Recent Advances in Efficient Lutein-Loaded Zein-Based Solid Nano-Delivery Systems: Establishment, Structural Characterization, and Functional Properties
Abstract
:1. Introduction
2. Structural Properties and Transport and Transport Functions of Zein
2.1. Physicochemical Properties of Zein
2.2. Embedding and Transport Functions of Zein
3. Establishment of Lutein Nanoparticle Delivery System Supported by Zein Carrier
3.1. Preparation of Zein-Loaded Lutein Nanoparticles
3.1.1. Nano-Lipidation Technology
3.1.2. Anti-Solvent Nanoparticles Technology
3.1.3. Supercritical Carbon Dioxide Preparation Technology
3.2. Using Modified Zein to Obtain Lutein-Supported Nanoparticles
3.3. Using Composite Zein to Obtain Lutein-Supported Nanoparticles
4. Structural Characterization of Zein-Loaded Lutein Nanoparticles
4.1. Particle Size, Potential Analysis and Detection of Zein-Loaded Lutein Nanoparticles
4.2. Microstructural Analysis of Zein-Loaded Lutein Nanoparticles
4.3. Chemical Structure Analysis of Zein-Loaded Nanoparticles by FTIR and XRD
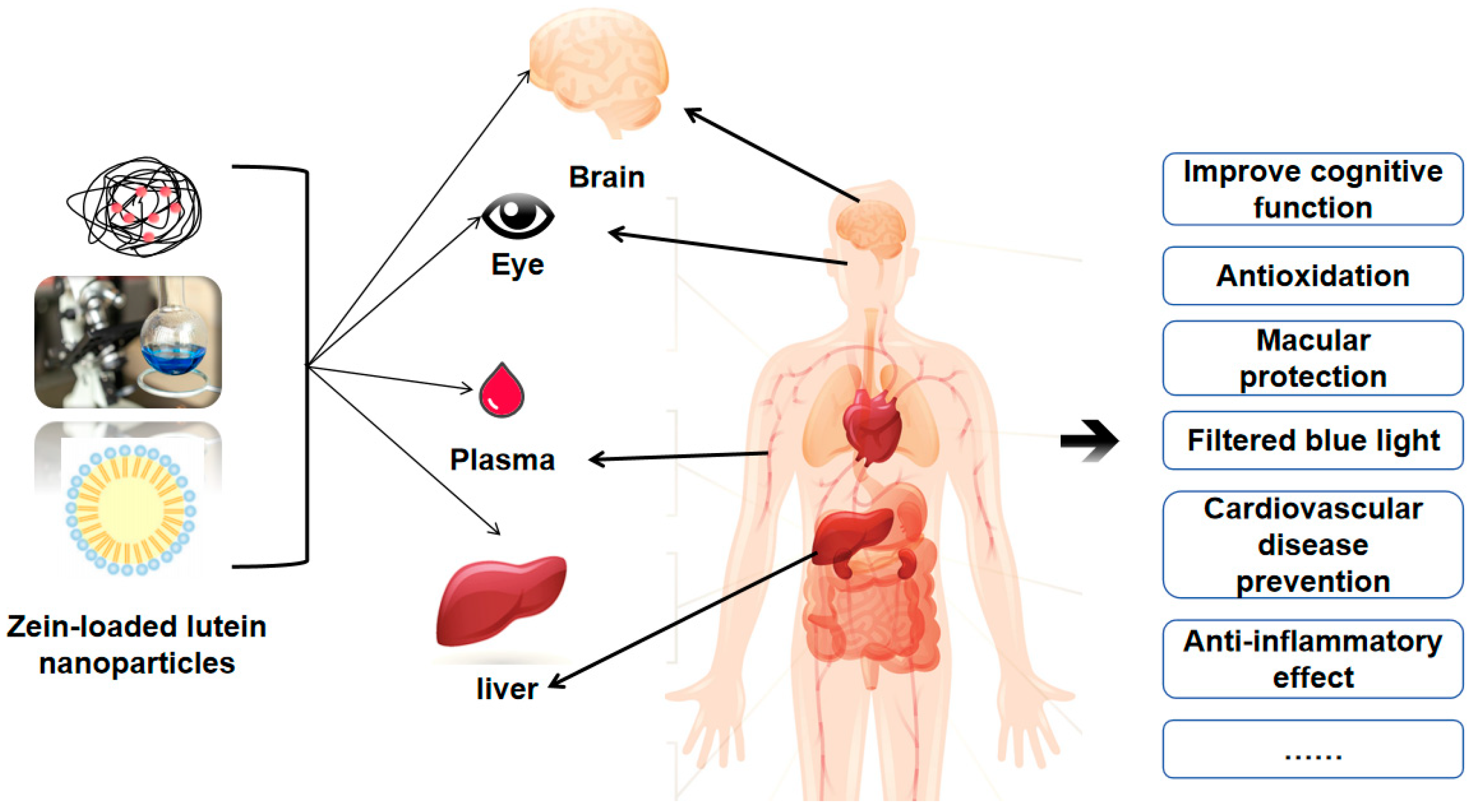
5. Functional Properties of Zein-Loaded Lutein Nanosystems
5.1. Stability of Zein-Loaded Lutein Nanoparticles
5.2. Solubility of Zein-Loaded Lutein Nanoparticles
5.3. Antioxidant Activity of Zein-Loaded Lutein Nanoparticles
5.4. Targeted Controlled Release of Zein-Loaded Lutein Nanoparticles
5.5. Bioaccessibility of Zein-Loaded Lutein Nanoparticles
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mora-Gutierrez, A.; Attarie, R.; Núñez de González, M.T.; Jung, Y.; Woldesenbe, S. Complexes of lutein with bovine and caprine caseins and their impact on lutein chemical stability in emulsion systems: Effect of arabinogalactan. J. Dairy Sci. 2018, 101, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.Q.; You, T.T.; Wang, J.; Jiang, Y.; Niu, J.C. Research progress on construction of lutein-loaded nano delivery system and their improvements on the bioactivity. Coatings 2022, 12, 1449. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, H.; Xu, L.; Zhao, S.; Hu, S.; Ma, A.; Ma, Y. Lutein can alleviate oxidative stress, inflammation, and apoptosisInduced by excessive alcohol to ameliorate reproductive damage in male rats. Nutrients 2022, 14, 2385. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.C.; Yen, G.C. Antioxidative and anti-inflammatory activity of functional foods. Curr. Opin. Food Sci. 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Montuori, E.; Lima, S.; Marchese, A.; Scargiali, F.; Lauritano, C. Lutein Production and Extraction from Microalgae: Recent Insights and Bioactive Potential. Int. J. Mol. Sci. 2024, 25, 2892. [Google Scholar] [CrossRef] [PubMed]
- Jardim, F.E.; Rósula, P.M.; Sotelo, B.M.; Cardoso, B.V.; Machado, A.S.; Aparecida, S.M.E.; Santos, D.C.A.; Vitória, L.F.; Hess, G.O.; Piaia, R.B.; et al. Exposure to lutein-loaded nanoparticles attenuates Parkinson’s model-induceddamage in Drosophila melanogaster: Restoration of dopaminergic and cholinergic system and oxidative stress indicators. Chem. Biol. Interact. 2021, 340, 109431. [Google Scholar]
- Yang, J.; Li, D.K.; Zhang, Y.Q.; Liao, Z.Y.; Aihemaitijiang, S.; Hou, Y.M.; Zhan, Z.J.; Xie, K.; Zhang, Z.F. Lutein protected the retina from light induced retinal damage by inhibiting increasing oxidative stress and inflammation. J. Funct. Foods 2020, 73, 104107. [Google Scholar] [CrossRef]
- Wilson, L.M.; Tharmarajah, S.; Jia, Y.X.; Semba, R.D.; Schaumberg, D.A.; Robinson, K.A. The effect of lutein/zeaxanthin intake on human macular pigment optical density: A systematic review and meta-analysis. Adv. Nutr. 2021, 12, 2244–2254. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.K.; Liu, X.S.; Wang, M.C.; Wang, P.; Yang, J.B.; Zhang, S.F. Lutein inhibits proliferation, invasion and migration of hypoxic breast cancer cells via downregulation of HES1. Int. J. Oncol. 2018, 52, 2119–2129. [Google Scholar] [CrossRef]
- Gansukh, E.; Mya, K.K.; Jun, M.n.; Keum, Y.S.; Kim, D.H.; Saini, R.K. Lutein derived from marigold (Tagetes erecta) petals triggers ROS generation and activates Bax and caspase-3 mediated apoptosis of human cervical carcinoma (HeLa) cells. Food Chem. Toxicol. 2019, 127, 11–18. [Google Scholar] [CrossRef]
- Maradagi, T.; Kumar, R.; Ponesakki, G. Hyperglycaemia-induced human hepatocellular carcinoma (HepG2) cell proliferation through ROS-mediated P38 activation is effectively inhibited by a xanthophyll carotenoid, lutein. Diabet. Med. 2022, 39, e14713. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Liu, D.; Zhao, J.; Li, X.; Yan, Y.; Wu, Z.J.; Wang, H.; Wang, C.T. Lutein attenuates oxidative stress and inhibits lipid accumulation in free fatty acids-induced HepG2 cells by activating the AMPK pathway. J. Funct. Foods 2019, 60, 103445. [Google Scholar] [CrossRef]
- Liu, B.; Teng, Z.H.; Wang, J.F.; Lu, G.J.; Deng, X.M.; Li, L. Inhibition of listeriolysin O oligomerization by lutein prevents Listeria monocytogenes infection. Fitoterapia 2017, 116, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Elkholy, N.S.; Shafaa, M.W.; Mohammed, H.S. Cationic liposome-encapsulated carotenoids as a potential treatment for fibromyalgia in an animal model. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166150. [Google Scholar] [CrossRef] [PubMed]
- Padmanabha, S.; Vallikannan, B. Fatty acids modulate the efficacy of lutein in cataract prevention: Assessment of oxidative and inflammatory parameters in rats. Biochem. Biophys. Res. Commun. 2018, 500, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Steiner, B.M.; McClements, J.D.; Davidov-Pardo, G. Encapsulation systems for lutein: A review. Trends Food Sci. Technol. 2018, 82, 71–81. [Google Scholar] [CrossRef]
- Qv, X.Y.; Zeng, Z.P.; Jiang, J.G. Preparation of lutein microencapsulation by complex coacervation method and its physicochemical properties and stability. Food Hydrocoll. 2011, 25, 1596–1603. [Google Scholar] [CrossRef]
- Ranganathan, A.; Keelara, V.H.P.; Vallikannan, B. Promising interaction between nanoencapsulated lutein with low molecular weight chitosan: Characterization and bioavailability of lutein in vitro and in vivo. Food Chem. 2013, 141, 327–337. [Google Scholar]
- Fu, Y.J.; Yang, J.D.; Jiang, L.W.; Ren, L.L.; Zhou, J. Encapsulation of Lutein into Starch Nanoparticles to Improve Its Dispersity in Water and Enhance Stability of Chemical Oxidation. Starch-Starke 2019, 71, 1800248. [Google Scholar] [CrossRef]
- Barreras-Urbina, C.G.; Ramírez-Wong, B.; López-Ahumada, G.A.; Burruel-Ibarra, S.E.; Martínez-Cruz, O.; Tapia-Hernández, J.A.; Rodriguez Felix, F. Nano- and Micro-Particles by nanoprecipitation: Possible application in the food and agricultural industries. Int. J. Food Prop. 2016, 19, 1912–1923. [Google Scholar] [CrossRef]
- Bhunchu, S.; Rojsitthisak, P.; Rojsitthisak, P. Effects of preparation parameters on the characteristics of chitosan-alginate nanoparticles containing curcumin diethyl disuccinate. J. Drug Deliv. Sci. Technol. 2015, 28, 64–72. [Google Scholar] [CrossRef]
- Yang, Y.H.; Mao, J.W.; Tan, X.L. Research progress on the source, production, and anti-cancer mechanisms of paclitaxel. Chin. J. Nat. Med. 2021, 18, 890–897. [Google Scholar] [CrossRef]
- Yuba, E. Development of functional liposomes by modification of stimuli-responsive materials and their biomedical applications. J. Mater. Chem. B 2020, 8, 1093–1107. [Google Scholar] [CrossRef] [PubMed]
- Fereydouni, N.; Astaneh, M.E. Fabrication of a Zein membrane containing cerium oxide nanoparticles: Physical, chemical and biological properties as a potential wound dressing. J. Mol. Struct. 2023, 1291, 136006. [Google Scholar] [CrossRef]
- Corradini, E.; Curti, P.S.; Meniqueti, A.B.; Martins, A.F.; Rubira, A.F.; Muniz, E.C. Recent Advances in Food-Packing, Pharmaceutical and Biomedical Applications of Zein and Zein-Based Materials. Int. J. Mol. Sci. 2014, 15, 22438–22470. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Velikov, K.P. Zein as a source of functional colloidal nano- and microstructures. Curr. Opin. Colloid Interface Sci. 2014, 19, 450–458. [Google Scholar] [CrossRef]
- Kim, S.; Peterson, S.C. Optimal conditions for the encapsulation of menthol into zein nanoparticles. LWT Food Sci. Technol. 2021, 144, 111213. [Google Scholar] [CrossRef]
- Matsushima, N.; Danno, G.; Takezawa, H.; Izumi, Y. Three-dimensional structure of maize α-zein proteins studied by small-angle X-ray scattering. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1997, 1339, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Khan, M.A.; Cheng, H.; Liang, L. Co-encapsulation of α-tocopherol and resveratrol within zein nanoparticles: Impact on antioxidant activity and stability. J. Food Eng. 2019, 247, 9–18. [Google Scholar] [CrossRef]
- Derya, B.; Gianmarco, I.; Gozde, S.S.; Derya, A.; Silvia, T.; Flavia, P.; Stefano, F.; Ahmet, Y. Development of flexible antimicrobial zein coatings with essential oils for the inhibition of critical pathogens on the surface of whole fruits: Test of coatings on inoculated melons. Food Packag. Shelf Life 2019, 20, 100316. [Google Scholar]
- Sareh, B.; Seyed, M.H.H.; Gholamhossein, Y.; Masoud, R.; Ali-Mohammad, T.; Paul, V.D.M. The stability of triphasic oil-in-water Pickering emulsions can be improved by physical modification of hordein- and secalin-based submicron particles. Food Hydrocoll. 2019, 89, 649–660. [Google Scholar]
- Chen, L.Y.; Remondetto, G.E.; Subirade, M. Food protein-based materials as nutraceutical delivery systems. Trends Food Sci. Technol. 2006, 17, 272–283. [Google Scholar] [CrossRef]
- Gagliardi, A.; Bonacci, S.; Paolino, D.; Celia, C.; Procopio, A.; Fresta, M.; Cosco, D. Paclitaxel-loaded sodium deoxycholate-stabilized zein nanoparticles: Characterization and in vitro cytotoxicity. Heliyon 2019, 5, e02422. [Google Scholar] [CrossRef] [PubMed]
- Da Rosa, C.G.; De Melo, A.P.Z.; Sganzerla, W.G.; Machado, M.H.; Nunes, M.R.; Maciel, M.V.D.O.B.; Bertoldi, F.C.; Barreto, P.L.M. Application in situ of zein nanocapsules loaded with Origanum vulgare Linneus and Thymus vulgaris as a preservative in bread. Food Hydrocoll. 2019, 99, 105339. [Google Scholar] [CrossRef]
- Huang, X.L.; Dai, Y.Q.; Cai, J.X.; Zhong, N.J.; Xiao, H.; McClements, D.J.; Hu, K. Resveratrol encapsulation in core-shell biopolymer nanoparticles Impact on antioxidant and anticancer activities. Food Hydrocoll. 2017, 64, 157–165. [Google Scholar] [CrossRef]
- Shi, Q.K.; Wang, X.Y.; Tang, X.D.; Zhen, N.; Wang, Y.P.; Luo, Z.J.; Zhang, H.; Liu, J.S.; Zhou, D.F.; Huang, K.K. In vitro antioxidant and antitumor study of zein/SHA nanoparticles loaded with resveratrol. Food Sci. Nutr. 2021, 9, 3530–3537. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Wan, Z.H.; Yang, X.Q.; Wang, J.M.; Guo, J.; Lin, Y. Colloidal complexation of zein hydrolysate with tannic acid: Constructing peptides-based nanoemulsions for alga oil delivery. Food Hydrocoll. 2015, 54, 40–48. [Google Scholar] [CrossRef]
- Spinella, M. Formation and characterization of zein-caseinate-pectin complex nanoparticles for encapsulation of eugenol. LWT-Food Sci. Technol. 2018, 89, 596–603. [Google Scholar]
- Ahmadzadeh, S.; Ubeyitogullari, A. Lutein encapsulation into dual-layered starch/zein gels using 3D food printing: Improved storage stability and in vitro bioaccessibility. Int. J. Biol. Macromol. 2024, 266, 131305. [Google Scholar] [CrossRef]
- Babazadeh, A.; Ghanbarzadeh, B.; Hamishehkar, H. Formulation of food grade nanostructured lipid carrier (NLC) for potential applications in medicinal-functional foods. J. Drug Deliv. Sci. Technol. 2017, 39, 50–58. [Google Scholar] [CrossRef]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Nutraceutical nanodelivery: An insight into the bioaccessibility/bioavailability of different bioactive compounds loaded within nanocarriers. Crit. Rev. Food Sci. Nutr. 2020, 61, 3031–3065. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Wang, F.L.; Pu, C.F.; Tang, W.T.; Sun, Q.J. Nanoencapsulation of lutein within lipid-based delivery systems: Characterization and comparison of zein peptide stabilized nano-emulsion, solid lipid nanoparticle, and nano-structured lipid carrier. Food Chem. 2021, 358, 129840. [Google Scholar] [CrossRef] [PubMed]
- Sahar, S.; Ashkan, M. Two-step sequential cross-linking of sugar beet pectin for transforming zein nanoparticle-based Pickering emulsions to emulgels. Carbohydr. Polym. 2015, 136, 738–743. [Google Scholar]
- Kim, S.; Xu, J. Aggregate formation of zein and its structural inversion in aqueous ethanol. J. Cereal Sci. 2008, 47, 1–5. [Google Scholar] [CrossRef]
- Cheng, C.J.; Ferrizzi, M.; Jones, O.G. Fate of lutein-containing zein nanoparticles following simulated gastric and intestinal digestion. Food Hydrocoll. 2018, 87, 229–236. [Google Scholar] [CrossRef]
- Jiao, Y.; Han, H.; Chang, Y.; Li, C.; Gao, J.W. Preparation and characterization of zein loaded lutein nanoparticles. Food Mach. 2019, 35, 7–12. [Google Scholar]
- Gong, K.N.; Rehman, I.H.U.; Darr, J.A. Characterization and drug release investigation of amorphous drug–hydroxypropyl methylcellulose composites made via supercritical carbon dioxide assisted impregnation. J. Pharm. Biomed. Anal. 2008, 48, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, E.; Cesaro, A.M.D.; Ferreira, S.R.S.; Oliveira, V. Precipitation of beta-carotene microparticles from SEDS technique using supercritical CO2. J. Food Eng. 2009, 95, 656–663. [Google Scholar] [CrossRef]
- Hu, D.D.; Lin, C.C.; Liu, L.; Li, S.N.; Zhao, Y.P. Preparation, characterization, and in vitro release investigation of lutein/zein nanoparticles via solution enhanced dispersion by supercritical fluids. J. Food Eng. 2012, 109, 545–552. [Google Scholar] [CrossRef]
- Zhou, L.J.; Xu, G.R.; Zhang, Z.; Li, H.X.; Yao, P. Surface activity and safety of deamidated zein peptide. Colloids Surf. A Physicochem. Eng. Asp. 2018, 540, 150–157. [Google Scholar] [CrossRef]
- Jin, D.X.; Liu, X.L.; Zheng, X.Q.; Wang, X.J.; He, J.F. Preparation of antioxidative corn protein hydrolysates, purification and evaluation of three novel corn antioxidant peptides. Food Chem. 2016, 204, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.T.; Liu, M.Y.; Luo, Y.X.; Cui, H.W.; Fu, Z.Y.; Zhang, J.; Sun, Q.J.; Pu, C.F. Influence of covalent conjugates of zein peptide-phenolic acids with different hydrophobicity on performance of resultant lutein-loaded emulsion gels. J. Food Eng. 2024, 361, 111740. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, X.Q.; Chang, Y.; Li, D.J.; Sun, X.H.; Liu, X.L. Zein-derived peptides as nanocarriers to increase the water solubility and stability of lutein. Food Funct. 2018, 9, 117–123. [Google Scholar] [CrossRef]
- Jiao, Y.; Han, H.; Chang, Y.; Li, D.J.; Riaz, A. Improvement in Entrapment Efficiency and In Vitro Digestion Stability of Lutein by Zein Nanocarriers with Pepsin Hydrolysis. J. Food Qual. 2020, 2020, 4696587. [Google Scholar] [CrossRef]
- Li, H.; Yuan, Y.K.; Zhu, J.X.; Wang, T.; Wang, D.F.; Xu, Y. Zein/soluble soybean polysaccharide composite nanoparticles for encapsulation and oral delivery of lutein. Food Hydrocoll. 2020, 103, 105715. [Google Scholar] [CrossRef]
- Ma, M.J.; Yuan, Y.K.; Yang, S.; Wang, Y.H.; Lv, Z.H. Fabrication and characterization of zein/tea saponin composite nanoparticles as delivery vehicles of lutein. LWT Food Sci. Technol. 2020, 125, 109270. [Google Scholar] [CrossRef]
- Chang, Y.; Jiao, Y.; Li, D.J.; Liu, X.L.; Han, H. Glycosylated zein as a novel nanodelivery vehicle for lutein. Food Chem. 2020, 376, 131927. [Google Scholar] [CrossRef] [PubMed]
- De Boer, F.Y.; Imhof, A.; Velikov, K.P. Photo-stability of lutein in surfactant-free lutein-zein composite colloidal particles. Food Chem. X 2020, 5, 100071. [Google Scholar] [CrossRef]
- Chuacharoen, T.; Sabliov, C.M. Stability and Controlled Release of Lutein Loaded in Zein Nanoparticles with and without Lecithin and Pluronic F127 Surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2016, 503, 11–18. [Google Scholar] [CrossRef]
- Yuan, Y.K.; Li, H.; Liu, C.Z.; Zhang, S.Z.; Xu, Y.; Wang, D.F. Fabrication and Characterization of Lutein-Loaded Nanoparticles Based on Zein and Sophorolipid: Enhancement of Water Solubility, Stability, and Bioaccessibility. J. Agric. Food Chem. 2019, 67, 11977–11985. [Google Scholar] [CrossRef]
- Yeap, S.P.; Lim, J.; Ngang, H.P.; Ooi, B.S.; Ahmad, A.L. Role of Particle-Particle Interaction Towards Effective Interpretation of Z-Average and Particle Size Distributions from Dynamic Light Scattering (DLS) Analysis. J. Nanosci. Nanotechnol. 2018, 18, 6957–6964. [Google Scholar] [CrossRef]
- Tong, Z.; Zhang, L.; Liao, W.Y.; Wang, Y.; Gao, Y.X. Extraction, identification and application of gliadin from gluten: Impact of pH on physicochemical properties of unloaded- and lutein-loaded gliadin nanoparticles. Int. J. Biol. Macromol. 2023, 253, 126638. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, X.; Chen, Z.X.; Wang, T.; Wang, L.; Zhong, Q.X. Biological macromolecule delivery system fabricated using zein and gum arabic to control the release rate of encapsulated tocopherol during in vitro digestion. Food Res. Int. 2018, 114, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.X.; Dai, L.; Gao, Y.X. Interaction and formation mechanism of binary complex between zein and propylene glycol alginate. Carbohydr. Polym. 2017, 157, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Eaton, P.; Quaresma, P.; Soares, C.; Neves, C.; Almeida, M.P.D.; Pereira, E.; West, P. A direct comparison of experimental methods to measure dimensions of synthetic nanoparticles. Ultramicroscopy 2017, 182, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.L.; Yu, S.N. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, D.F.; Liu, C.Z.; Zhu, J.X.; Fan, M.H.; Sun, X.; Wang, T.; Xu, Y.; Cao, Y.P. Fabrication of stable zein nanoparticles coated with soluble soybean polysaccharide for encapsulation of quercetin. Food Hydrocoll. 2018, 87, 342–351. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
- Hasni, I.; Bourassa, P.; Tajmir-Riahi, H.A. Binding of cationic lipids to milk beta-Lactoglobulin. J. Phys. Chem. B 2011, 115, 6683–6690. [Google Scholar] [CrossRef]
- Wei, Y.; Yu, Z.P.; Lin, K.S.; Dai, L.; Yang, S.F.; Mao, L.K.; Yuan, F.; Gao, Y.X. Fabrication and characterization of resveratrol loaded zein-propylene glycol alginate-rhamnolipid composite nanoparticles: Physicochemical stability, formation mechanism and in vitro digestion. Food Hydrocoll. 2019, 95, 336–348. [Google Scholar] [CrossRef]
- Chen, S.; Li, Q.K.; McClements, D.J.; Han, Y.H.; Dai, L.; Mao, L.K.; Gao, Y.X. Co-delivery of curcumin and piperine in zein-carrageenan core–shell nanoparticles: Formation, structure, stability and in vitro gastrointestinal digestion. Food Hydrocoll. 2019, 99, 105334. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, L.; Yu, Z.P.; Lin, K.S.; Yang, S.F.; Dai, L.; Liu, J.F.; Mao, L.K.; Yuan, F.; Gao, Y.X. Enhanced stability, structural characterization and simulated gastrointestinal digestion of coenzyme Q10 loaded ternary nanoparticles. Food Hydrocoll. 2019, 94, 333–344. [Google Scholar] [CrossRef]
- Sun, C.X.; Dai, L.; Gao, Y.X. Formation and characterization of the binary complex between zein and propylene glycol alginate at neutral pH. Food Hydrocoll. 2017, 64, 36–47. [Google Scholar] [CrossRef]
- Du, X.J.; Wang, S.; Lou, Z.X.; Jiang, C.Y.; Wang, H.X. Preparation, characterization and functional properties of ternary composite nanoparticles for enhanced water solubility and bioaccessibility of lutein. Food Hydrocoll. 2023, 144, 109039. [Google Scholar] [CrossRef]
- Livney, Y.D. Milk proteins as vehicles for bioactives. Curr. Opin. Colloid Interface Sci. 2010, 15, 73–83. [Google Scholar] [CrossRef]
- Shukla, R.; Cheryan, M. Zein: The industrial protein from corn. Ind. Crops Prod. 2001, 13, 171–192. [Google Scholar] [CrossRef]
- Gombač, Z.; Osojnik, Č.I.G.; Skrt, M.; Istenič, K.; Knez, K.A.; Pravst, I.; Poklar, U.N. Stabilisation of Lutein and Lutein Esters with Polyoxyethylene Sorbitan Monooleate, Medium-Chain Triglyceride Oil and Lecithin. Foods 2021, 10, 500. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Dawson, R.; Kong, L.Y.; Tan, L.B. Lutein supplementation for early-life health and development: Current knowledge, challenges, and implications. Crit. Rev. Food Sci. Nutr. 2024, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Begoña, O.A.; Fernando, G.L.; Julio, C.F.; Carmen, H.B.; Inmaculada, B.N.; Rocío, E.S. Bioavailability of Lutein from Marigold Flowers (Free vs. Ester Forms): A Randomised Cross-Over Study to Assess Serum Response and Visual Contrast Threshold in Adults. Nutrients 2024, 16, 1415. [Google Scholar] [CrossRef]
- Dai, Z.Q.; Nie, M.M.; Chen, Y.; Song, J.F.; Xu, Y.Y.; Zhang, Z.Y.; Zhang, G.D.; Yan, S.M.; Zhang, X.; Li, D.J. Lutein-stevioside nanoparticle attenuates H2O2-induced oxidative damage in ARPE cells. Food Sci. Hum. Wellness 2024, 13, 1628–1635. [Google Scholar] [CrossRef]
- Toragall, V.; Srirangam, P.; Jayapala, N.; Baskaran, V. Lutein encapsulated oleic-linoleic acid nanoemulsion boosts oral bioavailability of the eye protective carotenoid lutein in rat model. Mater. Today Commun. 2021, 28, 102522. [Google Scholar] [CrossRef]
- Liu, P.; Bai, X.Y.; Gao, X.T.; Liu, K.; Li, A.X.; Lyu, Z.J.; Li, Q.H. Preparation, characterization, and properties of lutein block polyethylene glycol copolymer loading with lutein nanoparticles. Macromol. Res. 2023, 31, 233–243. [Google Scholar] [CrossRef]
- Amorim-Carrilho, K.T.; Cepeda, A.; Fente, C.; Regal, P. Review of methods for analysis of carotenoids. Trac-Trends Anal. Chem. 2014, 56, 49–73. [Google Scholar] [CrossRef]
- Yao, K.F.; Chen, W.J.; Song, F.L.; McClements, D.J.; Hu, K. Tailoring zein nanoparticle functionality using biopolymer coatings: Impact on curcumin bioaccessibility and antioxidant capacity under simulated gastrointestinal conditions. Food Hydrocoll. 2018, 79, 262–272. [Google Scholar] [CrossRef]
- Ji, N.; Hong, Y.; Gu, Z.B.; Cheng, L.; Li, Z.F.; Li, C.M. Preparation and Characterization of Insulin-Loaded Zein/Carboxymethylated Short-Chain Amylose Complex Nanoparticles. J. Agric. Food Chem. 2018, 66, 9335–9343. [Google Scholar] [CrossRef]
- Shwetha, H.J.; Arathi, B.P.; Beto, M.M.; Ambedkar, R.; Shivaprasad, S.; Raichur, A.M.; Lakshminarayana, R. Zein-Alginate-Phosphatidylcholine Nanocomplex Efficiently Delivers Lycopene and Lutein over Dietary-Derived Carotenoid Mixed Micelles in Caco-2 Cells. J. Agric. Food Chem. 2022, 70, 15474–15486. [Google Scholar] [CrossRef]
- Jiao, Y.; Shi, L.; Li, D.J.; Chang, Y. Lutein-loaded glycosylated zein nanoparticles-Preparation, characterization, and stability in functional drink. LWT-Food Sci. Technol. 2023, 187, 115308. [Google Scholar] [CrossRef]
- Hao, S.; John, M.N.; Róisín, F.; Alfonso, P.C. Beyond food colouring: Lutein-food fortification to enhance health. Food Biosci. 2024, 59, 104085. [Google Scholar]
- Zheng, J.Y.; Hong, B.V.; Agus, J.K.; Tang, X.Y.; Klebaner, N.R.; Chen, S.Y.; Guo, F.; Harvey, D.J.; Lebrilla, C.B.; Zivkovic, A.M. Lutein and Zeaxanthin Enhance, Whereas Oxidation, Fructosylation, and Low pH Damage High-Density Lipoprotein Biological Functionality. Antioxidants 2024, 13, 616. [Google Scholar] [CrossRef]
- Toragall, V.; Baskaran, V. Chitosan-sodium alginate-fatty acid nanocarrier system: Lutein bioavailability, absorption pharmacokinetics in diabetic rat and protection of retinal cells against H2O2 induced oxidative stress in vitro. Carbohydr. Polym. 2021, 254, 117409. [Google Scholar] [CrossRef]



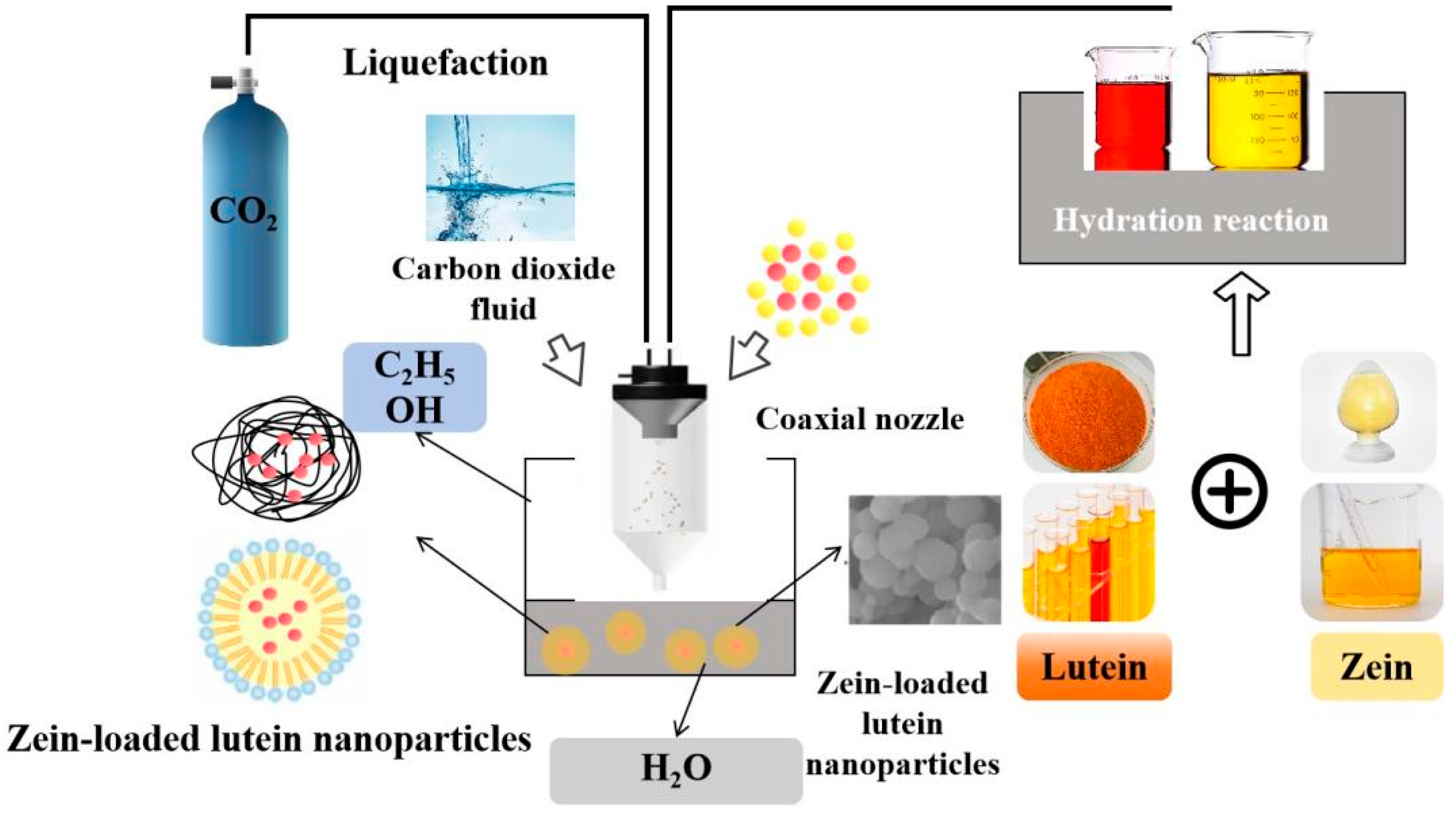
| Type | Concentration | Molecular Mass/kDa |
|---|---|---|
| α-zein | 75–85% | 19, 22 |
| β-zein | 10–15% | 24, 22, 14 |
| γ-zein | 5–10% | 16, 27, 50 |
| δ-zein | 1–5% | 10, 18 |
| Structural Characterization | Method | Application | Reference |
|---|---|---|---|
| Encapsulation efficiency | Chromatography–mass spectroscopy | Hu et al. measured the encapsulation rate of Zein-LUT by liquid chromatography at 83.15%. De Boer et al. measured the encapsulation rate of nanoparticles by ultraviolet spectrophotometer. | [49,58] |
| Nanoparticle size | Particle size and zeta potential analysis | The particle size of zein-loaded lutein was measured by a particle size analyzer, and the surface zeta potential was 27.4–29.4 mv. The particle size potential analysis could reflect the dispersion and stability of the nanoparticles. | [58] |
| Microstructure | TEM | Chuacharoen observed that the surfactant-containing particles were spherical with a rough surface, and some of the particles were connected in the surfactant lattice. The size of the nanoparticles without surfactants was smaller and more spherical but uneven in size and more prone to agglomeration. | [59] |
| SEM | Jiao et al. observed that Zein and its derived peptides were spherical and that the size of Zein was larger than that of its derived peptides. When lutein is loaded into Zein nanoparticles, the size of the nanoparticles decreases and aggregates. | [53] | |
| AFM | Cheng et al. observed a variety of distinct two-dimensional amorphous components, particles ranging in size from 10 to 700 nm in diameter, and particle aggregates. | [45] | |
| Structural characteristic | FTIR | Liu et al. analyzed the structural information of the samples and found that more hydrogen bonds were formed after the hydrolysis of Zein, and the secondary structures of hydrolysates obtained by different enzymes were significantly different. | [42] |
| XRD | Li et al. found that after encapsulation, the typical diffraction peak of lutein did not appear in the ZS-LNPs spectrum of nanoparticles formed by Zein, indicating that lutein may be distributed in the nanoparticles in an amorphous manner. | [55] | |
| Structural stability | PDI | Li et al. found that the size of Zein nanoparticles Z-LNP and ZS-LNP was significantly different (p < 0.05), indicating the interaction between Zein and lutein, while soy protein existed in the form of single molecules and associated aggregates. | [55] |
| Protein modification | Accessories: ascorbic acid, tea saponin, soy protein, sophorolipid, glucosamine, lecithin, Pluronic F127 emulsifier, etc. | [55,56,57,58,59,60] |
| Nature | Modified Product | Application | Reference |
|---|---|---|---|
| Stability | Ascorbic acid | The degradation rate of lutein by photodegradation was significantly decreased | [58] |
| The Planik F127 | After 10 h of ultraviolet radiation, more lutein was still retained | [59] | |
| Tea saponin | It has good stability under high-salt concentration | [56] | |
| Solubility | Glucose | Zein binds to glucosamine through an enzymatic reaction | [57] |
| Soybean polysaccharide | The solubility of lutein was increased by more than 30 times | [55] | |
| Oxidation resistance | Glucosamine chitosan | The encapsulation of Zein and glycosylated Zein enhanced the antioxidant activity of lutein in vitro | [57] |
| The glycosylated Zein carrier protects and enhances the antioxidant activity of lutein | [87] | ||
| Targeted controlled release | Biological enzyme hydrolysis | Protect lutein from massive degradation or loss in the simulated artificial gastric fluid environment and complete digestion in the simulated artificial intestinal fluid, allowing lutein to complete targeted, controlled release and digestion in the intestine | [55] |
| Biological accessibility | Alginate | Compared to direct dietary uptake of lutein, the Zein carrier significantly increased lutein bioavailability, cellular uptake, and basolateral secretion | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, H.; Chang, Y.; Jiao, Y. Recent Advances in Efficient Lutein-Loaded Zein-Based Solid Nano-Delivery Systems: Establishment, Structural Characterization, and Functional Properties. Foods 2024, 13, 2304. https://doi.org/10.3390/foods13142304
Han H, Chang Y, Jiao Y. Recent Advances in Efficient Lutein-Loaded Zein-Based Solid Nano-Delivery Systems: Establishment, Structural Characterization, and Functional Properties. Foods. 2024; 13(14):2304. https://doi.org/10.3390/foods13142304
Chicago/Turabian StyleHan, He, Ying Chang, and Yan Jiao. 2024. "Recent Advances in Efficient Lutein-Loaded Zein-Based Solid Nano-Delivery Systems: Establishment, Structural Characterization, and Functional Properties" Foods 13, no. 14: 2304. https://doi.org/10.3390/foods13142304
APA StyleHan, H., Chang, Y., & Jiao, Y. (2024). Recent Advances in Efficient Lutein-Loaded Zein-Based Solid Nano-Delivery Systems: Establishment, Structural Characterization, and Functional Properties. Foods, 13(14), 2304. https://doi.org/10.3390/foods13142304






