Antispasmodic Activity of Light-Roasted Coffee Extract and Its Potential Use in Gastrointestinal Motility Disorders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials
2.3. High-Performance Liquid Chromatography (HPLC) Analysis
2.4. Animals
2.5. Experimental Protocol
2.6. Statistical Analysis
3. Results
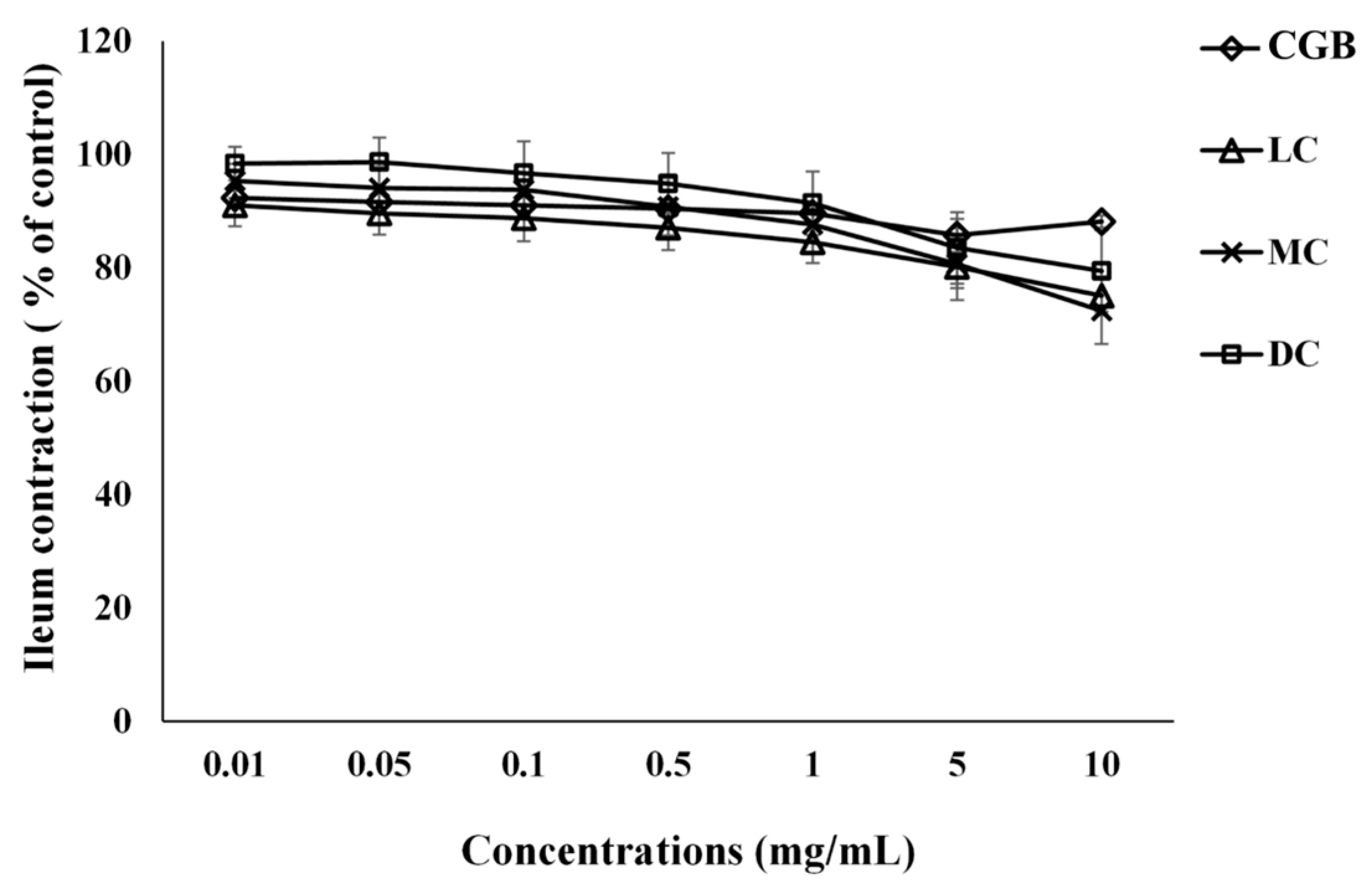
3.1. The Effect of Coffee Bean Extracts on Ileum Contraction Induced by KCl
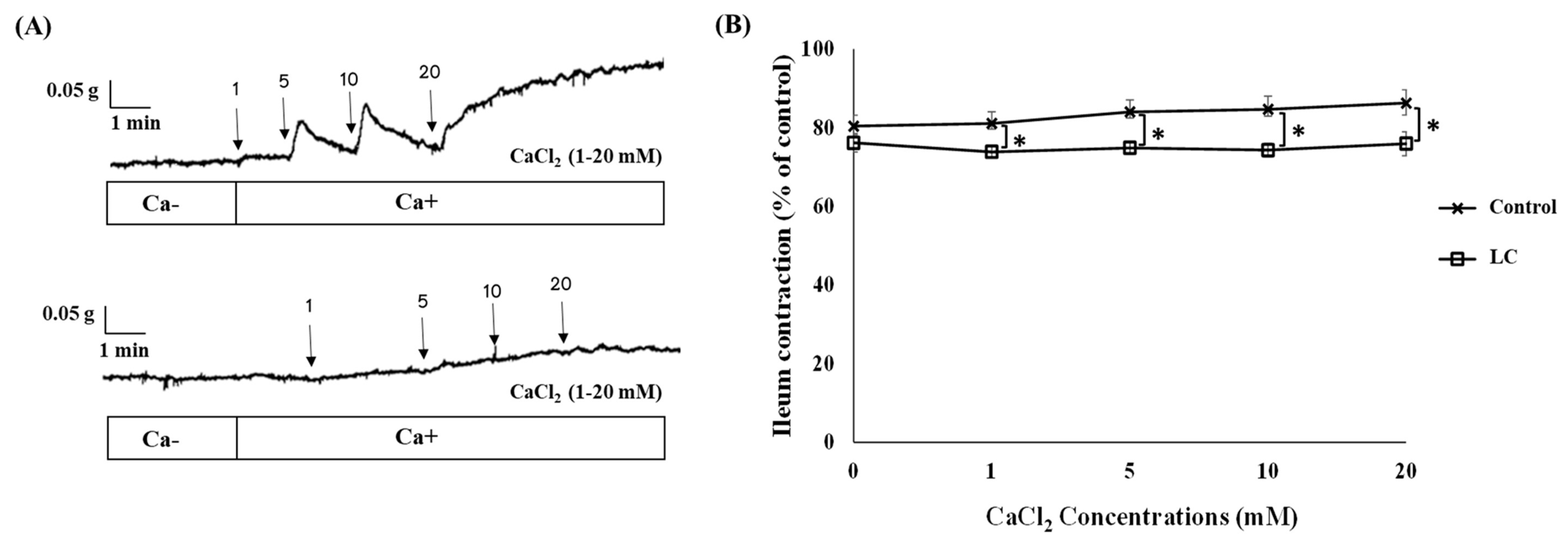
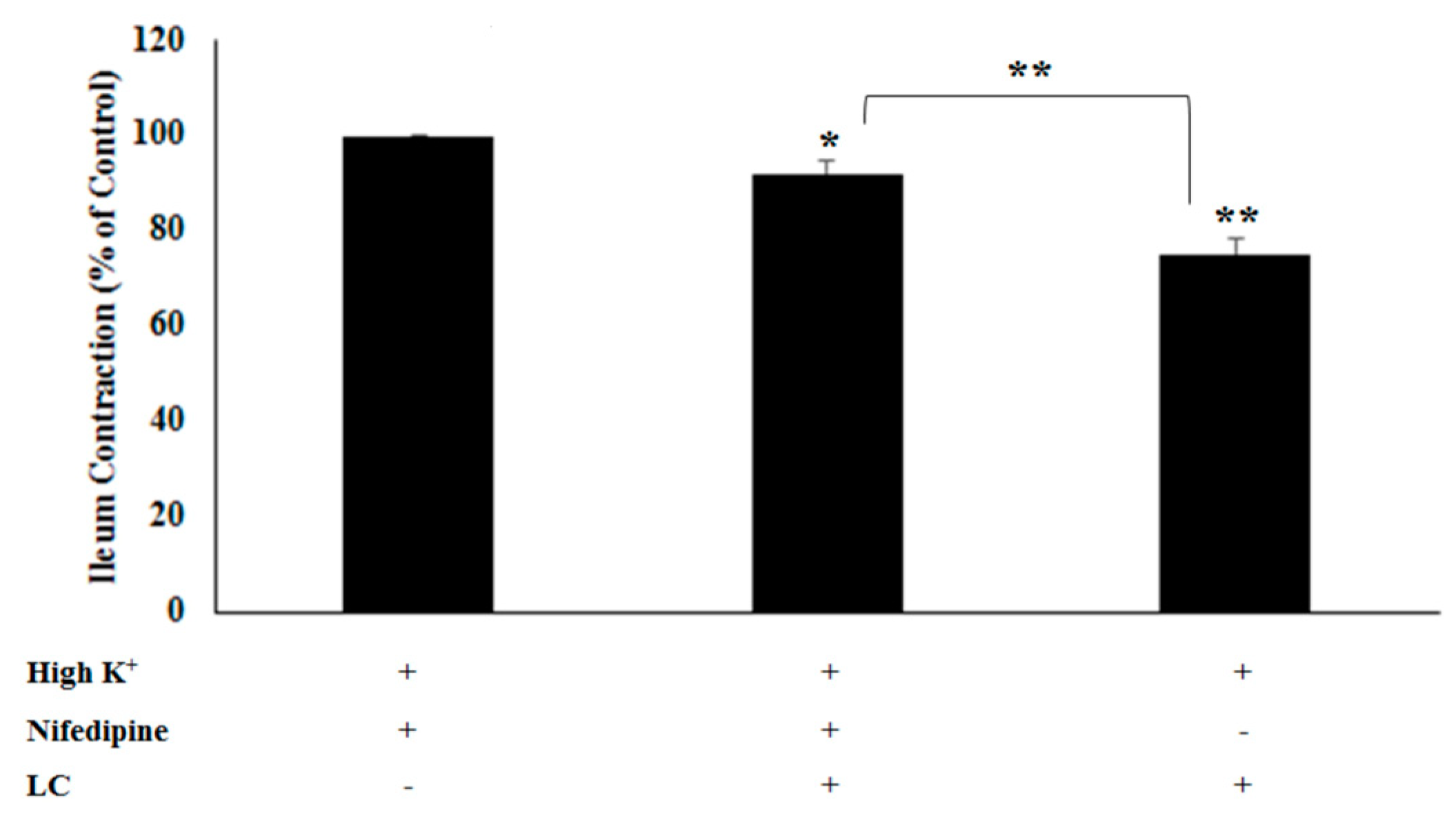
3.2. The Antispasmodic Activity of LC Extract in the Presence of CaCl2 and Nifedipine
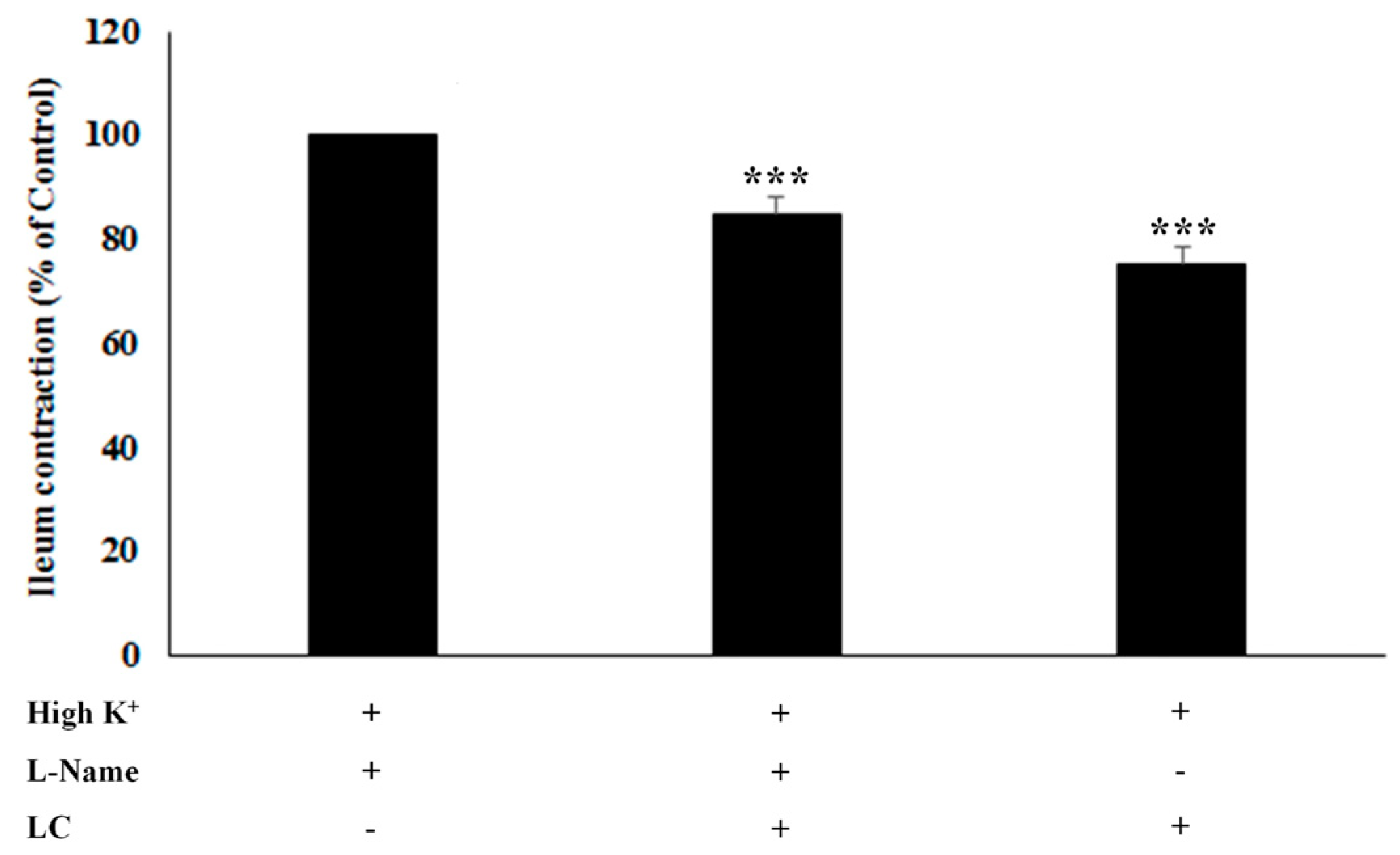
3.3. The Antispasmodic Activity of LC Extract in the Presence of Nitric Oxide Synthase [NOS] Inhibitor
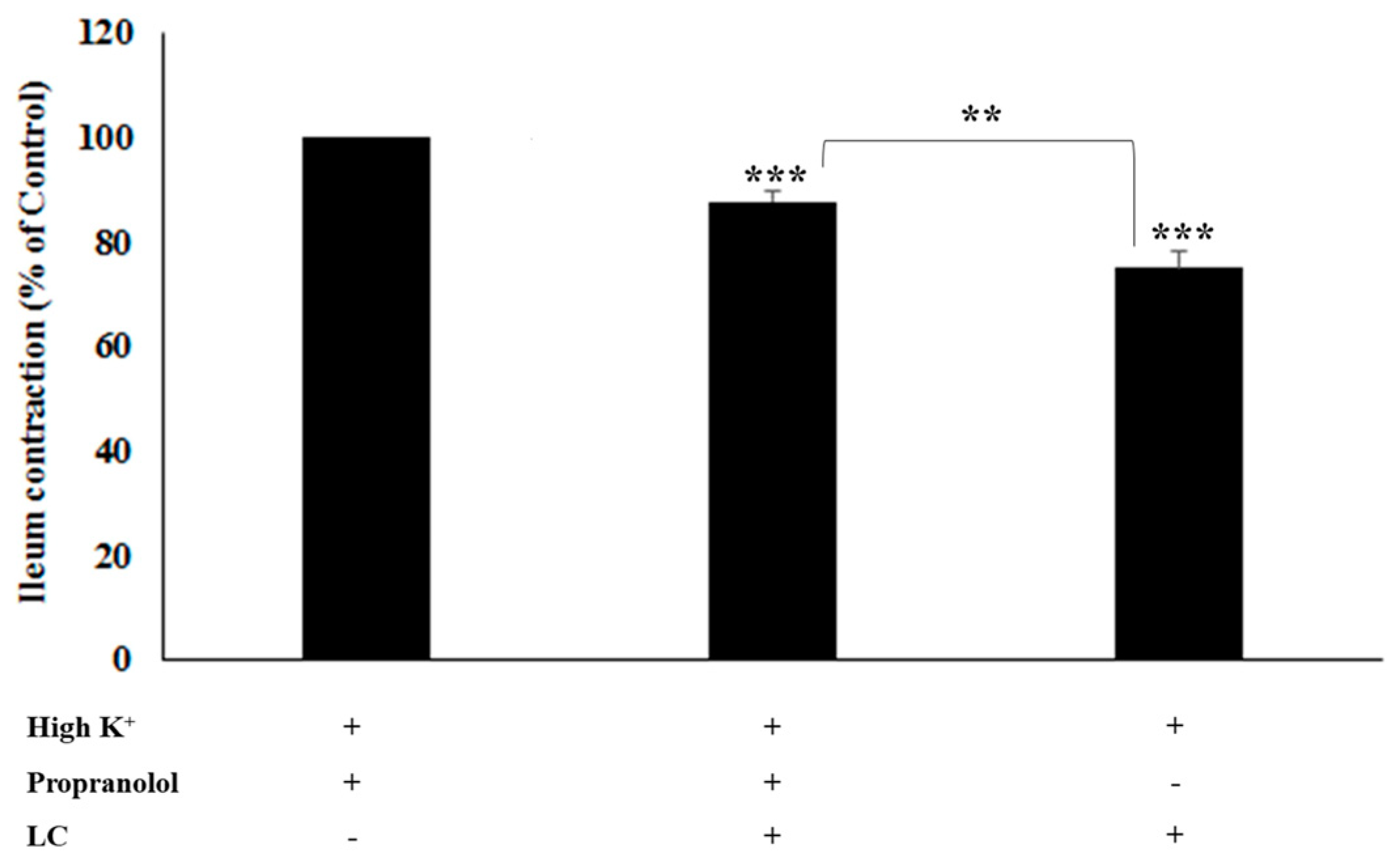
3.4. The Antispasmodic Activity of LC Extract in the Presence of β-Adrenergic Antagonist
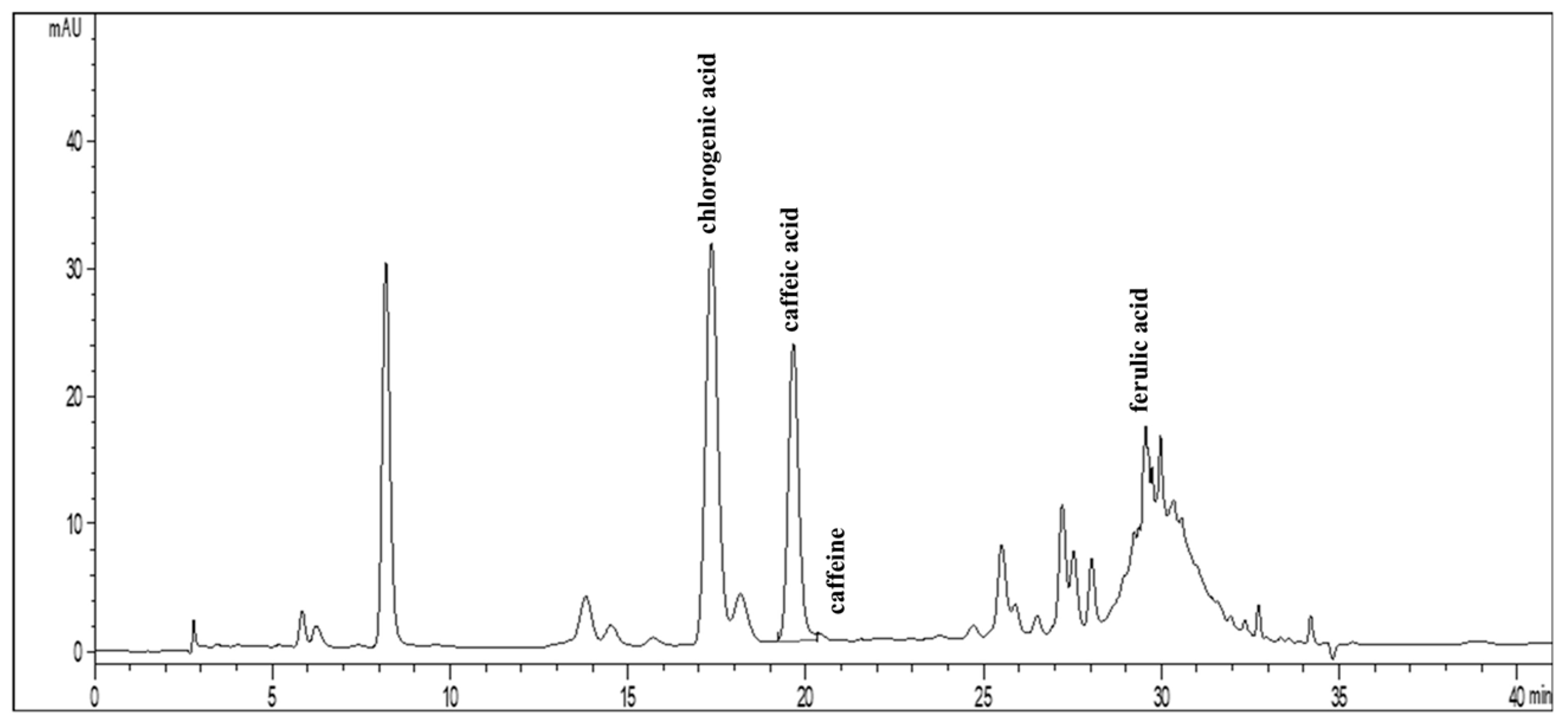
3.5. Determination of Active Compounds in Coffee Bean Extract by HPLC
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, V.A.; Ovalle Hernández, A.F. Rol de los antiespasmódicos en el manejo del síndrome de intestino irritable. Rev. Colomb. Gastroenterol. 2019, 34, 269–276. [Google Scholar] [CrossRef]
- Heghes, S.C.; Vostinaru, O.; Rus, L.M.; Mogosan, C.; Iuga, C.A.; Filip, L. Antispasmodic Effect of Essential Oils and Their Constituents: A Review. Molecules 2019, 24, 1675. [Google Scholar] [CrossRef] [PubMed]
- Bundy, R.; Walker, A.F.; Middleton, R.W.; Marakis, G.; Booth, J.C. Artichoke leaf extract reduces symptoms of irritable bowel syndrome and improves quality of life in otherwise healthy volunteers suffering from concomitant dyspepsia: A subset analysis. J. Altern. Complement. Med. 2004, 10, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pérez, E.F.; Juárez, Z.N.; Hernández, L.R.; Bach, H. Natural Antispasmodics: Source, Stereochemical Configuration, and Biological Activity. Biomed Res. Int. 2018, 2018, 3819714. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Uranga, J.A.; Del Castillo, M.D.; Abalo, R. Effects of coffee and its components on the gastrointestinal tract and the brain–gut axis. Nutrients 2020, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.L.; Wang, X.; Zhang, L.; Qiu, M.H. The sources and mechanisms of bioactive ingredients in coffee. Food Funct. 2019, 10, 3113–3126. [Google Scholar] [CrossRef] [PubMed]
- Tazzeo, T.; Bates, G.; Roman, H.N.; Lauzon, A.M.; Khasnis, M.D.; Eto, M.; Janssen, L.J. Caffeine relaxes smooth muscle through actin depolymerization. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 303, L334–L342. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, C.; Yamamoto, H.; Hirano, K.; Kobayashi, S.; Kanaide, H. Mechanisms of caffeine-induced contraction and relaxation of rat aortic smooth muscle. J. Physiol. 1992, 456, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, T.; Sasaki, N.; Urakawa, N.; Shimizu, K. Effects of chlorogenic acid on carbachol-induced contraction of mouse urinary bladder. J. Pharmacol. Sci. 2018, 136, 26–30. [Google Scholar] [CrossRef]
- Herawati, D.; Giriwono, P.E.; Dewi, F.N.A.; Kashiwagi, T.; Andarwulan, N. Critical roasting level determines bioactive content and antioxidant activity of Robusta coffee beans. Food Sci. Biotechnol. 2019, 28, 7–14. [Google Scholar] [CrossRef]
- Duangjai, A.; Goh, B.H.; Lee, L.H.; Saokaew, S. Relaxant effects of Azadirachta indica A. Juss var. siamensis Valeton flower extract on isolated rat ileum contractions and the mechanisms of action. J. Tradit. Complement. Med. 2018, 8, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Koh, S.D.; Ro, S.; Ward, S.M. Regulation of gastrointestinal motility—Insights from smooth muscle biology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Im, S.S.; Song, D.K.; Bae, J.H. Effects of chlorogenic acid on intracellular calcium regulation in lysophosphatidylcholine-treated endothelial cells. BMB Rep. 2017, 50, 323–328. [Google Scholar] [CrossRef]
- Missiaen, L.; Parys, J.B.; De Smedt, H.; Himpens, B.; Casteels, R. Inhibition of inositol trisphosphate-induced calcium release by caffeine is prevented by ATP. Biochem. J. 1994, 300, 81–84. [Google Scholar] [CrossRef]
- Martin, C.; Dacquet, C.; Mironneau, C.; Mironneau, J. Caffeine-induced inhibition of calcium channel current in cultured smooth cells from pregnant rat myometrium. Br. J. Pharmacol. 1989, 98, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Kochar, N.I.; Chandewal, A.V.; Bakal, R.L.; Kochar, P.N. Nitric Oxide and the Gastrointestinal Tract. Int. J. Pharmacol. 2011, 7, 31–39. [Google Scholar] [CrossRef]
- Lincoln, T.M.; Dey, N.; Sellak, H. Invited Review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: From the regulation of tone to gene expression. J. Appl. Physiol. 2001, 91, 1421–1430. [Google Scholar] [CrossRef]
- Tom, E.N.L.; Girard-Thernier, C.; Demougeot, C. The Janus face of chlorogenic acid on vascular reactivity: A study on rat isolated vessels. Phytomedicine 2016, 23, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Yamamoto, M.; Jokura, H.; Fujii, A.; Tokimitsu, I.; Hase, T.; Saito, I. Ferulic acid restores endothelium-dependent vasodilation in aortas of spontaneously hypertensive rats. Am. J. Hypertens. 2007, 20, 508–513. [Google Scholar] [CrossRef]
- Ozaki, H.; Kasai, H.; Hori, M.; Sato, K.; Ishihara, H.; Karaki, H. Direct inhibition of chicken gizzard smooth muscle contractile apparatus by caffeine. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1990, 341, 262–267. [Google Scholar] [CrossRef]
- Echeverri, D.; Montes, F.R.; Cabrera, M.; Galán, A.; Prieto, A. Caffeine’s Vascular Mechanisms of Action. Int. J. Vasc. Med. 2010, 2010, 834060. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Horinouchi, T.; Koike, K. New insights into beta-adrenoceptors in smooth muscle: Distribution of receptor subtypes and molecular mechanisms triggering muscle relaxation. Clin. Exp. Pharmacol. Physiol. 2005, 32, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Boekema, P.J.; Samsom, M.; van Berge Henegouwen, G.P.; Smout, A.J. Coffee and gastrointestinal function: Facts and fiction. A review. Scand. J. Gastroenterol. Suppl. 1999, 230, 35–39. [Google Scholar] [CrossRef]
- Boekema, P.J.; Samsom, M.; Roelofs, J.M.; Smout, A.J. Effect of coffee on motor and sensory function of proximal stomach. Dig. Dis. Sci. 2001, 46, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Lien, H.C.; Chen, G.H.; Chang, C.S.; Kao, C.H.; Wang, S.J. The effect of coffee on gastric emptying. Nucl. Med. Commun. 1995, 16, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Welsh, C.; Pan, J.; Belik, J. Caffeine impairs gastrointestinal function in newborn rats. Pediatr. Res. 2015, 78, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Patay, É.B.; Bencsik, T.; Papp, N. Phytochemical overview and medicinal importance of Coffea species from the past until now. Asian Pac. J. Trop. Med. 2016, 9, 1127–1135. [Google Scholar] [CrossRef]
- Oztekin, Ç.V.; Gur, S.; Abdulkadir, N.A.; Kartal, M.; Karabakan, M.; Akdemir, A.O.; Gökkaya, C.S.; Cetinkaya, M. Analysis of pomegranate juice components in rat corpora cavernosal relaxation. Int. J. Impot. Res. 2014, 26, 45–50. [Google Scholar] [CrossRef]
| Concentration (mg/mL) | Ileal Contractions (% of Control) | |||
|---|---|---|---|---|
| CGB Mean ± SEM | LC Mean ± SEM | MC Mean ± SEM | DC Mean ± SEM | |
| Control | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 |
| 0.01 | 92.39 ± 1.25 (p < 0.001) | 91.05 ± 3.64 (p < 0.05) | 95.36 ± 3.01 | 98.40 ± 2.96 |
| 0.05 | 91.62 ± 1.42 (p < 0.001) | 89.60 ± 3.72 (p < 0.05) | 94.12 ± 3.81 | 98.66 ± 4.33 |
| 0.1 | 91.05 ± 1.42 (p < 0.001) | 88.78 ± 4.05 (p < 0.05) | 93.81 ± 4.24 | 96.70 ± 5.63 |
| 0.5 | 90.48 ± 1.41 (p < 0.001) | 87.07 ± 3.88 (p < 0.005) | 90.80 ± 5.06 | 94.91 ± 5.34 |
| 1 | 89.62 ± 1.34 (p < 0.001) | 84.57 ± 3.68 (p < 0.001) | 87.67 ± 5.26 (p < 0.05) | 91.43 ± 5.59 (p < 0.05) |
| 5 | 85.76 ± 2.90 (p < 0.001) | 80.18 ± 3.73 (p < 0.001) | 80.73 ± 6.35 (p < 0.05) | 83.55 ± 6.30 (p < 0.005) |
| 10 | 88.20 ± 1.07 (p < 0.001) | 75.12 ± 3.42 (p < 0.001) | 72.43 ± 5.81 (p < 0.001) | 79.50 ± 7.19 (p < 0.005) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duangjai, A.; Rawangkan, A.; Yosboonruang, A.; Ontawong, A.; Saokaew, S.; Goh, B.-H.; Suganuma, M.; Phisalprapa, P. Antispasmodic Activity of Light-Roasted Coffee Extract and Its Potential Use in Gastrointestinal Motility Disorders. Foods 2024, 13, 2307. https://doi.org/10.3390/foods13152307
Duangjai A, Rawangkan A, Yosboonruang A, Ontawong A, Saokaew S, Goh B-H, Suganuma M, Phisalprapa P. Antispasmodic Activity of Light-Roasted Coffee Extract and Its Potential Use in Gastrointestinal Motility Disorders. Foods. 2024; 13(15):2307. https://doi.org/10.3390/foods13152307
Chicago/Turabian StyleDuangjai, Acharaporn, Anchalee Rawangkan, Atchariya Yosboonruang, Atcharaporn Ontawong, Surasak Saokaew, Bey-Hing Goh, Masami Suganuma, and Pochamana Phisalprapa. 2024. "Antispasmodic Activity of Light-Roasted Coffee Extract and Its Potential Use in Gastrointestinal Motility Disorders" Foods 13, no. 15: 2307. https://doi.org/10.3390/foods13152307







