mucosae NK41 and Bifidobacterium longum NK46. Microorganisms 2024, 12, 776. [Google Scholar] [CrossRef]
- Halder, D.; Mandal, M.; Chatterjee, S.S.; Pal, N.K.; Mandal, S. Indigenous probiotic Lactobacillus isolates presenting antibiotic like activity against human pathogenic bacteria. Biomedicines 2017, 5, 31. [Google Scholar] [CrossRef]
- Carasi, P.; Díaz, M.; Racedo, S.M.; De Antoni, G.; Urdaci, M.C.; Serradell, M.d.l.Á. Safety characterization and antimicrobial properties of kefir-isolated Lactobacillus kefiri. Biomed. Res. Int. 2014, 2014, 208974. [Google Scholar] [CrossRef]
- Zamani, H.; Rahbar, S.; Garakoui, S.R.; Afsah Sahebi, A.; Jafari, H. Antibiofilm potential of Lactobacillus plantarum spp. cell free supernatant (CFS) against multidrug resistant bacterial pathogens. Pharm. Biomed. Res. 2017, 3, 39–44. [Google Scholar] [CrossRef]
- Son, S.-H.; Jeon, H.-L.; Yang, S.-J.; Sim, M.-H.; Kim, Y.-J.; Lee, N.-K.; Paik, H.-D. Probiotic lactic acid bacteria isolated from traditional Korean fermented foods based on β-glucosidase activity. Food Sci. Biotechnol. 2018, 27, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Bengoa, A.A.; Zavala, L.; Carasi, P.; Trejo, S.A.; Bronsoms, S.; de los Ángeles Serradell, M.; Garrote, G.L.; Abraham, A.G. Simulated gastrointestinal conditions increase adhesion ability of Lactobacillus paracasei strains isolated from kefir to Caco-2 cells and mucin. Food Res. Int. 2018, 103, 462–467. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, G.M.D.; de Abreu, L.R.; do Egito, A.S.; Salles, H.O.; da Silva, L.M.F.; Nero, L.A.; Todorov, S.D.; Dos Santos, K.M.O. Functional properties of Lactobacillus mucosae strains isolated from Brazilian goat milk. Probiotics Antimicrob. Proteins 2017, 9, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Fajardo-Cavazos, P.; Nicholson, W.L. Shelf life and simulated gastrointestinal tract survival of selected commercial probiotics during a simulated round-trip journey to Mars. Front. Microbiol. 2021, 12, 748950. [Google Scholar] [CrossRef]
- da Silva, M.N.; Tagliapietra, B.L.; do Amaral Flores, V.; dos Santos Richards, N.S.P. In vitro test to evaluate survival in the gastrointestinal tract of commercial probiotics. Curr. Res. Food Sci. 2021, 4, 320–325. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Kang, C.-H. Immunostimulatory activity of lactic acid bacteria cell-free supernatants through the activation of NF-κB and MAPK signaling pathways in RAW 264.7 cells. Microorganisms 2022, 10, 2247. [Google Scholar] [CrossRef] [PubMed]
- Krausova, G.; Hyrslova, I.; Hynstova, I. In vitro evaluation of adhesion capacity, hydrophobicity, and auto-aggregation of newly isolated potential probiotic strains. Fermentation 2019, 5, 100. [Google Scholar] [CrossRef]
- García-Cayuela, T.; Korany, A.M.; Bustos, I.; de Cadiñanos, L.P.G.; Requena, T.; Peláez, C.; Martínez-Cuesta, M.C. Adhesion abilities of dairy Lactobacillus plantarum strains showing an aggregation phenotype. Food Res. Int. 2014, 57, 44–50. [Google Scholar] [CrossRef]
- Tuo, Y.; Yu, H.; Ai, L.; Wu, Z.; Guo, B.; Chen, W. Aggregation and adhesion properties of 22 Lactobacillus strains. J. Dairy Sci. 2013, 96, 4252–4257. [Google Scholar] [CrossRef]
- Collado, M.C.; Surono, I.; Meriluoto, J.; Salminen, S. Indigenous dadih lactic acid bacteria: Cell-surface properties and interactions with pathogens. J. Food Sci 2007, 72, M89–M93. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jeong, H.; Lee, H.; Ahn, J. Assessment of cell surface properties and adhesion potential of selected probiotic strains. Lett. Appl. Microbiol 2009, 49, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Meriluoto, J.; Salminen, S. Adhesion and aggregation properties of probiotic and pathogen strains. Eur. Food. Res. Technol. 2008, 226, 1065–1073. [Google Scholar] [CrossRef]
- Vlková, E.; Rada, V.; Šmehilová, M.; Killer, J. Auto-aggregation and co-aggregation ability in bifidobacteria and clostridia. Folia Microbiol. 2008, 53, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Popat, K.C.; Eltgroth, M.; LaTempa, T.J.; Grimes, C.A.; Desai, T.A. Decreased Staphylococcus epidermis adhesion and increased osteoblast functionality on antibiotic-loaded titania nanotubes. Biomaterials 2007, 28, 4880–4888. [Google Scholar] [CrossRef]
- Puckett, S.D.; Taylor, E.; Raimondo, T.; Webster, T.J. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials 2010, 31, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Y.; Cui, H.; Li, Y.; Sun, Y.; Qiu, H.-J. Characterization of lactic acid bacteria isolated from the gastrointestinal tract of a wild boar as potential probiotics. Front. Vet. Sci. 2020, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Patrone, V.; Al-Surrayai, T.; Romaniello, F.; Fontana, A.; Milani, G.; Sagheddu, V.; Puglisi, E.; Callegari, M.L.; Al-Mansour, H.; Kishk, M.W. Integrated phenotypic-genotypic analysis of candidate probiotic Weissella cibaria strains isolated from dairy cows in Kuwait. Probiotics Antimicrob. Proteins 2021, 13, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kang, C.-H. Probiotics alleviate oxidative stress in H2O2-exposed hepatocytes and t-BHP-induced C57BL/6 mice. Microorganisms 2022, 10, 234. [Google Scholar] [CrossRef]
- Park, J.-Y.; Lee, J.Y.; Kim, Y.; Kang, C.-H. Limosilactobacillus fermentum MG5091 and Lactococcus lactis MG4668 and MG5474 Suppress Muscle Atrophy by Regulating Apoptosis in C2C12 Cells. Fermentation 2023, 9, 659. [Google Scholar] [CrossRef]
- Inturri, R.; Trovato, L.; Volti, G.L.; Oliveri, S.; Blandino, G. In vitro inhibitory activity of Bifidobacterium longum BB536 and Lactobacillus rhamnosus HN001 alone or in combination against bacterial and Candida reference strains and clinical isolates. Heliyon 2019, 5, e02891. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhabi, N.A.; Valan Arasu, M.; Vijayaraghavan, P.; Esmail, G.A.; Duraipandiyan, V.; Kim, Y.O.; Kim, H.; Kim, H.-J. Probiotic and antioxidant potential of Lactobacillus reuteri LR12 and Lactobacillus lactis LL10 isolated from pineapple puree and quality analysis of pineapple-flavored goat milk yoghurt during storage. Microorganisms 2020, 8, 1461. [Google Scholar] [CrossRef]
- Arrioja-Bretón, D.; Mani-López, E.; Bach, H.; López-Malo, A. Antimicrobial activity of protein-containing fractions isolated from Lactobacillus plantarum NRRL B-4496 culture. Braz. J. Microbiol. 2020, 51, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Mekky, A.F.; Hassanein, W.A.; Reda, F.M.; Elsayed, H.M. Anti-biofilm potential of Lactobacillus plantarum Y3 culture and its cell-free supernatant against multidrug-resistant uropathogen Escherichia coli U12. Saudi J. Biol. Sci. 2022, 29, 2989–2997. [Google Scholar] [CrossRef] [PubMed]
- Yeu, J.-E.; Lee, H.-G.; Park, G.-Y.; Lee, J.; Kang, M.-S. Antimicrobial and antibiofilm activities of Weissella cibaria against pathogens of upper respiratory tract infections. Microorganisms 2021, 9, 1181. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Meadows, P.; Mullins, B.; Patel, K. Gas production by Escherichia coli in selective lactose fermentation media. FEMS Microbiol. Lett. 1980, 8, 17–21. [Google Scholar] [CrossRef]
- Monteiro, C.R.; do Carmo, M.S.; Melo, B.O.; Alves, M.S.; Dos Santos, C.I.; Monteiro, S.G.; Bomfim, M.R.Q.; Fernandes, E.S.; Monteiro-Neto, V. In vitro antimicrobial activity and probiotic potential of Bifidobacterium and Lactobacillus against species of Clostridium. Nutrients 2019, 11, 448. [Google Scholar] [CrossRef]




| Strains | Initial Counts (log CFU/mL) | SGF (log CFU/mL) | SIF (log CFU/mL) | Survival Rate (%) |
|---|---|---|---|---|
| L. plantarum LC27 | 7.04 ± 0.08 | 2.98 ± 0.09 * | 2.96 ± 0.11 * | 42.04 ± 2.01 c |
| L. reuteri NK33 | 7.52 ± 0.02 | 7.48 ± 0.13 | 7.47 ± 0.03 * | 99.27 ± 0.12 a |
| L. rhamnosus NK210 | 7.33 ± 0.02 | 6.72 ± 0.04 * | 6.56 ± 0.02 * | 89.54 ± 0.38 b |
| B. longum NK46 | 7.84 ± 0.04 | 3.24 ± 0.11 * | 3.20 ± 0.13 * | 40.82 ± 1.41 c |
| B. bifidum NK175 | 7.15 ± 0.03 | 2.73 ± 0.05 * | 2.19 ± 0.01 * | 30.65 ± 0.06 d |
| Adhesion | Auto-Aggregation | Co-Aggregation | Hydrophobicity | ||||
|---|---|---|---|---|---|---|---|
| S. aureus ATCC 25923 | E. coli ATCC 25922 | Xylene | Chloroform | Ethyl Acetate | |||
| Adhesion | 1 | ||||||
| Auto-aggregation | 0.048 | 1 | |||||
| Co-aggregation | |||||||
| S. aureus ATCC 25923 | 0.048 | 0.734 ** | 1 | ||||
| E. coli ATCC 25922 | 0.113 | 0.879 ** | 0.634 * | 1 | |||
| Hydrophobicity | |||||||
| Xylene | 0.028 | 0.483 | 0.807 ** | 0.344 | 1 | ||
| Chloroform | −0.106 | 0.570 * | 0.887 ** | 0.489 | 0.934 ** | 1 | |
| Ethyl acetate | −0.163 | −0.183 | 0.418 | −0.234 | 0.716 ** | 0.672 ** | 1 |
| Strains | R Value (mm) | |
|---|---|---|
| S. aureus ATCC 25923 | E. coli ATCC 25922 | |
| L. plantarum LC27 | 11.33 ± 0.76 (High) | 14.17 ± 0.29 (High) |
| L. reuteri NK33 | 6.17 ± 0.76 (High) | 8.83 ± 0.58 (High) |
| L. rhamnosus NK210 | 11.67 ± 0.29 (High) | 14.00 ± 0.50 (High) |
| B. longum NK46 | 7.33 ± 0.29 (High) | 9.83 ± 0.29 (High) |
| B. bifidum NK175 | 6.17 ± 0.29 (High) | 8.33 ± 0.58 (High) |
| Strains | MIC (mg/mL) | |
|---|---|---|
| S. aureus ATCC 25923 | E. coli ATCC 25922 | |
| L. plantarum LC27 | 20 | 20 |
| L. reuteri NK33 | 30 | 30 |
| L. rhamnosus NK210 | 20 | 30 |
| B. longum NK46 | 30 | 30 |
| B. bifidum NK175 | 50 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Jo, J.; Wan, J.; Seo, H.; Han, S.-W.; Shin, Y.-J.; Kim, D.-H. In Vitro Evaluation of Probiotic Properties and Anti-Pathogenic Effects of Lactobacillus and Bifidobacterium Strains as Potential Probiotics. Foods 2024, 13, 2301. https://doi.org/10.3390/foods13142301
Lee J, Jo J, Wan J, Seo H, Han S-W, Shin Y-J, Kim D-H. In Vitro Evaluation of Probiotic Properties and Anti-Pathogenic Effects of Lactobacillus and Bifidobacterium Strains as Potential Probiotics. Foods. 2024; 13(14):2301. https://doi.org/10.3390/foods13142301
Chicago/Turabian StyleLee, Jaekoo, Jaehyun Jo, Jungho Wan, Hanseul Seo, Seung-Won Han, Yoon-Jung Shin, and Dong-Hyun Kim. 2024. "In Vitro Evaluation of Probiotic Properties and Anti-Pathogenic Effects of Lactobacillus and Bifidobacterium Strains as Potential Probiotics" Foods 13, no. 14: 2301. https://doi.org/10.3390/foods13142301






